Submitted:
22 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Soybean Cultivars and Nematode Source
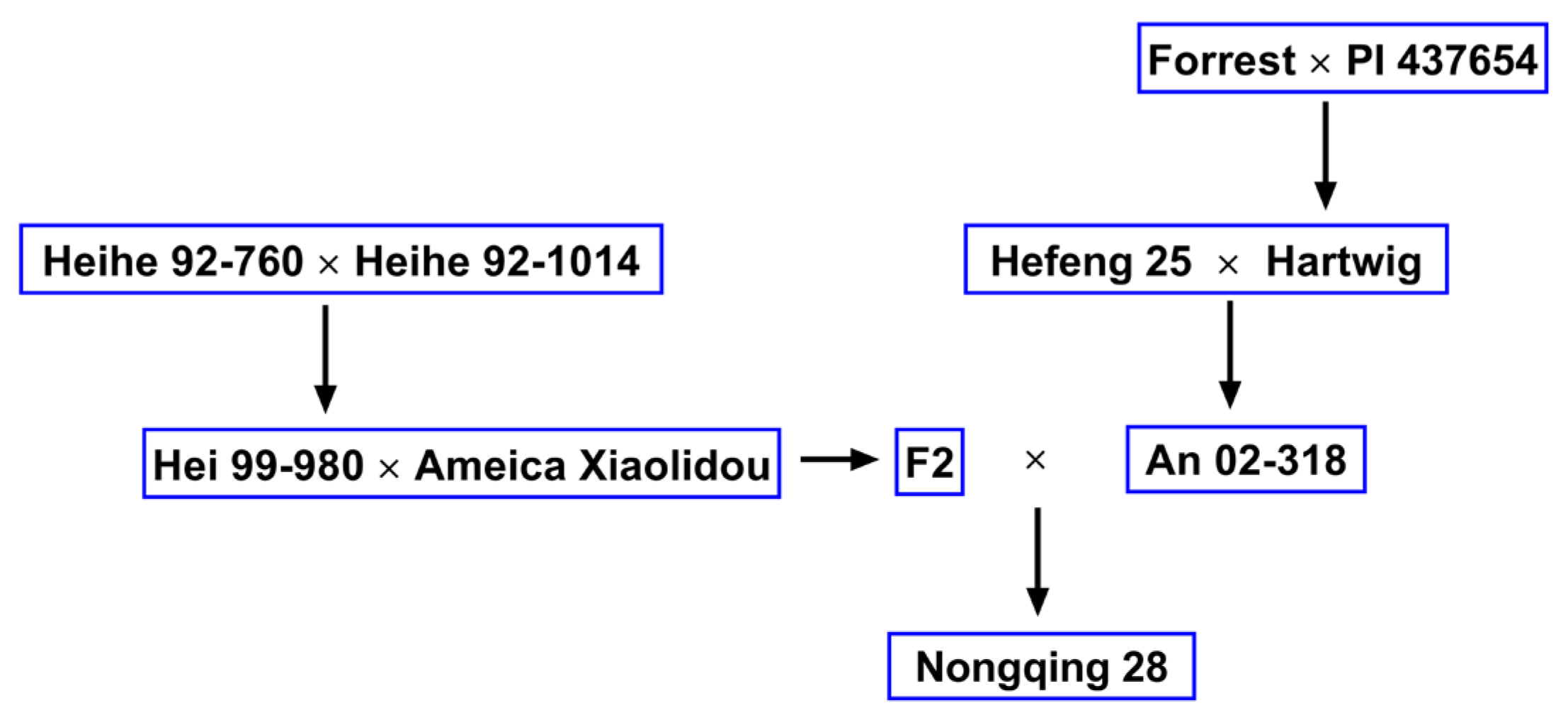
2.2. Breeding Nongqing 28 and Selection
2.3. Assessment of SCN Resistance
2.4. Analysis of Seed Protein and Fat Contents
2.5. RNA-Seq Analysis
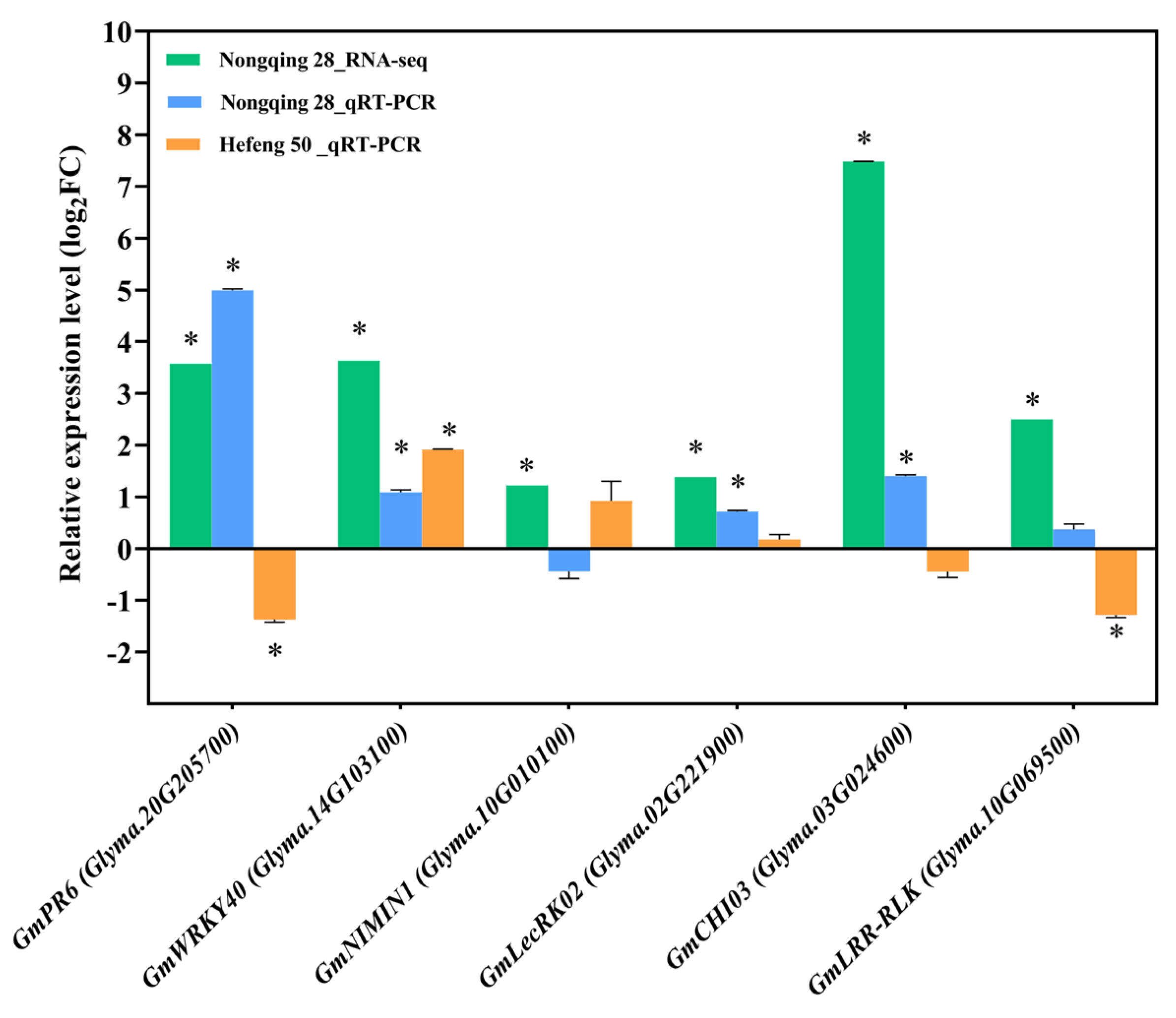
2.6. Quantitative Real-Time (qRT)-PCR Analysis
2.7. Data Analysis
3. Results
3.1. Main Characters of Nongqing 28
3.2. SCN Resistance of Nongqing 28
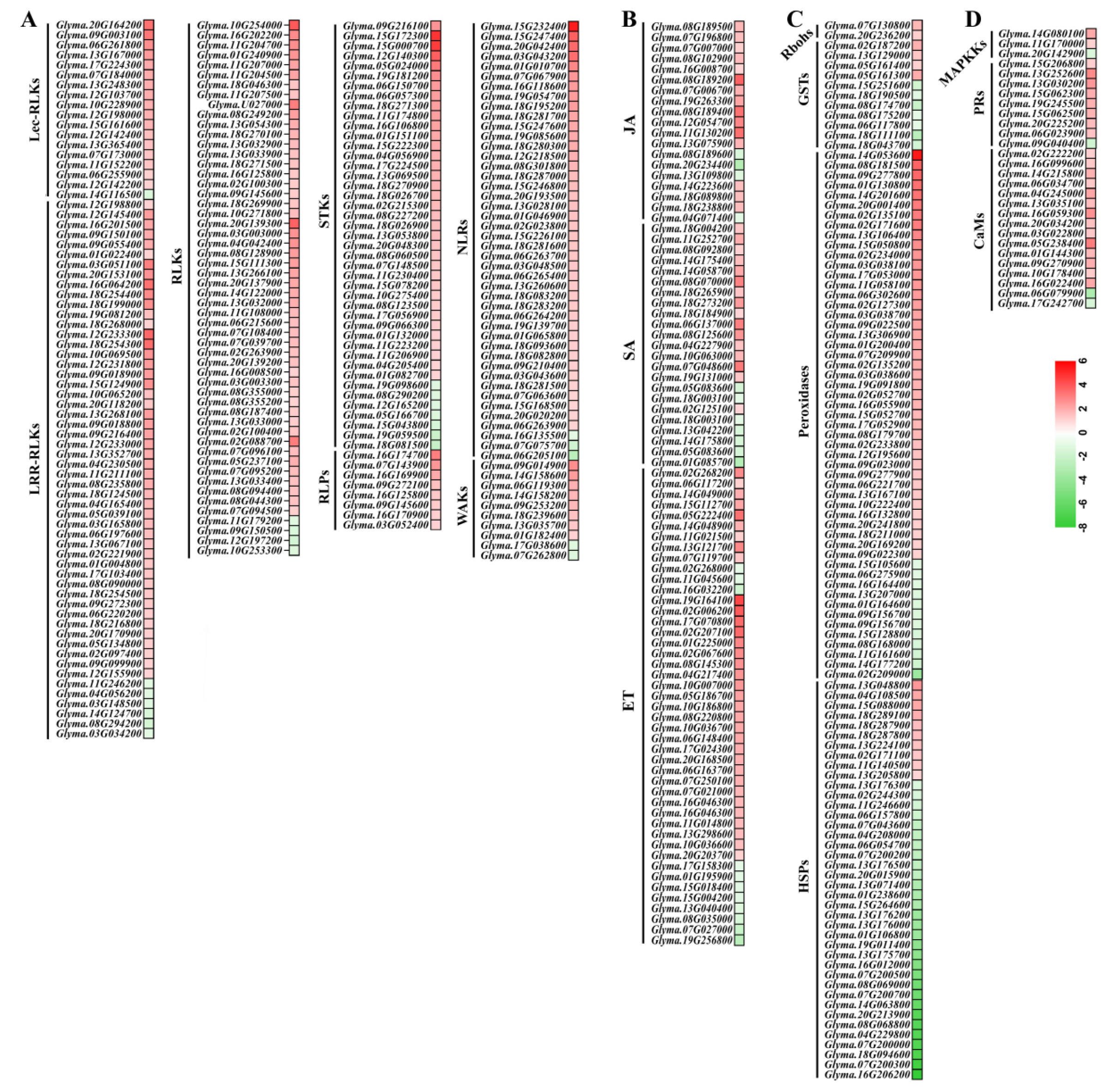
3.3. Transcription changes in Nongqing 28 in response to SCN infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Peng, D.L.; Jiang, R.; Peng, H.; Liu, S.M. Soybean cyst nematodes: a destructive threat to soybean production in China. Phytopathol Res 2021, 3, 19. [Google Scholar] [CrossRef]
- Tylka, G.L.; Marett, C.C. Known Distribution of the Soybean Cyst Nematode, Heterodera glycines, in the United States and Canada in 2020. Plant Health Progress. 2021, 22, 72–74. [Google Scholar] [CrossRef]
- Bandara, A.Y.; Weerasooriya, D.K.; Bradley, C.A.; Allen, T.W.; Esker, P.D. Dissecting the economic impact of soybean diseases in the United States over two decades. PLoS One. 2020, 15, e0231141. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Koch, G.; Bo, D.; Wang, J.; Nguyen, H.T.; Li, C.; Lu, W.G. The Spatial Distribution and Genetic Diversity of the Soybean Cyst Nematode, Heterodera glycines, in China: It Is Time to Take Measures to Control Soybean Cyst Nematode. Front Plant Sci. 2022, 13, 927773. [Google Scholar] [CrossRef]
- You, J.; Wang, J.J.; Tian, R.Z.; Wang, S.R.; Yu, Y.; Xu, L.J.; Zhou, C.J.; Pan, F.J.; Chen, J.S.; Sui, Y.Y.; Hu, Y.F. Survey of Heterodera glycines population abundance and virulence phenotypes during 2021-2022 in Heilongjiang Province. Plant Dis 2024. [Google Scholar] [CrossRef] [PubMed]
- Arjoune, Y.; Sugunaraj, N.; Peri, S.; Nair, S.V.; Skurdal, A.; Ranganathan, P.; Johnson, B. Soybean cyst nematode detection and management: A review. Plant Methods. 2022, 18, 6–12. [Google Scholar] [CrossRef]
- Brucker, E.; Carlson, S.; Wright, E.; Niblack, T.; Diers, B. Rhg1 alleles from soybean PI 437654 and PI 88788 respond diferentially to isolates of Heterodera glycines in the greenhouse. Theor Appl Genet. 2005, 111, 44–49. [Google Scholar] [CrossRef]
- Kofsky, J.; Zhang, H.; Song, B.H. Novel resistance strategies to soybean cyst nematode (SCN) in wild soybean. Sci Rep. 2021, 11, 1–13. [Google Scholar]
- Cook, D.E.; Lee, T.G.; Guo, X.; Melito, S.; Wang, K.; Bayless, A.M.; Wang, J.; Hughes, T.J.; Willis, D.K.; Clemente, T.E.; Diers, B.W.; Jiang, J.; Hudson, M.E.; Bent, A.F. Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science. 2012, 338, 1206–1209. [Google Scholar] [CrossRef]
- Liu, S.; Kandoth, P.K.; Warren, S.D.; Yeckel, G.; Heinz, R.; Alden, J.; Yang, C.; Jamai, A.; El-Mellouki, T.; Juvale, P.S.; Hill, J.; Baum, T.J.; Cianzio, S.; Whitham, S.A.; Korkin, D.; Mitchum, M.G.; Meksem, K. A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature. 2012, 492, 256–260. [Google Scholar] [CrossRef]
- Vuong, T.D.; Sleper, D.A.; Shannon, J.G.; Nguyen, H.T. Novel quantitative trait loci for broad-based resistance to soybean cyst nematode (Heterodera glycines Ichinohe) in soybean PI 567516C. Theor Appl Genet. 2021, 121, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Chen, J.S.; Zhang, F.; Li, Z.Y.; Chen, H.F.; Zhang, C.J.; Chen, L.M.; Yuan, S.L.; Li, R.; Cao, D.; Hao, Q.N.; Chen, S.L.; Shan, Z.H.; Yang, Z.L.; Zhang, X.J.; Qiu, Z.; You, Q.B.; Dai, W.J.; Zhou, X.A.; Shen, X.J.; Jiao, Y.Q. Characterization of Pingliang xiaoheidou (ZDD 11047), a soybean variety with resistance to soybean cyst nematode Heterodera glycines. Plant Mol Biol. 2020, 103, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Lian, Y.; Li, J.; Li, H.; Song, Q.; Wu, Y.; Lei, C.; Wang, S.; Zhang, H.; Wang, J.; Lu, W. Identification of Candidate Genes Controlling Soybean Cyst Nematode Resistance in “Handou 10” Based on Genome and Transcriptome Analyzes. Front Plant Sci 2022, 13, 860034. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Li, J.Q.; Zhang, D.S. History and status of soybean cyst nematode in China. Int J Nematol. 1997, 7, 18–25. [Google Scholar]
- Wang, J.; Kong, L.; Zhang, L.; Shi, X.; Yu, B.; Li, J.; Zhang, B.; Gao, M.; Liu, X.; Li, X.; Gao, Y.; Peng, D.; Liu, S. Breeding a Soybean Cultivar Heinong 531 with Peking-Type Cyst Nematode Resistance, Enhanced Yield, and High Seed-Oil Contents. Phytopathology. 2022, 112, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Y.; Wang, Y.; Fan, H.; Liu, X.; Wang, D.; Zhao, D.; Duan, Y.; Zhu, X.; Chen, L. Characterization of Virulence Phenotypes of Heterodera glycines in Heilongjiang, Northeast China. Plant Dis. 2021, 105, 2056–2060. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Gao, G.J.; Zhou, C.J.; Du, Z.Q.; Wu, Y.K.; Wang, M.Z.; Yang, L.; Li, J.Y. Study on the variation of soybean cyst nematode. Soybean Sci. 2007, 26, 290–292. [Google Scholar]
- Ma, Y.S.; Wang, W.H.; Wang, L.X.; Ma, F.M.; Wang, P.W.; Chang, R.Z.; Qiu, L.J. Genetic diversity of soybean and the establishment of a core collection focused on resistance to soybean cyst nematode. J Integr Plant Biol. 2006, 48, 722–731. [Google Scholar] [CrossRef]
- Meng, F.L.; Yu, J.Y.; Li, C.J.; Huang, M.H.; Zhao, L.; Wang, X.; Jiang, Y.; Qin, R.F.; Wang, C.L. Research progress on occurrence and management of soybean cyst nematode in Northeast China. Journal of Northeast Agricultural University 2022, 53, 87–94. (in Chinese). [Google Scholar]
- Wang, J.; Kong, L.; Zhang, L.; Shi, X.; Yu, B.; Li, J.; Zhang, B.; Gao, M.; Liu, X.; Li, X.; Gao, Y.; Peng, D.; Liu, S. Breeding a Soybean Cultivar Heinong 531 with Peking-Type Cyst Nematode Resistance, Enhanced Yield, and High Seed-Oil Contents. Phytopathology. 2022, 112, 1345–1349. [Google Scholar] [CrossRef]
- Riggs, R.D.; Schmitt, D.P. Complete Characterization of the Race Scheme for Heterodera glycines. J Nematol. 1988, 20, 392–395. [Google Scholar] [PubMed]
- Niblack, T.L.; Arelli, P.R.; Noel, G.R.; Opperman, C.H.; Orf, J.H. A revised classification scheme for genetically diverse populations of Heterodera glycines. J Nematol. 2002, 34, 279–288. [Google Scholar] [PubMed]
- Byrd, D.W.; Kirkpatrick, T.; Barker, K.R. An improved technique for clearing and staining plant tissues for detection of nematodes. J Nematol. 1983, 15, 142–143. [Google Scholar]
- Sun, M.M.; Wang, P.; Lv, S.X. New soybean varieties released in Heilongjiang Province in 2015. Soybean Sci 2015, 34, 918–920, (article in Chinese). [Google Scholar]
- Li, Y.H.; Li, X.B.; Tian, Z.Y.; Gao, J.G.; Cai, J.L.; Du, Z.Q. Study on the race about continuous plant varieties with resistance to SCN. Soybean Sci. 1998, 17, 370–372, (article in Chinese). [Google Scholar]
- Yu, B.S.; Wang, J.J.; Cui, L.W. Effects of Continuous Planting of Resistant SoybeanCultivars on Races of Soybean Cyst Nematode. Heilongjiang Agricultural Science 1999, 3, 5–7, (article in Chinese). [Google Scholar]
- Song, X.J. New soybean varieties released in Heilongjiang Province in 2012. Soybean Sci. 2012, 31, 504–510, (article in Chinese). [Google Scholar]
- Anand, S.C. Registration of ‘Hartwig’ soybean. Crop Sci. 1992, 32, 1069–1070. [Google Scholar] [CrossRef]
- Klink, V.P.; Overall, C.C.; Alkharouf, N.W.; Macdonald, M.H.; Matthews, B.F. Microarray detection call methodology as a means to identify and compare transcripts expressed within syncytial cells from soybean (Glycine max) roots undergoing resistant and susceptible reactions to the soybean cyst nematode (Heterodera glycines). J Biomed Biotechnol. 2010, 2010, 491217. [Google Scholar] [CrossRef]
- Klink, V.P.; Hosseini, P.; Matsye, P.D.; Alkharouf, N.W.; Matthews, B.F. Differences in gene expression amplitude overlie a conserved transcriptomic program occurring between the rapid and potent localized resistant reaction at the syncytium of the Glycine max genotype Peking (PI 548402) as compared to the prolonged and potent resistant reaction of PI 88788. Plant Mol Biol. 2011, 75, 141–165. [Google Scholar]
- Mazarei, M.; Liu, W.; Al-Ahmad, H.; Arelli, P.R.; Pantalone, V.R.; Stewart, Jr. C.N. Gene expression profiling of resistant and susceptible soybean lines infected with soybean cyst nematode. Theor Appl Genet. 2011, 123, 1193–1206. [Google Scholar] [CrossRef]
- Wan, J.; Vuong, T.; Jiao, Y.; Joshi, T.; Zhang, H.; Xu, D.; Nguyen, H.T. Whole-genome gene expression profiling revealed genes and pathways potentially involved in regulating interactions of soybean with cyst nematode (Heterodera glycines Ichinohe). BMC Genomics. 2015, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kjemtrup-Lovelace, S.; Li, C.; Luo, Y.; Chen, L.P.; Song, B.H. Comparative RNA-Seq Analysis Uncovers a Complex Regulatory Network for Soybean Cyst Nematode Resistance in Wild Soybean (Glycine soja). Scientific reports. 2017, 7, 9699. [Google Scholar] [CrossRef] [PubMed]
- Khatri, R.; Pant, S.R.; Sharma, K.; Niraula, P.M.; Lawaju, B.R.; Lawrence, K.S.; Alkharouf, N.W.; Klink, V.P. Glycine max Homologs of DOESN’T MAKE INFECTIONS 1, 2, and 3 Function to Impair Heterodera glycines Parasitism While Also Regulating Mitogen Activated Protein Kinase Expression. Front Plant Sci. 2022, 13, 842597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, Q.; Tan, Y.; Deng, M.; Zhang, L.; Cao, Y.; Guo, X. Mitogen-activated protein kinases MPK3 and MPK6 phosphorylate receptor-like cytoplasmic kinase CDL1 to regulate soybean basal immunity. Plant Cell. 2024, 36, 963–986. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Mazarei, M.; Zhao, N.; Zhu, J.J.; Zhuang, X.; Liu, W.; Pantalone, V.R.; Arelli, P.R.; Stewart, C.N. Jr.; Chen, F. Overexpression of a soybean salicylic acid methyltransferase gene confers resistance to soybean cyst nematode. Plant Biotechnol J. 2013, 11, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Mazarei, M.; Zhao, N.; Hatcher, C.N.; Wuddineh, W.A.; Rudis, M.; Tschaplinski, T.J.; Pantalone, V.R.; Arelli, P.R.; Hewezi, T.; Chen, F.; Stewart, C.N., Jr. Transgenic soybean overexpressing GmSAMT1 exhibits resistance to multiple-HG types of soybean cyst nematode Heterodera glycines. Plant Biotechnol J. 2016, 14, 2100–2109. [Google Scholar] [CrossRef]
- Matthews, B.F.; Beard, H.; Brewer, E.; Kabir, S. MacDonald MH, Youssef RM. Arabidopsis genes, AtNPR1, AtTGA2 and AtPR-5, confer partial resistance to soybean cyst nematode (Heterodera glycines) when overexpressed in transgenic soybean roots. BMC Plant Biol 2014, 14, 96. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, F.; Bao, A.; You, Q.; Li, Z.; Chen, J.; Cheng, Y.; Zhao, W.; Shen, X.; Zhou, X.; Jiao, Y. The soybean Rhg1 amino acid transporter gene alters glutamate homeostasis and jasmonic acid-induced resistance to soybean cyst nematode. Mol Plant Pathol. 2019, 20, 270–286. [Google Scholar] [CrossRef]
- Tucker, M.L.; Xue, P.; Yang, R. 1-Aminocyclopropane-1-carboxylic acid (ACC) concentration and ACC synthase expression in soybean roots, root tips, and soybean cyst nematode (Heterodera glycines)-infected roots. J Exp Bot. 2010, 61, 463–472. [Google Scholar] [CrossRef]


| Year | Trial locations | Height (cm) |
Seed yield (Kg/ha) |
Increase (%) | Oil contents | Protein contents |
|---|---|---|---|---|---|---|
| 2018 | Anda Dapeng Seeds Co.Ltd. | 92 | 2471.2 | 1.3 | 18.93 | 41.26 |
| Longjiang Branch of Qishan Seeds Co.Ltd. | 105 | 2725.0 | 6.2 | 22.03 | 38.41 | |
| Daqing Seeds Management Bureau | 91 | 2250.0 | 0.7 | 22.97 | 37.53 | |
| Dumeng Seeds Management Bureau | 90 | 2956.7 | 7.9 | 21.24 | 40.13 | |
| Tailai Seeds Station | 92 | 2426.0 | 9.1 | 20.78 | 39.21 | |
| Qiqihar Branch of Heilongjiang Academy of Agricultural Sciences | 91 | 2421.2 | -0.5 | 19.67 | 40.17 | |
| mean | 93.5 ± 2.3 | 2542 ± 103.9 | 4.1 ± 1.7 | 20.9 ± 0.6 | 39.5 ± 0.6 | |
| 2019 | Anda Dapeng Seeds Co.Ltd. | 107 | 2677.9 | 11.8 | 22.41 | 36.69 |
| Qiqihar Branch of Heilongjiang Academy of Agricultural Sciences | 109 | 2623.3 | 1.3 | 20.17 | 37.93 | |
| Longjiang Branch of Qishan Seeds Co.Ltd. | 82 | 2732.4 | 14.4 | 21.72 | 36.43 | |
| Qinggang Experimental Station of Beifeng Seeds Co.Ltd. | 109 | 2550.0 | 1.9 | 22.01 | 35.36 | |
| Tailai Seeds Station | 101 | 2521.3 | 7.0 | 21.61 | 36.73 | |
| Mean | 101.6 ± 5.1 | 2621 ± 39.1 | 7.3 ± 2.6 | 21.6 ± 0.4 | 36.6 ± 0.4 | |
| Total | 97.2 ± 2.8 | 2593 ± 61.9 | 6.2 ± 1.5 | 21.2 ± 0.4 | 38.2 ± 0.6 |
| Trial locations | Seed yield (kg/ha) | Increase (%) |
|---|---|---|
| Anda Dapeng Seeds Co.Ltd. | 2523.3 | 12.7 |
| Qiqihar Branch of Heilongjiang Academy of Agricultural Sciences | 2663.8 | 1.5 |
| Longjiang Branch of Qishan Seeds Co.Ltd. | 2467.5 | 11.4 |
| Qinggang Experimental Station of Beifeng Seeds Co.Ltd. | 2764.4 | 5.7 |
| Tailai Seeds Station | 2880 | 9.3 |
| Total | 2660 ± 75.9 | 8.1 ± 2.0 |
| soybean cultivars |
2018 | 2019 | 2020 | 2021 | ||||
|---|---|---|---|---|---|---|---|---|
| Cystsa | FI (%)b | Cystsa | FI (%)b | Cystsa | FI (%)b | Cystsa | FI (%)b | |
| Lee | 63 ± 4.15A | 100 | 65 ± 5.57A | 100 | 69 ± 4.74A | 100 | 68 ± 6.53A | 100 |
| Hefeng 50 | 47 ± 2.55A | 74.60 | 52 ± 4.16A | 80.00 | 67 ± 4.06A | 97.1 | 58 ± 3.90A | 85.29 |
| Nongqing 28 | 3 ± 0.42B | 4.76 | 2 ± 0.43B | 3.08 | 3 ± 0.50B | 4.35 | 3 ± 0.46B | 4.41 |
| PI 437654 | 1 ± 0.33B | 1.59 | 2 ± 0.60B | 3.08 | 1 ± 0.42B | 1.45 | 2 ± 0.72B | 2.94 |
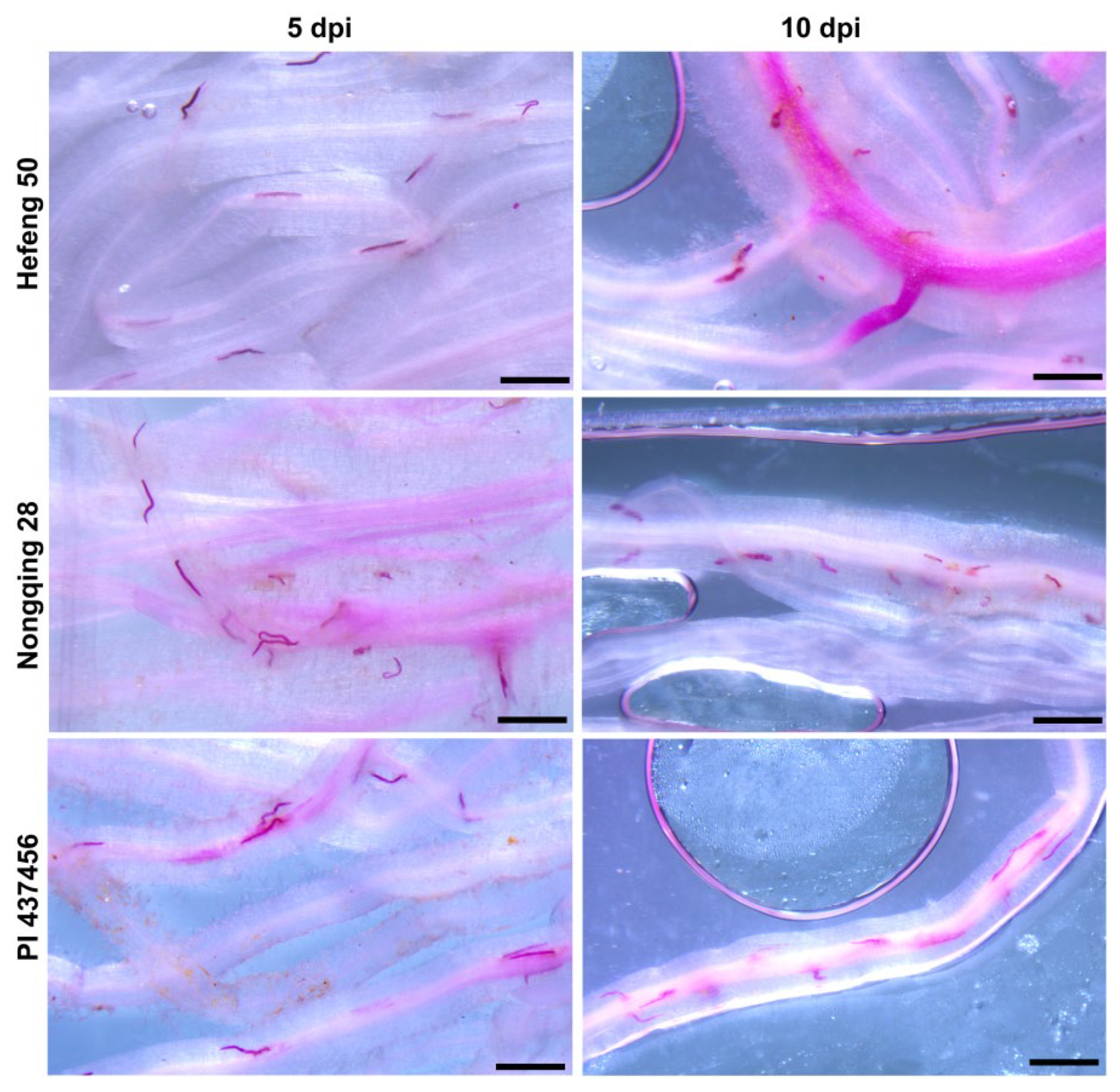
| Soybean cultivars | 5 dpi | 10 dpi | 21 dpi | |||
|---|---|---|---|---|---|---|
| J2 | J3 | J2 | J3 | J4 | Female | |
| Hefeng 50 | 205 ± 8.14 a | 18 ± 3.21 a | 107 ± 8.77 a | 73 ± 3.31 a | 5 ± 0.53 a | 51 ± 5.93 a |
| Nongqing 28 | 193 ± 6.51 a | 12 ± 1.53 b | 95 ± 9.69 a | 41 ± 3.01 b | 2 ± 0.43 a | 13 ± 1.73 b |
| PI 437654 | 165 ± 4.84 b | 3 ± 0.88 c | 83 ± 4.67 a | 23 ± 2.92 c | 0 ± 0 b | 0 ± 0.10 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




