Submitted:
23 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- evaluate peri-operative outcomes and late survival of AAAD repair
- compare limited versus extended resections.
2. Materials and Methods
Population
Operative Techniques
Statistical and Propensity Score Analysis
3. Results
3.1. Operative Results
3.2. Comment
- a)
- propensity matched in-hospital mortality and composite outcomes were similar for ER and LR.
- b)
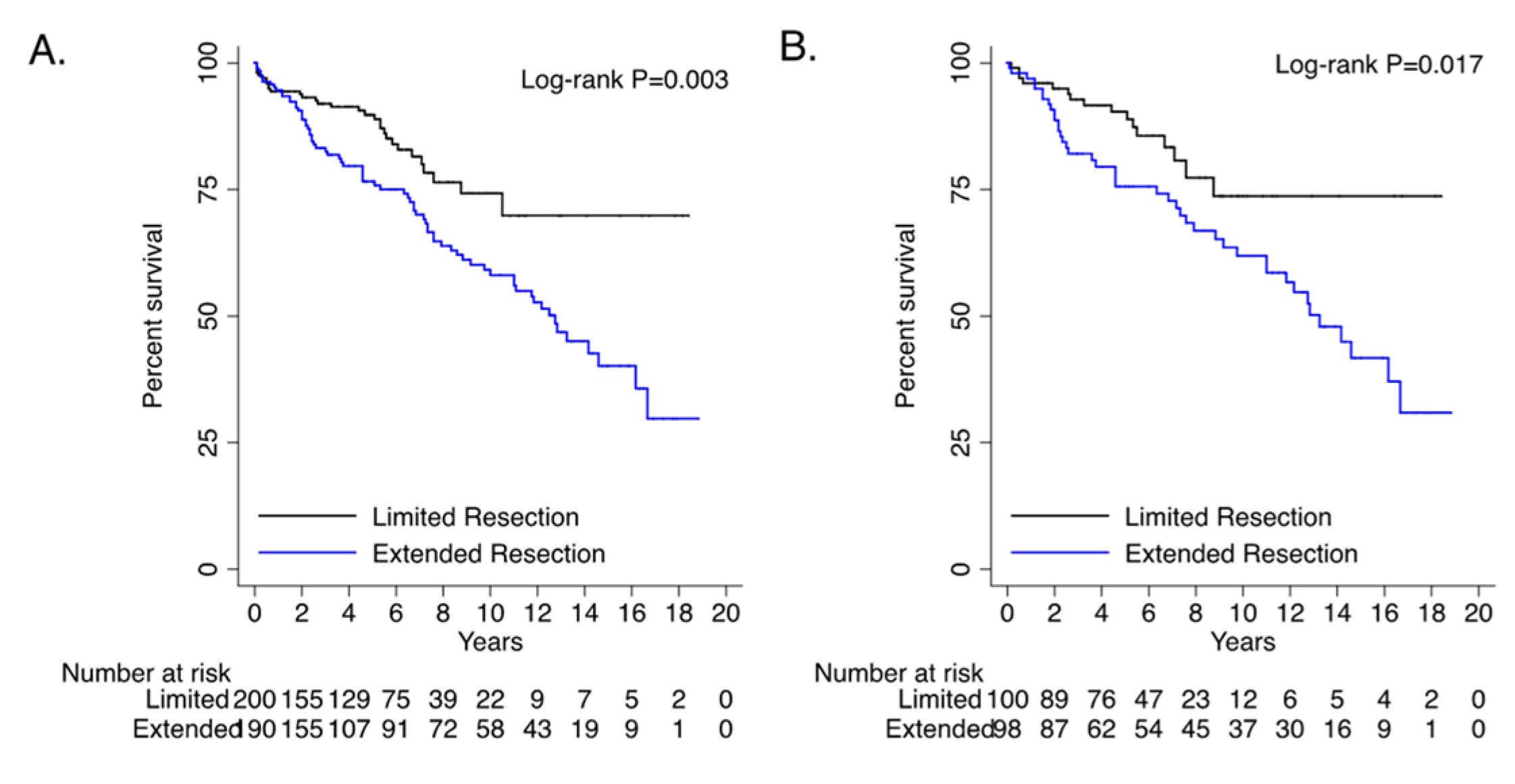
- long term survival for extended resections was worse than limited resections.
- c)
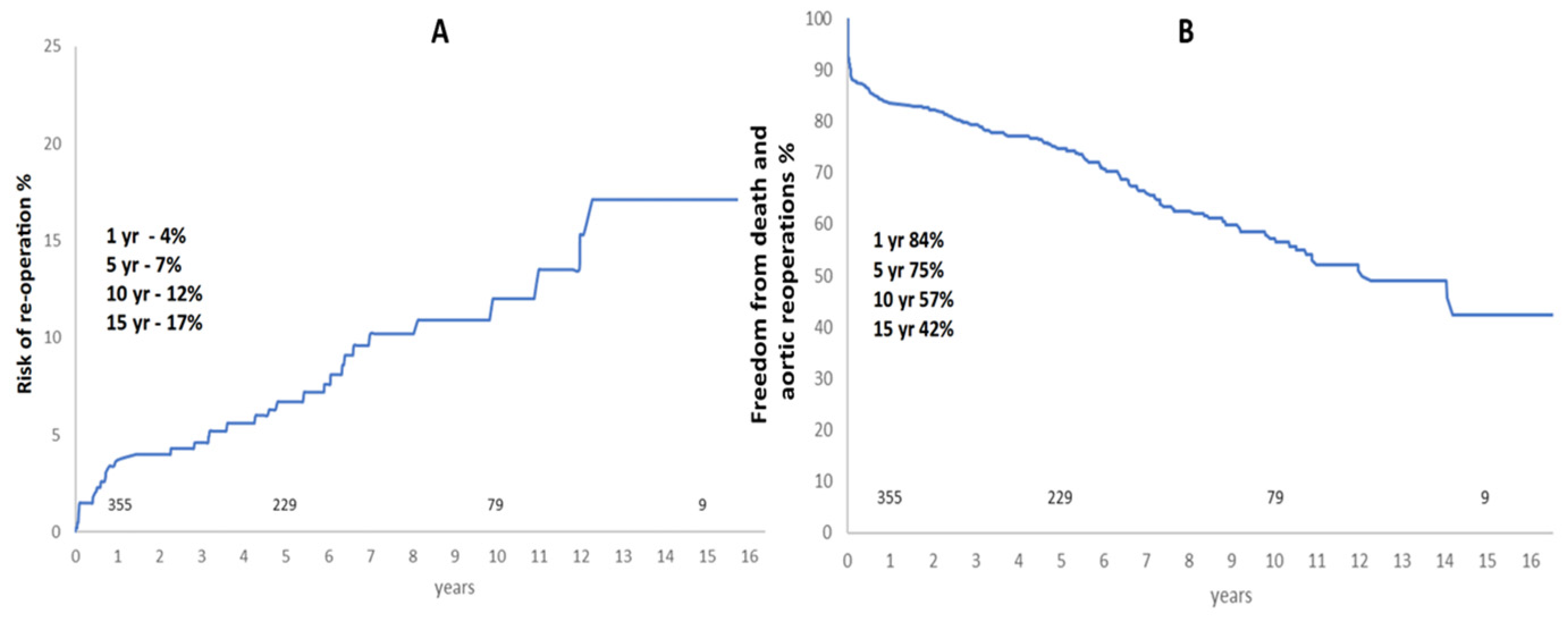
- risk of aortic re-operation was very low at 10% at 10 years and 17% at 15 years.
4. Limitations
5. Conclusions
6. Future Directions
Supplementary Materials
References
- Conzelmann LO, Weigang E, Mehlhorn U, Abugameh A, Hoffmann I, Blettner M, Etz CD, Czerny M, Vahl CF; GERAADA Investigators. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg. 2016, 49, e44–e52. [Google Scholar] [CrossRef] [PubMed]
- Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, Myrmel T, Larsen M, Harris KM, Greason K, Di Eusanio M, Bossone E, Montgomery DG, Eagle KA, Nienaber CA, Isselbacher EM, O’Gara P. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015, 66, 350–8. [Google Scholar]
- Malvindi PG, Modi A, Miskolczi S, Kaarne M, Velissaris T, Barlow C, Ohri SK, Tsang G, Livesey S. Open and closed distal anastomosis for acute type A aortic dissection repair. Interact Cardiovasc Thorac Surg. 2016, 22, 776–83. [Google Scholar] [CrossRef] [PubMed]
- Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, Ehrlich MP, Trimarchi S, Braverman AC, Myrmel T, Harris KM, Hutchinson S, O’Gara P, Suzuki T, Nienaber CA, Eagle KA; IRAD Investigators. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation. 2018, 137, 1846–1860. [Google Scholar] [CrossRef] [PubMed]
- Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A et al. Presentation, diagnosis, and outcome of acute aortic dissection: 17-year trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol, 2015; 66, 350–358.
- Reutersberg B, Salvermoser M, Trenner M, Geisbüsch S, Zimmermann A, Eckstein HH, Kuehnl A. Hospital Incidence and In-Hospital Mortality of Surgically and Interventionally Treated Aortic Dissections: Secondary Data Analysis of the Nationwide German Diagnosis-Related Group Statistics From 2006 to 2014. J Am Heart Assoc. 2019, 8, e011402.
- Abe T, Yamamoto H, Miyata H, Motomura N, Tokuda Y, Tanemoto K, Usui A, Takamoto S. Patient trends and outcomes of surgery for type A acute aortic dissection in Japan: an analysis of more than 10 000 patients from the Japan Cardiovascular Surgery Database. Eur J Cardiothorac Surg. 2020, 57, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi M, Sundt TM, Cameron DE, et al. Factors associated with acute stroke after type A aortic dissection repair: An analysis of the Society of Thoracic Surgeons National Adult Cardiac Surgery Database. J Thorac Cardiovasc Surg. 2020, 159, 2143–2154.e3. [Google Scholar] [CrossRef] [PubMed]
- Helder MRK, Schaff HV, Day CN, Pochettino A, Bagameri G, Greason KL, Lansman SL, Girardi LN, Storlie CB, Habermann EB. Regional and Temporal Trends in the Outcomes of Repairs for Acute Type A Aortic Dissections. Ann Thorac Surg. 2020, 109, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yoshitake A, Tochii M, Tokunaga C, Hayashi J, Takazawa A, Yamashita K, Chubachi F, Hori Y, Nakajima H, Iguchi A, Gatate Y, Nakano S, Asakura T. Early and long-term results of total arch replacement with the frozen elephant trunk technique for acute type A aortic dissection. Eur J Cardiothorac Surg. 2020, 58, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Smith HN, Boodhwani M, Ouzounian M, Saczkowski R, Gregory AJ, Herget EJ, Appoo JJ. Classification and outcomes of extended arch repair for acute Type A aortic dissection: a systematic review and metaanalysis. Interact Cardiovasc Thorac Surg. 2017, 24, 450–459. [Google Scholar]
- Luthra S, Tsang GM. Concurrent stabilization of “downstream” aorta during acute type A aortic dissection repair. J Thorac Cardiovasc Surg. 2023, 165, 586–588. [Google Scholar] [CrossRef] [PubMed]
- Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ; ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014, 35, 2873–926. [Google Scholar]
- Dib B, Seppelt PC, Arif R, Weymann A, Veres G, Schmack B, Beller CJ, Ruhparwar A, Karck M, Kallenbach K. Extensive aortic surgery in acute aortic dissection type A on outcome - insights from 25 years single center experience. J Cardiothorac Surg. 2019, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Lansman, SL. Commentary: Is more always better? J Thorac Cardiovasc Surg. 2019, 158, 1282. [Google Scholar] [CrossRef] [PubMed]
- Yan Y, Xu L, Zhang H, Xu ZY, Ding XY, Wang SW, Xue X, Tan MW. Proximal aortic repair versus extensive aortic repair in the treatment of acute type A aortic dissection: a meta-analysis. Eur J Cardiothorac Surg. 2016, 49, 1392–401. [Google Scholar] [CrossRef] [PubMed]
- Kim JH, Lee SH, Lee S, Youn YN, Yoo KJ, Joo HC. The Impact of a Reentry Tear After Open Repair of Nonsyndromic Acute Type I Aortic Dissection. Ann Thorac Surg. 2019 Dec 17. pii: S0003-4975(19)31876-4.
- Lin FY, Tseng YH, Huang JW, Hsieh CC, Chen HM, Chiu CC, Chen YF. Fate of distal aorta after acute type A aortic dissection repair: Change and persistency of postoperative false lumen status. Int J Cardiol. 2018, 266, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Halstead JC, Meier M, Etz C, Spielvogel D, Bodian C, Wurm M, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2007, 133, 127–35. [Google Scholar] [CrossRef] [PubMed]
- Kimura N, Itoh S, Yuri K, Adachi K, Matsumoto H, Yamaguchi A, Adachi H. Reoperation for enlargement of the distal aorta after initial surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg. 2015, 149, S91–S98. [Google Scholar] [CrossRef] [PubMed]
| UNMATCHED | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (n=440) |
Limited Resection (n=215) |
Extended Resection (n=225) | ||||||||||||
| n | % or IQR | N | % or IQR | N | % or IQR | P value | ||||||||
| Bypass time | 187 | [130-249] | 173 | [121-256] | 191 | [145.5-245.5] | 0.225 | |||||||
| Cross clamp time | 99 | [75-130] | 99 | [74-131] | 99 | [76-128] | 0.862 | |||||||
| Circulatory arrest time | 27 | [75-130] | 26 | [19-33] | 27 | [18-33.5] | 0.545 | |||||||
| LOS days[IQR] | 12 | [8-20] | 12 | [8-20] | 11 | [7-17] | 0.012 | |||||||
| 30-day survival | 390 | 88.6 | 200 | 93.0 | 190 | 84.4 | 0.005 | |||||||
| Composite outcome | 126 | 28.6 | 53 | 24.7 | 73 | 32.4 | 0.071 | |||||||
| Stroke/TIA | 81 | 18.4 | 35 | 16.3 | 46 | 20.4 | 0.474 | |||||||
| In-hospital death | 44 | 10.0 | 13 | 6.0 | 31 | 13.8 | 0.007 | |||||||
| Overall survival | 286 | 65.0 | 169 | 78.6 | 117 | 52.0 | <0.001 | |||||||
| LOS: length of stay, TIA: transient ischemic attack | ||||||||||||||
| PROPENSITY MATCHED | ||||||||||||||
|
Overall (n=218) |
Limited Resections (n=109) | Extended Resection (n=109) | ||||||||||||
| N | % or IQR | n | % or IQR | N | % or IQR | P value | ||||||||
| Bypass time | 188 | [125-249] | 172 | [119-254] | 192 | [129-240] | 0.828 | |||||||
| Cross clamp time | 97 | [76-130] | 97 | [76-130] | 97 | [78-132] | 0.994 | |||||||
| Circulatory arrest time | 26 | [17-35] | 26 | [17-36] | 27 | [18-34] | 0.848 | |||||||
| LOS days [IQR] | 12 | [8-18] | 12 | [9-20] | 11 | [8-17.5] | 0.537 | |||||||
| 30-day mortality | 20 | 9.2 | 9 | 8.3 | 11 | 10.1 | 0.815 | |||||||
| Composite outcome | 58 | 26.6 | 24 | 22.0 | 34 | 31.2 | 0.133 | |||||||
| Stroke/TIA | 34 | 15.6 | 12 | 11.0 | 22 | 20.2 | 0.064 | |||||||
| In-hospital death | 16 | 7.3 | 8 | 7.3 | 8 | 7.3 | 1.000 | |||||||
| Overall survival | 140 | 64.2 | 84 | 77.1 | 56 | 51.4 | <0.001 | |||||||
| LOS: length of stay, TIA: transient ischemic attack | ||||||||||||||
| OR | 95% CI (lower, upper) |
P value | OR | 95% CI (lower, upper) |
P value | |||
|---|---|---|---|---|---|---|---|---|
| Extended Resection | 1.47 | 0.97 | 2.23 | 0.071 | 1.52 | 0.92 | 2.49 | 0.101 |
| Male gender | 1.04 | 0.67 | 1.59 | 0.868 | ||||
| CCS III-IV | 1.37 | 0.77 | 2.43 | 0.282 | ||||
| NYHA III-IV | 1.33 | 0.75 | 2.36 | 0.326 | ||||
| Prior cardiac surgery | 1.28 | 0.65 | 2.51 | 0.48 | ||||
| Diabetes | 1.03 | 0.42 | 2.54 | 0.953 | ||||
| Hypertension | 1.29 | 0.84 | 1.98 | 0.246 | 1.38 | 0.83 | 2.31 | 0.217 |
| Smoking history | 0.96 | 0.63 | 1.47 | 0.859 | ||||
| Renal disease | 1.40 | 0.46 | 4.26 | 0.553 | ||||
| Pulmonary disease | 1.37 | 0.73 | 2.58 | 0.321 | ||||
| PVD | 1.27 | 0.47 | 3.46 | 0.641 | ||||
| LVEF moderate/poor | 1.58 | 0.94 | 2.64 | 0.081 | 1.49 | 0.81 | 2.75 | 0.203 |
| Hemodynamically unstable | 1.47 | 0.86 | 2.51 | 0.159 | 1.40 | 0.74 | 2.65 | 0.299 |
| Salvage surgery | 2.26 | 1.12 | 4.55 | 0.023 | 2.94 | 1.22 | 7.08 | 0.016 |
| Obese (BMI >28) | 0.84 | 0.54 | 1.31 | 0.451 | ||||
| Bypass time | 1.00 | 1.00 | 1.01 | <0.001 | 1.01 | 1.00 | 1.01 | 0.001 |
| Cross clamp time | 1.00 | 1.00 | 1.01 | 0.044 | 1.00 | 0.99 | 1.00 | 0.724 |
| Circulatory arrest time | 1.01 | 0.99 | 1.92 | 0.471 | ||||
| Additional cardiac procedures | 1.19 | 0.72 | 1.96 | 0.51 | ||||
| Aortic arch procedure | 1.41 | 0.87 | 2.30 | 0.167 | 1.03 | 0.56 | 1.91 | 0.928 |
| Log euroscore | 1.03 | 0.99 | 1.07 | 0.163 | 1.01 | 0.97 | 1.06 | 0.632 |
| Age >70 | 1.63 | 1.07 | 2.48 | 0.023 | 1.85 | 1.11 | 3.07 | 0.017 |
| OR: odds ratio, CI: confidence interval, CCS: Canadian cardiovascular score, NYHA: New York Heart Association, PVD: peripheral vascular disease, LVEF: left ventricular ejection fraction, BMI: body mass index | ||||||||
| HR |
95% CI (lower, upper) |
P value | HR |
95% CI (lower, upper) |
P value | |||
| Extended Resection | 1.90 | 1.24 | 2.92 | 0.003 | 2.06 | 1.31 | 3.25 | 0.002 |
| Male gender | 1.22 | 0.82 | 1.81 | 0.323 | ||||
| CCS III-IV | 1.38 | 0.80 | 2.38 | 0.253 | ||||
| NYHA III-IV | 2.03 | 1.28 | 3.22 | 0.003 | 2.07 | 1.28 | 3.35 | 0.003 |
| Prior cardiac surgery | 1.66 | 0.99 | 2.79 | 0.057 | 2.01 | 1.17 | 3.45 | 0.012 |
| Diabetes | 0.69 | 0.28 | 1.69 | 0.411 | ||||
| Hypertension | 1.59 | 1.05 | 2.40 | 0.027 | 1.45 | 0.94 | 2.22 | 0.092 |
| Smoking history | 1.10 | 0.75 | 1.62 | 0.628 | ||||
| Renal disease | 1.72 | 0.70 | 4.23 | 0.238 | 1.89 | 0.75 | 4.76 | 0.175 |
| Pulmonary disease | 0.84 | 0.44 | 1.62 | 0.613 | ||||
| PVD | 1.13 | 0.46 | 2.77 | 0.796 | ||||
| LVEF moderate/poor | 1.34 | 0.84 | 2.12 | 0.22 | 1.42 | 0.88 | 2.30 | 0.153 |
| Hemodynamically unstable | 0.85 | 0.48 | 1.50 | 0.574 | ||||
| Salvage surgery | 0.79 | 0.32 | 1.94 | 0.602 | ||||
| Obese (BMI >28) | 0.92 | 0.61 | 1.39 | 0.682 | ||||
| Bypass time | 1.00 | 0.99 | 1.00 | 0.707 | ||||
| Cross clamp time | 0.99 | 0.99 | 1.00 | 0.136 | 1.00 | 0.99 | 1.00 | 0.341 |
| Circulatory arrest time | 0.99 | 0.98 | 1.01 | 0.343 | ||||
| Additional cardiac procedures | 0.68 | 0.35 | 1.31 | 0.245 | 0.76 | 0.37 | 1.57 | 0.459 |
| Aortic arch procedure | 1.21 | 0.76 | 1.93 | 0.415 | ||||
| Log euroscore | 1.02 | 0.98 | 1.06 | 0.283 | ||||
| Age >70 | 2.10 | 1.42 | 3.12 | <0.001 | 2.00 | 1.32 | 3.03 | 0.001 |
| OR: odds ratio, CI: confidence interval, CCS: Canadian cardiovascular score, NYHA: New York Heart Association, PVD: peripheral vascular disease, LVEF: left ventricular ejection fraction, BMI: body mass index | ||||||||
| Aortic procedures after AAAD repair | ||
| Aortic valve and root | (47.2%) | |
| Aortic root repair/replacement | 10 | |
| Aortic valve replacement +/- redo Interposition graft | 7 | |
| Arch | (13.9%) | |
| Arch resection +/- Frozen elephant trunk | 5 | |
| Descending thoracic aorta | (27.8%) | |
| Descending thoracic aortic aneurysm repair | 8 | |
| Thoracic endovascular aneurysm repair | 2 | |
| Abdominal aortic aneurysm repair | (8.3%) | |
| Abdominal aortic aneurysm repair (open) | 2 | |
| Endovascular aneurysm repair (EVAR) | 1 | |
| Total | 36 | (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





