Submitted:
22 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Population and Methods
Study Design
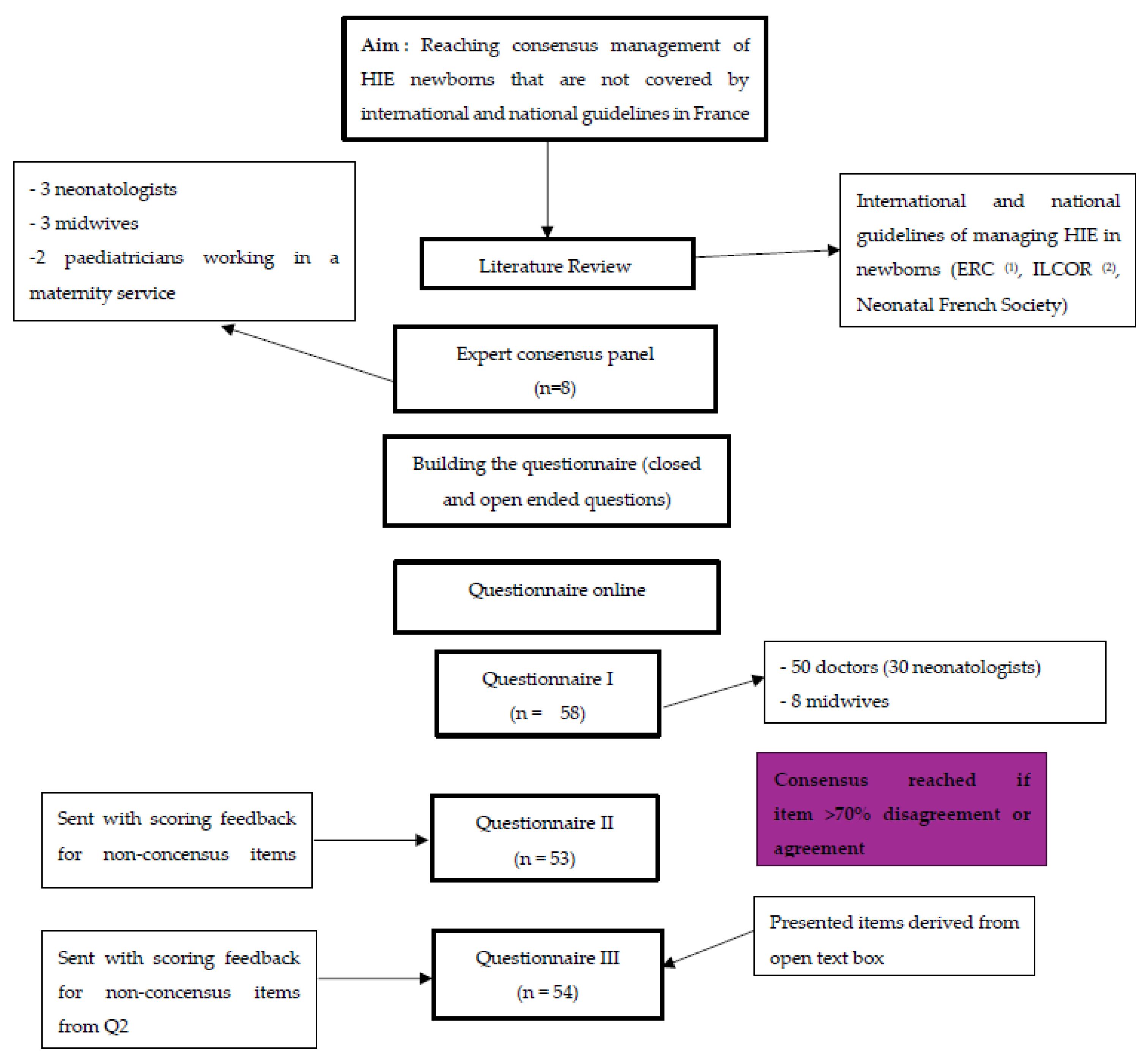
Informations Sources
Building the Questionary
Consensus Process
Ethical and Legal Considerations
3. Results
3.1. Characteristics
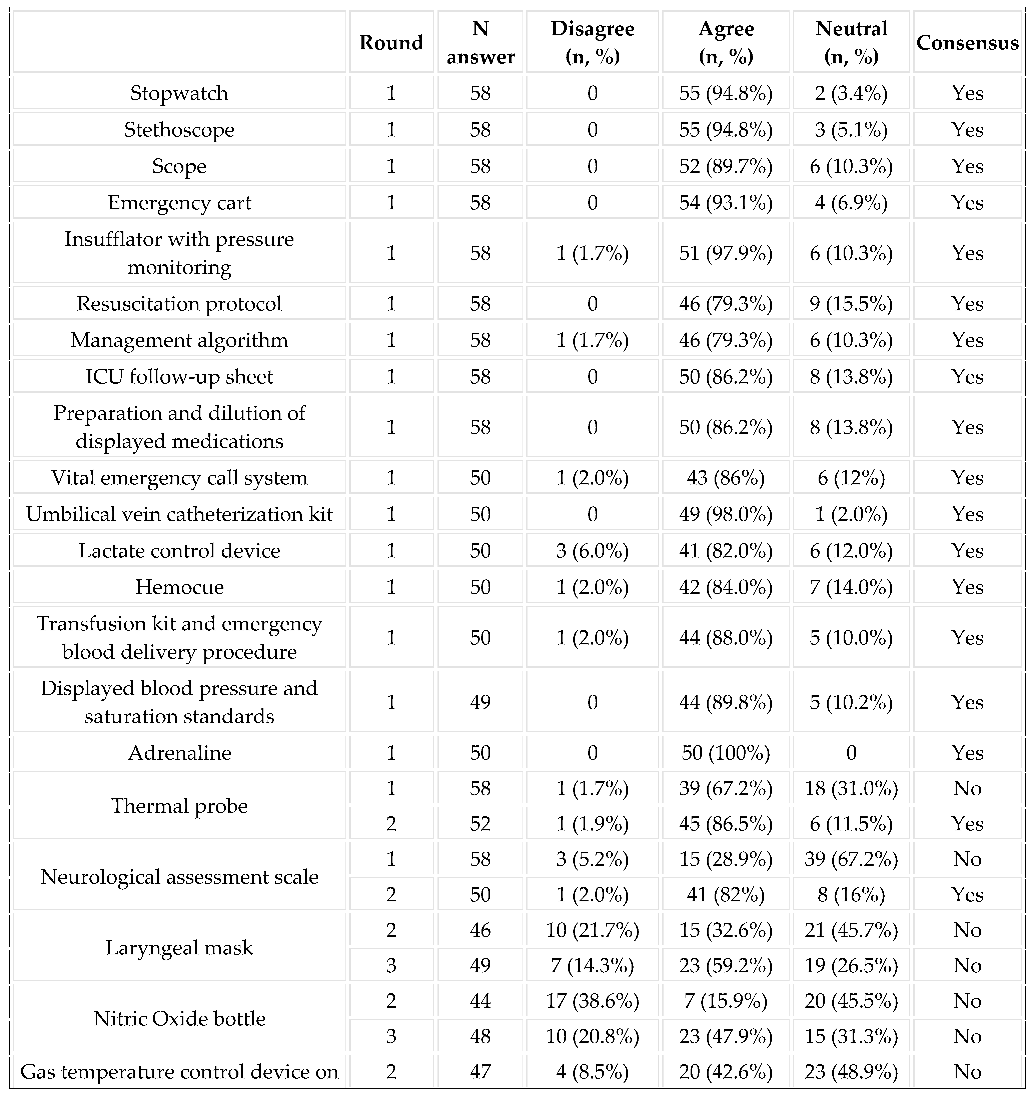
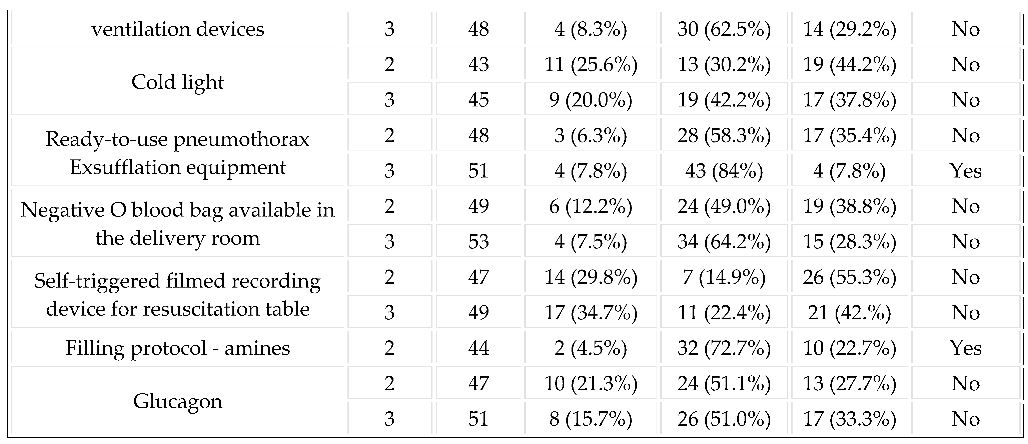
3.2. Equipment in the Delivery Room
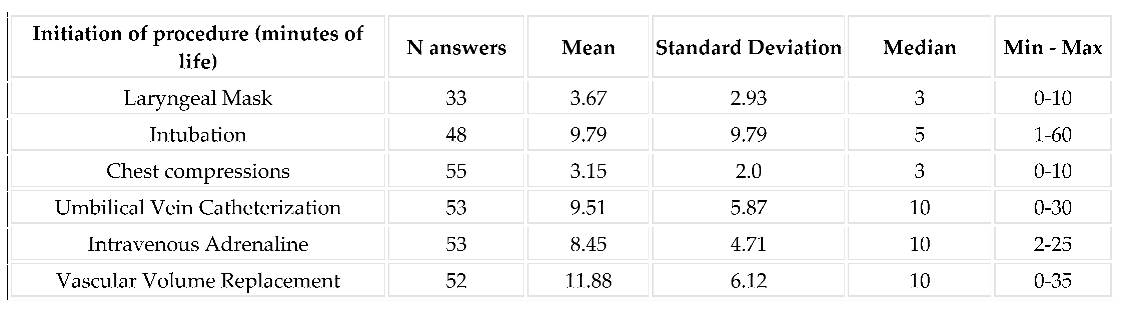
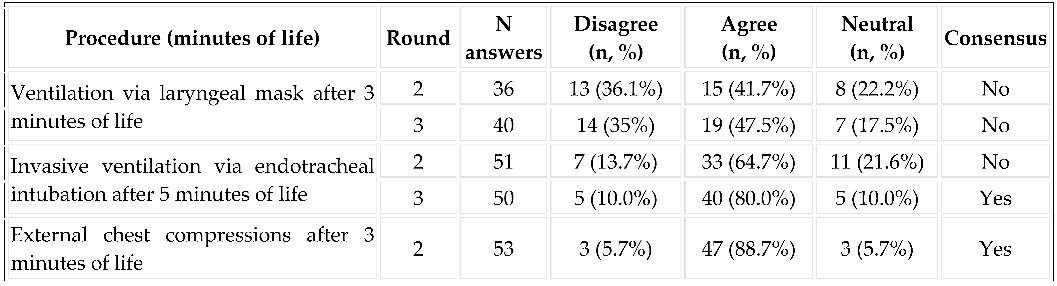
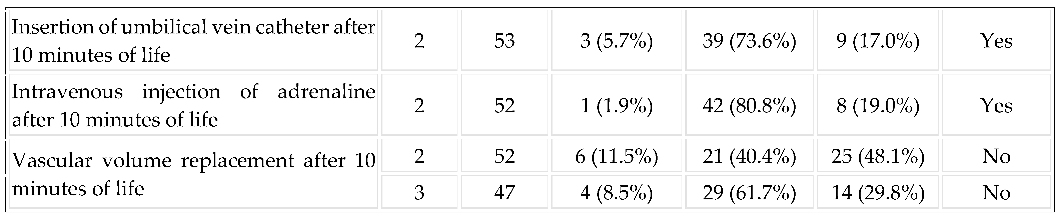
3.3. Implementation of Resuscitation:
3.4. Calling the Pediatrician by the Midwife
3.5. Care in the Delivery Room during the First Hours of Life
3.5.1. Systematic Sedation and Analgesia in the Delivery Room
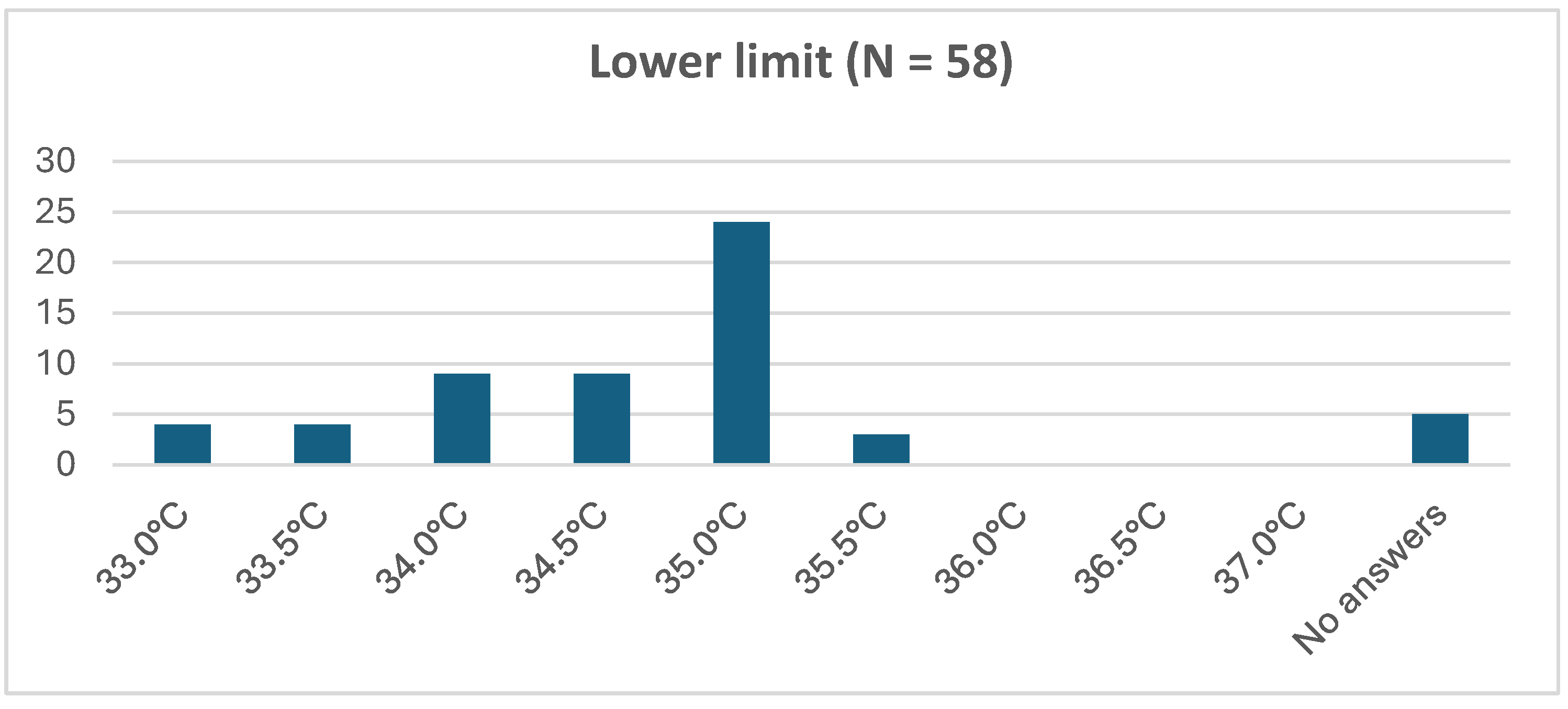
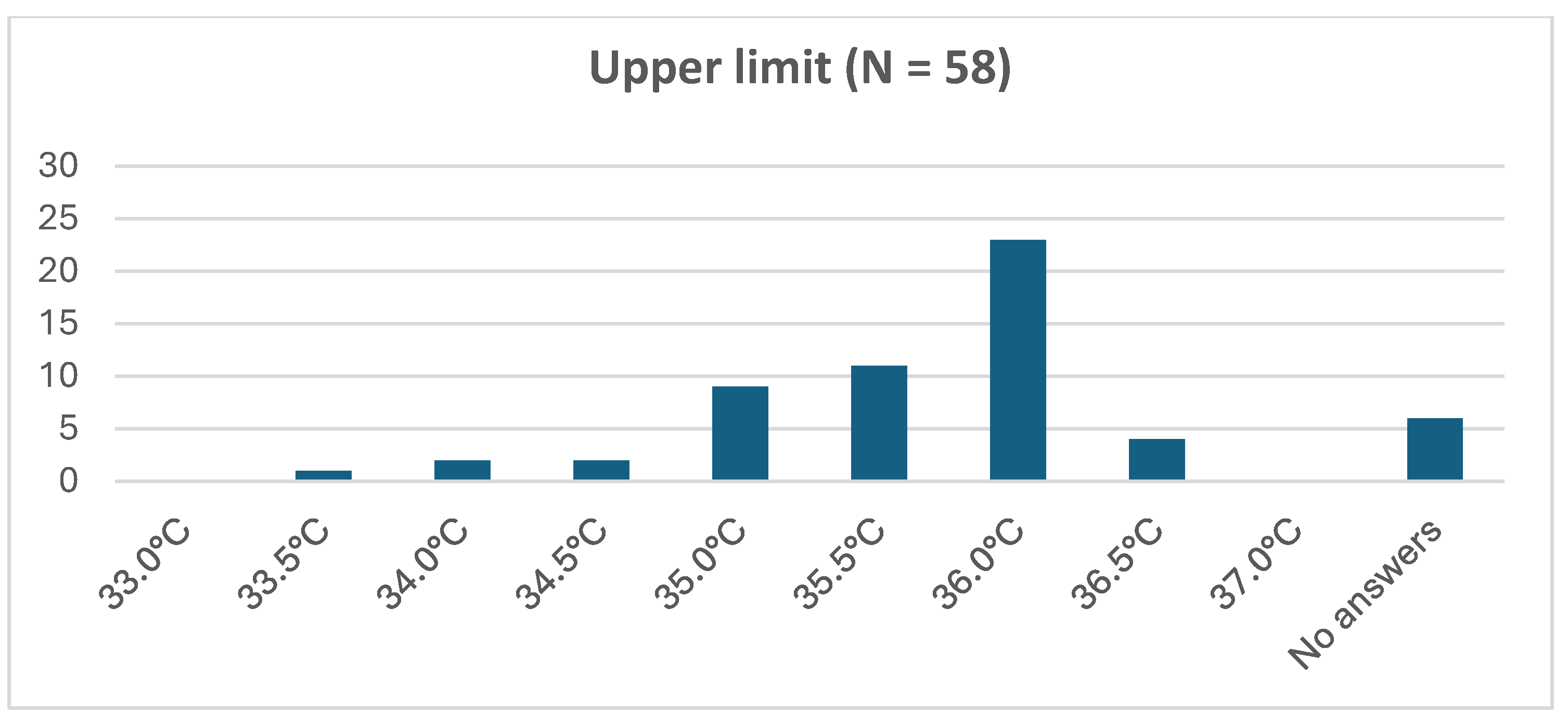
3.5.2. Passive Hypothermia in the Delivery Room and during Transport:
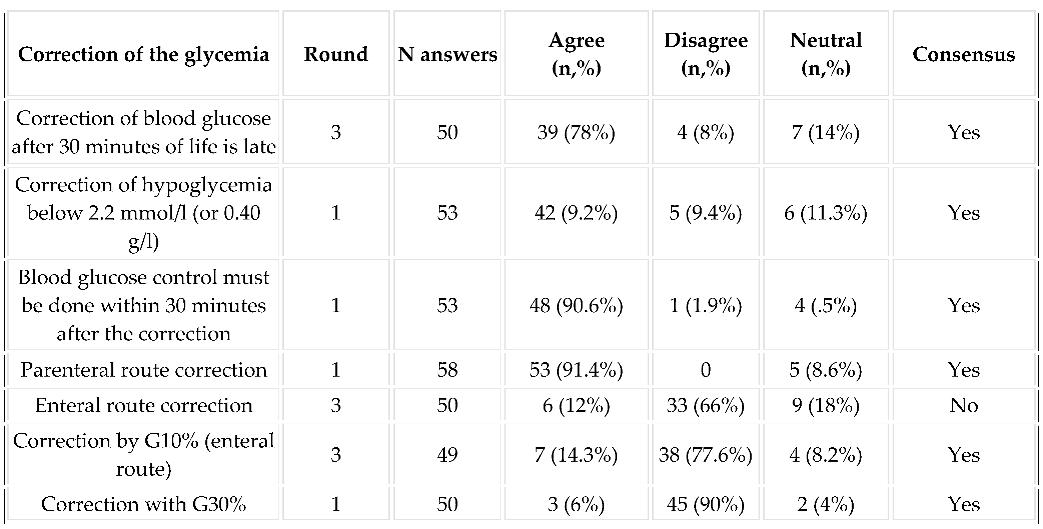
3.5.3. Regarding the Management of Glycemia:
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vega-Del-Val, C.; Arnaez, J.; Caserío, S.; Gutiérrez, E.P.; Castañón, L.; Benito, M.; et al. Adherence to hypothermia guidelines in newborns with hypoxic-ischemic encephalopathy. An Pediatr. 2022, 97, 30–39. [Google Scholar] [CrossRef]
- Lundgren, C.; Brudin, L.; Wanby, A.S.; Blomberg, M. Ante- and intrapartum risk factors for neonatal hypoxic ischemic encephalopathy. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2018, 31, 1595–1601. [Google Scholar] [CrossRef]
- Zupan Simunek, V. Définition de l’asphyxie intrapartum et conséquences sur le devenir. J Gynécologie Obstétrique Biol Reprod. 2008, 37, S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Palsdottir, K.; Dagbjartsson, A.; Thorkelsson, T.; Hardardottir, H. [Birth asphyxia and hypoxic ischemic encephalopathy, incidence and obstetric risk factors]. Laeknabladid. 2007, 93, 595–601. [Google Scholar] [PubMed]
- Zupan Simunek, V. Asphyxie périnatale à terme : Diagnostic, pronostic, éléments de neuroprotection. Arch Pédiatrie. 2010, 17, 578–582. [Google Scholar] [CrossRef]
- McKinlay, C.J.D.; Alsweiler, J.M.; Anstice, N.S.; Burakevych, N.; Chakraborty, A.; Chase, J.G.; et al. Association of Neonatal Glycemia With Neurodevelopmental Outcomes at 4. 5 Years. JAMA Pediatr. 2017, 171, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.D.; Brocklehurst, P.; Gunn, A.J.; Halliday, H.; Juszczak, E.; Levene, M.; Strohm, B.; Thoresen, M.; Whitelaw, A.; Azzopardi, D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ. 2010, 340, c363. [Google Scholar] [CrossRef]
- van Handel, M.; Swaab, H.; de Vries, L.S.; Jongmans, M.J. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: A review. Eur J Pediatr. 2007, 166, 645–654. [Google Scholar] [CrossRef]
- Goswami, I.; Guillot, M.; Tam, E.W.Y. Predictors of Long-Term Neurodevelopmental Outcome of Hypoxic-Ischemic Encephalopathy Treated with Therapeutic Hypothermia. Semin Neurol. 2020, 40, 322–334. [Google Scholar] [CrossRef]
- Armstrong-Wells, J.; Bernard, T.J.; Boada, R.; Manco-Johnson, M. Neurocognitive outcomes following neonatal encephalopathy. NeuroRehabilitation. 2010, 26, 27–33. [Google Scholar] [CrossRef]
- Lou, H.C. Etiology and pathogenesis of attention-deficit hyperactivity disorder (ADHD): Significance of prematurity and perinatal hypoxic-haemodynamic encephalopathy. Acta Paediatr Oslo Nor 1992. 1996, 85, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rüdiger, M.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation. 2021, 161, 291–326. [Google Scholar] [CrossRef] [PubMed]
- Perlman, J.M.; Wyllie, J.; Kattwinkel, J.; Wyckoff, M.H.; Aziz, K.; Guinsburg, R.; et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015, 132 (Suppl. 1), S204–241. [Google Scholar] [CrossRef] [PubMed]
- Berglund, S.; Norman, M.; Grunewald, C.; Pettersson, H.; Cnattingius, S. Neonatal resuscitation after severe asphyxia--a critical evaluation of 177 Swedish cases. Acta Paediatr. 2008, 97, 714–719. Acta Paediatr. 2008, 97, 714–719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gale, C.; Longford, N.T.; Jeyakumaran, D.; Ougham, K.; Battersby, C.; Ojha, S.; et al. Feeding during neonatal therapeutic hypothermia, assessed using routinely collected National Neonatal Research Database data: A retrospective, UK population-based cohort study. Lancet Child Adolesc Health. 2021, 5, 408–416. [Google Scholar] [CrossRef] [PubMed]
- The International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/l (54 mg/dl) should be reported in clinical trials: A joint position statement of the American Diabetes Association and the Europian Association for the Study of Diabetes. Diabetologia. 2017, 60, 3–6.
- Roeper, M.; Hoermann, H.; Kummer, S.; Meissner, T. Neonatal hypoglycemia: Lack of evidence for a safe management. Front Endocrinol. 2023, 14, 1179102. [Google Scholar] [CrossRef]
- Giouleka, S.; Gkiouleka, M.; Tsakiridis, I.; Daniilidou, A.; Mamopoulos, A.; Athanasiadis, A.; et al. Diagnosis and Management of Neonatal Hypoglycemia: A Comprehensive Review of Guidelines. Child Basel Switz. 2023, 10, 1220. [Google Scholar] [CrossRef]
- Pinchefsky, E.F.; Schneider, J.; Basu, S.; Tam, E.W.Y.; Gale, C. , Newborn Brain Society Guidelines and Publications Committee*. Nutrition and management of glycemia in neonates with neonatal encephalopathy treated with hypothermia. Semin Fetal Neonatal Med. 2021, 26, 101268. [Google Scholar]
- Cornblath, M.; Ichord, R. Hypoglycemia in the neonate. Semin Perinatol. 2000, 24, 136–149. [Google Scholar] [CrossRef]
- Taze, D.; Hartley, C.; Morgan, A.W.; Chakrabarty, A.; Mackie, S.L.; Griffin, K.J. Developing consensus in Histopathology: The role of the Delphi method. Histopathology. 2022, 81, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Humphrey-Murto, S.; Varpio, L.; Gonsalves, C.; Wood, T.J. Using consensus group methods such as Delphi and Nominal Group in medical education research. Med Teach. 2017, 39, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Romero-Collado, A. Essential elements to elaborate a study with the (e)Delphi method. Enferm Intensiva. 2021, 32, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Fanaroff, J.M.; Ross, M.G.; Donn, S.M. , Newborn Brain Society Guidelines and Publications Committee*. Medico-legal considerations in the context of neonatal encephalopathy and therapeutic hypothermia. Semin Fetal Neonatal Med. 2021, 26, 101266. [Google Scholar] [PubMed]
- Srinivasan, G.; Pildes, R.S.; Cattamanchi, G.; Voora, S.; Lilien, L.D. Plasma glucose values in normal neonates: A new look. J Pediatr. 1986, 109, 114–117. [Google Scholar] [CrossRef]
- Hoermann, H.; Mokwa, A.; Roeper, M.; Salimi Dafsari, R.; Koestner, F.; Hagenbeck, C.; et al. Reliability and Observer Dependence of Signs of Neonatal Hypoglycemia. J Pediatr. 2022, 245, 22–29.e2. [Google Scholar] [CrossRef] [PubMed]
- Warchoł, A.; Kwinta, P. Nutrition of Newborns with Hypoxic-Ischaemic Encephalopathy during Therapeutic Hypothermia - A Survey of Practice in Polish Neonatal Care Units. J Mother Child. 2024, 28, 8–13. [Google Scholar] [PubMed]
- Wassink, G.; Gunn, E.R.; Drury, P.P.; Bennet, L.; Gunn, A.J. The mechanisms and treatment of asphyxial encephalopathy. Front Neurosci. 2014, 8, 40. [Google Scholar] [CrossRef]
- Chiang, M.C.; Jong, Y.J.; Lin, C.H. Therapeutic hypothermia for neonates with hypoxic ischemic encephalopathy. Pediatr Neonatol. 2017, 58, 475–483. [Google Scholar] [CrossRef]
- Russ, J.B.; Simmons, R.; Glass, H.C. Neonatal Encephalopathy: Beyond Hypoxic-Ischemic Encephalopathy. NeoReviews. 2021, 22, e148–e162. [Google Scholar] [CrossRef]
- Torre Monmany, N.; Behrsin, J.; Leslie, A. Servo-controlled cooling during neonatal transport for babies with hypoxic-ischaemic encephalopathy is practical and beneficial: Experience from a large UK neonatal transport service. J Paediatr Child Health. 2019, 55, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, G.; Agudelo-Pérez, S.; Maldonado, N.T.; Becerra, M.P. Relationship of passive hypothermia during transport with the incidence of early multiorgan compromise in newborns with perinatal asphyxia. Early Hum Dev. 2023, 187, 105902. [Google Scholar] [CrossRef] [PubMed]
- Bellini, C.; Ramenghi, L.A.; Gente, M. Effective Passive Cooling During Neonatal Transport. Ther Hypothermia Temp Manag. 2022, 12, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Kendall, G.S.; Kapetanakis, A.; Ratnavel, N.; Azzopardi, D.; Robertson, N.J. ; Cooling on Retrieval Study Group. Passive cooling for initiation of therapeutic hypothermia in neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010, 95, F408–F412. [Google Scholar] [CrossRef] [PubMed]
- Momin, S.; Thomas, S.; Zein, H.; Scott, J.N.; Leijser, L.M.; Vayalthrikovil, S.; Yusuf, K.; Paul, R.; Howlett, A.; Mohammad, K. Comparing Three Methods of Therapeutic Hypothermia Among Transported Neonates with Hypoxic-Ischemic Encephalopathy. Ther Hypothermia Temp Manag. 2023, 13, 141–148. [Google Scholar] [CrossRef]
- Laptook, A.; Tyson, J.; Shankaran, S.; McDonald, S.; Ehrenkranz, R.; Fanaroff, A.; et al. Elevated temperature after hypoxic-ischemic encephalopathy: Risk factor for adverse outcomes. Pediatrics. 2008, 122, 491–499. [Google Scholar] [CrossRef]
- Troncoso, G.; Agudelo-Pérez, S.; Thorin, N.; Diaz, C.; Vargas, A. Corrigendum correcting the paper "Short-term neurological injury in newborns infants with overcooling in passive hypothermia and transferred to reference hospital in Colombia". Acta Paediatr. 2024, 113, 366–367. [Google Scholar] [CrossRef]
 |
 |
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





