Submitted:
23 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Diagnostic Criteria
2.3. Histopathological Correlation
2.4. Statistechal Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, R.; Naran, S.; Lallu, S.; Fauck, R. The diagnostic value of fine needle aspiration cytology (FNAC) in the assessment of palpable supraclavicular lymph nodes: a study of 218 cases. Cytopathology 2003, 14, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Hafez, N.H.; Tahoun, N.S. Reliability of fine needle aspiration cytology (FNAC) as a diagnostic tool in cases of cervical lymphadenopathy. Journal of the Egyptian National Cancer Institute 2011, 23, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Makarenko, V.V.; DeLelys, M.E.; Hasserjian, R.P.; Ly, A. Lymph node FNA cytology: diagnostic performance and clinical implications of proposed diagnostic categories. Cancer Cytopathology 2022, 130, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cordero, R. Challenges and opportunities in lymph node FNA: Insights from the DELYCYUS study. Cancer Cytopathology 2023, 131, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, R.; Wilkinson, A. Utility of fine needle aspiration cytology of lymph nodes. IOSR J Dent Med Sci 2013, 8, 13–18. [Google Scholar] [CrossRef]
- Zhou, J.; Li, F.; Meng, L.; Hao, F.; Liu, X.; Zhao, C.; Zhang, K.; Dong, A. Fine needle aspiration cytology for lymph nodes: a three-year study. British Journal of Biomedical Science 2016, 73, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.; Ciliberti, V.; D'Antonio, A.; D'Ardia, A.; Fumo, R.; Giudice, V.; Pezzullo, L.; Sabbatino, F.; Zeppa, P. Real-world experience with the Sydney system on 1458 cases of lymph node fine needle aspiration cytology. Cytopathology 2022, 33, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Kanhe, R.; Tummidi, S.; Kothari, K.; Agnihotri, M. Utility of the proposed Sydney system for classification of fine-needle aspiration cytopathology of lymph node: a retrospective study at a tertiary care center. Acta Cytologica 2023, 67, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Al-Abbadi, M.A.; Barroca, H.; Bode-Lesniewska, B.; Calaminici, M.; Caraway, N.P.; Chhieng, D.F.; Cozzolino, I.; Ehinger, M.; Field, A.S.; Geddie, W.R. A proposal for the performance, classification, and reporting of lymph node fine-needle aspiration cytopathology: the Sydney system. Acta Cytologica 2020, 64, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Saradva, N.; Trivedi, D.P. Lymph node fine needle aspiration cytology reporting using Sydney system at tertiary care center.
- Sreelekshmi, J.R.; Joseph, T. Structured reporting of lymph node cytopathology using the 2020 sydney system guidelines-a retrospective study. National Journal of Laboratory Medicine 2023, 12, 39–44. [Google Scholar] [CrossRef]
- Zeppa, P.; Cozzolino, I.; Caraway, N.P.; Al-Abbadi, M.A.; Barroca, H.; Bode-Lesniewska, B.; Calaminici, M.; Chhieng, D.F.; Ehinger, M.; Geddie, W.R. Announcement: the international system for reporting lymph node cytopathology. Acta Cytologica 2020, 64, 299–305. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: the kappa statistic. Biochemia medica 2012, 22, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Goswami, B.; Chakrabarti, S.; Giri, A.; Pramanik, R. Fine needle aspiration cytology of lymph nodes-An institutional study of 1448 cases over a five year period. Journal of Cytology 2004, 21, 187–190. [Google Scholar] [CrossRef]
- Pangarkar, M.A. The Bethesda System for reporting cervical cytology. Cytojournal 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Aziz Khan, A.; Ahuja, R.; Ahuja, P.; Zaheer, S. Systematic Review and Meta-Analysis of the Diagnostic Accuracy of the Sydney System for Reporting Lymph Node Fine-Needle Aspiration Biopsy in Diagnosing Malignancy. Acta Cytologica 2024, 68, 13–25. [Google Scholar] [CrossRef]
- Vigliar, E.; Acanfora, G.; Iaccarino, A.; Mascolo, M.; Russo, D.; Scalia, G.; Della Pepa, R.; Bellevicine, C.; Picardi, M.; Troncone, G. A novel approach to classification and reporting of lymph node fine-needle cytology: application of the proposed Sydney system. Diagnostics 2021, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Torres Rivas, H.E.; Villar Zarra, K.; Pérez Pabón, L.A.; González Gutierréz, M.d.l.P.; Zapico Ortiz, N.; Olmo Fernández, M.d.M.; Nieto Llanos, S.; Antoranz Álvarez, N.; Gómez Martín, Á.; Fernández Fernández, L.M. Ultrasound-guided fine-needle aspiration of superficial lymphadenopathy performed by interventional pathologists: the applicability of the Sydney system from 2 years of experience and 363 cases. Acta Cytologica 2021, 65, 453–462. [Google Scholar] [CrossRef] [PubMed]
- GV, N.; Indoria, P.; Bijjaragi, S. Application of the proposed Sydney system of reporting lymphnode cytopathology: A retrospective study in a tertiary institute. Onkologia i Radioterapia 2023, 17. [Google Scholar]
- Gupta, P.; Gupta, N.; Kumar, P.; Bhardwaj, S.; Srinivasan, R.; Dey, P.; Rohilla, M.; Bal, A.; Das, A.; Rajwanshi, A. Assessment of risk of malignancy by application of the proposed Sydney system for classification and reporting lymph node cytopathology. Cancer Cytopathology 2021, 129, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.S.; Saldanha, C. An approach to classification and reporting lymph node cytopathology using Sydney system and evaluating the likelihood of malignancy. Biomedicine 2023, 43, 701–705. [Google Scholar] [CrossRef]
- Juanita, J.; Ikram, D.; Sungowati, N.K.; Purnama, I.P.; Amalia, A.; Ningrati, A.F.; Miskad, U.A. Diagnostic accuracy of lymph nodes fine needle aspiration biopsy based on the Sydney system for reporting lymph node cytology. Asian Pacific Journal of Cancer Prevention: APJCP 2023, 24, 1917. [Google Scholar] [CrossRef]
| Category | The cytomorphologic features |
|---|---|
| L1: Inadequate/Insufficient | Scant cellularity; Extensive necrosis; Technical limitations that cannot be overcome |
| L2: Benign | Suppurative and granulomatous inflammation; Heterogeneous lymphoid population with small lymphocytes predominating, and often germinal centers with dendritic cells and tingible body macrophages |
| L3: Atypical (Cells) Undetermined Significance/Atypical Lymphoid (Cells) of Uncertain Significance (ALUS/AUS) | Heterogeneous lymphoid population, features suggest a reactive process, follicular lymphoma cannot be excluded; Excess of large cells (centroblasts or immunoblasts) or immature small lymphoid cells or cases where the atypical cells are not lymphoid cells. |
| L4: Suspicious | Small and/or medium-sized, monomorphic atypical lymphoid cells suspicious of lymphoma, but the cytomorphology alone is not sufficient; Polymorphous lymphoid smears, few Hodgkin- or Reed-Sternberg-like cells are detected; Large cell or Burkitt lymphomas scantly cellular; Smears in which atypical cells suspicious for metastasis are detected, but are too scant to be diagnostic |
| L5: Malignant | NHL; HL: Appropriate cellular background and diagnostic Hodgkin and Reed-Sternberg cells; Metastatic neoplasms. |
| Variable | No. (%) |
|---|---|
| Age | |
| ≤ 50 years | 79 (42) |
| > 50 years | 109 (58) |
| Age (years) (Mean ± SD) | 51.22 ± 16.4 |
| Gender | |
| Female | 117 (62.2) |
| Male | 71 (37.8) |
| Specimen source | |
|---|---|
| Axillary LN | 72 (38.3) |
| Cervical LN | 20 (10.6) |
| Hilar mass | 9 (4.8) |
| Inguinal LN | 2 (1.1) |
| Neck LN | 2 (1.1) |
| Paratracheal LN | 18 (9.6) |
| Peripancreatic LN | 1 (0.5) |
| Subcarinal LN | 22 (11.7) |
| Submandibular LN | 8 (4.3) |
| Supraclavicular LN | 3 (1.6) |
| Thyroid | 1 (0.5) |
| NA | 30 (16) |
| Tissue correlation | |
| No | 1 (0.5) |
| Yes | 187 (99.5) |
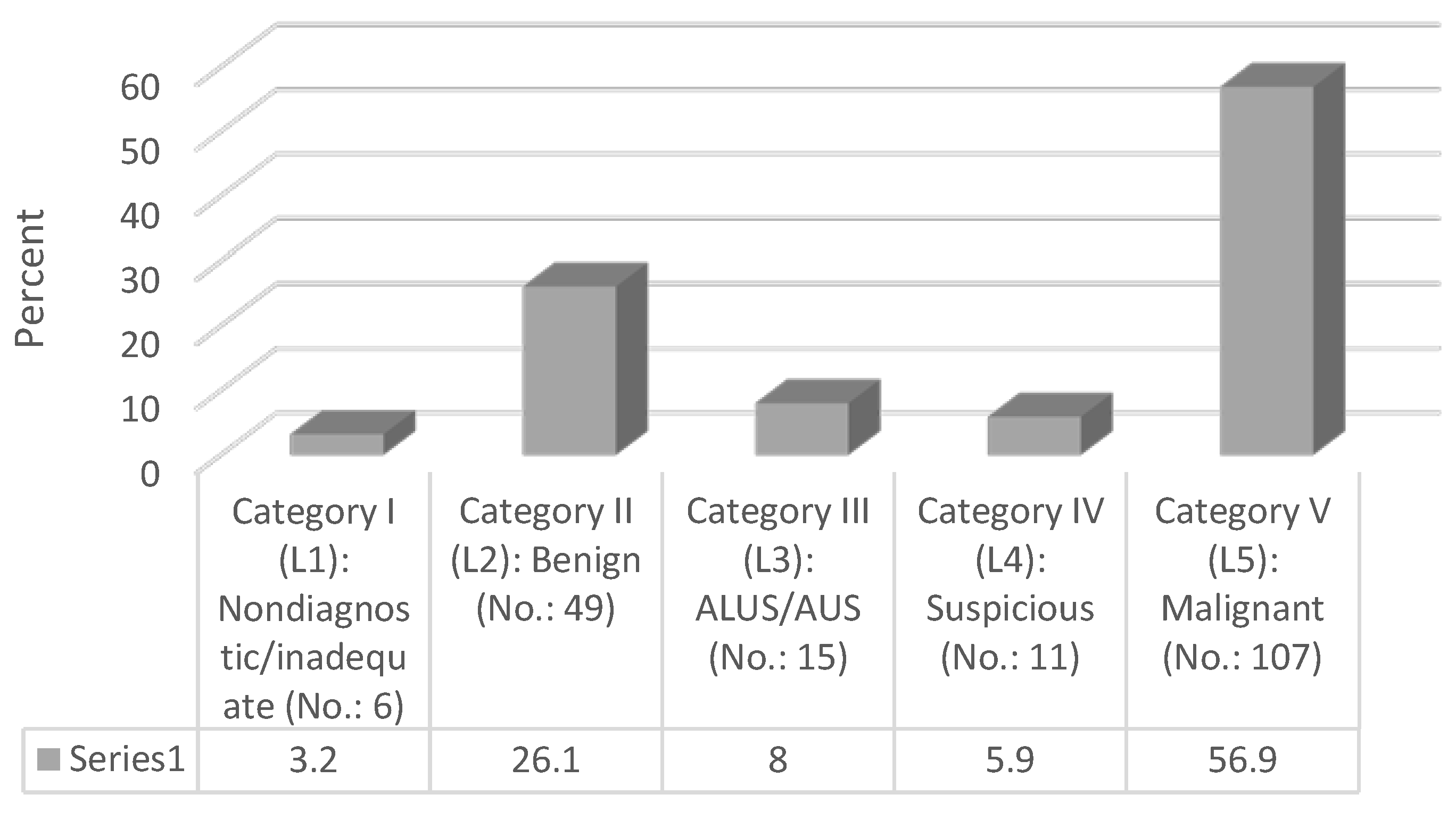
| Sydney system Category | |
| Category I (L1): Nondiagnostic/inadequate | 6 (3.2) |
| Category II (L2): Benign | 49 (26.1) |
| Category III (L3): ALUS/AUS | 15 (8) |
| Category IV (L4): Suspicious | 11 (5.9) |
| Category V (L5): Malignant | 107 (56.9) |
| Final surgical specimen diagnosis | |
| Benign | 67 (35.6) |
| Malignant | 121 (64.4) |
| Variable | Parameter | Estimate |
|---|---|---|
| Sydney System Category classification results compared to the final surgical specimen diagnosis | True positive | 95 (50.5) |
| True negative | 55 (29.3) | |
| False positive | 12 (6.4) | |
| False negative | 26 (13.8) | |
| Sensitivity | 78.5% | |
| Specificity | 82% | |
| Positive predictive value (PPV) | 88.7% | |
| Negative predictive value (NPV) | 67.9% | |
| Accuracy rate | 79.7% |
|
Cytologic category as per the proposed Sydney system for reporting lymph node cytopathology |
Total | Final surgical specimen diagnosis | χ2 | p-value | |
|---|---|---|---|---|---|
| No. (%) | Benign No. (%) |
Malignant No. (%) |
|||
| L1: Inadequate/Insufficient | 6 (3.2) | 4 (6) | 2 (1.7) | 13.95 | <0.001 |
| L2: Benign | 49 (26.1) | 42 (62.7) | 7 (5.8) | ||
| L3: Atypical (Cells) Undetermined | 15 (8) | 5 (7.5) | 10 (8.3) | ||
| L4: Suspicious | 11 (5.9) | 4 (6) | 7 (5.8) | ||
| L5: Malignant | 107 (56.9) | 12 (17.9) | 95 (78.5) | ||
| Cytologic category as per the proposed Sydney system for reporting lymph node Cytopathology |
Total no. of cases with histopathologic diagnosis in each diagnostic category (No.:188) | Total no. of cases reported as malignant on histopathology (No.: 121) | Overall risk of malignancy (ROM) (%) |
|---|---|---|---|
| Category I (L1): Nondiagnostic/inadequate | 6 (3.2) | 2 | 33.3 |
| Category II (L2): Benign | 49 (26.1) | 7 | 14.2 |
| Category III (L3): ALUS/AUS | 15 (8) | 10 | 66.6 |
| Category IV (L4): Suspicious | 11 (5.9) | 7 | 63.6 |
| Category V (L5): Malignant | 107 (56.9) | 95 | 88.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




