1. Introduction
Chagas disease (CD), a neglected tropical parasitic disease caused by the protozoan
Trypanosoma cruzi, is endemic in 21 Latin American countries. Due to increased migration, it is now also commonly detected in non-endemic areas, making CD a global issue [
1,
2]. In Brazil, it is estimated that 1.9 to 4.6 million people are infected every year, translating to a prevalence of 1.0 to 2.4% of the population [
3]. Approximately 30% of those infected with
T. cruzi will have cardiac and/or digestive complications [
4].
The only drugs prescribed for the etiological treatment of chagasic patients are the nitroheterocyclic compounds nifurtimox (BAYER, Germany) and benznidazole (LAFEPE, Brazil). These drugs have a very limited effectiveness, particularly in the chronic phase of the disease, in which the cure depends on the susceptibility of the etiological agent, the stage of the disease and the physiology of the host. In addition, they have undesirable side effects that often lead to treatment interruption [
5]. Despite the incomplete understanding of the mechanisms that lead to the pathogenesis of CD, there has been a growing consensus that the chagasic pathology is strictly dependent on the presence of the parasite in affected organs and that the demonstrated autoimmunity is a consequence of the tissue destruction caused by the infection [
6]. All these facts have led to an increased interest in discovering new drugs and better treatments for CD, evidenced by several clinical trials using benznidazole, nifurtimox or new compounds that have been launched in recent years [
1,
7].
Chagas disease is a highly neglected disease and, as such, arouses little interest in the pharmaceutical industry, which hampers the development of new chemotherapeutic agents. It is thus an urgent task to search for more efficient and less toxic compounds that can be used in the etiological treatment of this important disease. Guanidine compounds have been previously studied due to their great biological potential with a broad spectrum of action against several protozoan parasites such as
Trypanosoma brucei,
T. cruzi,
Plasmodium falciparum and
Leishmania spp. Guanidines constitute a broad class of compounds widely found in nature, where they are found in microorganisms, fungi, plants, and animals. Guanidines have already demonstrated several biological activities, such as anti-inflammatory [
8,
9], antibacterial [
10,
11], antifungal [
12], and antiprotozoal effects [
13,
14,
15,
16,
17], and have even been studied for use in diabetes [
18]. It was demonstrated that guanidines act as cysteine protease inhibitors with potential for the treatment of CD [
19,
20].
The present study reports the synthesis of a series of guanidine compounds, followed by in vitro trypanocidal and cytotoxicity evaluations performed following the criteria established by the Fiocruz Program for Research and Technological Development on Chagas Disease (PIDC/Fiocruz) and the Drugs for Neglected Diseases initiative (DNDi) for the identification of lead compounds to be used in the development of new therapies for CD [
21].
2. Results and Discussion
The synthesis and chemical characterization of the compounds LQOF-G1 to LQOF-G8 has been reported in detail in a previous study [
16]. The new compound, LQOF-G29, was characterized by HRESI-(+)MS, HPLC with UV/Vis detection, and NMR.
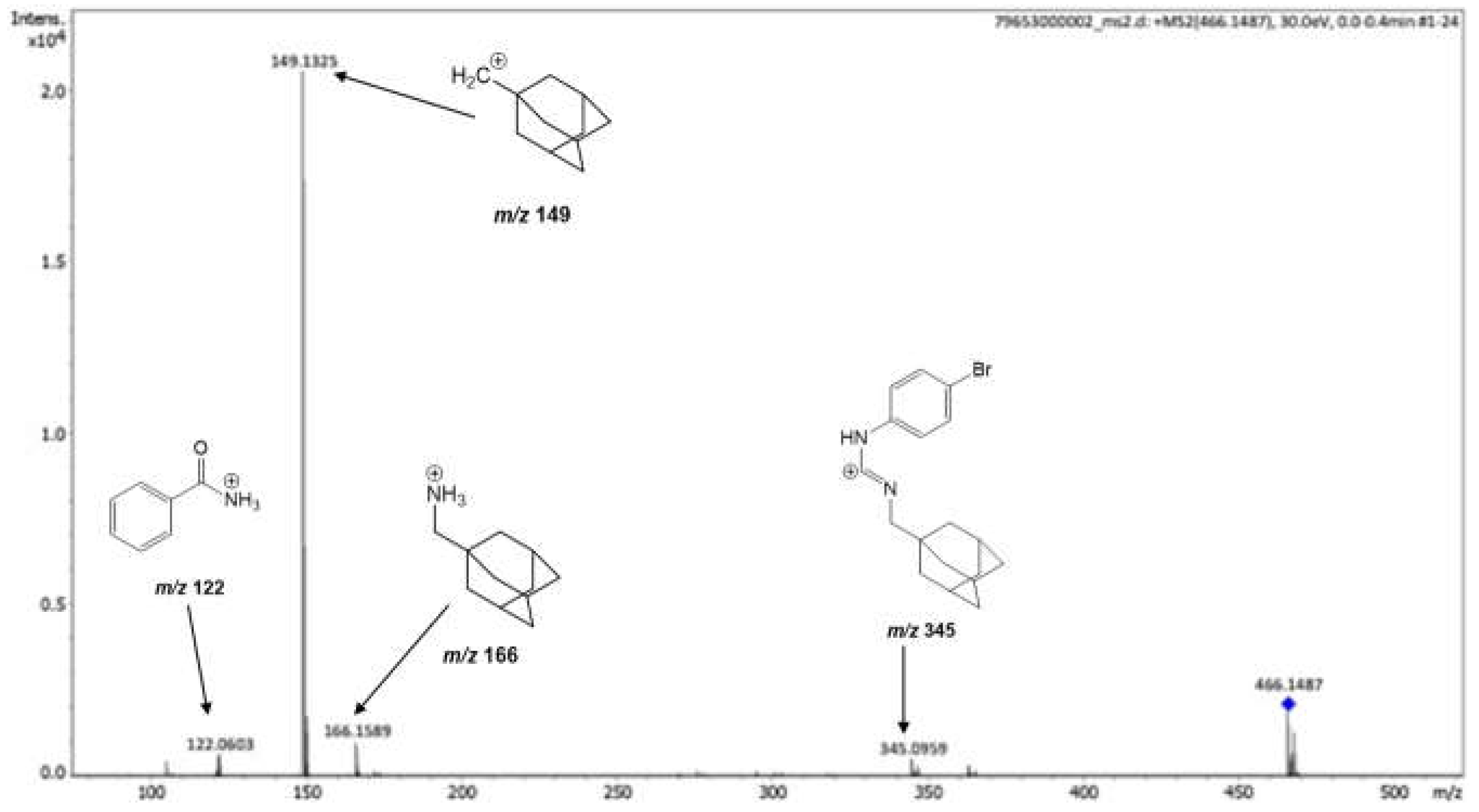
In the HRESI-(+) MS analysis, the guanidine LQOF-G29 was identified and confirmed by the protonated molecule, which was selected and further fragmented via HRESI(+)–MS/MS experiments (see also Supplementary material). The collision-induced fragmentation of the protonated guanidine yielded fragment ions ions at
m/z 122.0603,
m/z 149.1325,
m/z 166.1589 and
m/z 345.0959 (
Figure 1). The most abundant fragment ion was at
m/z 149.1325, corresponding to the 1-methyl adamantane carbocation. The ions at
m/z 122.0603 and
m/z 166.1589 are attributed to protonated benzamide and adamantanemethylamine, respectively.
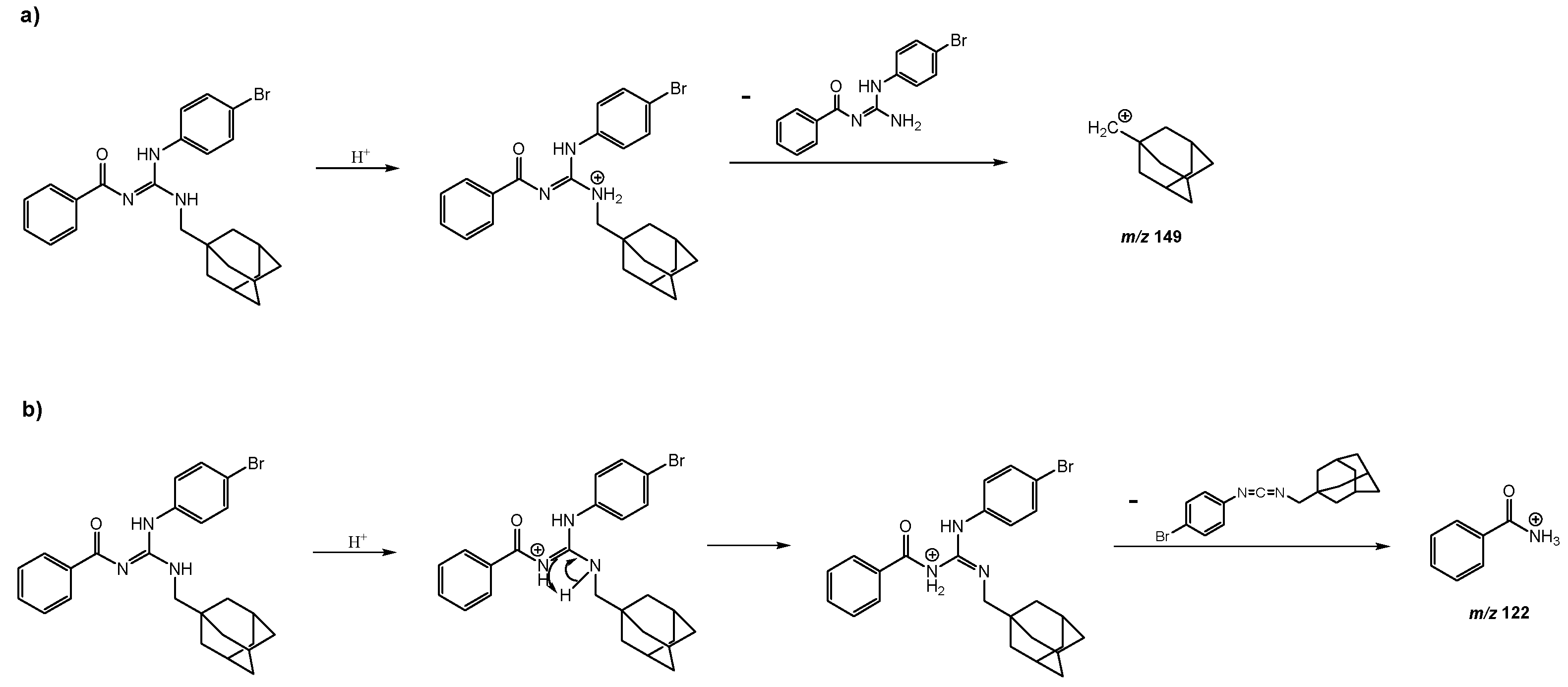
The proposed mechanisms for the formation of the fragment ions with
m/z 149 and
m/z 122 are presented below (
Scheme 1) [
14].
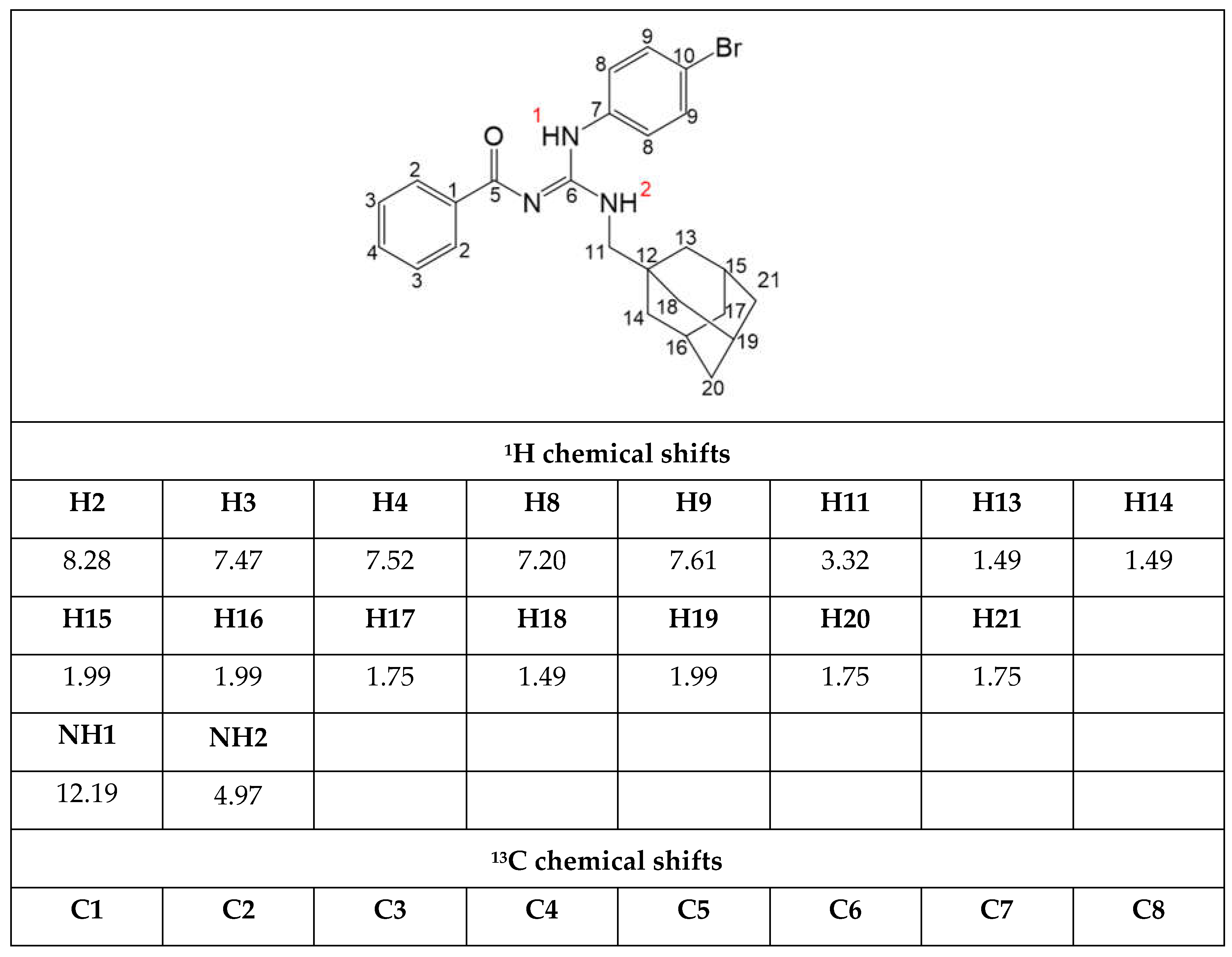
Furthermore, analyses of NMR and HPLC-UV/Vis data of the new compound
LQOF-G29 were performed, confirming the structure and yielding an HPLC purity of 99.73%. NMR studies were performed at 243 K, all NMR spectra are placed in supplementary material and the most relevant data is highlighted in
Table 1.
NMR analyses showed the signals corresponding to all protons and carbon atoms of the LQOF-G29 structure. The 1H signals of the two NH hydrogens observed at around 12 and 5 ppm suggested the preferential Z conformation of this compound. The signal of the aliphatic methylenic hydrogens (H11), was observed at 3.3 ppm and signals of the aliphatic hydrogens of the adamantyl group were observed in the 1.2 to 2.1 ppm region. Aromatic hydrogens resonance was observed in the region of 7.0 to 8.5 ppm as expected.
For the 13C NMR spectra, the DEPTq pulse sequence was used, differentiating primary and tertiary from secondary and quaternary carbons. Therefore, the aliphatic carbon with a negative phase observed at 52.4 ppm was attributed to N-bound C11. The adamantyl carbons were detected between 27.8 and 40.1 ppm, differentiated by the positive (CH) and negative (CH2) phases. Signals from all remaining carbons were observed between 120.3 and 177.3 ppm. Overall, the NMR analyses fully confirmed the structure and Z conformation of compound LQOF-G29.
To assess the anti-
T. cruzi activity of all six investigated guanidines, we used an enzymatic phenotypic assay that evaluates the effects on both amastigote and trypomastigote forms simultaneously. This better simulates the conditions encountered in vertebrate hosts. The toxicity of the compounds on the L929 host cells was determined using alamarBlue, a fluorometric/colorimetric indicator of cell viability. Based on the IC
50 values on the parasites and the CC
50 values on the L929 cells values, the selectivity index (SI) of each compound was determined. This index reflects how much stronger each compound is active against the parasite without inducing toxicity towards the host cell. The IC
50 values on
T. cruzi, CC
50 values on L929 cells, and resulting selectivity index of the different guanidines are shown in
Table 2.
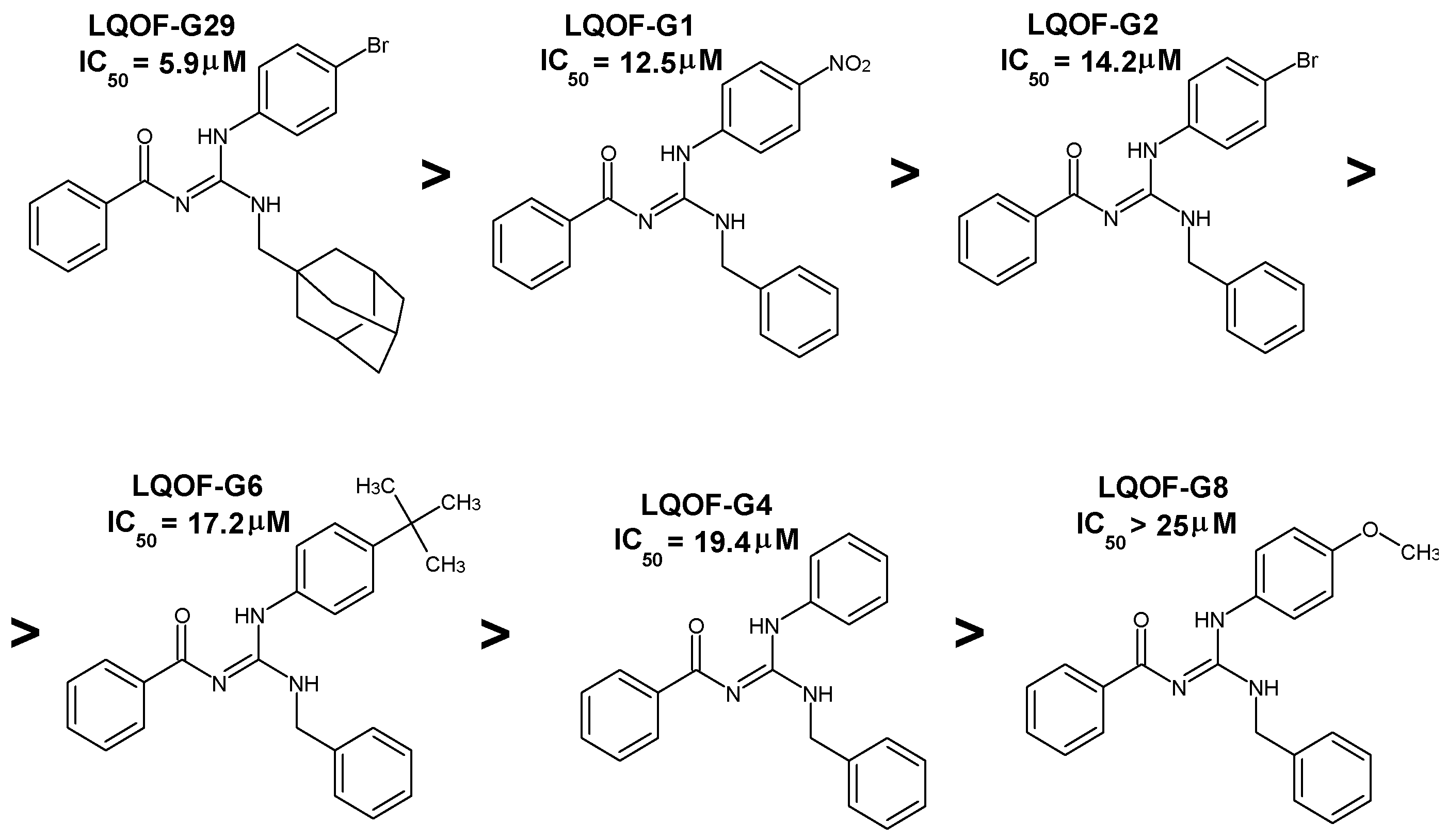
Amongst the 6 compounds tested, five displayed significant trypanocidal activity with IC
50 values in the range of 5.8 to 20 μM. However, none of the guanidines was more active than the reference drug benznidazole (IC
50 = 3.0 ± 1.4 μM). Since a compound should show a selective index (SI) equal or greater than 50 to be considered promising for treatment of CD [
21], only guanidine LQOF-G1 meets the criteria for in vivo tests. However, since LQOF-G29 was the most active guanidine (IC
50 = 5.8 ± 1.0 μM) and still showed an SI of 31.8, we decided to include it in the in vivo assessment. The very low SIs of the remaining guanidines (2.4, 5.1 and >6.3) preclude its use in in vivo assays.
In a previous study, LQOF-G1, LQOF-G2, and LQOF-G6 were evaluated against
Leishmania (Viannia) braziliensis [
15], along with other tri-substituted guanidines. LQOF-G1 showed a high activity against
L. braziliensis strains (IC
50-promastigote= 4.6 ± 0.5 and IC
50-amastigote= 3.6 ± 0.2) [
15]. Moreover, LQOF-G1 was shown to promote an increase in pro-inflammatory cytokines in the Th1 response and a decrease in anti-inflammatory cytokines in the Th2 response, thus inducing a host-protective cytokine response. It also stimulated an increase in nitric oxide (NO) and reactive oxygen species (ROS) levels, which are assumed to contribute to the parasite death [
22].
For
T. cruzi strains, it is known that the toll-like receptors (TLRs) recognize the parasite [
23]. TLRs play an important role in the host’s defense by activing the innate immune system and stimulating the Th1 response, i.e., activating cytokine production (IFN-γ, TNF-α and IL-12). In this way, the LQOF-G1 activity against
T. cruzi can be also associated with an increase in pro-inflammatory cytokines [
15,
23].
Furthermore, it should be highlighted that LQOF-G1, as benznidazole (BZN) and nifurtimox, has a nitro group and likely acts by a similar mechanism. BZN and nifurtimox are nitroheterocycles and prodrugs that need to be activated by nitro reductase enzymes of
T. cruzi [
24,
25,
26]. For BZN, the most accepted mechanism involves the conversion of the nitro group to the amine, whereby a radical intermediate (R-NO
2-) is produced that reacts with macromolecules of the parasite such as DNA, lipids and proteins, causing a toxic effect against
T. cruzi [
27].
Although LQOF-G1 presented the highest SI value (66.7), the most active compound was LQOF-G29 (IC
50 = 5.8 ± 1.0 μM), which has no nitro but a bromine and a methyladamantane group in the structure. According to
Table 2, the order of activity is:
LQOF-G29 >
LQOF-G1 >
LQOF-G2 >
LQOF-G6 >
LQOF-G4 >
LQOF-G8.
The bulky adamantyl group, characterized by its hydrophobic nature, can restrict or modulate intramolecular reactivity [
28,
29,
30,
31,
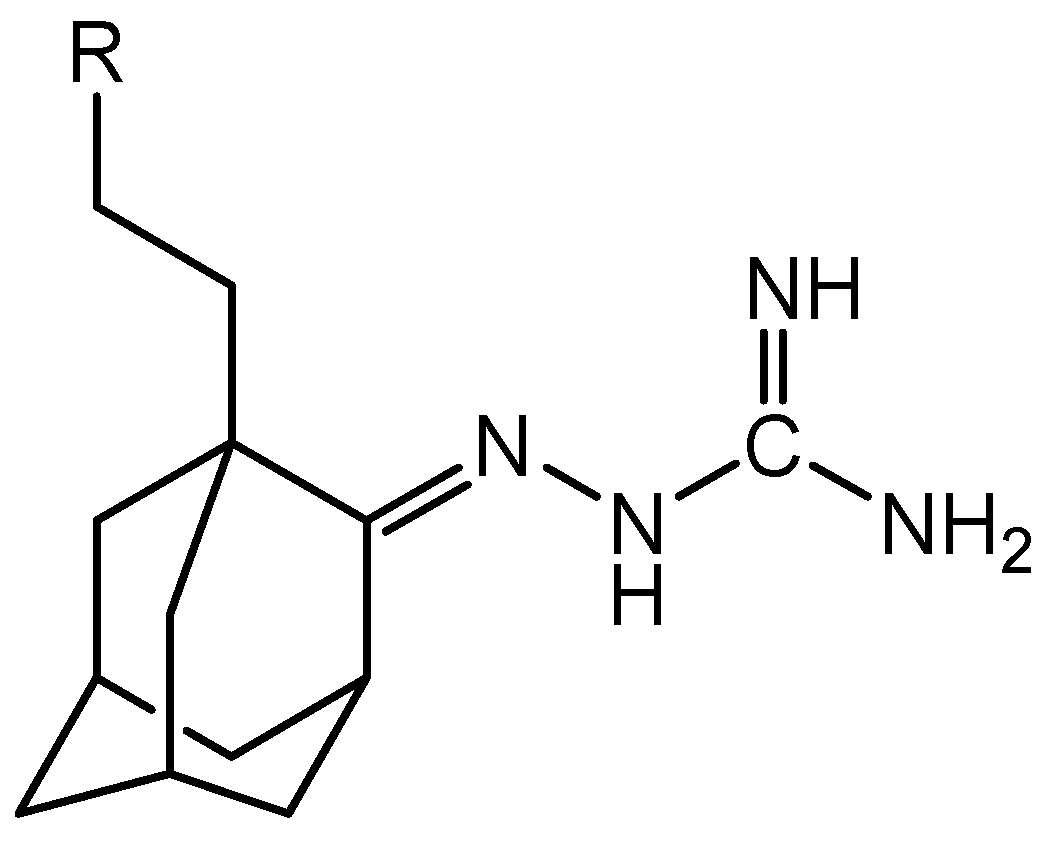
32]. Studies referring to this class of compounds highlight lipophilicity as an important property for the transfer of the active compound across the cell membrane. In 2008, researchers published a monosubstituted guanidine with an adamantylamine group (
Figure 3), which showed antitrypanosomal activity with an IC
50 of 0.09±0.02 μM and an IC
90 of 0.11±0.00 μM. This compound acts by blocking ion channels in the cell membrane [
30,
31].
It is noteworthy that benznidazole and nifurtimox have a low tolerability profile and a high rate of treatment interruption, due to adverse reactions that usually occur in less than 30 days [
33]. The most common side effects include cutaneous hypersensitivity, digestive disorders, fever, neurological disorders, depression, anxiety or insomnia, and dyspnea [
33]. LQOF-G29, because it does not have a nitro group, but rather an adamantly moiety, is a promising lead compound for the treatment of Chagas disease.
3. Experimental
3.1. Materials
Benzoylchloride, ammonium isothiocyanate, 4-nitroaniline, 4-bromoaniline, 4-tertbutylaniline, 4-fluoroaniline, adamantanemethylamine, 4-methoxyaniline, benzylamine, triethylamine, penta-hydrated bismuth nitrate. Solvents: N, N-dimethylformamide, acetonitrile, dichloromethane, petroleum ether, diethyl ether.
3.2. Synthesis
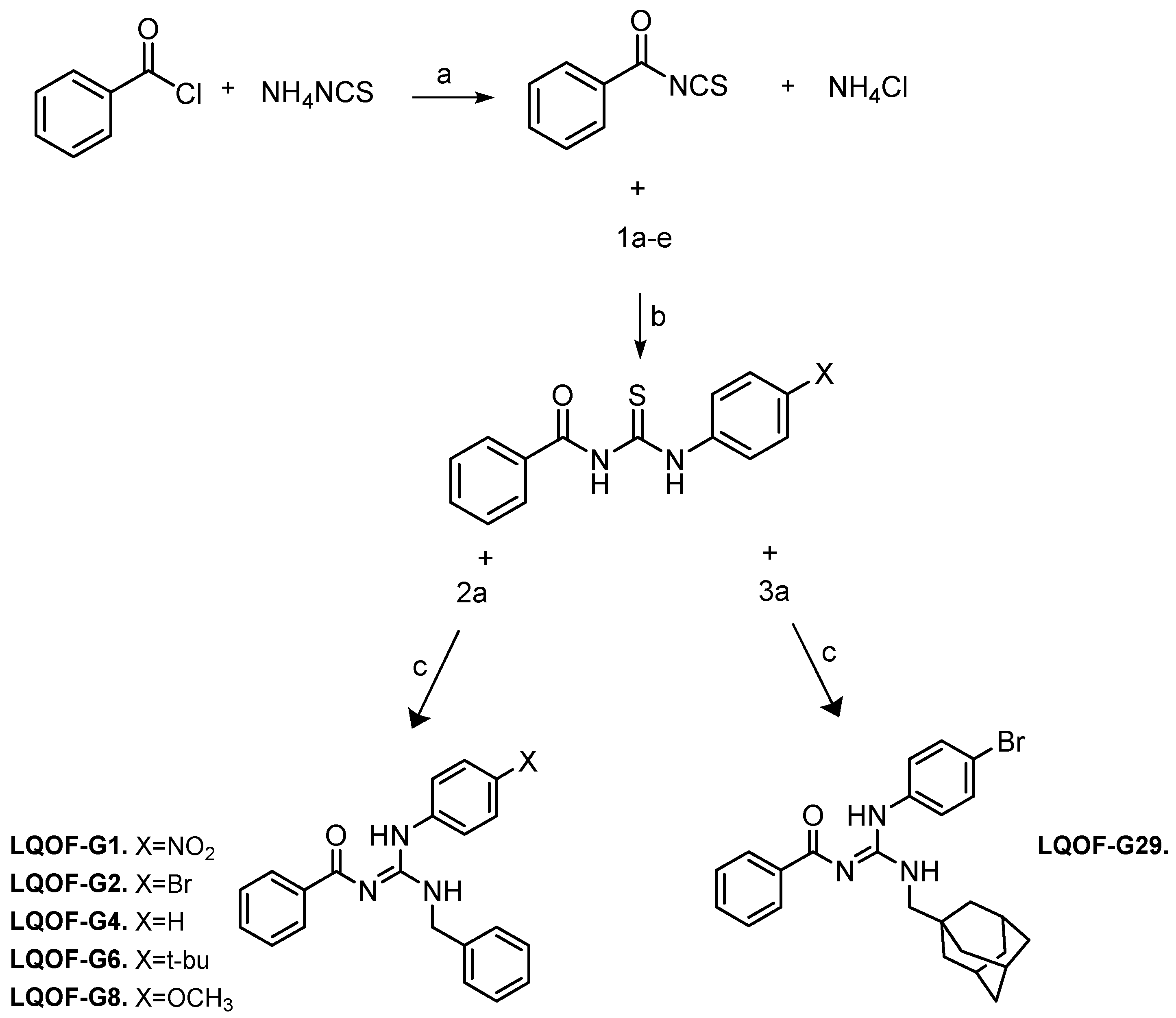
The synthesis of the compounds was performed following the previously reported method [
16,
34,
35]. The synthesis is first based on the preparation of the thiourea intermediate, which, after reacting with an aniline, forms a guanidine as the final product (
Scheme 2).
a) Into a round bottomed flask, containing a solution of ammonium isothiocyanate (0.76 g, 10 mmol) in acetonitrile (20mL), benzoyl chloride (1.40 g, 10 mmol) was added, and the resulting mixture was refluxed for 1h at a temperature of 85°C. Afterwards, the resulting solution was filtered off using a Buchner funnel with porous plate to remove the ammonium chloride (solid) from the filtrate of benzoylisothiocyanate.
b) The benzoylisothiocyanate solution was mixed with 10 mmol of the respective aniline and the reaction mixture was refluxed for 2h at 85°C. The product was filtered in a Buchner funnel and washed with cold acetonitrile to produce the thioureas. The reaction yield was about 90 to 95%, depending on the aniline.
c) Into a round bottom flask with 1 mmol of thiourea intermediate dissolved in 5 mL of N,N-dimethylformamide, 2 mmol of benzylamine or adamantanemethylamine, triethylamine (0.404g, 4 mmol) and Bi(NO3)3.5H2O (0.485g, 1 mmol) was added. The reaction mixture was heated for 24h at ~120°C. Then, the resulting suspension was filtered using a pad of celite and the pad was washed with 20 mL of dichloromethane. The polar impurities in the filtrate were removed by extraction with water (4 x 15 mL). The organic layer was dried over anhydrous MgSO4, filtered and the solvent evaporated. The crude residue was recrystallized from 4:1 Et2O/petroleum ether or hexane. The reaction yield was 62% for LQOF-G1, 85% for LQOF-G2, 89% for LQOF-G4, 65% for LQOF-G6, 85% for LQOF-G8 and 62% for LQOF-G29.
3.3. NMR Measurements
1H NMR spectra were obtained at 400.13 MHz. 13C NMR spectra were obtained at 100.61 MHz. Chemical shifts for 1H NMR and 13C NMR were referenced to TMS, analysis performed in CDCl3 and all NMR peaks were reported in ppm. Data are presented as follows: chemical shift, multiplicity (s = singlet, d = doublet, dd = doublet of doublet, t = triplet, qua = quadruplet, qu = quintuplet, m = multiplet, br s = broad singlet), integration, and coupling constants (in Hertz).
3.4. Electron Ionization (EI) Mass Spectrometry (MS)
The EI mass spectra of the guanidines were acquired by direct introduction (DI) into a Shimadzu QP-2010 Plus instrument equipped with an EI source. The parameters used for the analysis were: Interface temperature: 240°C; Ionization chamber: 300°C; Time to solvent cut; 0.5 min; Initial time: 0.7 min; Final time: 25 min; DI temperature program: Initial temperature: 50°C, with heating by 20°C/min to 350°C and standby time 10 min. The analysis was performed using acetonitrile and dichloromethane as a solvents. The energy used for ionization was 70 eV.
3.5. High-Resolution Electrospray Ionization (HRESI)-MS
HRESI-(+) MS spectra were obtained on a maXis UHR ESI-Qq-TOF mass spectrometer (Bruker Daltonics, Bremen, Germany). Samples were dissolved and directly infused into the ESI source at a flow rate of 3 μL/min with a syringe pump. The ESI ion source was operated as follows: capillary voltage: 4.5 kV, nebulizer: 0.4 bar (N2), dry gas flow: 4 L/min (N2), and dry temperature: 180 °C. Mass spectra were recorded in the range of m/z 50–1900 in the positive-ion mode. The sum formulas were determined using Bruker Compass DataAnalysis 4.1 based on the mass accuracy (Δm/z ≤ 5 ppm) and isotopic pattern matching (SmartFormula algorithm).
3.6. High-Performance Liquid Chromatography (HPLC) UV/Vis Analysis
The HPLC analyses were performed on an UltiMate 3000 series system equipped with a VWD detector (Dionex/Thermo Fisher Scientifc). Separation was carried out on an Acclaim 120 C18, 4.6×250 mm, 5 µm HPLC column using water and acetonitrile, both modified with 0.1% formic acid, as mobile phase A and B, respectively. The sample components were separated and eluted with a linear gradient from 10 to 90% B in 25 min followed by an isocratic column cleaning (5 min at 90% B) and re-equilibration step (9 min at 10% B). The flow rate was 0.75 mL/min and the column oven temperature was set to 25 °C. The purity was determined from the UV chromatogram (254 nm) as the ratio of the peak area of the compound to the total peak area (i.e., the sum of the areas of all peaks that were not present in the solvent blank).
3.7. Melting Point
The melting point was obtained using a WRS-2 Micro Processor Melting-point apparatus. The samples were placed in a capillary tube, and pre-heating and final ramp temperatures were selected as 60°C and 250°C, respectively. The heating rate used was 2.0°C/min.
3.8. Biological Assays
3.8.1. Compounds
The guanidine derivatives were dissolved in DMSO (stock solution, 20 mg/mL) and immediately added to the biological assays (trypanocidal or cytotoxicity assay) in different concentrations. The reference drug, benznidazole (Roche), were dissolved in DMSO at 0.4 mg/mL. The maximum final concentration of DMSO never exceeded 1%.
3.8.2. In Vitro Trypanocidal Activity Test of Compounds
This assay was performed as previously described [
36], with modifications [
21], using
T. cruzi (Tulahuen strain) expressing the
Escherichia coli β-galactosidase gene. Infective trypomastigote forms were obtained through culture in monolayers of mouse L929 fibroblasts in RPMI-1640 medium (pH 7.2-7.4), without phenol red (Gibco BRL), containing 10% foetal bovine serum and 2 mM glutamine. For the bioassay, 4,000 L929 cells in 80 μL of supplemented medium were added to each well of a 96-well microtiter plate. After an overnight incubation, 40,000 trypomastigotes in 20 μL were added to the cells and the cells are incubated for 2 h. Medium containing parasites that did not penetrate the cells was replaced with 200 μL of fresh medium and the plate was incubated for an additional 48 h to establish infection. The medium was then replaced with solutions of compounds at different concentrations in fresh medium (200 μL) and the plate was incubated for 96 h at 37°C. After this period, 50 μL of 500 μM CPRG (chlorophenol red beta-D-galactopyranoside) in 0.5% Nonidet P40 was added to each well and the plate was incubated for 18 h at 37°C, after which the absorbance at 570 nm was measured. Controls with uninfected cells, untreated infected cells, infected cells treated with benznidazole at 3.8 μM (positive control) or DMSO 1% were used. The results were expressed as the percentage of
T. cruzi growth inhibition in compound-tested cells as compared to the infected cells and untreated cells. The compound concentration that inhibits 50% of the growth of the amastigote and trypomastigote forms (IC
50) was determined. Quadruplicates were run in the same plate, and the experiments were repeated at least once.
3.8.3. In Vitro Cytotoxic Activity Test of Compounds over L929 Cell Line
For this bioassay, 4,000 L929 cells in 200 μL of RPMI-1640 medium (pH 7.2-7.4) (Gibco BRL) plus 10% foetal bovine serum and 2 mM glutamine were added to each well of a 96-well microtiter plate that was incubated for three days at 37°C [
21]. The medium was then replaced, and the cells were exposed to compounds at increasing concentrations starting at IC
50 value for
T. cruzi. After 96 h of incubation with the compounds, the alamarBlue (Invitrogen) was added and the absorbance at 570 and 600 nm was measured after 4-6 h. Controls with untreated and DMSO 1%-treated cells were run in parallel. The results were expressed as the percent difference in the reduction between treated and untreated cells using the manufacturer’s recommended equation. The compound concentration that inhibits 50% of the L929 cell viability (CC
50) was determined. Quadruplicates were run in the same plate and the experiments were repeated at least once.
3.8.4. IC50, CC50 and Selectivity Index Determinations and Statistical Analysis
Half maximum inhibitory (IC
50) and half maximum cytotoxicity concentration (CC
50) values were determined by linear interpolation [
37] using the Excel software (Microsoft Corporation, USA) and the selectivity index (SI) was calculated by the ratio of CC
50 L929 cells/ IC
50 T. cruzi. Then, using the GraphPad InSat 3.06 software, the values were compared by analysis of variance (ANOVA) followed by Tukey post-hoc test to perform the paired comparisons.
Author Contributions
Conceptualization, Policarpo Ademar Sales Junior, Silvane Maria Fonseca Murta, Predrag Kalaba, Gert Lubec and Eduardo Gonzalez; Data curation, Eduardo Zampieri, Alexandre Prado-Roller, Natalie Gajic, Martin Zehl, Anna Fabisikova, Luana Ribeiro dos Anjos and Luana Passianoto Gushiken; Formal analysis, Eduardo Zampieri, Alexandre Prado-Roller, Natalie Gajic, Martin Zehl, Anna Fabisikova, Luana Ribeiro dos Anjos and Luana Passianoto Gushiken; Funding acquisition, Policarpo Ademar Sales Junior, Silvane Maria Fonseca Murta and Eduardo Gonzalez; Investigation, Eduardo Zampieri, Natalie Gajic, Anna Fabisikova, Luana Ribeiro dos Anjos and Luana Passianoto Gushiken; Supervision, Eduardo Gonzalez; Writing – review & editing, Eduardo Zampieri, Policarpo Ademar Sales Junior, Silvane Maria Fonseca Murta, Martin Zehl, Luana Ribeiro dos Anjos and Eduardo Gonzalez.