Submitted:
24 July 2024
Posted:
24 July 2024
You are already at the latest version
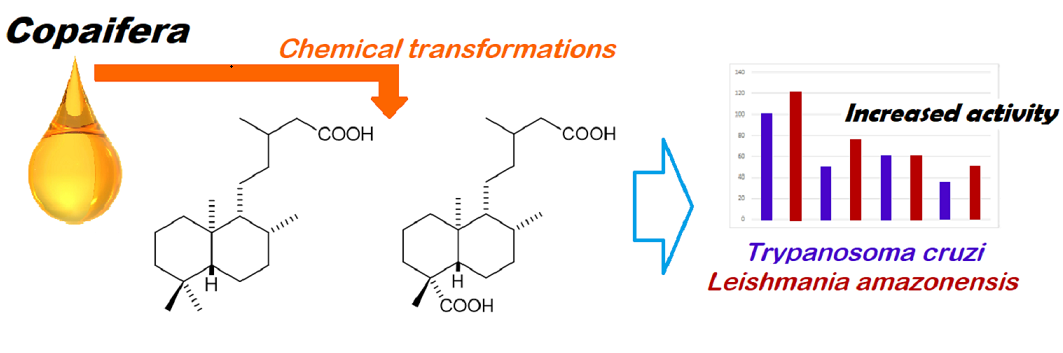
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. Plant Material
2.1.2. Extraction and Isolation
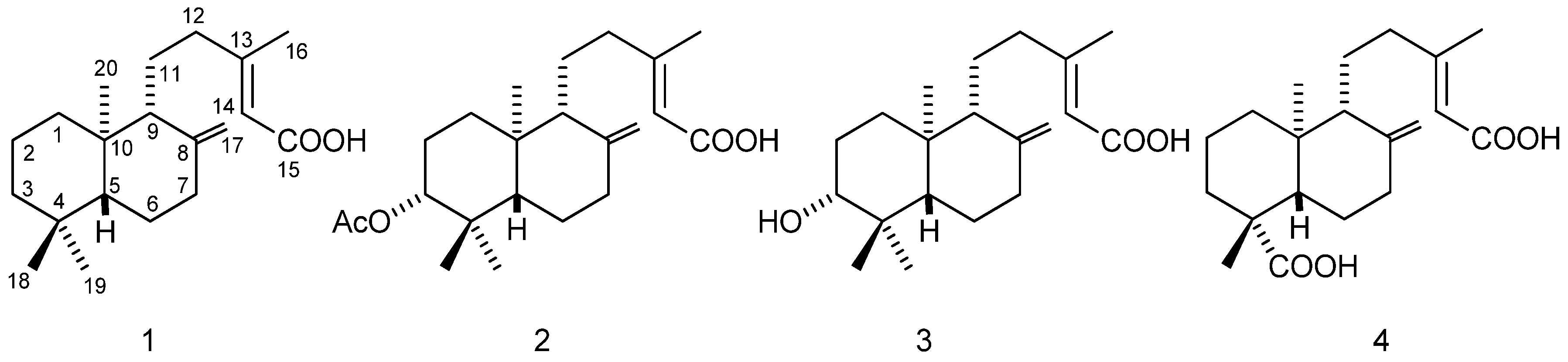
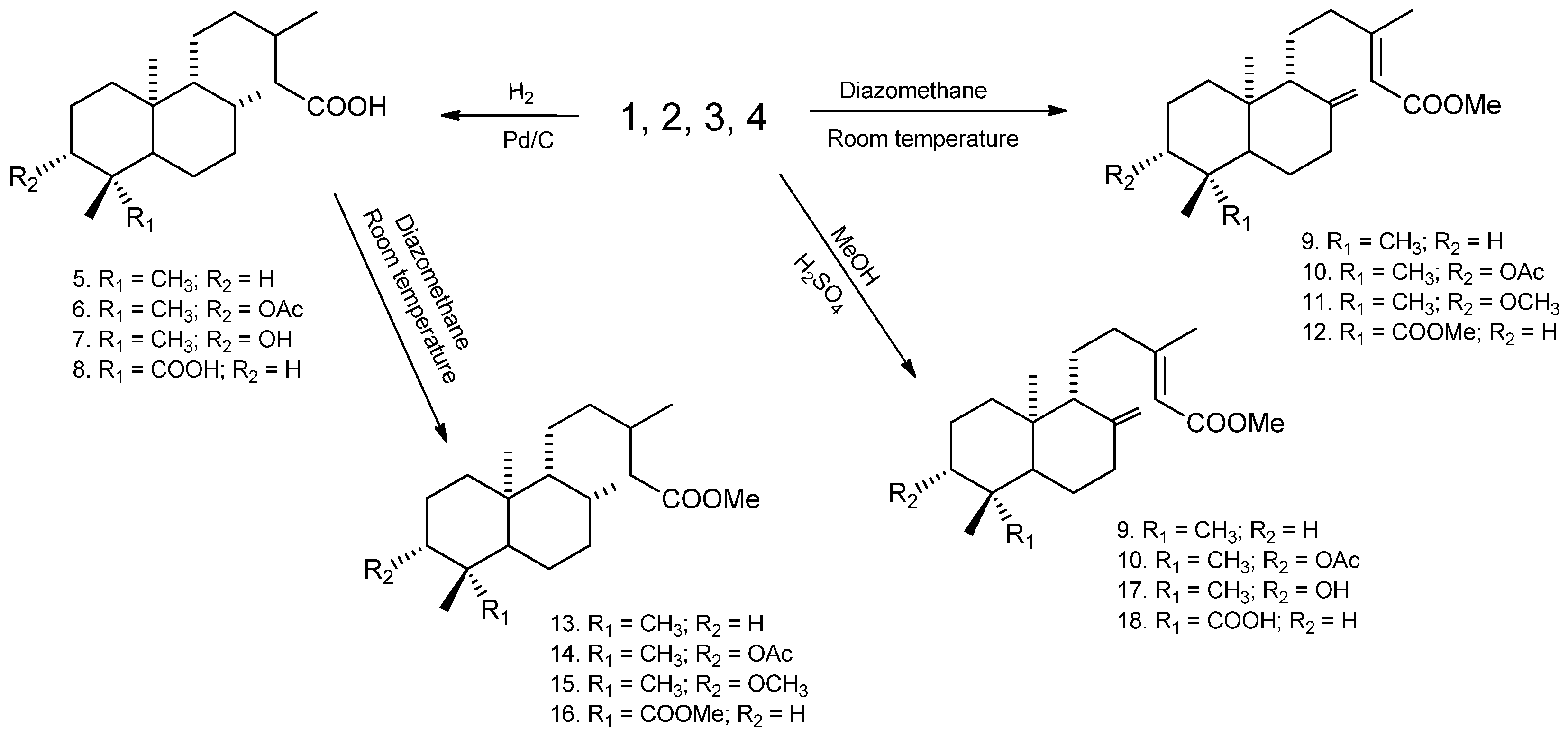
2.1.3. Semi-Synthetic Derivatives
2.1.4. Hydrogenation
2.1.5. Esterification with Diazomethane
2.1.6. Esterification with Methanol
2.2. In Vitro Trypanocidal Evaluation
2.3. In Vitro Leishmanicidal Evaluation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO) Chagas disease (also known as American trypanosomiasis) 10 March 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 20 June 2024).
- DNDi CHAGAS DISEASE IN SEARCH OF SHORTER, BETTER TREATMENTS TO STOP A SILENT KILLER. Available online: https://www.dndi.org/wp-content/uploads/2019/09/Factsheet2019_ChagasDisease.pdf (accessed on 20 June 2024).
- Sales, P. A.; Molina, I.; Murta, S. M. F.; Sánchez-Montalvá, A.; Salvador, F.; Corrêa-Oliveira, R.; Carneiro, C. M. Experimental and Clinical Treatment of Chagas Disease: A Review. Am. J. Trop. Med. Hyg. 2017, 97, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J. A. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches; Acta Trop. 2010, 115, 55-68.
- Antonio Marin-Neto, J.; Rassi, A.; Avezum, A.; Mattos, A. C.; Rassi, A. The BENEFIT trial: testing the hypothesis that trypanocidal therapy is beneficial for patients with chronic Chagas heart disease. Mem. Inst. Oswaldo Cruz 2009, 104, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Coura, J. R.; Borges-Pereira, J. Chagas disease. What is known and what should be improved: a systemic review. Rev. Soc. Bras. Med. Trop. 2012, 45, 286–296. [Google Scholar] [CrossRef]
- Jackson, Y.; Alirol, E.; Getaz, L.; Wolff, H.; Combescure, C.; Chappuis, F. Tolerance and safety of nifurtimox in patients with chronic chagas disease. Clin. Infect. Dis. 2010, 51, e69–75. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, M. J.; Guerrero, L.; Posada, E.; Rodríguez, E.; Soy, D.; Gascon, J. Benznidazole-Related Adverse Drug Reactions and Their Relationship to Serum Drug Concentrations in Patients with Chronic Chagas Disease. Antimicrob. Agents Chemother. 2013, 57, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Perin, L.; Da Silva, R. M.; Da Silva Fonseca, K.; De Oliveira Cardoso, J. M.; Mathias, F. A. S.; Reis, L. E. S.; Molina, I.; Correa-Oliveira, R.; De Abreu Vieira, P. M.; Carneiroa, C. M. Pharmacokinetics and Tissue Distribution of Benznidazole after Oral Administration in Mice. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Urbina, J. A. Specific treatment of Chagas disease: current status and new developments. Curr. Opin. Infect. Dis. 2001, 14, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J. A.; Docampo, R. Specific chemotherapy of Chagas disease: controversies and advances; Trends Parasitol. 2003, 19, 495-501.
- Andrade, S.G.; Magalhaes, J.B.; Pontes, A.L. Evaluation of chemotherapy with benznidazole and nifurtimox in mice infected with Trypanosoma cruzi strains of different types. Bull. World Health Organ. 1985, 63, 721–726. [Google Scholar]
- Filardi, L. S.; Brener, Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 755–759. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO) Cutaneous Leishmaniasis The Mark of Neglect. Available online: https://dndi.org/publications/2019/cutaneous-leishmaniasis-factsheet/ (accessed on 20 June 2024).
- Reithinger, R.; Aadil, K.; Lolaczinski, J.; Mohsen, M.; Hami, S. Social Impact of Leishmaniasis, Afghanistan. Emerg. Infect. Dis. 2005, 11, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, S.; Caljon, G.; Maes, L. Need for sustainable approaches in antileishmanial drug discovery. Parasitol. Res. 2019, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Charlton, R.L.; Rossi-bergmann, B.; Denny, P.W.; Steel, P.G. Repurposing as a strategy for the discovery of new anti-leishmanials: the-state-of-the-art; Parasitology 2018, 145, 219-236.
- Ghorbani, M.; Farhoudi, R. Leishmaniasis in humans: drug or vaccine therapy? Drug Des. Devel. Ther. 2018, 12, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.G.; Almeida, J.R.G.S.; Macêdo, R.O.; Barbosa-Filho, J.M. A review of natural products with antileishmanial activity; Phytomedicine 2005, 12, 514-535.
- Soares, A. C. F.; Matos, P. M.; Dias, H. J.; Aguiar, G. D. P.; Dos Santos, E. S.; Martins, C. H. G.; Veneziani, R. C. S.; Ambrósio, S. R.; Heleno, V. C. G. Variability of the antibacterial potential among analogue diterpenes against Gram-positive bacteria: considerations on the structure–activity relationship. Can. J. Chem. 2019, 97, 568–575. [Google Scholar] [CrossRef]
- Jiao, W.; Wan, Z.; Chen, S.; Lu, R.; Chen, X.; Fang, D.; Wang, J.; Pu, S.; Huang, X.; Gao, H.; Shao, H. Lathyrol diterpenes as modulators of P-glycoprotein dependent multidrug resistance: structure-activity relationship studies on Euphorbia factor L3 derivatives. J. Med. Chem. 2015, 58, 3720–3738. [Google Scholar] [CrossRef]
- Pardo-vargas, A.; Ramos, F. A.; Cirne-santos, C. C.; Stephens, R.; Christina, I.; Paixão, P.; Teixeira, V. L.; Castellanos, L.; Pardo-vargas, A.; Ramos, F. A.; Cirne-santos, C. C.; Roberto, P.; Christina, I.; Paixão, P.; Laneuville, V.; Castellanos, L. Semi-synthesis of oxygenated dolabellane diterpenes with highly in vitro anti-HIV-1 activity. Bioorg. Med. Chem. Lett. 2014, 24, 4381–4383. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F. D. S.; De Almeida, P. D. O.; Aranha, E. S. P.; Boleti, A. P. D. A.; Newton, P.; De Vasconcellos, M. C.; Veiga, V. F.; Lima, E. S. Biological Activities and Cytotoxicity of Diterpenes from Copaifera spp. Oleoresins; Molecules 2015, 20, 6194–6210. [Google Scholar] [CrossRef] [PubMed]
- Mota, S. F.; Oliveira, D. F.; Heleno, V. C. G.; Soares, A. C. F.; Midiwo, J. O.; Souza, E. A. Methyl and p-Bromobenzyl Esters of Hydrogenated Kaurenoic Acid for Controlling Anthracnose in Common Bean Plants. J. Agric. Food Chem. 2017, 65, 1489–1495. [Google Scholar] [CrossRef]
- Silva, A.; Soares, A. C.; Cabral, M.; de Andrade, A.; da Silva, M.; Martins, C.; Veneziani, R.; Ambrósio, S.; Bastos, J.; Heleno, V. Antitubercular Activity Increase in Labdane Diterpenes from Copaifera Oleoresin through Structural Modification. J. Braz. Chem. Soc. 2017, 28, 1106–1112. [Google Scholar] [CrossRef]
- Alegre-Gómez, S.; Sainz, P.; Simões, M.F.; Rijo, P.; Moiteiro, C.; González-Coloma, A.; Martínez-Díaz, R.A. Antiparasitic Activity of Diterpenoids Against Trypanosoma cruzi; Planta Med. 2017, 83, 306-311.
- Souza, A. B.; Martins, C. H. G.; Souza, M. G. M.; Furtado, N. A. J. C.; Heleno, V. C. G.; De Sousa, J. P. B.; Rocha, E. M. P.; Bastos, J. K.; Cunha, W. R.; Veneziani, R. C. S.; Ambrõsio, S. R. Antimicrobial Activity of Terpenoids from Copaifera langsdorffii Desf. Against Cariogenic Bacteria. Phyther. Res. 2011, 25, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, A.; Yan, L. T.; Ito, S.; Edatsugi, H.; Iwata, D.; Komoda, Y. The isolation and in vivo potent antitumor activity of clerodane diterpenoid from the oleoresin of the Brazilian medicinal plant, Copaifera langsdorfii Desfon. Bioorg. Med. Chem. 1994, 4, 2889–2892. [Google Scholar] [CrossRef]
- Zdero, C.; Bohlmann, F.; King, R.M. Diterpenes and norditerpenes from the Aristeguetza group; Phytochemistry 1991, 30, 2991-3000.
- De Andrade, P. M.; De Melo, D. C.; Alcoba, A. E. T.; Ferreira Júnior, W. G.; Pagotti, M. C.; Magalhães, L. G.; Dos Santos, T. C. L.; Crotti, A. E. M.; Alves, C. C. F.; Miranda, M. L. D. Chemical composition and evaluation of antileishmanial and cytotoxic activities of the essential oil from leaves of Cryptocarya aschersoniana Mez. (Lauraceae Juss.). An. Acad. Bras. Cienc. 2018, 90, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Chávez, K.; Compagnone, R. S.; Álvarez, A.; Figarella, K.; Galindo-Castro, I.; Marsiccobetre, S.; Triviño, J.; Arocha, I.; Taddei, A.; Orsini, G.; Tillett, S.; Suárez, A. I. Synthesis and biological evaluation of caracasine acid derivatives. Bioorg. Med. Chem. 2015, 23, 3687–3695. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, Y.; Fujiwara, K.; Yamazaki, A.; Sugawara, N.; Yano, R.; Fukaya, H.; Hitotsuyanagi, Y.; Takeya, K.; Ishiyama, A.; Iwatsuki, M.; Otoguro, K.; Yamada, H.; Omura, S. Semisynthesis of salviandulin E analogues and their antitrypanosomal activity. Bioorganic Med. Chem. Lett. 2014, 24, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Pertino, M.; Vega, C.; Rolón, M.; Coronel, C.; Rojas de Arias, A.; Schmeda-Hirschmann, G. Antiprotozoal Activity of Triazole Derivatives of Dehydroabietic Acid and Oleanolic Acid; Molecules 2017, 22(3), 369.
- Ullah, Asad; Barattto, L.C.; Paula, R.C.; Silva, L.H.V.; Soares, M.J.; Oliveira, B. H.; Preparation of Derivatives of Betulinic Acid, Steviol and Isosteviol and Evaluation of Antitrypanosomal and Antimalarial Activities; J. Braz. Chem. Soc. 2016, 27, 1245-1253.
- Sartorelli, P.; Salomone Carvalho, C.; Quero Reimão, J.; Lorenzi, H.; Tempone, A.G. Antitrypanosomal Activity of a Diterpene and Lignans Isolated from Aristolochia cymbifera; Planta Med. 2010, 76, 1454-1456.
- Scio, E.; Ribeiro, A.; Alves, T.M.A.; Romanha, A.J.; Dias de Souza Filho, J.; Cordell, G.A.; Zani, C.L. Diterpenes from Alomia myriadenia (Asteraceae) with cytotoxic and trypanocidal activity; Phytochemistry 2010, 64, 1125-1131.
- Varela, J.; et al. Bioactive-guided Identification of Labdane Diterpenoids from Aerial Parts of Aristeguietia glutinosa as anti-Trypanosoma cruzi agents. Nat. Prod. Commun. 2012, 7, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Farhoudi, R. Leishmaniasis in humans: drug or vaccine therapy? Drug Des. Devel. Ther. 2018, 12, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, M.; Srivedavyasasri, R.; Asekun, O. T.; Familoni, O. B.; Orishadipe, A.; Zulfiqar, F.; Ibrahim, M. A.; Ross, S. A. Phytochemical study of Piliostigma thonningii, a medicinal plant grown in Nigeria. Med. Chem. Res. 2018, 27, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Fokialakis, N.; Kalpoutzakis, E.; Tekwani, B. L.; Skaltsounis, A. L.; Duke, S. O. Antileishmanial Activity of Natural Diterpenes from Cistus sp. and Semisynthetic Derivatives Thereof. Biol. Pharm. Bull. 2006, 29, 1775–1778. [Google Scholar] [CrossRef]
| Compounds |
L. amazonensis IC50 (μM) |
T. cruzi IC50 (μM) |
Compounds |
L. amazonensis IC50 (μM) |
T. cruzi IC50 (μM) |
|---|---|---|---|---|---|
| 1 | 30.65 | >100 | 10 | 14.65 | >100 |
| 2 | 87.49 | >100 | 11 | 8.27 | 15.05 |
| 3 | 89.47 | 0.425 | 12 | 8.73 | >100 |
| 4 | >100 | >100 | 13 | 26.39 | >100 |
| 5 | >100 | >100 | 14 | 11.35 | 13.31 |
| 6 | 5.94 | >100 | 15 | 8.88 | >100 |
| 7 | >100 | >100 | 16 | 5.31 | >100 |
| 8 | >100 | >100 | 17 | 61.25 | 68.36 |
| 9 | 17.20 | >100 | 18 | >100 | >100 |
| Anfothericin B | 0.043 | --- | Anfothericin B | 0.043 | --- |
| Benznidazole | --- | 13.12 | Benznidazole | --- | 13.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





