1. Introduction
Breast cancer continues to be a challenge for health services around the world. Despite advances in prevention, diagnosis, and treatment, the incidence and mortality rates of this disease continue to rise from year to year. Breast cancer is the most frequently reported malignant neoplasm worldwide. The 2.3 million new cases of breast cancer diagnosed around the world in 2020 constituted 11.7% of new cases of all cancers, representing a higher rate of incidence than that found for lung, colon, or prostate cancer [
1], according to data provided by the International Agency for Research on Cancer (IARC, 2020).
Two major difficulties diminish the effectiveness of breast cancer treatment. Firstly, the diagnosis is often made in the advanced stages of the disease, at which time the patients are undergoing metastasis. Under this condition, there are limited therapeutic options capable of eliminating cancer cells. Secondly, cancer cells commonly acquire resistance mechanisms to chemotherapy drugs, causing some patients who initially respond well to chemotherapy to become insensitive to treatment [
2].
2. Classification of Breast Cancer
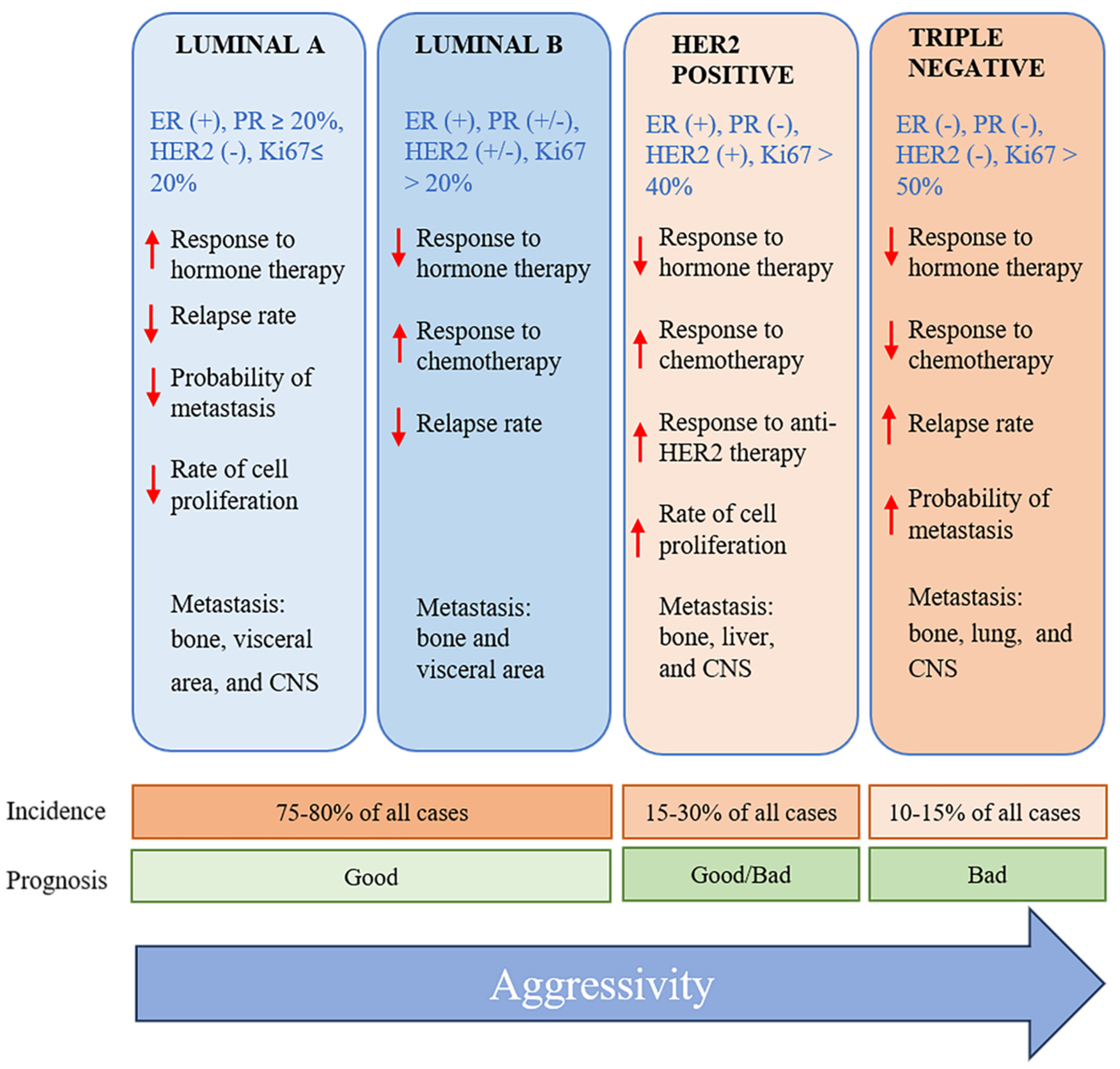
Breast cancer has been classified by using diverse criteria, including histology, molecular characteristics, and the expression of specific biomarkers. The establishment of the specific characteristics of breast cancer tumors allows for the selection of the most effective treatment. Regarding the molecular perspective, among the various subtypes of breast cancer are luminal A, luminal B, normal-like, HER2-enriched, basal-like, and triple-negative [
3,
4] (
Figure 1). The triple-negative subtype comprises 10-20% of all cases of breast cancer. It is found at a greater prevalence in Afro-descendants, women under 40 years of age, and/or those with a mutation in the BCRA1gene [
5].
3. Triple-Negative Breast Cancer: Characteristics and New Perspectives for Treatment
Triple-negative breast cancer (TNBC) is characterized by the absence or low expression of estrogen receptors (ERs), progesterone receptors (PRs), and human epidermal growth factor 2 (HER2) receptors. Since these receptors are the targets of many chemotherapy drugs, their limited expression makes TNBC the breast tumor type with the least number of therapeutic options available [
7].
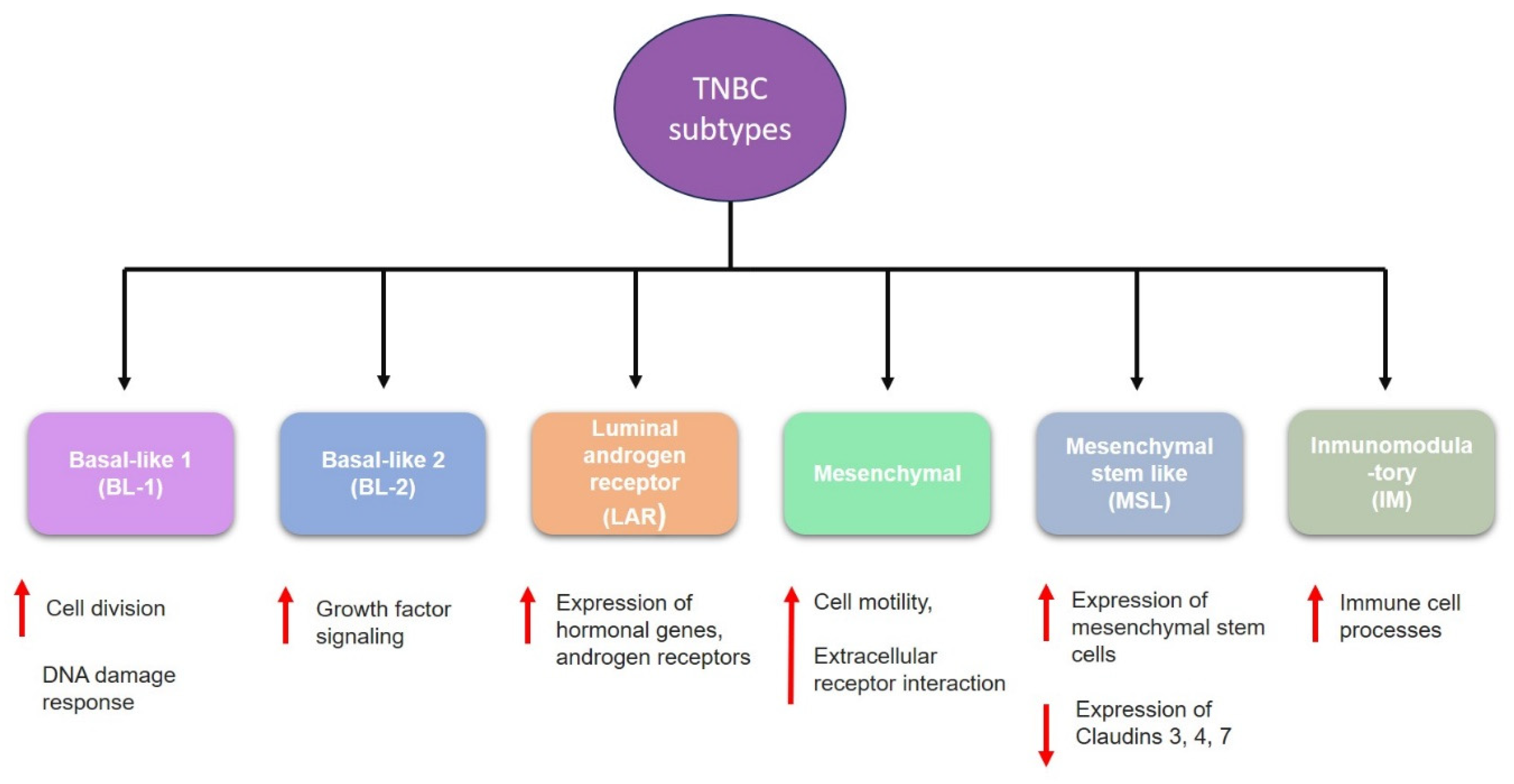
TNBC is highly heterogeneous and classified into six subtypes (
Figure 2); these subtypes have different molecular and biological characteristics that could influence prognosis and treatment response. These subtypes are:
- (1)
Basal-like 1 (BL-1) is characterized by increased cell division, and elevated DNA damage response pathways (ATR/BCA) [
8,
9].
- (2)
Basal-like 2 (BL-2) involved increased growth factor signaling pathways such as epidermal growth factor (EGF), nerve growth factor (NGF), and Wnt/β-catenin [
8,
10].
- (3)
Luminal androgen receptor (LAR) is characterized by high levels of androgen receptor genes and alterations of PI3K pathway genes [
8,
11,
12].
- (4)
Mesenchymal (M) has elevated expression of genes involved in epithelial-mesenchymal-transition (EMT), and it is enriched in components involved in cell motility [
8,
13].
- (5)
Mesenchymal stem-like (MLS) shared the overexpression of genes related to EMT; however, it is also characterized by enrichment in genes involved in angiogenesis, including VEGFR2 and high expression of stem cell genes [
8,
14].
- (6)
Immunomodulatory (IM) is enriched within genes that regulate immune response and antigen processing [
8].
Chemotherapy with cytotoxic agents affords the greatest rate of success for TNBC patients, above all if administered in the early stages of the disease. Some of the most common drugs for TNBC are anthracyclines (doxorubicin), taxanes (paclitaxel), and alkylating agents (cyclophosphamide). Treatment in the advanced stages involves antimetabolites (gemcitabine and capecitabine), inhibitors of microtubules (eribulin), and platinum derivatives (carboplatin) [
7]. Although the initial response to chemotherapy is good in patients with TNBC, 90% of cases acquire resistance to pharmacological treatment. Indeed, drug resistance in tumors is the main cause of therapeutic failure [
2].
Nanomedicine is an alternative to conventional treatments and is one of the areas of science presently showing the most rapid growth. It involves the integration of several branches of research, including medicine, pharmacology, molecular biology, and gene therapy. In recent years, nanoparticles have been developed as systems for the transport and release of drugs in order to achieve greater efficiency in the treatment of diverse diseases (e.g., cancer). In various preclinical studies, nanoparticles have proven to be advantageous as a form of encapsulation and transport, being capable of selectively delivering drugs to target receptors. Such studies carried out during the last few years have generally shown a better pharmacological response to treatment with new therapeutic strategies based on nanotechnology than with conventional treatments.
Regarding TNBC, the recent development of nanoparticle systems gives rise to new therapeutic alternatives with the potential of being more efficacious and personalized. Current investigation in this field is focused on optimizing this potential to improve the quality of life of TNBC patients.
4. Nanoparticles: Advances and Potential Therapeutic Uses
Nanoparticles have attracted the attention of scientists due to their capacity for diagnosis, medical imaging, and treatment of cancer in a single system. They are defined as particles with a diameter of 1-1,000 nanometers (nm) [
15]. However, a size range of 10-100 nm is considered most suitable for cancer therapy. Whereas nanoparticles with a diameter less than 10 nm are able to filter through the kidneys [
16,
17], those with a diameter greater than 100 nm have a higher probability of being eliminated by phagocytosis [
18]. Also affecting biodistribution is the shape of a nanoparticle, which can be cubical, spherical, rod-shaped, disc-shaped, or hexagonal [
19,
20,
21]. Spherical nanoparticles are reported to be eliminated from the organism more slowly [
20]. Additionally, the coating of these particles with different materials [e.g., polyethylene glycol (PEG)] reduces their opsonization, leading to a longer life in the circulatory system and thus an increase in their bioavailability (
Figure 3) [
21].
Recent studies have demonstrated that nanoparticles have great potential as drug delivery systems due to their capacity to encapsulate hydrophilic as well as hydrophobic compounds. This encapsulation improves bioavailability and protects against biological, physical, and chemical degradation. Moreover, nanoparticles can be directed at a particular organ and because of their size are able to cross biological barriers. They can transport a wide variety of molecules and provide a controlled and targeted release. Compared to conventional therapies, nanosystems are more stable, less toxic to the organism, and more selective for cancer cells [
22].
5. Structure and Characteristics of Solid Lipid Nanoparticles (SLNs)
Distinct types of nanoparticles are continually being developed to enhance the efficiency of current treatments for TNBC. Within this context, solid lipid nanoparticles (SLNs) are a new generation of nanocarriers with great promise for therapy. Their structure, which is a matrix of solid lipids, has good efficiency of encapsulation and stability as well as low toxicity, according to various studies [
23,
24,
25].
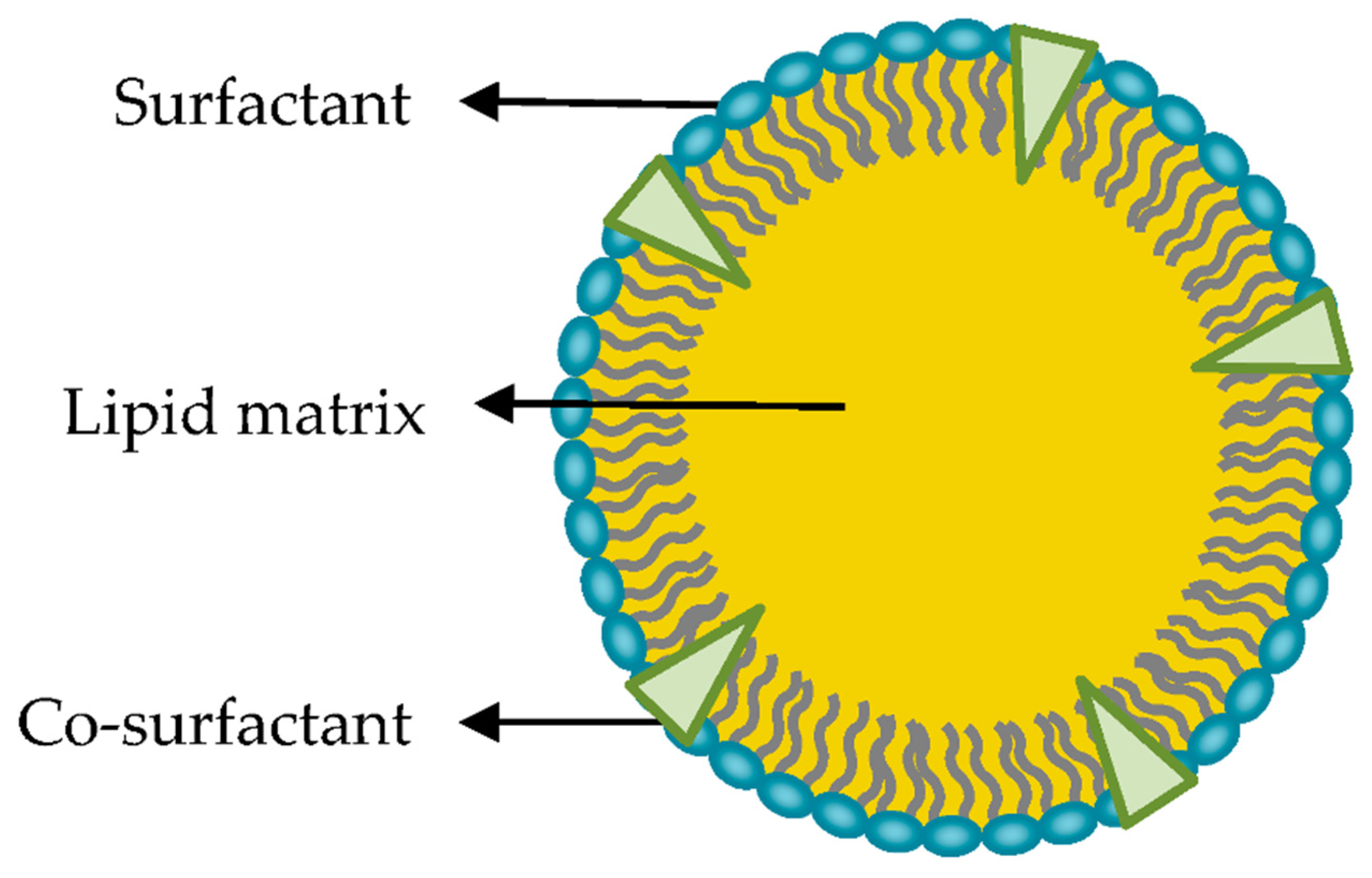
SLNs are colloidal particles of submicron size, from 50 to 1,000 nm in diameter. They consist of a matrix of crystalline lipids that are solid at ambient temperature and some tensoactive agents that allow them to accommodate molecules between their chains of fatty acids, either in the lipid nucleus or on the surface (
Figure 4). They may be spherical, ellipsoidal, or disc-like. The surface charge of an SLN is determined by its constituents and the pH of the surrounding medium [
24,
26,
27].
The quality of the formulations of SLNs depends on an adequate proportion of the lipid components. These components should be selected based on the nature of the drug to be incorporated. The drug must be solubilized in the lipid matrix in order to have good efficiency of encapsulation. The substances most commonly used to form the lipid nucleus are mono-, di- and triglycerides, fatty acids, fatty alcohols, and waxes, which are all biocompatible [
26]. The lipid components of the solid matrix are in solid form at room temperature and body temperature. Consequently, they are safe and viable for low-cost mass production. The majority are super-purified waxes and complex emulsions of glycerides [
28].
The formation of a solid matrix is essential because it determines the controlled release of the drug. Moreover, the mobility of a drug in a solid lipid matrix is substantially less than that found in other components. As a system of transport of different active agents, SLNs offer various advantages compared to conventional systems of administration (
Table 1) [
25,
26,
29]. The unique properties of SLNs, contingent on their extremely small size and their capacity to incorporate drugs, provide the opportunity to create new therapeutic approaches involving a targeted and controlled release of drugs [
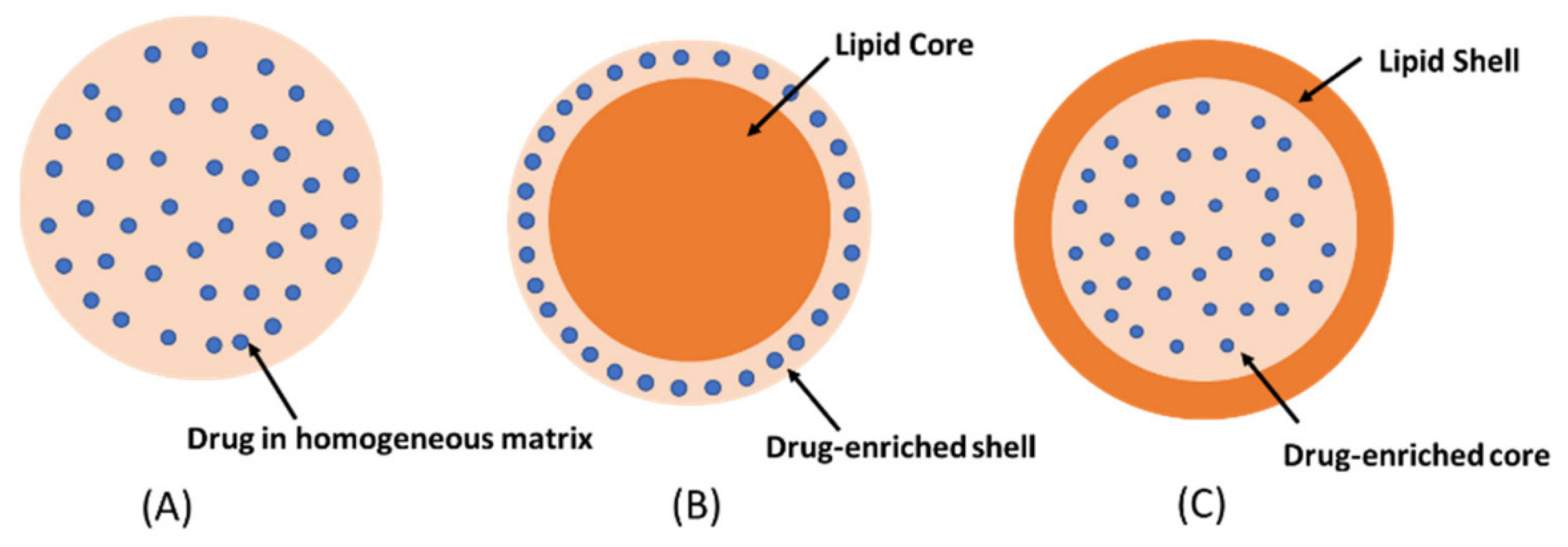
30]. Depending on the incorporation of the drug into SNL, it may be classified into three models: A) Drug into the homogeneous matrix, which is formed when the drugs are homogenously dispersed, B) Drug-enriched shell, which is characterized by the drug enclosed by an outer shell and C) Drug-enriched core, is obtained when the drugs crystalized before the lipid (
Figure 5) [
31,
32].
It has been known that SLN are stable in aqueous dispersion. To obtain adequate structure and stability, the method of preparation of SLNs is crucial. A transfer of energy to the SLN system is required to generate very small and stable particles in aqueous environments. This energy can originate from distinct sources, as is exhaustively described in the literature: high-pressure or high-speed homogenization, cold or hot homogenization, sonication, and emulsification [
24].
6. Nanotoxicological Classification System
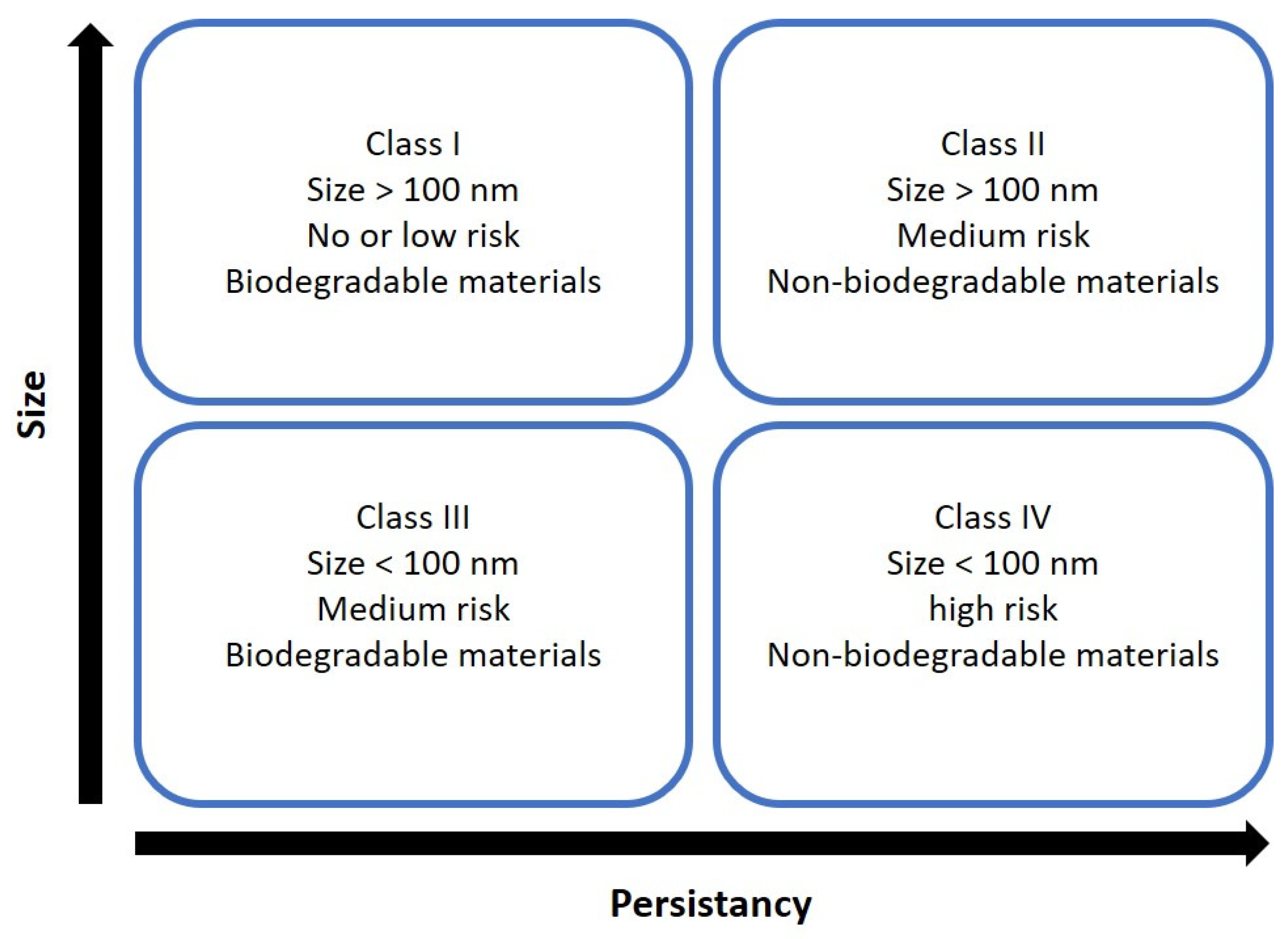
Nanosystems are placed in four categories according to size and biodegradability (
Figure 6). SLNs are composed of lipids, natural substances in the body, and are considered biodegradable. Therefore, SLNs are typically classified as members of classes I or III [
24]. Class I refers to nanosystems composed of biodegradable materials with a size above 100 nm. Since SLN are made from physiologically compatible lipids such as fatty acids, glycerides, and sterols. Class III, conversely, is composed of biodegradable lipids, but their size is above 100 nm [
33].
It has been reported that SLNs remain stable in aqueous dispersion, and
in-vitro studies have shown that SLNs are acceptable at
˂ 1 mg/ml concentration of lipids, and nanosystems with high quantities of surfactants have more risk of cytotoxicity [
33,
34]; however, it is necessary more nanotoxicology studies to understand the effects of SLN on the body.
6. Delivery Mechanism of SLNs
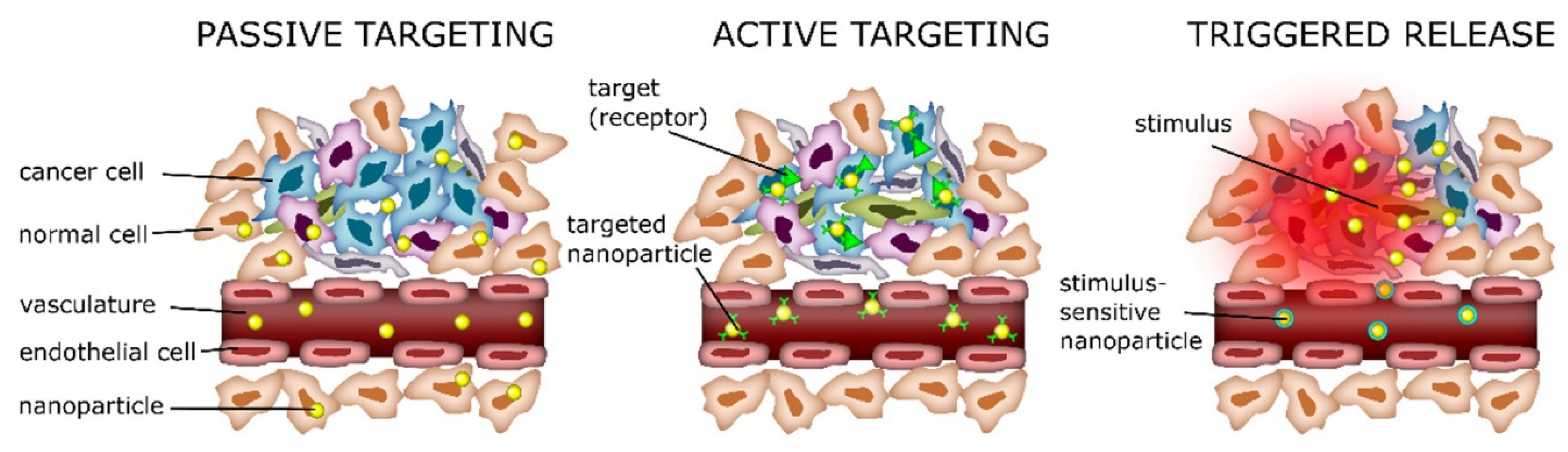
It has been reported that lipid composition, pH, temperature, and the site of the drug where is encapsulated influence the drug delivery mechanisms, either through passive or active accumulation.
6.1. Passive Delivery
In passive delivery, the properties of SLN, such as size, composition lipid, and surface characteristics, play a crucial role. One of the main characteristics of tumors is the stimulation of angiogenesis to promote increased nutrients and oxygen. However, blood vessel formation resulted in a defective architecture with fenestrations (from 200-1200nm) [
24,
35]. This pathology is the enhanced permeability and retention effect (EPR). Therefore, SLNs accumulate in the tumor through passive diffusion via the EPR effect, penetrating through the fenestrations and remaining there due to poor lymphatic drainage nanoparticles, with sizes ranging from 50–200 nm, which can reach passively the tumor site. Surface modification of SLNs with hydrophilic polymers such as polyethylene glycol (PEG) can enhance their circulation time by reducing opsonization and clearance by the reticuloendothelial system (RES), thus promoting passive accumulation at the target site. PEGylation is the most important surface modification of nanoparticles. PEG increases time circulation, and it has been reported that it enhances particle permeation in tumors [
36].
6.2. Active Delivery
There have been several studies on active delivery; generally, nanoparticles are functionalized by adjusting the composition of SLNs; they can be readily functionalized with various targeting methods to facilitate active delivery against specific cell types of interest. This modification aims to enhance the targeting efficiency of nanoparticles and reduce the side effects [
37]. Active delivery involves modifying the surface of nanoparticles with several ligands that are overexpressed on the tumor cells, such as small molecules, peptides, antibodies, polysaccharides, hormones, and nucleic acids. These molecules are recognized by cell receptors or transporters [
38,
39]. The specific interaction between the surface ligands of nanoparticles and their receptor expression on the target cells could facilitate the internalization process through endocytosis. It has been reported that nanoparticles modified with folate for active targeting showed enhanced cellular uptake by endocytosis [
40,
41].
6.3. Triggered Release
Triggered release mechanisms play a crucial role in drug delivery systems. These drug release mechanisms depend on various stimuli in the tumor microenvironment or external agents to control the release [
42]. Some of the critical stimuli and their associated responses include pH; it is known that tumors often have a lower pH compared to healthy tissues, and SLN can be designed to respond to this acidic pH to release therapeutic agents. Other stimuli are magnetic field, ultrasonic, and temperature. Magnetic solid lipid nanoparticles use an external magnetic field to control their location, thus avoiding the disadvantages of systemic administration. Once magnetic drug carriers accumulate within a tumor, an externally applied alternating magnetic field (AMF) can increase the drug release and temperature [
43]. In the case of ultrasound, waves can trigger the release of drugs from nanoparticles in the tumor area without affecting healthy tissue. It has been reported that exposure to ultrasound produces a reversible permeability on cell membranes and increases the drug into the tumor [
44,
45]. Hyperthermia is a common strategy used in cancer therapy, where tumors are heated to temperatures slightly higher than normal body temperature, and nanoparticles release their therapeutic agents in tumors [
46].
Figure 7.
Schematic representation of drug targeting via passive (EPR effect), active, and triggered release. Image from Hafeez, et al. [
42].
Figure 7.
Schematic representation of drug targeting via passive (EPR effect), active, and triggered release. Image from Hafeez, et al. [
42].
6. SLNs as a Promising Alternative for Treating TNBC
Several studies are investigating using SLNs to enhance the therapeutic effect and overcome high proliferation rates and drug resistance.
During the last few years, SLNs have been elaborated with the encapsulation of different compounds, and these have been evaluated on TNBC cells. According to Paiva et al. (2022), SLNs loaded with docetaxel enhance the cytotoxic effect of the drug on MDA-MB-231 cells, a TNBC cell line [
47]. This finding highlights the nanocarrier capability of SLNs, which are able to maintain and increase the antineoplastic activity of several drugs.
Chemoresistance during the TNBC treatment is a frequent event, and it has been associated with the presence of drug efflux pumps in tumor cells. One of the primary drug efflux pumps related to the development of acquired resistance is P-glycoprotein (P-gp/ABCB1) [
48], so its inhibition has become a research focus. It has been reported that curcumin is an inhibitor of P-gp [
49]; however, it is limited by low solubility and poor bioavailability [
50]. One of the tools used to improve these physicochemical characteristics is encapsulating curcumin in SLN. Fathy et al. reported that combining curcumin-NSL and doxorubicin effectively overcome P-gp-mediated chemoresistance. Their study demonstrated that the formulation of curcumin-SLN increased 2-fold more the intracellular accumulation of doxorubicin in the MDA-MB-231 cell line, this enhanced accumulation of doxorubicin is attributed to reduced P-gp mRNA because curcumin decreases intracellular ROS and consequent down-regulation of Akt/IKK-α/β/NF-kB pathway [
51].
Recently, quercetin has been tested as a treatment for breast cancer. In diverse studies, quercetin has exhibited low availability to tumor cells. One of the advantages of encapsulating quercetin in SLNs is the consequent increase in sustained release, thus improving the bioavailability of the compound in tumor cells. Hatami et al. reported that the encapsulation of quercetin in SLN triggered a higher rate of apoptosis in an MDA-MD-231 cell line by regulating the expression of Bax and Bcl-2 genes [
52].
The new generations of nanoparticles developed in the last few decades have allowed for the co-encapsulation of drugs with distinct types of molecules, such as peptides with genetic material. The addition of peptides or ligands to nanoparticles is reported to potentiate the targeting of tumor cells, achieving a greater accumulation of the encapsulated drug in the therapeutic target.
TNBC cells and tumor neovascular endothelial cells are known to overexpress various types of receptors, among which are those of α(v)β(3) integrins. The latter receptors play an important role in tumor invasion and metastasis. Several studies on the arginine-glycine-aspartate peptide (RGD) have evidenced its competitive binding to α(v)β(3) integrins, showing lower expression. Shan et al. demonstrated that the functionalization of SLNs with the RGD peptide increased the concentration of nanoparticles in tumor cells, leading to the inhibition of MDA-MB-231 cell adhesion and invasion [
53].
Due to the low therapeutic response observed in TNBC, different alternatives are being investigated, including natural compounds such as DATS (diallyl trisulfide), an antioxidant derived from garlic. It has been reported that DATS has a high cytotoxic effect on the MDA-MB-231 cell line [
54]; however, this compound has limited bioavailability and a short half-life. Recently, De,
et al., reported a cytotoxic effect of DATS in SLN (DATS-SLN) on the MDA-MB-231 cell line. Nevertheless, when DATS-SLN was functionalized with folic acid (FA) to target TNBC cells, a more significant cytotoxic effect was observed compared to DATS-SLN. FA-DATS-SLNs significantly reduced Bcl-2 and increased caspase 9 expression, thereby enhancing apoptosis [
41]. The authors demonstrated that directing SLN with FA improved the efficacy of DATS, paving the way for the development of targeted therapies with natural compounds.
Metastasis is the main cause of death in patients with TNBC. Although ongoing research has been carried out for many years on different strategies for improving the prevention and treatment of metastasis in cancer patients, conventional therapeutic methods have not produced a significant decrease in metastasis or the survival rate (22%) 5 years after the onset of metastasis. According to Da Rocha
et al., the antitumor efficiency of docetaxel was improved by encapsulating it in SLNs, resulting in reduced spontaneous pulmonary metastasis in an orthotopic model of breast cancer [
55]. Overall, the aforementioned findings support the use of SLNs as a promising alternative for the treatment of TNBC.
7. SLNs as a System for the Release of Nucleic Acids
Nucleic acids, such as small interfering RNA (siRNA) and microRNA (miRNA), have recently been applied to the treatment of diverse diseases. When administered with conventional approaches, they have a short half-life and are quickly eliminated from systemic circulation. However, genetic material encapsulated and transported in SLNs has displayed increased circulation time.
As one example of the versatility of SLNs, they have been synthesized with a net positive charge on their surface. These cationic SLNs (cSLNs) have been achieved by utilizing surfactant agents or ionic lipids with positively charged structural groups [
56]. Among the lipids most commonly employed in such nanoformulations are didodecyldimethylammonium bromide (DDAB) and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) [
57].
cSLNs are one of the most efficient nanosystems for the transport of hydrophilic drugs and genetic material with low solubility in water. They hold promise for the targeted delivery of genes to treat a wide variety of diseases. Since the surface of cell membranes and serum proteins have a negative charge, they tend to interact better with cSLNs than other nanoparticles [
56,
58]. Yu et al. developed cSLNs loaded with paclitaxel and plasmid encoding an enhanced green fluorescent protein (pEGFP). This plasmid contains only the EGFP gene. The evaluation of their antineoplastic activity when using in vitro and in vivo models of breast cancer revealed an efficiency superior to that of conventional formulations. Furthermore, hyaluronic acid was added to the SLNs because the distinct pH of tumor cells reacts with it to stimulate the release of the drug and genetic material. Therefore, there was a greater accumulation in tumor tissue, leading to better biodistribution and reduced tumor size [
59].
Liu et al. demonstrated that cSLNs are able to function as nanocarriers of miRNA, protecting it from degradation. They targeted breast cancer stem cells after co-encapsulating paclitaxel with miRNA 200C, observing an enhanced cytotoxic response compared to the same substances administered in conventional form [
60]. Hence, cSLNs can protect nucleic acids from degradation and deliver them to their target.
Another strategy that has been recently investigated is the use of aptamers. Aptamers are single-stranded DNA or RNA sequences that recognize a specific target with high affinity [
61]. Due to the TNBC is highly heterogeneous, different therapeutic targets have been explored, including CD44 and the epidermal growth factor receptor (EGFR). CD44 is a transmembrane glycoprotein overexpressed in TNBC cells, playing a significant role in cell proliferation, migration, and invasion [
62]. Thus, CD44 has become an essential therapeutic target in TNBC research. Darabi,
et al., developed and characterized SLN with anti-CD44 and the epidermal growth factor receptor (EGFR) aptamers to release Doxorubicin. The targeted Doxorubicin delivery system showed a high cytotoxic effect on the MDA-MB-468 cell line compared to nanoparticles not decorated with anti-EGFR and CD44 [
63]. Their results indicated that using aptamers in combination with chemotherapy in SLN could be a promising strategy for TNBC treatment. Recently, it has been a Phase 1 clinical trial (NCT03739931) to evaluate the safety and tolerability of a lipid nanoparticle encapsulating mRNA-2752 (mRNA encoding OX40L T cell co-stimulator, IL-23 and IL-36γ pro-inflammatory cytokines) in advanced solid tumors and lymphoma; the authors reported that the nanoparticle with mRNA-2752 is safe and tolerable when combined with durvalumab. Enrollment is ongoing in expansion cohorts of TNBC, urothelial cancer, lymphoma, immune-checkpoint refractory melanoma, and NSCLC [
64].
8. Future Directions
Several preclinical and clinical studies have investigated using SLNs as a delivery nanosystem in breast cancer. SLN offers several advantages: stability, low cost, and scalable production methods. Furthermore, SLN can co-delivery multiple therapeutic agents, such as antineoplastic drugs, nucleic acids, or several target therapies. The future direction of SLN in TNBC is promising; research efforts will likely focus on refining SLN formulations, optimizing targeting strategies for TNBC tumor cells to decrease side effects, and advancing their clinical translation to benefit patients.
9. Conclusions
SLNs are novel drug transport systems with advantages over other colloidal and polymeric nanocarriers. Their capacity to encapsulate therapeutic agents allows for a controlled and targeted release, thus improving the efficiency of cancer treatment and minimizing adverse effects. The co-encapsulation of drugs with genetic material has been achieved in some SLNs. Additionally, the surface of SLNs has been modified to enhance affinity for tumor cells. The ongoing development of SLNs as a therapeutic strategy opens new perspectives for treating TNBC. Although various studies have shown the great potential of SLNs as an alternative treatment for TNBC, further research is needed on the long-term safety and efficiency of these nanoparticles to be applied in clinical trials.
Author Contributions
Conceptualization, P.G-L and M.L-M.; investigation, M. V-L.; writing, P.G-L. and M L-M; supervision, P.G-L.; funding acquisition, P.G-L and M.L-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially financed by CONAHCYT (Mexico City, Mexico) through grant number: CF-2023-I-2771 and CF-2023-I-2075.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request to the corresponding author.
Acknowledgments
We thank Bruce Allan Larsen for proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209-249. [CrossRef]
- Nedeljkovic, M.; Damjanovic, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8. [CrossRef] [PubMed]
- Rakha, E.A.; Tse, G.M.; Quinn, C.M. An update on the pathological classification of breast cancer. Histopathology 2023, 82, 5-16. [CrossRef] [PubMed]
- Mohamad Hanif, E.A.; Shah, S.A. Overview on Epigenetic Re-programming: A Potential Therapeutic Intervention in Triple Negative Breast Cancers. Asian Pac J Cancer Prev 2018, 19, 3341-3351. [CrossRef] [PubMed]
- MacDonald, I.; Nixon, N.A.; Khan, O.F. Triple-Negative Breast Cancer: A Review of Current Curative Intent Therapies. Curr Oncol 2022, 29, 4768-4778. [CrossRef] [PubMed]
- Al-Thoubaity, F.K. Molecular classification of breast cancer: A retrospective cohort study. Ann Med Surg (Lond) 2020, 49, 44-48. [CrossRef] [PubMed]
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front Mol Biosci 2022, 9, 836417. [CrossRef] [PubMed]
- Diana, A.; Carlino, F.; Franzese, E.; Oikonomidou, O.; Criscitiello, C.; De Vita, F.; Ciardiello, F.; Orditura, M. Early Triple Negative Breast Cancer: Conventional Treatment and Emerging Therapeutic Landscapes. Cancers 2020, 12, 819. [CrossRef] [PubMed]
- Nandini, D.; Jennifer, A.; Pradip, D. Therapeutic Strategies for Metastatic Triple-Negative Breast Cancers: From Negative to Positive. Pharmaceuticals (Basel) 2021, 14. [CrossRef] [PubMed]
- Ramos-Solis, N.; Yeh, E.S. 6.04 - Triple Negative Breast Cancer. In Comprehensive Pharmacology, Kenakin, T., Ed.; Elsevier: Oxford, 2022; pp. 35-48.
- Khadela, A.; Chavda, V.P.; Soni, S.; Megha, K.; Pandya, A.J.; Vora, L. Anti-Androgenic Therapies Targeting the Luminal Androgen Receptor of a Typical Triple-Negative Breast Cancer. Cancers 2023, 15, 233.
- Gerratana, L.; Basile, D.; Buono, G.; De Placido, S.; Giuliano, M.; Minichillo, S.; Coinu, A.; Martorana, F.; De Santo, I.; Del Mastro, L.; et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treatment Reviews 2018, 68, 102-110. [CrossRef] [PubMed]
- Grosse-Wilde, A.; Fouquier d’Hérouël, A.; McIntosh, E.; Ertaylan, G.; Skupin, A.; Kuestner, R.E.; del Sol, A.; Walters, K.A.; Huang, S. Stemness of the hybrid Epithelial/Mesenchymal State in Breast Cancer and Its Association with Poor Survival. PLoS One 2015, 10, e0126522. [CrossRef] [PubMed]
- Wu, Y.; Shum, H.C.E.; Wu, K.; Vadgama, J. From Interaction to Intervention: How Mesenchymal Stem Cells Affect and Target Triple-Negative Breast Cancer. Biomedicines 2023, 11, 1182. [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 2018, 9, 1050-1074. [CrossRef] [PubMed]
- Venturoli, D.; Rippe, B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. Am J Physiol Renal Physiol 2005, 288, F605-613. [CrossRef] [PubMed]
- Ma, Y.; Cai, F.; Li, Y.; Chen, J.; Han, F.; Lin, W. A review of the application of nanoparticles in the diagnosis and treatment of chronic kidney disease. Bioact Mater 2020, 5, 732-743. [CrossRef] [PubMed]
- Decuzzi, P.; Pasqualini, R.; Arap, W.; Ferrari, M. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res 2009, 26, 235-243. [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 2015, 33, 941-951. [CrossRef] [PubMed]
- Gentile, F.; Chiappini, C.; Fine, D.; Bhavane, R.C.; Peluccio, M.S.; Cheng, M.M.; Liu, X.; Ferrari, M.; Decuzzi, P. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J Biomech 2008, 41, 2312-2318. [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg 2019, 6, 23. [CrossRef]
- Smolkova, B.; Dusinska, M.; Gabelova, A. Nanomedicine and epigenome. Possible health risks. Food Chem Toxicol 2017, 109, 780-796. [CrossRef] [PubMed]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines (Basel) 2021, 9. [CrossRef] [PubMed]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front Mol Biosci 2020, 7, 587997. [CrossRef] [PubMed]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Optimization of the Conditions of Solid Lipid Nanoparticles (SLN) Synthesis. Molecules 2022, 27. [CrossRef] [PubMed]
- Bayon-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials (Basel) 2019, 9. [CrossRef] [PubMed]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv 2020, 10, 26777-26791. [CrossRef] [PubMed]
- Hallan, S.S.; Sguizzato, M.; Esposito, E.; Cortesi, R. Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics 2021, 13. [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci 2018, 13, 288-303. [CrossRef] [PubMed]
- Tekade, R.K.; Maheshwari, R.; Tekade, M.; Chougule, M.B. Solid lipid nanoparticles for targeting and delivery of drugs and genes. In Nanotechnology-based approaches for targeting and delivery of drugs and genes; Elsevier: 2017; pp. 256-286.
- Chutoprapat, R.; Kopongpanich, P.; Chan, L.W. A Mini-Review on Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Topical Delivery of Phytochemicals for the Treatment of Acne Vulgaris. Molecules 2022, 27, 3460. [CrossRef]
- Balamurugan K, C.P. Lipid nano particulate drug delivery: An overview of the emerging trend. Pharma Innovation 2018, 7, 779-789.
- Keck, C.M.; Müller, R.H. Nanotoxicological classification system (NCS) – A guide for the risk-benefit assessment of nanoparticulate drug delivery systems. European Journal of Pharmaceutics and Biopharmaceutics 2013, 84, 445-448. [CrossRef] [PubMed]
- Campos, J.R.; Severino, P.; Santini, A.; Silva, A.M.; Shegokar, R.; Souto, S.B.; Souto, E.B. Chapter 1 - Solid lipid nanoparticles (SLN): prediction of toxicity, metabolism, fate and physicochemical properties. In Nanopharmaceuticals, Shegokar, R., Ed.; Elsevier: 2020; pp. 1-15.
- Huang, D.; Sun, L.; Huang, L.; Chen, Y. Nanodrug Delivery Systems Modulate Tumor Vessels to Increase the Enhanced Permeability and Retention Effect. Journal of Personalized Medicine 2021, 11, 124. [CrossRef] [PubMed]
- Niidome, T.; Yamagata, M.; Okamoto, Y.; Akiyama, Y.; Takahashi, H.; Kawano, T.; Katayama, Y.; Niidome, Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release 2006, 114, 343-347. [CrossRef] [PubMed]
- Kenchegowda, M.; Rahamathulla, M.; Hani, U.; Begum, M.Y.; Guruswamy, S.; Osmani, R.A.M.; Gowrav, M.P.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A.; et al. Smart Nanocarriers as an Emerging Platform for Cancer Therapy: A Review. Molecules 2022, 27, 146. [CrossRef] [PubMed]
- Liu, Y.; Hui, Y.; Ran, R.; Yang, G.Z.; Wibowo, D.; Wang, H.F.; Middelberg, A.P.J.; Zhao, C.X. Synergetic Combinations of Dual-Targeting Ligands for Enhanced In Vitro and In Vivo Tumor Targeting. Adv Healthc Mater 2018, 7, e1800106. [CrossRef] [PubMed]
- Roncato, F.; Rruga, F.; Porcù, E.; Casarin, E.; Ronca, R.; Maccarinelli, F.; Realdon, N.; Basso, G.; Alon, R.; Viola, G.; et al. Improvement and extension of anti-EGFR targeting in breast cancer therapy by integration with the Avidin-Nucleic-Acid-Nano-Assemblies. Nat Commun 2018, 9, 4070. [CrossRef] [PubMed]
- Son, J.; Yang, S.M.; Yi, G.; Roh, Y.J.; Park, H.; Park, J.M.; Choi, M.-G.; Koo, H. Folate-modified PLGA nanoparticles for tumor-targeted delivery of pheophorbide a in vivo. Biochemical and Biophysical Research Communications 2018, 498, 523-528. [CrossRef] [PubMed]
- De, A.; Roychowdhury, P.; Bhuyan, N.R.; Ko, Y.T.; Singh, S.K.; Dua, K.; Kuppusamy, G. Folic Acid Functionalized Diallyl Trisulfide-Solid Lipid Nanoparticles for Targeting Triple Negative Breast Cancer. Molecules 2023, 28. [CrossRef]
- Hafeez, M.N.; Celia, C.; Petrikaite, V. Challenges towards Targeted Drug Delivery in Cancer Nanomedicines. Processes 2021, 9, 1527. [CrossRef]
- Ying, X.-Y.; Du, Y.-Z.; Hong, L.-H.; Yuan, H.; Hu, F.-Q. Magnetic lipid nanoparticles loading doxorubicin for intracellular delivery: Preparation and characteristics. Journal of Magnetism and Magnetic Materials 2011, 323, 1088-1093. [CrossRef]
- Sundaram, J.; Mellein, B.R.; Mitragotri, S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys J 2003, 84, 3087-3101. [CrossRef]
- Lammertink, B.; Deckers, R.; Storm, G.; Moonen, C.; Bos, C. Duration of ultrasound-mediated enhanced plasma membrane permeability. Int J Pharm 2015, 482, 92-98. [CrossRef]
- Spoială, A.; Ilie, C.-I.; Motelica, L.; Ficai, D.; Semenescu, A.; Oprea, O.-C.; Ficai, A. Smart Magnetic Drug Delivery Systems for the Treatment of Cancer. Nanomaterials 2023, 13, 876. [CrossRef] [PubMed]
- Paiva, K.L.R.; Radicchi, M.A.; Bao, S.N. In Vitro Evaluation of NLS-DTX Activity in Triple-Negative Breast Cancer. Molecules 2022, 27. [CrossRef] [PubMed]
- Abd El-Aziz, Y.S.; Spillane, A.J.; Jansson, P.J.; Sahni, S. Role of ABCB1 in mediating chemoresistance of triple-negative breast cancers. Biosci Rep 2021, 41. [CrossRef]
- Lopes-Rodrigues, V.; Sousa, E.; Vasconcelos, M.H. Curcumin as a Modulator of P-Glycoprotein in Cancer: Challenges and Perspectives. Pharmaceuticals (Basel) 2016, 9. [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25. [CrossRef]
- Fathy Abd-Ellatef, G.E.; Gazzano, E.; Chirio, D.; Hamed, A.R.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Assem Said Marie, M.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics 2020, 12. [CrossRef]
- Hatami, M.; Kouchak, M.; Kheirollah, A.; Khorsandi, L.; Rashidi, M. Quercetin-loaded solid lipid nanoparticles exhibit antitumor activity and suppress the proliferation of triple-negative MDA-MB 231 breast cancer cells: implications for invasive breast cancer treatment. Mol Biol Rep 2023, 50, 9417-9430. [CrossRef] [PubMed]
- Shan, D.; Li, J.; Cai, P.; Prasad, P.; Liu, F.; Rauth, A.M.; Wu, X.Y. RGD-conjugated solid lipid nanoparticles inhibit adhesion and invasion of alphavbeta3 integrin-overexpressing breast cancer cells. Drug Deliv Transl Res 2015, 5, 15-26. [CrossRef]
- Marni, R.; Kundrapu, D.B.; Chakraborti, A.; Malla, R. Insight into drug sensitizing effect of diallyl disulfide and diallyl trisulfide from Allium sativum L. on paclitaxel-resistant triple-negative breast cancer cells. J Ethnopharmacol 2022, 296, 115452. [CrossRef] [PubMed]
- da Rocha, M.C.O.; da Silva, P.B.; Radicchi, M.A.; Andrade, B.Y.G.; de Oliveira, J.V.; Venus, T.; Merker, C.; Estrela-Lopis, I.; Longo, J.P.F.; Bao, S.N. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J Nanobiotechnology 2020, 18, 43. [CrossRef] [PubMed]
- Subhan, M.A.; Filipczak, N.; Torchilin, V.P. Advances with Lipid-Based Nanosystems for siRNA Delivery to Breast Cancers. Pharmaceuticals (Basel) 2023, 16. [CrossRef] [PubMed]
- Bondi, M.L.; Craparo, E.F. Solid lipid nanoparticles for applications in gene therapy: a review of the state of the art. Expert Opin Drug Deliv 2010, 7, 7-18. [CrossRef] [PubMed]
- Mendonca, M.C.P.; Radaic, A.; Garcia-Fossa, F.; da Cruz-Hofling, M.A.; Vinolo, M.A.R.; de Jesus, M.B. The in vivo toxicological profile of cationic solid lipid nanoparticles. Drug Deliv Transl Res 2020, 10, 34-42. [CrossRef] [PubMed]
- Yu, D.; Li, W.; Zhang, Y.; Zhang, B. Anti-tumor efficiency of paclitaxel and DNA when co-delivered by pH responsive ligand modified nanocarriers for breast cancer treatment. Biomed Pharmacother 2016, 83, 1428-1435. [CrossRef] [PubMed]
- Liu, J.; Meng, T.; Yuan, M.; Wen, L.; Cheng, B.; Liu, N.; Huang, X.; Hong, Y.; Yuan, H.; Hu, F. MicroRNA-200c delivered by solid lipid nanoparticles enhances the effect of paclitaxel on breast cancer stem cell. Int J Nanomedicine 2016, 11, 6713-6725. [CrossRef] [PubMed]
- Ospina, J.D. Aptamers as a novel diagnostic and therapeutic tool and their potential use in parasitology. Biomedica 2020, 40, 148-165. [CrossRef] [PubMed]
- Nabil, G.; Alzhrani, R.; Alsaab, H.O.; Atef, M.; Sau, S.; Iyer, A.K.; Banna, H.E. CD44 Targeted Nanomaterials for Treatment of Triple-Negative Breast Cancer. Cancers (Basel) 2021, 13. [CrossRef]
- Darabi, F.; Saidijam, M.; Nouri, F.; Mahjub, R.; Soleimani, M. Anti-CD44 and EGFR Dual-Targeted Solid Lipid Nanoparticles for Delivery of Doxorubicin to Triple-Negative Breast Cancer Cell Line: Preparation, Statistical Optimization, and In Vitro Characterization. Biomed Res Int 2022, 2022, 6253978. [CrossRef]
- Patel, M.; Jimeno, A.; Wang, D.; Stemmer, S.; Bauer, T.; Sweis, R.; Geva, R.; Kummar, S.; Reagan, P.; Perets, R.; et al. 539 Phase 1 study of mRNA-2752, a lipid nanoparticle encapsulating mRNAs encoding human OX40L/IL-23/IL-36γ, for intratumoral (ITu) injection +/- durvalumab in advanced solid tumors and lymphoma. Journal for ImmunoTherapy of Cancer 2021, 9, A569-A569. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).