1. Introduction
As one of the most common heart diseases in dogs, myxomatous mitral valve disease (MMVD) causes symptoms such as cough and increased respiratory effort. In dogs, MMVD causes progressive degeneration of the mitral valve. The characteristics of the mitral valve leaflet include elongated chordae tendinae, thickening, and shrinkage of the leaflets with bulging or prolapse toward the left atrium [
1]. The standard treatment for this disease is medication, which slows the progression of heart failure and the heart remodeling process. According to common treatment practices and ACVIM guidelines, individualized medication treatment is recommended [
2,
3].
In recent years, open heart surgical treatment has been performed for mitral valve repair in dogs, and mitral valve repair is possible and recommended for patients with stage B2 disease [
4]. However, similar to that in humans, open heart surgery in dogs is time-consuming and invasive. The surgical procedure requires heart and lung bypass and carries a high risk of complications such as bleeding and thrombosis. In addition, the health benefits and financial costs of open-heart surgery should be considered the need for cardiopulmonary bypass equipment [
5]. Percutaneous mitral valve repair using the MitraClip® system (Abbott Vascular, Abbott Park, IL, USA) was established in 2008. The use of the MitraClip for congestive heart failure treatment has been shown to be safe and less invasive, relieves clinical symptoms, and reduces the length of hospital admission [
6,
7]. Unfortunately, the MitraClip is not available for use in dogs due to the size of the device. A transcatheter edge-to-edge mitral valve repair (TEER) device called the ValveClamp was designed to treat structural mitral valve disease in humans and has been used in the clinical setting in China [
8]. A TEER device named the V-clamp was established for clinical use in dogs by HongYu Medical Company.
2. Materials and Methods
Laboratory Investigations
Blood samples were collected by venipuncture and submitted for routine hematological and biochemical investigations. All blood samples were then separated into two fractions. The first fraction was put into tubes containing potassium ethylene diamine tetra-acetic acid (EDTA) to evaluate the complete blood count (CBC) analysis. The second fraction was put in tubes containing silica dioxide to evaluate blood chemistry analysis (blood urea nitrogen (BUN), creatinine (Cre), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total protein (TP), albumin (ALB), and globulin (GLB)). This study was conducted at the Faculty of Veterinary Medicine, Kasetsart University Kamphaengsaen, written client consent to participate was obtained from each patient owner enrolled in this surgical treatment.
Thoracic Radiography
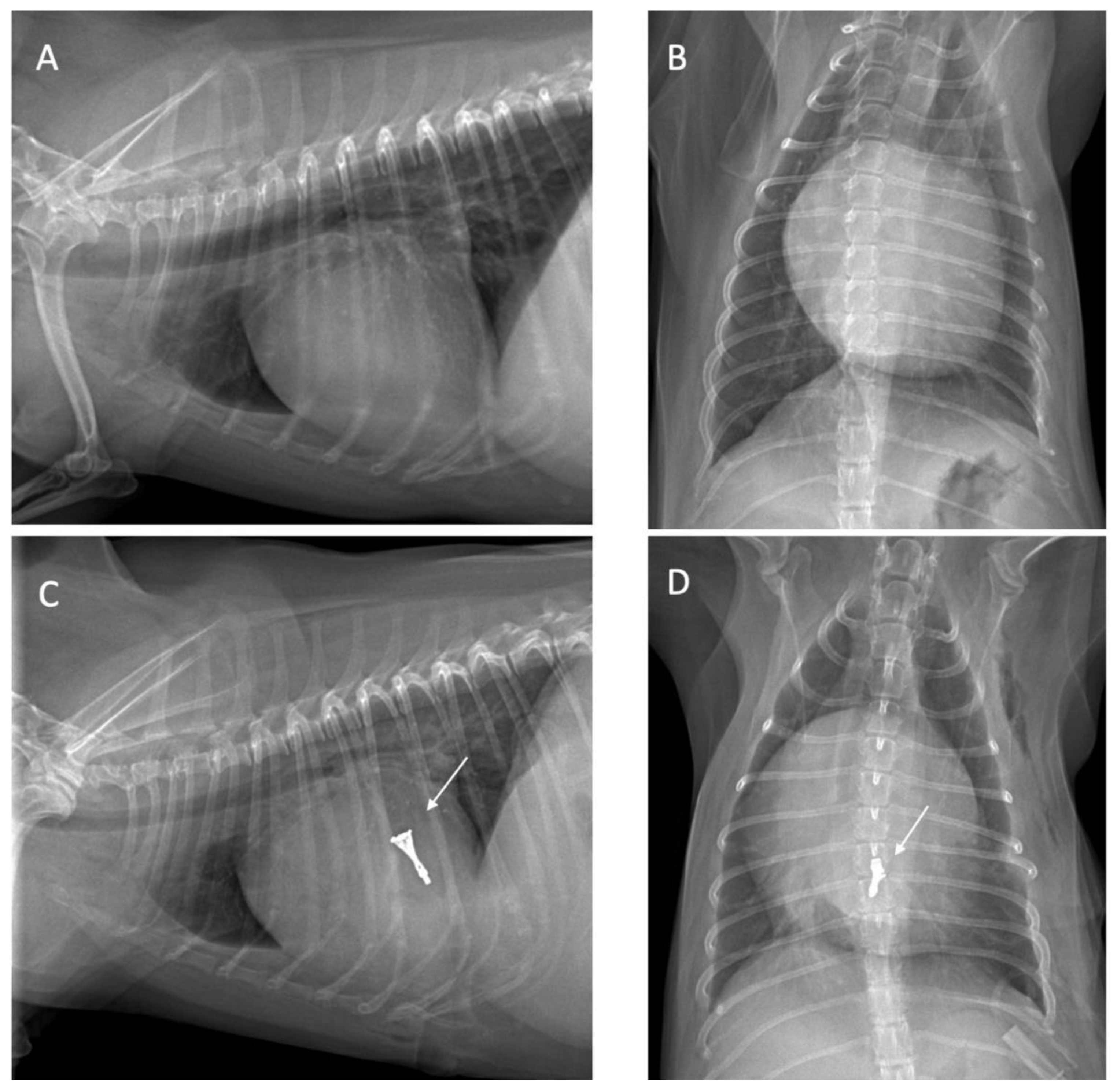
Thoracic radiography was performed to determine the heart position and to locate the cardiac apex, to ensure precise incision placement for minimally invasive surgery and thereby reducing procedure time. Thoracic radiography images were assessed both before and after surgery to evaluate radiographic features, such as alterations in the vascular pattern and pulmonary atelectasis, a common complication in patients undergoing heart surgery [
9] as illustrated in
Figure 1.
Echocardiography
The patients underwent transthoracic echocardiography (TTE) by using a General Electric Vivid 5s ultrasound system with continuous electrocardiography monitoring to evaluate the cardiac function. Echocardiography was performed at baseline, and every month until 3 months after the surgical intervention by two skilled sonographers. The measurement was performed in a right parasternal long axis and right parasternal short axis in a right lateral recumbent position, whereas and left apical four-chamber view was performed in a left lateral recumbent position without sedation. Echocardiographic images were captured and stored for offline analysis. Left ventricular wall structure and function were calculated by measuring the images from two-dimensional and M-mode planes. LV wall thickness, LV dimension, and LV function were evaluated by M-mode echocardiography in the right parasternal short axis (at the base of the heart and the level of the papillary muscles) [
10]. Ventricular wall thickness and dimensions were recorded during diastole and systole to obtain the parameters such as IVSd; diastolic interventricular septum thickness, IVSs; systolic interventricular septum thickness, LVIDd; left ventricular end-diastolic diameter, LVIDs; left ventricular end-systolic diameter, LVPWd; left ventricular wall diastolic thickness and LVPWs; left ventricular wall systolic thickness. All averaged M-mode chamber measurements were normalized by body weight using the Cornell allometric scale for dogs [
11]. In addition, M-mode echocardiography parameters were used to calculate the percentage of fractional shortening (%FS) and percentage ejection fraction (%EF) by using the Teicholz formula which was accomplished automatically by the echocardiographic equipment software. The right parasternal short-axis view was used to measure the left atrial dimension (LA) and aortic dimension (AO) in early diastole. Then the left atrial to aortic root ratio (LA: AO ratio) was calculated using the Swedish method. Moreover, the diastolic function indicated by the E-wave per A-wave ratio was performed in the left apical four-chamber view using a pulse wave Doppler echocardiography. Three consecutive beats of cardiac cycles were measured, and the average values were used for all echocardiographic parameters. Right ventricular dimensions and pulmonary artery diameters were observed. Pulmonary velocity was evaluated using a pulse wave Doppler echocardiography. A transesophageal echocardiogram (TEE) was obtained to plan the procedure and confirm the appearance of the heart valves, including the leak location.
Anesthetic and Analgesic Protocol
Anesthetics and analgesics play a crucial role in thoracic surgery for cardiac patients. Ensuring effective analgesia is also important for preventing hemodynamic changes caused by surgical pain [
12]. Patients were premedicated with Butorphanol (0.2 mg/kg) and then followed by etomidate (1-2 mg/kg) for induction and intercostal block with bupivacaine (at ribs 5, 6, 7, 8, and 9). The anesthesia was maintained by isoflurane at a 2-5% concentration in oxygen at 1.5-2 L/min. In our study, all patients underwent continuous rate infusion (CRI) of potent opioids (2-5 mcg/kg of Fentanyl) along with intercostal nerve block. However, monitoring for potential side effects of potent opioids, such as bradycardia, is essential. Low-dose atropine can be used as an intervention if necessary.
Surgical Intervention
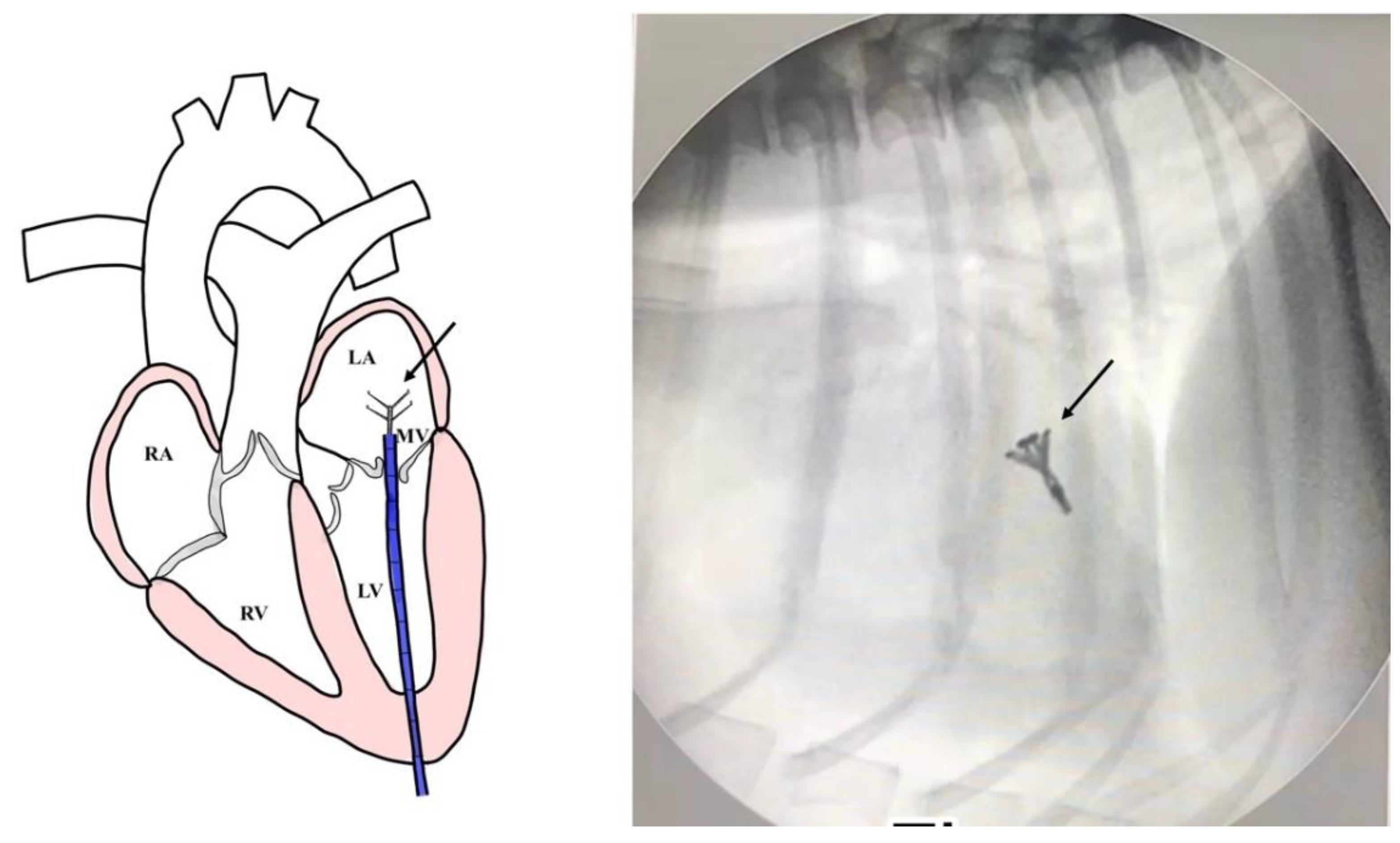
Surgical intervention was performed using a v-clamp device as shown in
Figure 2. A 4 French percutaneous sheath introducer (Merit Medical, USA.) was guided into the left ventricle, and the v-clamp device was inserted and positioned in the left ventricle under transesophageal echocardiography and 3D imaging (
Figure 3). Limb-lead electrocardiography was used to determine the cardiac rhythm. The mitral valve leaflet was repaired by v-clamp. After the mitral valve was clipped, the guiding catheter was removed and flushed, and then the skin was sutured with a surgical nylon. Patients were recovered in a cage filled with oxygen for 24-48 hours. For 24 hours after surgery, the chest drainage and the urinary catheter were removed. The blood profiles and thoracic radiographs were evaluated every day until discharge.
Evaluation
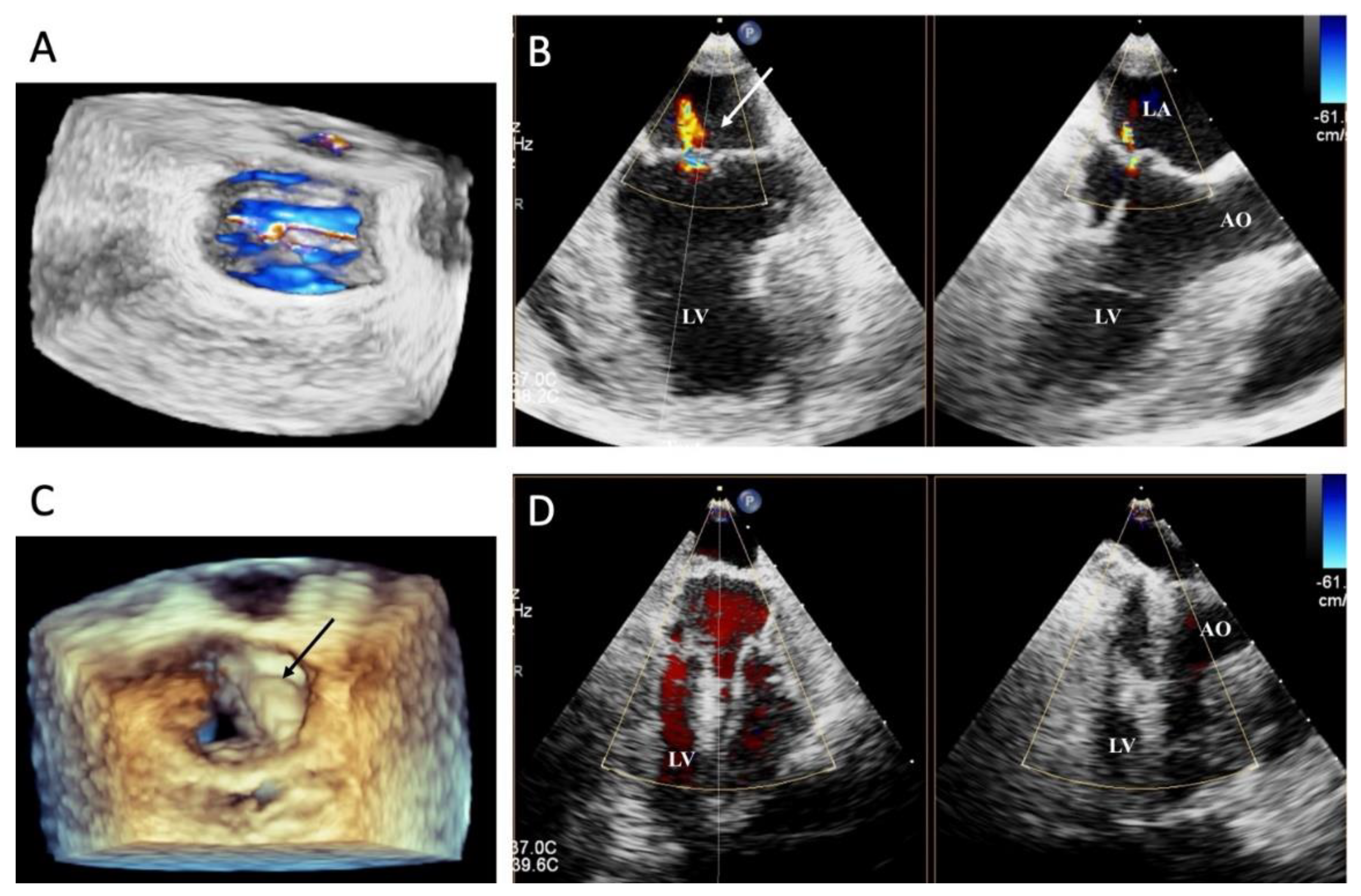
By performing a physical examination (inspection, palpation, percussion, and auscultation), all dogs were assessed for general health status. In this evaluation, mucous membranes, capillary refill time, rectal temperature, heart rate, heart sounds, pulse rate, and state of hydration were assessed. The dogs presented with a history of coughing, dyspnea, and exercise intolerance. Thoracic radiography revealed left heart enlargement (
Figure 2). The mitral valve structure was evaluated by 3D transesophageal echocardiography, as shown in
Figure 3.
The owners evaluated the questionnaires which were adapted from Minnesotans Living with Heart Failure and Validation [
13], to assess various aspects of quality of life. These questionnaires measured happiness, mental status, hygiene, and physical fitness, with higher scores indicating a lower quality of life as detailed in
Table 1. The dogs exhibited improvements in respiratory signs during the follow-up visit and showed enhanced happiness and overall quality of life after the surgical intervention as shown in
Table 1.
Prior to repairing the mitral valve, a biochemical blood profile revealed elevated liver enzymes in all dogs. However, the profiles, particularly the liver enzymes improved one month after the valve was repaired as shown in
Table 2.
3. Results
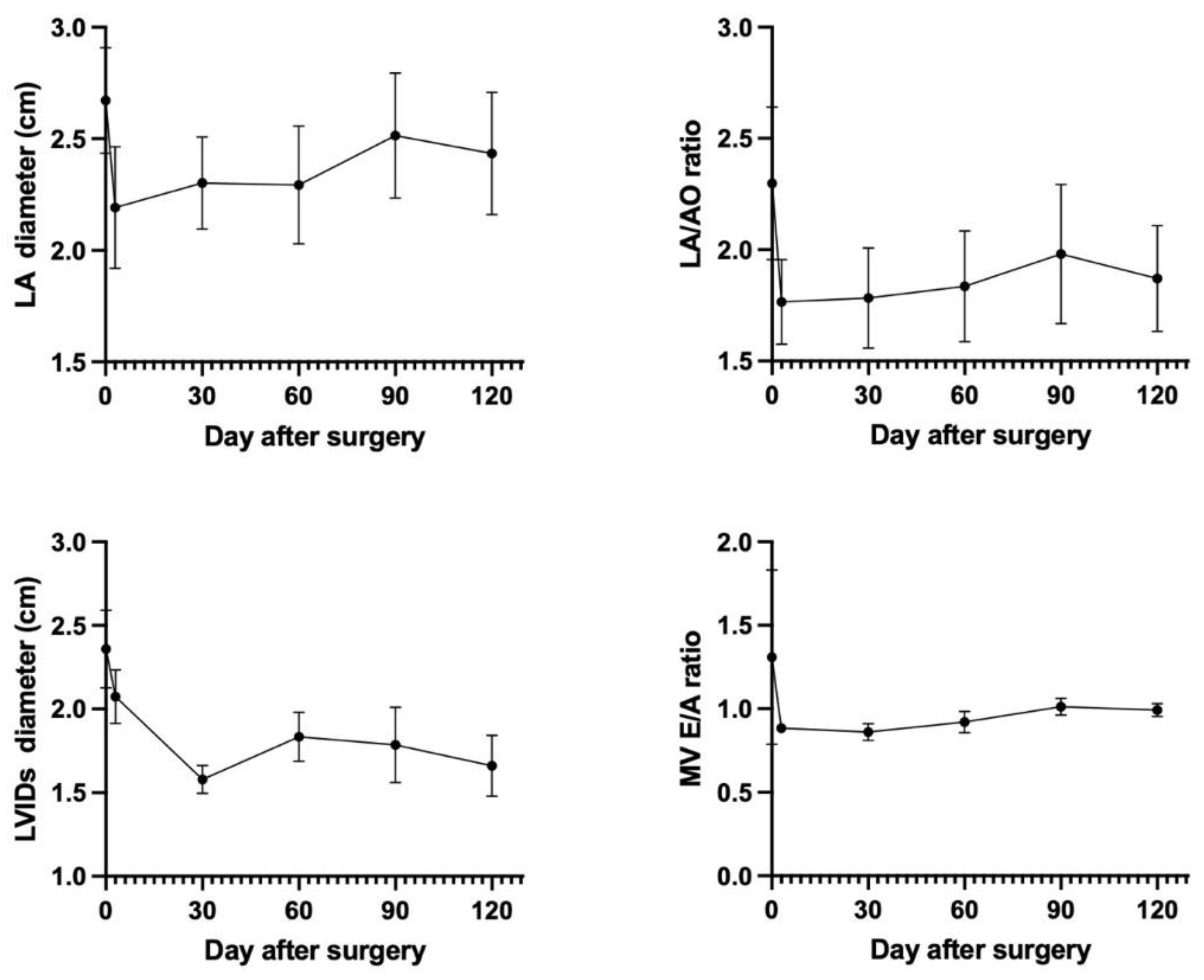
All 4 patients showed clinical stability at the 4-month follow-up visit, and no patients in our study were re-admitted due to decompensated heart failure. Clinical signs such as cough and dyspnea have resolved without diuretic medication. TTE showed preserved left ventricular fractional shortening of 40.02±14.24%. Echocardiography parameters such as the left atrium (LA) dimension, LA/AO ratio, left ventricular internal diameter during systole (LVIDs), and Mitral valve E/A ratio showed a rapid decrease within the initial 3 days following mitral valve repair. Subsequently, the LA size, LA/AO ratio, LVIDs, and Mitral valve E/A ratio exhibited a gradual increase over the next 3 months until it stabilized at a constant value after 4 months post-mitral valve repair as shown in
Figure 4. It was observed that the v-clamp device remained intact and properly positioned during this period.
Patient 1: Before the mitral valve repair, her prescription included 0.25 mg/kg pimobendane q12h, 1.0 mg/kg daily spironolactone, 2.0 mg/kg daily furosemide, and cardiac support. Transthoracic echocardiography revealed stage B2 characterized by LA dilation and mild mitral valve regurgitation. 3D transesophageal echocardiography was used to observe the main regurgitation orifice. Under general anesthesia, the center of the anterior leaflet (A2) and the center of the posterior leaflet (P2) were approximated using the v-clamp device to reduce the regurgitation. After mitral valve repair, the mean transmitral gradient was 1 mmHg. The dog was discharged 24 hours after the operation. At the 1-month follow-up examination, the dog exhibited remarkable clinical improvement from ACVIM stage B2 to B1. The size of the left atrium decreased by 13.7 %, 17.6 %, and 5.9 % at 1, 2, and 3 months after mitral valve repair, respectively.
Patient 2: Before the mitral valve repair, his prescription included 0.25 mg/kg pimobendane q12h, 1.0 mg/kg daily spironolactone, 2.0 mg/kg furosemide q12h, cardiac support, and liver support. Transthoracic echocardiography revealed stage C, characterized by LA and LV dilation and severe mitral valve regurgitation. 3D transesophageal echocardiography was used to observe the main regurgitation orifice. The anterior leaflet of the mitral valve was prolapsed with a 22.8 AP diameter, The length of the anterior leaflet was 13.2 millimeters, and the length of the posterior leaflet was 9.9 millimeters. Under general anesthesia, the center of the anterior leaflet (A2) and the center of the posterior leaflet (P2) were deployed by the v-clamp device to reduce the regurgitation. After mitral valve repair, the mean transmitral gradient was 4 mmHg. A ventricular escape rhythm with a heart rate of 120 bpm, was seen after mitral valve repair, a 2 mg/kg bolus of Lidocaine along with 2 mcg/kg/hr of Fentanyl was intravenously administered to control pain and the arrhythmias. The dog was discharged on postoperative day 5. At the 1-month follow-up examination, the dog exhibited remarkable clinical improvement in the heart dimension, the size of the LA decreased by 19.5 %, 17.9 %, and 6.7 % at 1, 2, and 3 months after mitral valve repair, respectively, and the ACVIM improved from stage C to B2.
Patient 3: Before mitral valve repair, her prescription included 0.25 mg/kg pimobendane q12h, 1.0 mg/kg daily spironolactone, and 2.0 mg/kg furosemide q12h and cardiac support. Transthoracic echocardiography revealed stage C disease, characterized by LA and LV dilation and severe mitral valve regurgitation. 3D transesophageal echocardiography was used to observe the main regurgitation orifice. No flail of the mitral valve was observed with a 21.7 AP diameter. The length of the anterior leaflet was 7.7 millimeters, and the length of the posterior leaflet was 5.8 millimeters. Under general anesthesia, the center of the anterior leaflet (A2) and the center of the posterior leaflet (P2) were deployed by the v-clamp device to reduce regurgitation. After mitral valve repair, the mean transmitral gradient was 1 mmHg. The dog was discharged 48 hours after surgery. At the 1-month follow-up examination, the dogs exhibited remarkable clinical improvement in the left ventricle dimension by 8.3%, 11.4%, and 25.4% at 1, 2, and 3 months after mitral valve repair, respectively. The patient exhibited a stable mitral valve V-clamping position, moderate regurgitation, and fractional shortening of 55.7%.
Patient 4: Before mitral valve repair, his prescription included 0.25 mg/kg pimobendane q12h, 1.0 mg/kg daily spironolactone, 2.0 mg/kg daily furosemide and cardiac support. Transthoracic echocardiography revealed stage 2 disease, characterized by LA dilation and moderate mitral valve regurgitation. 3D transesophageal echocardiography was used to observe the main regurgitation orifice. The anterior leaflet of the mitral valve was prolapsed with a 12.7 AP diameter. The length of the anterior leaflet was 7.91 millimeters, and the length of the posterior leaflet was 5.3 millimeters. Under general anesthesia, the center of the anterior leaflet (A2) and the center of the posterior leaflet (P2) were deployed by the v-clamp device to reduce regurgitation. After mitral valve repair, the mean transmitral gradient was 1 mmHg. The dog was discharged 48 hours after surgery. At the 1-month follow-up examination, the dog exhibited remarkable clinical improvement as the ACVIM stage improved from stage B2 to B1, and the size of the LA decreased at 1, 2, and 3 months after mitral valve repair.
Transthoracic Echocardiography Findings at 4 Months after Mitral Valve Repair
The transthoracic echocardiography (TTE) findings at 4 months after the v-clamp procedure are shown in
Table 3. TTE at the 4-month follow-up visits revealed a reduced LA/Ao ratio. The percentage of change in the LA/Ao ratio and LV internal diameter at systole was greater in patients with stage B2 disease than in those with stage C disease.
4. Discussion
This case series demonstrated the safety and feasibility of the v-clamp procedure for patients with stages B2 and C myxomatous mitral valve degeneration who are considered high-risk surgical patients. Left ventricular enlargement and degeneration of the mitral valve can impair the coaptation of the mitral leaflets, leading to mitral valve regurgitation, as observed in our patients. In general, medical therapy is recommended according to ACVIM guidelines, and mitral valve repair is recommended if necessary [
3]. However, many MMVD patients are considered high-risk surgical patients due to severe congestive heart failure with LV dysfunction and multiple concurrent diseases such as pancreatitis, and liver or kidney dysfunction, despite mitral valve repair being a treatment option. The patient's hydration status and electrolyte levels, particularly potassium, sodium, calcium, and magnesium should be monitored after the operation [
14].
Transcatheter edge-to-edge repair using a v-clamp device, a procedure similar to the human MitraClip technique is a current alternative treatment for mitral valve regurgitation in dogs and is considered an appropriate treatment for high-risk cardiac surgery patients [
15,
16,
17]. The technique involves a transapical puncture with an introducer sheath to enter the left ventricle and subsequently the mitral valve. However, there is a lack of data on post-operative care and management following the v-clamp procedure. In this study, cardiac muscle injury can result from transapical puncture during the v-clamp procedure, and acute ventricular arrhythmias may occur during post-operative recovery. Monitoring parameters such as NT-proBNP or cardiac troponin levels preoperatively and postoperatively can assist in evaluating the cardiac status, including cardiac stretch, dilation, and hypertrophy [
18].
Thoracic and heart surgery has been reported to be associated with significantly more pain than other surgical methods [
19,
20]. Pain after heart surgery is usually severe on postoperative days 1 and 2. Despite gradual reductions in pain throughout the recovery period, most patients continue to experience severe pain. Severe pain is primarily associated with the main surgical site on postoperative day 1, but from postoperative day 2 to postoperative day 4, pain is generally caused by coughing, movement, turning, and deep breathing [
21]. Local anesthesia such as bupivacaine has been reported to provide postoperative pain relief for up to 72 hours in dogs and cats due to the ability to reduce pain [
22]. Therefore, local anesthesia is recommended for use in the surgical, especially thoracic and heart surgery. Lidocaine is widely used to manage pain during and after cardiac surgery. Lidocaine can be safely administered intravenously, and intravenous infusion of lidocaine is significantly associated with reduced pain scores and decreased amount of fentanyl. However, no significant relationships were found between lidocaine administration and other parameters such as postoperative death and length of hospital stay [
23]. No data were reported for other secondary outcomes after surgery, including nausea, vomiting, or arrhythmias. Earlier research indicated that external sympathetic pathways play a dominant role in the nervous system of the heart. Preoperative and postoperative administration of nerve blockers containing lidocaine have been shown to be effective at decreasing recurrent arrhythmias and alleviating the burden of ventricular arrhythmias in patients [
19].
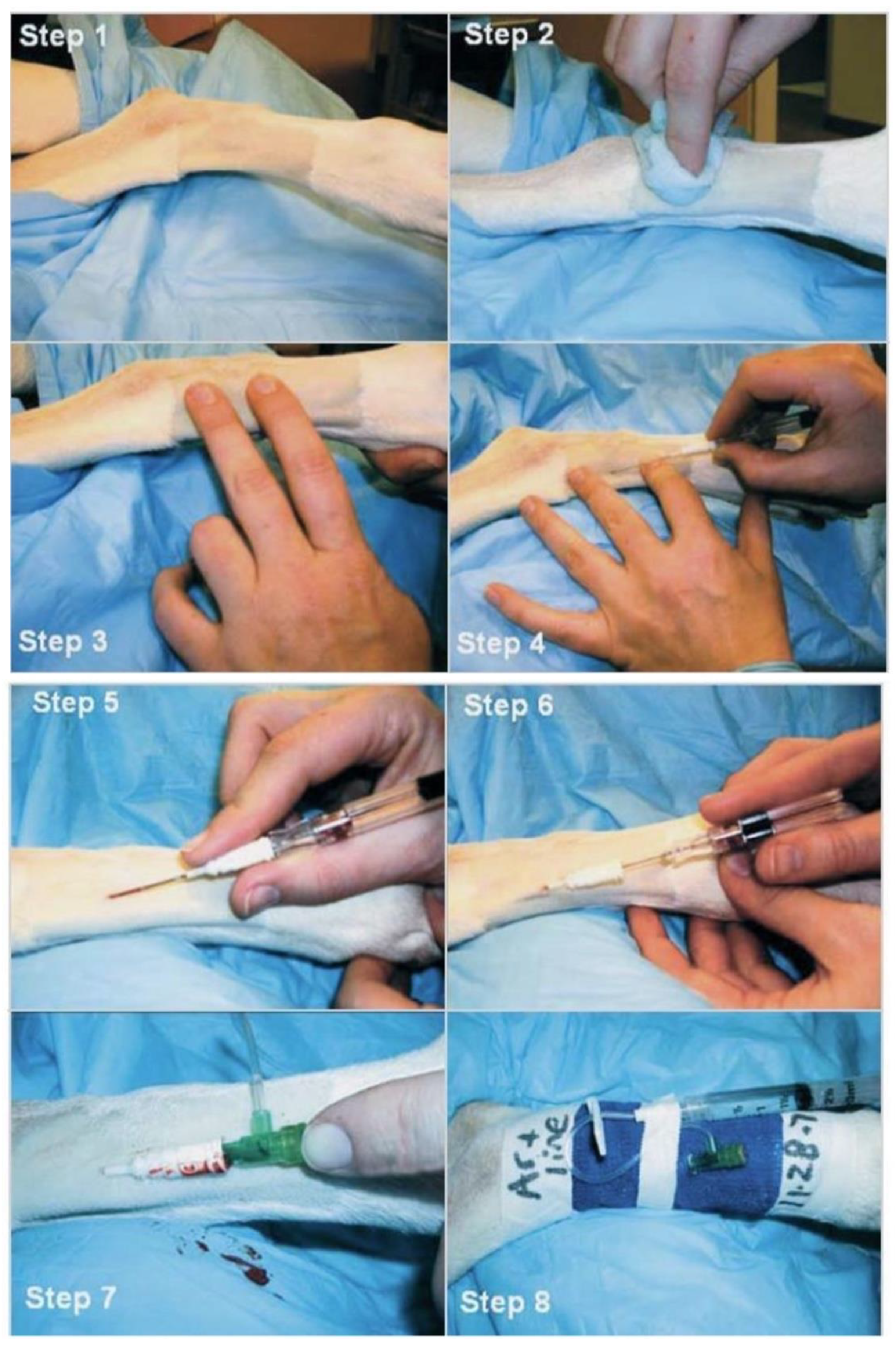
Additionally, performing an electrocardiogram (ECG) record before and after surgery can help to identify any abnormalities in heart rate and rhythm, including tachycardia, bradycardia, atrial fibrillation, premature ventricular contractions, and ventricular escape beats. Continuous monitoring using ECG or Holter monitoring during the perioperative period is vital for assessing hemodynamic impact and determining the necessity for therapeutic intervention. Moreover, obtaining an accurate baseline of the patient’s heart rate and blood pressure before surgery is essential for developing an appropriate anesthesia plan and documenting cardiovascular changes throughout the surgical procedure. Direct arterial blood pressure monitoring is the gold standard for blood pressure measurement in heart surgery. Continuous monitoring of systolic arterial pressure (SAP), diastolic arterial pressure (DAP), and mean arterial pressure (MAP) using an arterial catheter is recommended in the surgical procedure. Dorsal pedal artery catheterization in dogs is depicted in
Figure 5.
The findings of this study suggested that effective management of acute postoperative pain as well as electrocardiography and echocardiography assessments are important for monitoring and stabilizing electrophysiological and heart function.
Moreover, interventional cardiovascular procedures require post-operative anticoagulation management to prevent the formation of blood clots on catheters and devices and therefore prevent vascular complications [
24,
25]. Post-operative anticoagulation management is also essential due to the use of a v-clamp device in the left atrium, and the dilation of the left atrium with a low blood flow velocity. Therefore, the risk of thromboembolism is high, and management with antithrombotic drugs is crucial for post-operative care. In our study, an antithrombotic drug such as clopidogrel (total dose of 18.75 mg) was administered for at least 3 months to prevent post-operative thromboembolism of the v-clamp device.
Monitoring the left atrial pressure (LAP) and transmitral pressure gradient during mitral valve repair provides valuable insights into immediate hemodynamic effects and postoperative clinical outcomes. The LAP is typically elevated, and reductions are typically observed within 30 days after the procedures [
26,
27]. Conversely, an increased LAP following mitral valve repair indicates a high risk of both postoperative heart failure and all-cause mortality. Therefore, it is advisable to carefully monitor and adjust the device position if the pressure above the mitral valve exceeds 5 mmHg [
28]. However, there are limitations in our study, this single center involved a small number of patients, and the initial learning curve may have impacted the results. Additionally, the LAP measurements before and after repair were not included in the analysis. Consequently, the data for all patients was unavailable.
5. Conclusions
In this study, we share our perioperative experience, in managing patients before and after surgery as well as managing complications such as pain and arrhythmias.
Author Contributions
All the authors contributed to the manuscript. interpreted the results. Ratikorn Bootcha, Wannisa Meepoo, Kotchapol Jaturanratsamee, Chattida Panprom, and Soontaree Petchdee performed the surgery, Methodology, and validation Ratikorn Bootcha, Wannisa Meepoo, Kotchapol Jaturanratsamee, Chattida Panprom, Jing Lei and Soontaree Petchdee; formal analysis, Soontaree Petchdee.; investigation, Ratikorn Bootcha, Wannisa Meepoo, Kotchapol Jaturanratsamee, Chattida Panprom, Jing Lei and Soontaree Petchdee.; data curation, Soontaree Petchdee.; writing—original draft preparation, Soontaree Petchdee and Wanpitak Pongkan.; writing—review and editing, Soontaree Petchdee and Wanpitak Pongkan.; supervision, Soontaree Petchdee. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
The written informed consent was informed to the owner of the animals.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors express their gratitude to Kasetsart Veterinary Teaching Hospital, KamphaengSaen for providing the essential facilities, and extend their thanks to HongYu Medical for their assistance during the surgical procedure.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dal-Bianco, J.P.; Beaudoin, J.; Handschumacher, M.D.; Levine, R.A. Basic mechanisms of mitral regurgitation. The Canadian journal of cardiology 2014, 30, 971–981. [Google Scholar] [CrossRef]
- van Staveren, M.D.B.; Muis, E.; Szatmári, V. Self-Reported Utilization of International (ACVIM Consensus) Guidelines and the Latest Clinical Trial Results on the Treatment of Dogs with Various Stages of Myxomatous Mitral Valve Degeneration: A Survey among Veterinary Practitioners. Animals : An open access journal from MDPI 2024, 14, 772. [Google Scholar] [CrossRef] [PubMed]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. Journal of veterinary internal medicine 2019, 33, 1127–1140. https://doi.org/10.1111/jvim.15488Uechi M. Mitral valve repair in dogs. J Vet Cardiol. 2012, 14, 185–192. [CrossRef]
- Uechi, M.; Mizukoshi, T.; Mizuno, T.; Mizuno, M.; Harada, K.; Ebisawa, T.; Takeuchi, J.; Sawada, T.; Uchida, S.; Shinoda, A.; Kasuya, A.; Endo, M.; Nishida, M.; Kono, S.; Fujiwara, M.; Nakamura, T. Mitral valve repair under cardiopulmonary bypass in small-breed dogs: 48 cases (2006-2009). Journal of the American Veterinary Medical Association 2012, 240, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Mihara, K.; Kanemoto, I.; Sato, K.; Yasuhira, Y.; Watanabe, I.; Suzuki, Y.; Nagura, J.; Misumi, K. Effects of mitral valve repair on valvular geometry and hemodynamics in dogs with myxomatous mitral valve disease. Veterinary surgery : VS 2024, 53, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Nyman, C.B.; Mackensen, G.B.; Jelacic, S.; Little, S.H.; Smith, T.W.; Mahmood, F. Transcatheter Mitral Valve Repair Using the Edge-to-Edge Clip. Journal of the American Society of Echocardiography : Official publication of the American Society of Echocardiography 2018, 31, 434–453. [Google Scholar] [CrossRef]
- Pan, W.; Long, Y.; Guo, Y.; Pan, C.; Wei, L.; Liu, X.; Luo, J.; Chen, L.; Zhou, D.; Ge, J. Transapical Edge-to-Edge Repair System in High-Risk Patients With Degenerative Mitral Regurgitation: A Multicenter Trial (CLAMP-2). JACC. Cardiovascular interventions 2023, 16, 2340–2342. [Google Scholar] [CrossRef]
- Hu, M.C.; Yang, Y.L.; Chen, T.T.; Lee, C.I.; Tam, K.W. Recruitment maneuvers to reduce pulmonary atelectasis after cardiac surgery: A meta-analysis of randomized trials. The Journal of thoracic and cardiovascular surgery 2022, 164, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Moise, N.S.; Moses, B.L. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. Journal of veterinary internal medicine 1993, 7, 247–252. [Google Scholar] [CrossRef]
- Cornell, C.C.; Kittleson, M.D.; Della Torre, P.; Häggström, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric scaling of M-mode cardiac measurements in normal adult dogs. Journal of veterinary internal medicine 2004, 18, 311–321. [Google Scholar] [CrossRef]
- Sasaki, K.; Ma, D.; Mandour, A.S.; et al. Evaluation of Changes in the Cardiac Function Before and after Transcatheter Edge-to-Edge Mitral Valve Repair in Healthy Dogs: Conventional and Novel Echocardiography. Animals (Basel). 2021, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Naveiro-Rilo, J.C.; Diez-Juárez, D.M.; Blanco, A.R.; Rebollo-Gutierrez, F.; Rodriguez-Martinez, A.; Rodriguez-Garcia, M.A. Validation of the Minnesota living with heart failure questionnaire in primary care. Rev Esp Cardiol. 2010, 63, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Polderman, K.H.; Girbes, A.R. Severe electrolyte disorders following cardiac surgery: A prospective controlled observational study. Crit Care. 2004, 8, R459–R466. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.; Kar, S.; Rinaldi, M.; et al. Percutaneous mitral repair with the MitraClip system: Safety and midterm durability in the initial EVEREST (Endovascular Valve Edge to-Edge REpair Study) cohort. J Am Coll Cardiol. 2009, 54, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Whitlow, P.L.; Feldman, T.; Pedersen, W.R.; et al. Acute and 12-month results with catheterbased mitral valve leaflet repair: The EVEREST II (Endovascular Valve Edge-to-Edge Repair) high risk study. J Am Coll Cardiol. 2012, 59, 130–139. [Google Scholar] [CrossRef]
- Feldman, T.; Kar, S.; Elmariah, S.; et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol. 2015, 66, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Ruaux, C.; Scollan, K.; Suchodolski, J.S.; Steiner, J.M.; Sisson, D.D. Biologic variability in NT-proBNP and cardiac troponin-I in healthy dogs and dogs with mitral valve degeneration. Vet Clin Pathol. 2015, 44, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Sepolvere, G.; Scialdone, V.R. Chronic pain in cardiac surgery. Minerva Anestesiol. 2023, 89, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Feray, S.; Lemoine, A.; Aveline, C.; Quesnel, C. Pain management after thoracic surgery or chest trauma. Minerva Anestesiol. 2023, 89, 1022–1033. [Google Scholar] [CrossRef]
- Thompson-Brazill, K.A. Pain Control in the Cardiothoracic Surgery Patient. Crit Care Nurs Clin North Am. 2019, 31, 389–405. [Google Scholar] [CrossRef]
- Grubb, T.; Lobprise, H. Local and regional anaesthesia in dogs and cats: Overview of concepts and drugs (Part 1). Vet Med Sci. 2020, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Boswell, M.R.; Moman, R.N.; Burtoft, M.; et al. Lidocaine for postoperative pain after cardiac surgery: A systematic review. J Cardiothorac Surg. 2021, 16, 157. [Google Scholar] [CrossRef] [PubMed]
- Baumann Kreuziger, L.; Karkouti, K.; Tweddell, J.; Massicotte, M.P. Antithrombotic therapy management of adult and pediatric cardiac surgery patients. J Thromb Haemost. 2018, 16, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, H.; Xie, X.; et al. Long-term left atrial thrombi after mitral valve replacement. J Thromb Thrombolysis. 2021, 51, 129–135. [Google Scholar] [CrossRef] [PubMed]
- El Shaer, A.; Mahayni, A.; Simard, T.; et al. Baseline Left Atrial Pressure Predicts Mortality Following Transcatheter Edge-to-Edge Mitral Valve Repair. JACC Cardiovasc Interv. 2021, 14, 2306–2308. [Google Scholar] [CrossRef] [PubMed]
- Thaden, J.J.; Malouf, J.F.; Nkomo, V.T.; et al. Mitral Valve Anatomic Predictors of Hemodynamic Success With Transcatheter Mitral Valve Repair. J Am Heart Assoc. 2018, 7, e007315. [Google Scholar] [CrossRef] [PubMed]
- Neuss, M.; Schau, T.; Isotani, A.; Pilz, M.; Schöpp, M.; Butter, C. Elevated Mitral Valve Pressure Gradient After MitraClip Implantation Deteriorates Long-Term Outcome in Patients With Severe Mitral Regurgitation and Severe Heart Failure. JACC Cardiovasc Interv. 2017, 10, 931–939. [Google Scholar] [CrossRef]
- Ross, E.S.; Visser, L.C.; Sbardellati, N.; Potter, B.M.; Ohlendorf, A.; Scansen, B.A. Utility of vertebral left atrial size and vertebral heart size to aid detection of congestive heart failure in dogs with respiratory signs. J Vet Intern Med. 2023, 37, 2021–2029. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).