1. Introduction
Endoscopic ultrasound (EUS) and intraductal ultrasound (IDUS) have become indispensable modalities for the biliary region, being broadly applied in diagnosis and treatment. Because of their high spatial resolution, their diagnostic uses include diagnosis of choledocholithiasis [
1,
2], differential diagnosis of the stricture and wall thickness of the biliary tract, and evaluation of tumor invasion and horizontal spreading. Currently, in addition to conventional B-mode EUS, diagnostic EUS frequently makes use of techniques such as contrast-enhanced EUS (CE-EUS) and detective flow imaging (DFI). These advances have greatly contributed to improving differential diagnostic capabilities.
EUS-guided tissue acquisition (EUS-TA) has also recently been performed for biliary tract diseases, and with the application of precision medicine to biliary tract cancers playing an increasingly important role, EUS-TA is becoming more important. The reference standard treatment for obstructive jaundice is endoscopic retrograde cholangiopancreatography (ERCP)-guided biliary drainage. If ERCP is unsuccessful, treatments such as percutaneous transhepatic biliary drainage (PTBD) or surgical choledochoenterostomy were traditionally performed; however, the utility of EUS-guided biliary drainage has recently been reported. Here, we review the current literature with respect to the roles of EUS and IDUS in the diagnosis and treatment of biliary tract diseases.
2. Endoscopic Ultrasound (EUS)
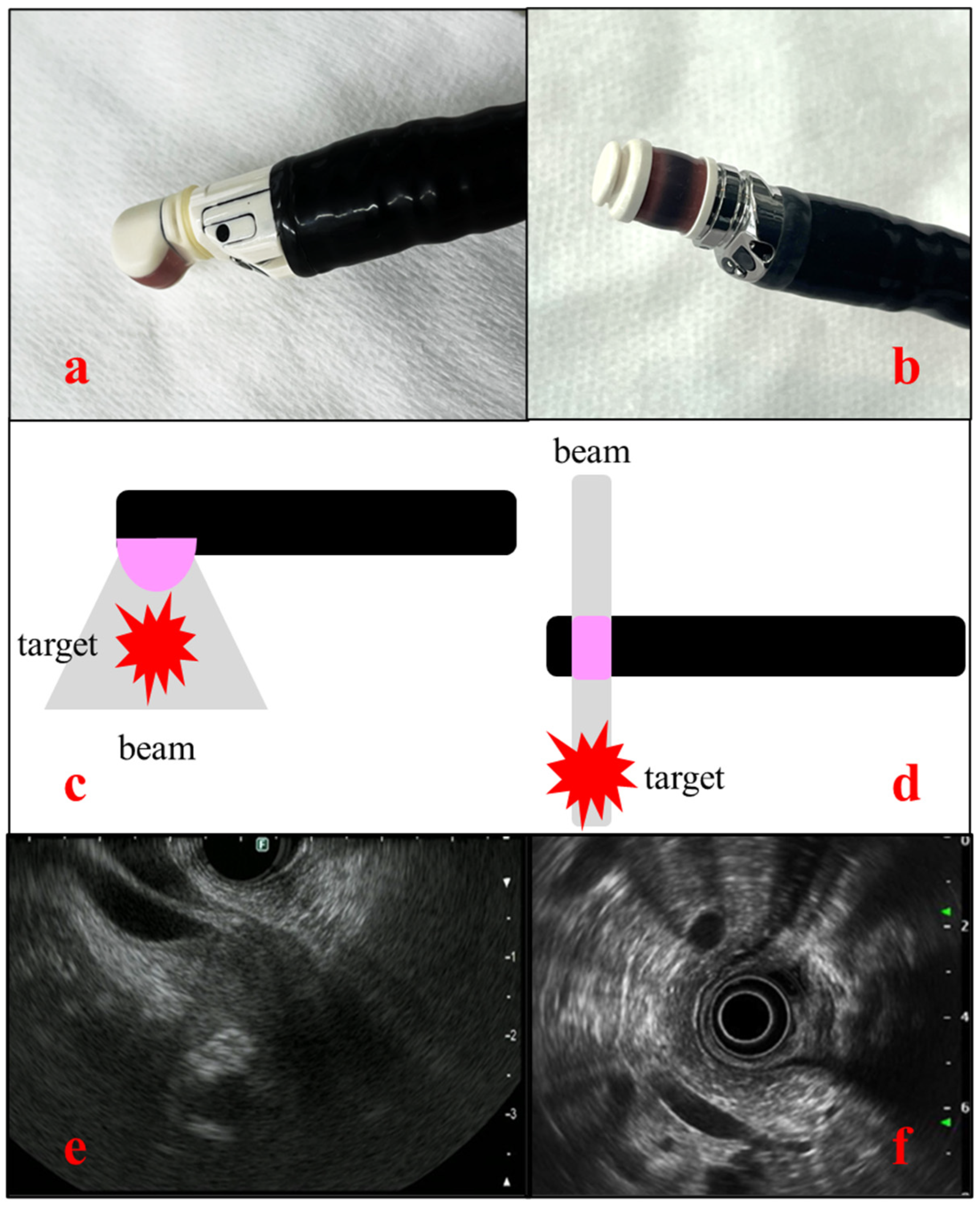
EUS uses an endoscope equipped with an ultrasound transducer at its tip. The frequency typically ranges from 5 to 20 MHz, allowing for switching according to the lesion. EUS is usually used for observing lesions of the digestive tract, biliary tract, pancreas, parts of the liver, and lymph nodes in the mediastinum and abdominal cavity. One of the important features of EUS is its high spatial resolution, which is achieved through close-up observation of target organs. Whereas transabdominal ultrasound has a limitation in observation of pancreatobiliary system due to gastrointestinal gas, thick subcutaneous fat, and bone, EUS enables visualization of organs from the gastrointestinal tract, such as from the stomach and duodenum, and is less affected by such intervening tissues. There are two types of EUS: the radial type, in which ultrasound images are acquired perpendicular to the endoscope, and the convex type (linear type), in which ultrasound images are acquired parallel to the endoscope. The former can depict a 360° field of view, making anatomical orientation easier. By contrast, the convex type has a 180° field of view, although it is useful for EUS-TA and interventional EUS [
Figure 1].
3. Intraductal Ultrasound (IDUS)
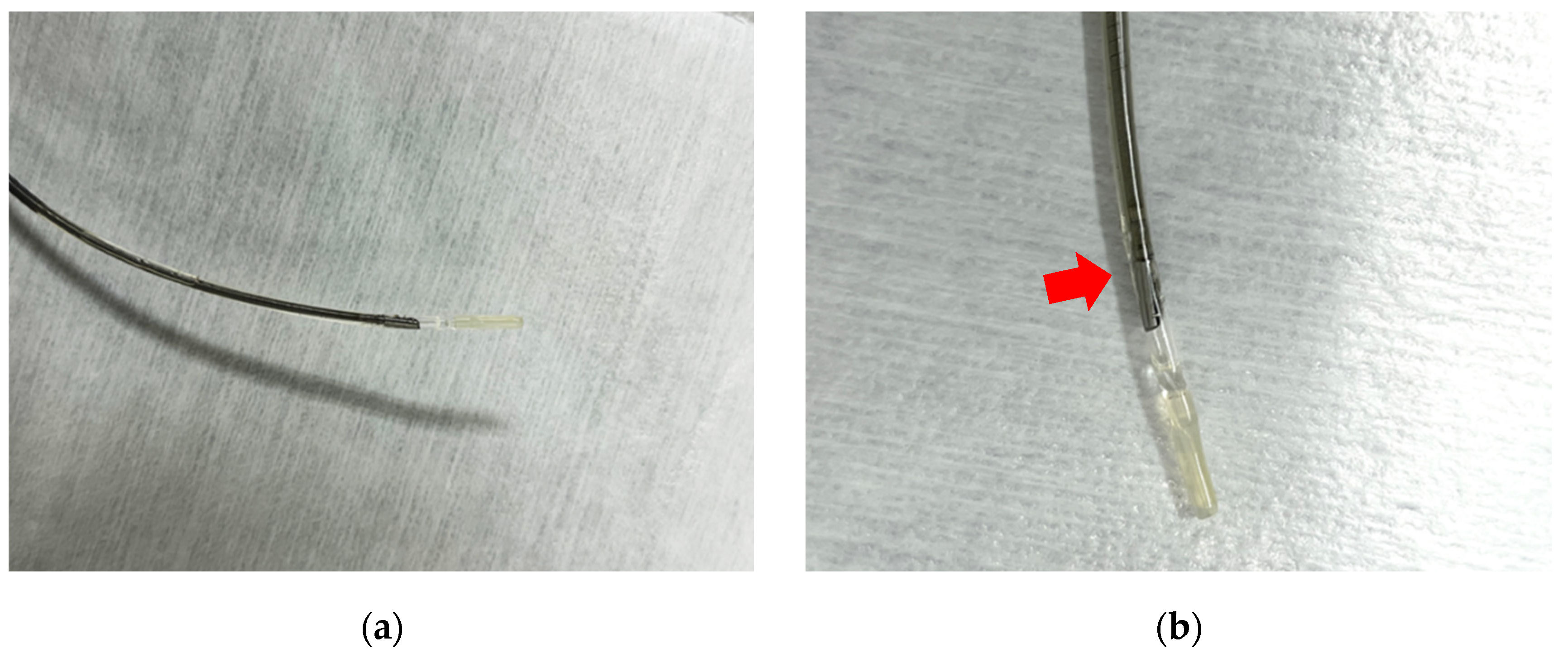
Intraductal ultrasound (IDUS) uses a thin ultrasound probe (diameter of 2–3 mm) with a frequency ranging from 15 to 30 MHz that can be inserted through the endoscope channel. The probe is sufficiently small to allow insertion into the bile duct or pancreatic duct, from where it can obtain ultrasound images. The ultrasound probe has high spatial resolution allowing for visualization of positional relationships with surrounding blood vessels, the bile duct, the pancreatic duct, and the sphincter of Oddi at major papillae [
Figure 2].
4. Contrast-Enhanced EUS
Contrast agents used in ultrasound have a structure consisting of microbubbles covered with carbohydrate or phospholipids. Commercial intravenous ultrasound contrast agents started with Levovist
® (Bayer Schering Pharma, Berlin, Germany), which is now referred to as a first-generation contrast agent, and have now developed to second-generation contrast agents such as Sonazoid
® (GE healthcare, Japan), SonoVue
® (Bracco, Italy), and Definity
® (BristolMyers Squibb Medical Imaging, USA), which make contrast-enhanced EUS (CE-EUS) possible even under low acoustic power. These agents enable stable imaging and prolonged observation with contrast harmonic imaging, allowing for real-time assessment of micro-scale blood flow. They also have a low risk of allergic reactions and are suitable for patients with renal dysfunction because the gas contained in the microbubbles is excreted through exhalation. [
3] They can also be used for those who are allergic to iodine-based contrast agents. It is also possible and economically viable to use them for repeat examinations.
5. EUS-Guided Tissue Acquisition
EUS-guided tissue acquisition (EUS-TA) is a procedure for obtaining tissue from lesions in submucosa or located outside the gastrointestinal tract. Convex-type EUS is used to visualize the target lesion, and a specialized needle is inserted through the scope channel to puncture the lesion and obtain tissue samples. Since its utility was first reported by Vilmann et al. [
4], EUS-TA has become widely adopted. Nowadays, it is the reference standard for diagnosis, and is useful for tumor staging, and for deciding on treatment strategy. The needles used for puncture are classified as aspiration needles (FNA needle) or biopsy needles (FNB needle) according to their shape and size (typically 19G, 22G, or 25G). FNB needles are superior to FNA needles for collecting the large tissue samples [
5,
6] that are useful for performing immunohistochemistry staining and comprehensive genome profiling [
7]. However, FNA needles offer superior puncture performance [
8] and allow samples to be obtained from smaller lesions [
9]. It is important to choose the appropriate needle according to the specific purpose.
6. Diagnosis of Biliary Duct Diseases
6.1. Choledocholithiasis
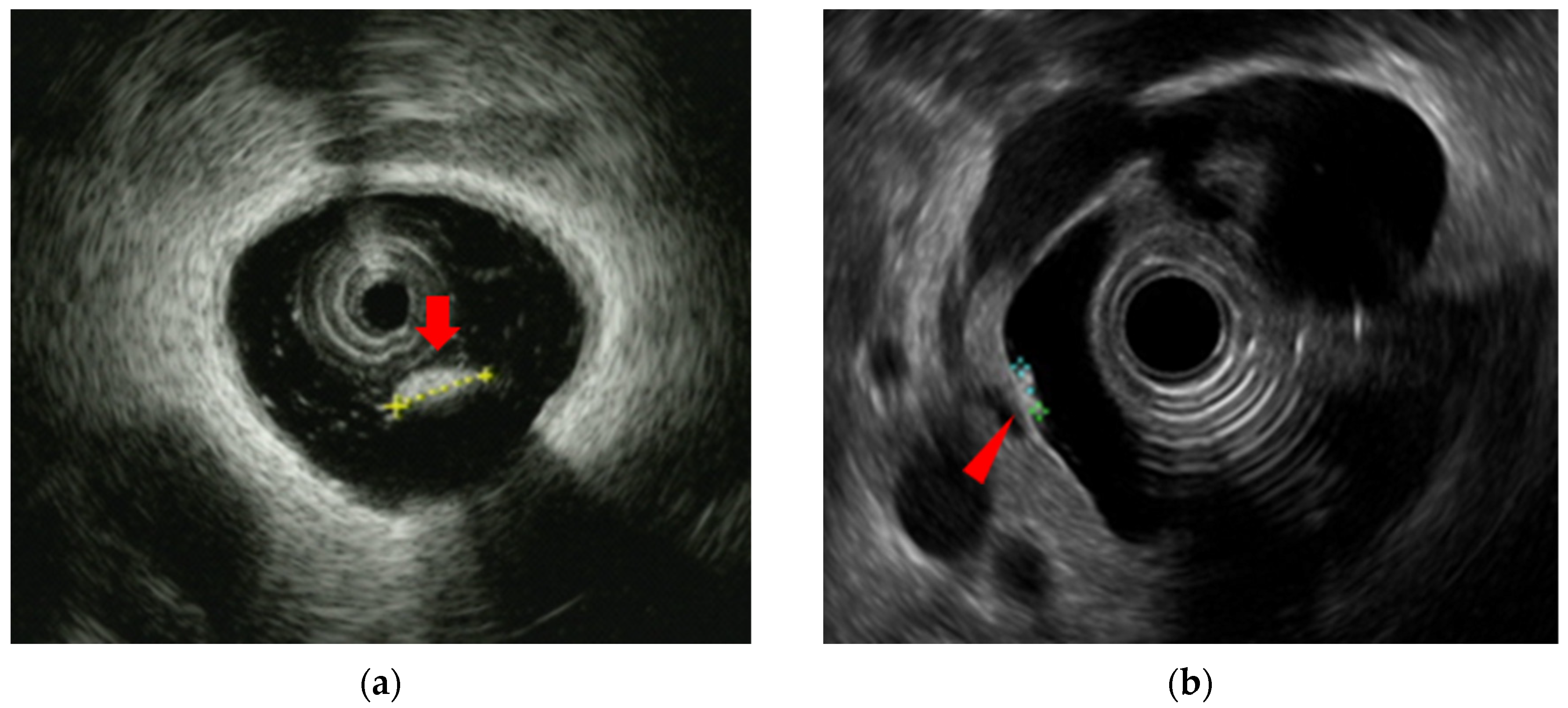
Choledocholithiasis is the most common benign biliary disease encountered in clinical practice, and can sometimes be complicated by severe acute cholangitis, which requires biliary drainage. Diagnostic modalities for choledocholithiasis include abdominal ultrasound (AUS), computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP), and EUS. In some cases, IDUS may also be employed. The sensitivity for detecting choledocholithiasis is 63% for AUS, 71% for CT, and 96% for EUS [
10]. AUS and CT tend to show particularly poor diagnostic ability for choledocholithiasis in patients with small stones or stones in a non-dilated common bile duct. The specificity of EUS for detecting choledocholithiasis is near to 100%, and is higher than that of AUS (95%) and CT (97%) [
10]. MRCP is also an effective modality for the diagnosis of choledocholithiasis; a meta-analysis found sensitivity and specificity for diagnosing choledocholithiasis of 96% and 92%, respectively, for EUS, and 85% and 90% for MRCP [
11]. Suzuki et al. [
12] also reported that EUS had superior diagnostic ability to MRCP for choledocholithiasis that was missed on CT, with the high spatial resolution of EUS making it the most reliable and efficient diagnostic modality for small lesions [
13,
14,
15,
16,
17] [
Figure 3].
IDUS is also known to be effective for detecting choledocholithiasis; Linghu et al. [
18] reported that its accuracy and sensitivity in the diagnosis of extrahepatic bile duct stones were both 100%. Another study reported [
19] that IDUS revealed residual stones in 38% of cases despite a normal cholangiography. Whether IDUS should be performed to confirm residual stones as a routine procedure is not clearly defined, and further investigation is needed.
6.2. Biliary Stricture
Biliary stricture is a commonly encountered condition, and distinguishing between benign and malignant lesions is crucial. Benign diseases include primary sclerosing cholangitis (PSC), IgG4-related sclerosing cholangitis (IgG4-SC), inflammatory stricture, postoperative stricture, and other secondary cholangitis, and their differentiation from biliary tract cancer is very important. A previous report showed that 8%–43% of biliary strictures suspected to be malignant and subsequently resected were actually benign [
20]. Therefore, since surgery for biliary tract cancer is highly invasive, accurate diagnosis is important to avoid unnecessary procedures. It is also important to diagnose IgG4-SC because it is curable if appropriate treatment with steroids is provided.
Previous studies reported that EUS and IDUS were effective for the differential diagnosis between IgG4-SC, PSC, and cholangiocarcinoma [
21,
22,
23].
Table 1. lists the typical findings and features of these diseases. A key point in the differential diagnosis concerns the wall thickness, which is observed in regions of non-stricture in IgG4-SC, and this sign is important for the differential diagnosis from cholangiocarcinoma (sensitivity 95%, specificity 91%, accuracy 94%) [
21]. Recently, the use of immune checkpoint inhibitors has increased, leading to more reports of cholangitis caused by immune-related adverse events. [
24,
25,
26,
27] The differential diagnosis of cholangiocarcinoma from IgG4-SC and PSC is necessary, and while it is reported that diffuse bile duct wall thickness is characteristic, it tends not to involve bile duct strictures, unlike in PSC and IgG4-SC. However, there are still few reported cases, and further accumulation of cases is needed.
As mentioned above, cholangiocarcinoma is the most important disease in biliary stricture, and it requires exact diagnosis. EUS and IDUS have been reported to be effective in the qualitative diagnosis of cholangiocarcinoma, including duct wall invasion, intraductal progression, and invasion into surrounding organs and vessels.
In qualitative diagnosis by EUS, a hypoechoic mass appearance completely occluding the lumen and heterogeneously increased irregular wall thickness in the distal bile duct were found to be highly predictive and sensitive for detecting malignancy originating from the distal bile duct, with reported sensitivity of 75.8% and 68.1%, respectively [
28]. By comparison, the sensitivity, specificity, and accuracy rates for qualitative diagnosis of bile duct strictures (wall thickening, irregular margins) by IDUS are high at 93.2%, 89.5%, and 91.4%, respectively [
29].
6.3. T-Staging
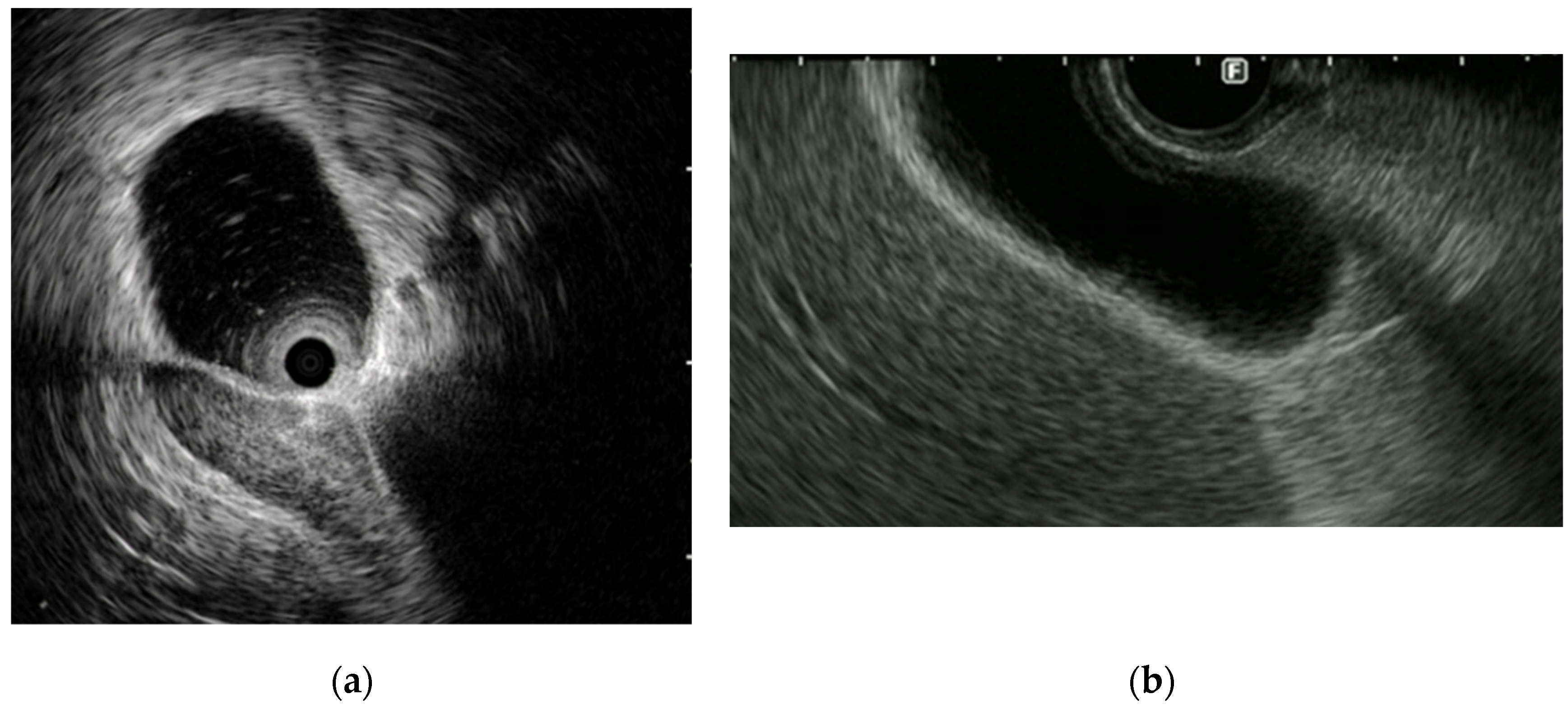
EUS and IDUS can clearly delineate the structure of the bile duct wall. The normal bile duct wall structure consists of two to three layers, with the inner layer being hypoechoic and the outer layer being hyperechoic [
Figure 4]. The inner hypoechoic layer reflects the mucosal layer, fibromuscular layer, and part of the subserosal layer. The outer hyperechoic layer corresponds to the subserosa and serosa. If this lateral hyperechoic layer is irregular or interrupted, it should be considered suspicious for invasion.
In the diagnosis of extrahepatic cholangiocarcinoma progression using EUS, the accuracy rates for diagnosis of invasion into the surrounding tissue, invasion into the pancreatic parenchyma, and invasion into the portal vein, were reported to be 67%–83%, 70%–83%, and 80%–92%, respectively [
30,
31]. Although these values indicate EUS to have high diagnostic performance, there is a weak point in that the diagnostic ability for vascular invasion is inferior from the hilar lesion to distal bile duct [
32].
The accuracy rates of IDUS for the diagnosis of invasion into the right hepatic artery, pancreatic parenchyma, and portal vein are 100%, 93%, and 93%, respectively [
33]. IDUS is reported to have higher accuracy rates for diagnosis and T-staging than EUS (IDUS: 77.7% vs. EUS: 54.1%) [
34].
CE-EUS is useful for the T-staging of biliary tract cancer. Otsuka et al. [
35] reported that CE-EUS is superior to contrast-enhanced CT and conventional EUS in the detection of invasion beyond the bile duct wall. Imazu et al. [
36] also reported that CE-EUS showed higher diagnostic accuracy for the depth of invasion of biliary tract cancer than did conventional B-mode EUS (accuracy rates: 92.4% vs. 69.2%, p<0.05). The diagnosis of longitudinal tumor extent is especially important in hilar cholangiocarcinoma because operative methods differ depending on the tumor extent, and preoperative misdiagnosis of tumor extent makes R0 resection impossible. The reported accuracy rates for the longitudinal extent of hilar cholangiocarcinoma on IDUS range from 85% to 90% [
37,
38].
6.4. N-Staging
The overall survival of lymph node metastasis-negative patients with cholangiocarcinoma is better than that of lymph node metastasis-positive patients [
39]. The detection of at least one malignant lymph node metastasis is associated with lower median survival and mortality [
40]. Therefore, it is important to detect any lymph node metastasis (N-staging). EUS is superior to cross-sectional imaging such as CT and MRI in the detection of lymph nodes (86% vs. 47%, p<0.001) [
40]. Miyata et al. reported that EUS findings are useful for distinguishing between benign and malignant lymph nodes, with findings suggesting malignancy including a long axis ≥ 20 mm, a round shape, sharp edge, hypoechogenicity, absence of central intranodal blood vessel, and heterogeneiety on contrast enhancement [
41].
6.5. EUS-TA
Recently, the effectiveness and safety of EUS-TA for biliary tract tumors has been frequently reported.
Table 2. shows several results concerning EUS-TA for malignant biliary stricture [
42,
43,
44,
45]. These results were validated in many cases, with EUS-TA demonstrating high diagnostic capability and few adverse events. In systematic reviews comparing the use of ERCP and EUS-TA for the diagnosis of malignant biliary stricture, the sensitivities of ERCP and EUS-TA for tissue diagnosis of malignant biliary stricture were 49% and 75%, respectively, the specificities were 96% and 100%, and the accuracy rates were 61% and 79% [
43]. Jo et al. [
44] compared the diagnostic performance of EUS-TA and ERCP-based tissue sampling for malignant biliary obstruction and revealed overall diagnostic sensitivity and accuracy rates of 74% and 76%, respectively, for EUS-TA, 57% and 61% for ERCP, and 86% and 87% for a combination of EUS and ERCP. However, we should be careful about the risks of peritonitis due to bile leakage and potential tumor seeding [
46]. We believe that in cases where a diagnosis cannot be obtained with ERCP, performing EUS-TA for biliary stricture should be considered with careful consideration of the risks and benefits.
7. Diagnosis of Gallbladder Diseases
7.1. Wall Thickening Lesions
The gallbladder wall consists of two layers: the inner layer includes the mucosa, the muscularis propria and a part of the subserosa, which appear hypoechoic; and the outer layer consists of a part of the subserosa and the serosa, which appear hyperechoic. EUS can usually depict these two layers [
47] [
Figure 4].
Wall thickening of the gallbladder is defined as thickened wall measuring more than 3 mm in diameter. Its differential diagnoses are varied, ranging from benign diseases such as chronic cholecystitis, xanthogranulomatous cholecystitis, and adenomyomatosis (ADM), to malignant diseases such as gallbladder carcinoma.
Characteristic EUS findings of ADM include comet-tail artifacts within the thickened wall and cystic anechoic spots, which are crucial for diagnosis [
48] [
Figure 5]. EUS can depict these findings in detail, whereas gallbladder carcinoma usually does not have such echoic findings. Findings suggestive of malignancy include wall thickening of 10 mm or more, heterogeneous internal echogenicity with regions of low echogenicity, and loss of layer structure [
49] [
Table 3].
However, it is necessary to carefully observe whether there are any irregularities on the surface of ADM because carcinoma can coexist with ADM [
50]. Xanthogranulomatous cholecystitis is a subtype of cholecystitis. Its characteristics include xanthoma cells with bile pigment forming granuloma in the gallbladder wall, and inflammatory infiltration extending to surrounding organs as if cancer was invading; however, it is difficult to distinguish it from gallbladder carcinoma. The characteristic ultrasound findings of xanthogranulomatous cholecystitis are reported to include a lack of wall disruption and intramural hypoechoic nodules [
51], but clinicians often face challenges in its differential diagnosis. It may be difficult to distinguish it from malignancy by EUS alone.
7.2. Protuberant Lesions
Protuberant lesions of the gallbladder encompass a wide range of non-neoplastic conditions such as cholesterol polyps, hyperplastic polyps, adenomyomatosis, and inflammatory polyps, as well as neoplastic conditions including adenomas and gallbladder carcinoma. Given this broad spectrum, differential diagnosis is crucial. EUS plays an important role in this differentiation, with it being reported to have high diagnostic ability for protuberant gallbladder lesions. Azuma et al. [
52] reported that EUS had high differential diagnostic ability for gallbladder lesions (less than 20 mm), and that it was superior to AUS. Their study showed sensitivity, specificity, and positive and negative predictive values for EUS and AUS in the diagnosis of malignancy of 91.7% vs. 54.2%, 87.7% vs. 53.8%, 75.9% vs. 54.2%, and 96.6% vs. 94.6%, respectively. However, for lesions smaller than 10 mm, the differentiation of malignancy by EUS can be challenging [
53]. In European guidelines, cholecystectomy is recommended for gallbladder polyps of more than 10 mm. However, even if polyps are of less than 10 mm, they can still be neoplastic lesions [
54,
55], and we must be careful. It was reported that polyps larger than 14 mm are useful for distinguishing between benign and malignant lesions [
56]. While it is easy to suggest that the larger the polyp, the higher the proportion of malignancy, there are also scoring systems that are useful for distinguishing between smaller polyps.
An EUS scoring system for diagnosing whether gallbladder polypoid lesions are benign or malignant has been suggested, with this system including features such as layer stricture, echo patterns, margin of polyp, stalk, and number of polyps; polyps with a score of six or greater are considered to be at high risk of malignancy (
Table 4) [
57]. This scoring system has utility for distinguishing whether gallbladder lesions (size 5–15 mm) are benign or malignant. Findings suggestive of malignancy include loss of layer structure, an isoechoic heterogeneous pattern, lobulated margin, sessile type, and presence of multiple polyps [
57].
Contrast-enhanced EUS (CE-EUS) is also useful for discriminating benign from malignant gallbladder lesions. The evaluation of tumor vascularity aids in distinguishing between benign and malignant lesions. Choi et al. [
58] reported that an irregular vessel pattern in the gallbladder polyp on CE-EUS could diagnose malignancy with a sensitivity and a specificity of 90.3% and 96.6%, respectively, and the presence of perfusion defects in the gallbladder polyp determined by CE-EUS could diagnose malignancy with a sensitivity and specificity of 90.3% and 94.9%, respectively. In point of diagnosis of gallbladder carcinoma with definitely determined, the sensitivity and a specificity of CE-EUS were 93.5% and 93.2%, respectively, slightly superior to conventional EUS (90.0% and 91.1%).
Yamashita et al. [
59] reported that a novel technique called detective flow imaging (DFI), which can visualize fine vessels and slow flow not detectable with conventional color Doppler or power Doppler techniques, was useful for distinguishing between benign and malignant gallbladder lesions. This technique does not require contrast agents or additional examination time and can be more easily evaluated. In the diagnosis of gallbladder carcinoma, the sensitivity, specificity, and accuracy of irregular vessels detected with DFI-EUS were 89%, 100%, and 92% respectively [
59].
In gallbladder carcinoma, EUS is useful not only for qualitative diagnosis, but also for staging and pathological diagnosis. It is important to evaluate the depth of gallbladder wall invasion and the extent of invasion into surrounding organs because these are involved in determining the treatment plan.
7.3. T-Staging
As mentioned above, the gallbladder wall is depicted as two layers. Because the outer layer consists of part of the subserosa and the serosa, thinning and disruption of the outer layer indicates suspicion of tumor invasion deeper than the subserosa. Sugimoto et al. reported that EUS can diagnose subserosal invasion of gallbladder carcinoma by focusing on the condition of the outer layer (sensitivity 97.1%, specificity 86.7%, accuracy 93.8%) [
60]. CE-EUS is reported to be useful for diagnosis of tumor invasion because it allows clear visualization of the layer structure [
36].
7.4. N-Staging
The presence or absence of lymph node metastasis in gallbladder cancer affects the surgical approach and patient prognosis, similar to the T-stage. Therefore, the detection of lymph node metastasis is very important. EUS is also useful for diagnosing the N-stage of gallbladder cancer [
61]. Mitake et al. reported that the sensitivity, specificity, and accuracy of EUS for detecting regional lymph node metastasis were 81.8%, 92.9%, and 89.7%, respectively [
62].
7.5. EUS-TA
EUS-TA is reported to be effective for pathological examination of gallbladder lesions. Giri et al. reported a systematic review and meta-analysis on the utility and safety of EUS-TA for gallbladder lesions [
63]. This showed that the sensitivity, specificity, and accuracy for the diagnosis of malignant lesions were 90%, 100%, and 94.1%, respectively. Adverse events showed a pooled incidence of 1.8%, but none of the patients had a serious adverse event. However, as when EUS-TA is used for biliary tract cancer, we cannot deny the risk of peritonitis due to bile leakage and potential tumor seeding. In operable cases, a first tissue sampling via ERCP is recommended. If the diagnosis is not established with ERCP, EUS-TA for lymph node metastases or liver metastases may be considered. For unresectable cases, it is preferable to consider EUS-TA from locations where tissue sampling can be reliably performed.
The number of chemotherapy drugs available for biliary tract cancers, including gallbladder carcinoma, is rather limited. Recently, comprehensive genome profiling (CGP) has been attracting attention in respect to devising treatment strategies, and EUS-TA plays an important role not only in pathological diagnosis, but also in the field of CGP [
64,
65]. In a meta-analysis by Yoon et al., the diagnostic sensitivity for biliary tract cancers was 67% with biopsy and 73.6% with EUS-TA [
66]. The sensitivity of transpapillary biopsy is not so high, which may be challenging for CGP. Yanaidani et al. reported the utility of EUS-TA for CGP of biliary tract cancers [
67]. They suggested that we should use an FNB needle of 22G or larger because it allows suitable specimens for CGP to be obtained, with such specimens having accuracy comparable to surgical specimens.
8. Diagnosis of Other Diseases
8.1. Pancreaticobiliary Maljunction
Pancreaticobiliary maljunction is a congenital anomaly where the pancreatic and bile ducts join outside the duodenal wall. This condition leads to the reflux of pancreatic juice and bile, which significantly increases the risk of bile duct gallbladder cancer and pancreatitis. Diagnosis can be confirmed by observing absence of the sphincter of Oddi’s influence at the junction of the pancreatic and bile ducts.
EUS is an effective modality for diagnosing pancreaticobiliary maljunction. It allows confirmation that the bile duct and pancreatic duct converge within the pancreatic parenchyma. The diagnostic accuracy of EUS for pancreaticobiliary maljunction is reported to be high, ranging from 88% to 100% [
68,
69,
70,
71,
72] [
Figure 6]. As a diagnostic method for pancreaticobiliary maljunction, direct cholangiography via ERCP is also useful. Additionally, measurement of amylase levels in bile is possible. IDUS is performed following ERCP, confirming the junction of the pancreatic and bile ducts outside the duodenal wall. However, these methods are invasive, and post-ERCP pancreatitis can be a concern.
8.2. Ampullary Tumors
Ampullary tumors can be classified into epithelial and non-epithelial types. Epithelial tumors include adenoma and adenocarcinoma. Non-epithelial tumors include neuroendocrine tumors among others. Treatment for ampullary tumors traditionally involved pancreaticoduodenectomy, which is highly invasive and associated with mortality rates and high postoperative adverse event rates [
73]. Therefore, the less invasive approach of endoscopic papillectomy has gained attention for ampullary adenoma and adenocarcinoma which do not invade the pancreatic or bile ducts. Endoscopic papillectomy for ampullary adenoma has obtained a consensus in guidelines [
74], but for adenocarcinoma, invasion up to T1a depth is acceptable. Evaluation of factors such as tumor invasion beyond the Oddi sphincter and involvement of the pancreatic or bile ducts is crucial for treatment decisions. Therefore, EUS and IDUS are essential modalities for assessing the depth of ampullary tumors.
8.3. T-Staging
A meta-analysis on the diagnostic accuracy of EUS for local staging of ampullary tumors reported sensitivity and specificity for T-staging of 89% and 87%, respectively, for T1, 76% and 91% for T2, 81% and 94% for T3, and 72% and 98% for T4 [
75]. By comparison, the sensitivity and specificity of IDUS for T-staging were reported to be 99% and 88%, respectively, for T1, 73% and 91% for T2, and 79% and 97% for T3.
8.4. N-Staging
A meta-analysis on the diagnostic accuracy of EUS and IDUS for N-staging of ampullary tumors reported the sensitivity and specificity were 61%, 77% and 61%, 92%, respectively [
75].
8.5. EUS-TA
Reports on EUS-TA for ampullary tumors are few [
Table 5] [
76,
77,
78], which may be due to the fact that diagnosis can often be achieved through conventional endoscopic biopsies. EUS-TA is expected to be useful for diagnosing ampullary tumors that cannot be diagnosed through conventional pathological examinations such as biopsies taken from within the papilla and/or brush cytology during ERCP.
9. Therapy of Biliary Duct Diseases
9.1. Classification of EUS-Guided Biliary Drainage
EUS-guided biliary drainage (EUS-BD) includes various procedures for creating fistulas between the gastrointestinal tract and bile ducts, such as EUS-guided hepaticogastrostomy (EUS-HGS) and EUS-guided choledochoduodenostomy (EUS-CDS), as well as adjunctive techniques like EUS-guided rendezvous, which involves accessing the bile ducts after fistula creation and passing a guidewire through the ampulla to assist in bile duct access, and EUS-guided antegrade, in which antegrade stent placement is performed. Additionally, there are procedures such as EUS-guided hepaticojejunostomy, in which a fistula is created between the bile duct and jejunum, EUS-guided hepaticoduodenostomy, in which a fistula is formed between the duodenum and right intrahepatic bile duct, and EUS-guided gallbladder drainage (EUS-GBD), in which a fistula is created between the duodenum and gallbladder. The choice of treatment should be tailored to the individual case.
9.2. Technical Procedures of EUS-Guided Biliary Drainage (EUS-BD)
The procedure for EUS-BD is as follows. First, the bile duct is visualized using EUS from the gastrointestinal tract. Then, Doppler imaging is used to avoid blood vessels along the puncture line and the bile duct is punctured and a guidewire inserted. After confirming intraductal placement with a contrast tube, the fistula is dilated using dilation devices. Finally, a stent is placed [
Figure 7]. The advantage of EUS-BD is its ability to achieve internal fistulization. However, there are potential drawbacks, including serious adverse events such as bile leakage, bile peritonitis, bleeding, and stent migration. The overall frequency of adverse events was reported to be 13.7%, with bile leakage at 2.2%, bleeding at 0.9%, and stent migration at 1.7% [
79].
9.2.1. Comparison of EUS-BD vs. PTBD
PTBD has traditionally been chosen as an alternative treatment for biliary drainage in cases where ERCP fails or is difficult. Several meta-analyses and systematic reviews have compared EUS-BD and PTBD, and the results of these are summarized in
Table 6 [
80,
81,
82,
83]. It is suggested that EUS-BD has a higher success rate, less adverse events, and fewer reinterventions than PTBD. However, these differences are still open to debate because prospective trials have not been conducted.
9.2.2. Comparison of EUS-BD vs. ERCP
Two previous studies [
84,
85] reported the technical and clinical success rates of EUS-BD and ERCP to be equivalent, and the incidence of adverse events was also comparable. In a study by Paik et al. [
86], although the technical and clinical success rates were similar between the two procedures, the incidence of adverse events was lower with EUS-BD (6.3% vs. 19.7%, p=0.03). In ERCP, there is always a risk of pancreatitis, but this concern does not exist with EUS-BD. We must not forget that EUS-BD also carries the risk of specific adverse events such as bile leakage, bile peritonitis, and stent migration. In the latest meta-analysis comparing ERCP and EUS-BD for malignant biliary obstruction, there were no significant differences between the two techniques in regard to technical success rate (OR=0.76; 95% CI: 0.30–1.91), clinical success (OR=1.45, 95% CI, 0.66–3.16), and adverse event rates (OR=0.75, 95% CI, 0.45–1.24) [
87]. EUS-BD also required less reinterventions than ERCP (OR=0.36, 95% CI, 0.15–0.86) [
87]. These findings suggest that EUS-BD could be considered as a safe and effective alternative treatment for biliary drainage. In the future, EUS-BD may become the first line therapy for biliary drainage [
88].
9.2.3. Comparison of EUS-CDS vs. EUS-HGS
In the field of EUS-BD, both EUS-CDS and EUS-HGS are commonly performed procedures. EUS-CDS is typically performed in cases of common bile duct obstruction or duodenal obstruction beyond the superior duodenal angle (SDA). By contrast, EUS-HGS is typically performed in cases of hilar bile duct obstruction, duodenal obstruction proximal to the SDA, or postoperative intestinal reconstruction. In studies comparing these two techniques [
Table 7] [
89,
90,
91], EUS-CDS and EUS-HGS were found to be comparable with respect to technical success rate, clinical success rate, and adverse events. However, EUS-HGS was reported to have a higher reintervention rate.
9.3. Indication and Technical Procedures of EUS-Guided Gallbladder Drainage (EUS-GBD)
When the surgical risk is not high, the first-choice treatment for acute cholecystitis is early cholecystectomy, which has gained a consensus [
92]. In cases of high surgical risk, gallbladder drainage becomes necessary, with procedures such as percutaneous transhepatic gallbladder drainage (PTGBD) or endoscopic transpapillary gallbladder drainage (ETGBD) being performed. PTGBD is not recommended in patients with an inability to puncture due to anatomical issues or ascites, and in elderly patients at high risk of tube self-removal. In such cases, consideration should be given to ETGBD. ETGBD offers significant benefits from the use of an internal fistula. However, the risk of post-ERCP pancreatitis and the relatively low success rate are problematic. In recent years, EUS-GBD has been reported as a new option for high-risk surgical cases of acute cholecystitis.
The procedure for EUS-GBD is as follows. First, the gallbladder is depicted using EUS from the duodenum. Then, Doppler imaging is used to avoid blood vessels along the puncture line and the gallbladder is punctured and contrast agent injected. After confirming the gallbladder under fluoroscopic guidance, a guidewire is inserted and the fistula is dilated using dilation devices. Finally, a stent is placed. As in EUS-BD, there is a risk of serious adverse events including bile leakage, bile peritonitis, and stent migration.
9.3.1. Comparison of EUS-GBD vs. PTGBD or ETGBD
Like PTGBD, EUS-GBD may encounter difficulties in patients undergoing antithrombotic therapies, those with substantial ascites, and in cases of postoperative intestinal reconstruction. However, it offers a higher success rate than ETGBD [
93], and is advantageous in terms of the achievement of an internal fistula, making it a valuable treatment option. In a systematic review and meta-analysis comparing EUS-GBD and PTGBD, the technical success rates, clinical success rates, adverse event rates, and recurrency rates of acute cholecystitis were 96.5% vs. 98.6%, 93.5% vs. 91.9%, 17.9% vs. 33.9% and 4.2% vs. 7.6%, respectively [
94]. Currently, EUS-GBD is performed only in certain advanced medical facilities, and for it to become an alternative treatment to PTGBD, further dissemination is necessary.
10. Conclusions and Future Directions
In conclusion, EUS and IDUS perform important roles in the diagnosis and treatment of biliary diseases. Because of their superior spatial resolution compared with other modalities, they enable us to detect small lesions and perform differential diagnosis and tumor staging of malignant tumors. Furthermore, with the emergence of new techniques, EUS-BD has had a significant impact on endoscopic biliary drainage procedures. Further increases in its application are expected in the future.
Author Contributions
Conceptualization, A.N.; Y.Y. and M.K.; writing—original draft preparation, A.N.; writing—review and editing, Y.Y.; supervision, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the review of previously published articles.
Data Availability Statement
Data supporting reported results can be found in the references at the end of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shivaraj Afzalpurkar, Suprabhat Giri, Sunil Kasturi, Sushrut Ingawale, Sridhar Sundaram. Magnetic resonance cholangiopancreatography versus endoscopic ultrasound for diagnosis of choledocholithiasis: an updated systematic review and meta-analysis. Surg Endosc. 2023, 37(4):2566-2573.
- Masato Suzuki, Yusuke Sekino, Kunihiro Hosono, Kouji Yamamoto, Kenichi Kawana, Hajime Nagase, Kensuke Kubota, Atsushi Nakajima. Endoscopic ultrasound versus magnetic resonance cholangiopancreatography for the diagnosis of computed tomography-negative common bile duct stone: Prospective randomized controlled trial. Dig Endosc. 2022, 34(5):1052-1059.
- Steinar Uran, Kristin Landmark, Per Trygve Normann, Petter-Arnt Hals, Kim Gunnar Toft, Tore Skotland. J Pharm Biomed Anal. 2005, 39(3-4): 746-751.
- P Vilmann, G K Jacobsen, F W Henriksen, S Hancke. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992, 38: 172-3.
- Ji Young Bang, Shantel Hebert-Magee, Udayakumar Navaneethan, Muhammad K Hasan, Robert Hawes, Shyam Varadarajulu. EUS-guided fine needle biopsy of pancreatic masses can yield true histology. Gut. 2018, 67(12):2081-2084.
- Masahiro Itonaga, Satoru Yasukawa, Nobuyasu Fukutake, Takeshi Ogura, Masanori Asada, Toshio Shimokawa, Osamu Inatomi, Yoshitaka Nakai, Hideyuki Shiomi, Hiroko Nebiki, Azumi Suzuki, Koh Kitagawa, Satoshi Asai, Masaaki Shimatani, Tsuyoshi Sanuki, Akira Kurita, Mamoru Takenaka, Motoyuki Yoshida, Noriyuki Hoki, Hiroaki Yasuda, Hirotsugu Maruyama, Hisakazu Matsumoto, Akio Yanagisawa, Masayuki Kitano. Comparison of 22-gauge standard and Franseen needles in EUS-guided tissue acquisition for diagnosing solid pancreatic lesions: a multicenter randomized controlled trial. Gastrointest Endosc. 2022,96(1):57-66.e2.
- Sherif Elhanafi, Nadim Mahmud, Norge Vergara, Michael L Kochman, Koushik K Das, Gregory G Ginsberg, Michael Rajala, Vinay Chandrasekhara. Comparison of endoscopic ultrasound tissue acquisition methods for genomic analysis of pancreatic cancer. J Gastroenterol Hepatol. 2019, 34(5):907-913.
- Julio Iglesias-Garcia, Jan-Werner Poley, Alberto Larghi, Marc Giovannini, Maria Chiara Petrone, Ihab Abdulkader, Genevieve Monges, Guido Costamagna, Paolo Arcidiacono, Katharina Biermann, Guido Rindi, Erwan Bories, Claudio Dogloni, Marco Bruno, J Enrique Dominguez-Muñoz. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011, 73:1189-96.
- Hiroyuki Uehara, Kenji Ikezawa, Natsuko Kawada, Nobuyasu Fukutake, Kazuhiro Katayama, Rena Takakura, Yasuna Takano, Osamu Ishikawa, Akemi Takenaka. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J Gastroenterol Hepatol. 2011, 26: 1256-61.
- M Sugiyama, Y Atomi. Endoscopic ultrasonography for diagnosing choledocholithiasis: a prospective comparative study with ultrasonography and computed tomography. Gastrointest Endosc. 1997, 45(2):143-6.
- Shivaraj Afzalpurkar, Suprabhat Giri, Sunil Kasturi, Sushrut Ingawale, Sridhar Sundaram. Magnetic resonance cholangiopancreatography versus endoscopic ultrasound for diagnosis of choledocholithiasis: an updated systematic review and meta-analysis. Surg Endosc. 2023, 37(4): 2566-2573.
- Masato Suzuki, Yusuke Sekino, Kunihiro Hosono, Kouji Yamamoto, Kenichi Kawana, Hajime Nagase, Kensuke Kubota, Atsushi Nakajima. Endoscopic ultrasound versus magnetic resonance cholangiopancreatography for the diagnosis of computed tomography-negative common bile duct stone: Prospective randomized controlled trial. Dig Endosc. 2022, 34(5): 1052-1059.
- M F Müller, C Meyenberger, P Bertschinger, R Schaer, B Marincek. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994, 190:745-751.
- Hiroki Sakamoto, Masayuki Kitano, Yoichiro Suetomi, Kiyoshi Maekawa, Yoshifumi Takeyama, Masatoshi Kudo. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med. Biol. 2008, 34: 525-32.
- Koji Yamaguchi, Takuji Okusaka, Kyoko Shimizu, Junji Furuse, Yoshinori Ito, Keiji Hanada, Tooru Shimosegawa, Kazuichi Okazaki. Clinical Practice Guidelines for Pancreatic Cancer 2016 From the Japan Pancreas Society: A Synopsis. Pancreas. 2017, 46: 595-604.
- Somashekar G Krishna, Bhavana B Rao, Emmanuel Ugbarugba, Zarine K Shah, Alecia Blaszczak, Alice Hinton, Darwin L Conwell, Phil A Hart. Diagnostic performance of endoscopic ultrasound for detection of pancreatic malignancy following an indeterminate multidetector CT scan: a systemic review and meta-analysis. Surg Endosc. 2017, 31: 4558-4567.
- Shintaro Kondo, Hiroyuki Isayama, Masaaki Akahane, Nobuo Toda, Naoki Sasahira, Yosuke Nakai, Natsuyo Yamamoto, Kenji Hirano, Yutaka Komatsu, Minoru Tada, Haruhiko Yoshida, Takao Kawabe, Kuni Ohtomo, Masao Omata. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol. 2005, 54: 271-275.
- En-Qiang Linghu, Liu-Fang Cheng, Xiang-Dong Wang, Zhi-Qiang Wang, Yun-Sheng Yang, Wen Li, Feng-Chun Cai, Hong-Zhi Wang, Hong Du, Jiang-Yun Meng. Intraductal ultrasonography and endoscopic retrograde cholangiography in diagnosis of extrahepatic bile duct stones: a comparative study. Hepatobiliary Pancreat Dis Int. 2004, 3(1): 129-32.
- Andrew Catanzaro, Patrick Pfau, Gerard A Isenberg, Richard C k Wong, Michael V Sivak Jr, Amitabh Chak. Clinical utility of intraductal US for evaluation of choledocholithiasis. Gastrointest Endosc. 2003, 57(6): 648-52.
- E Buc, M Lesurtel, J Belghiti. Is preoperative histological diagnosis necessary before referral to major surgery for cholangiocarcinoma?. HPB (Oxford). 2008, 10(2): 98-105.
- Itaru Naitoh, Takahiro Nakazawa, Hirotaka Ohara, Tomoaki Ando, Kazuki Hayashi, Hajime Tanaka, Fumihiro Okumura, Satoru Takahashi, Takashi Joh. Endoscopic transpapillary intraductal ultrasonography and biopsy in the diagnosis of IgG4-related sclerosing cholangitis. J Gastroenterol. 2009, 44(11): 1147-55.
- Itaru Naitoh, Takahiro Nakazawa, Kazuki Hayashi, Katsuyuki Miyabe, Shuya Shimizu, Hiromu Kondo, Yuji Nishi, Michihiro Yoshida, Shuichiro Umemura, Yasuki Hori, Akihisa Kato, Fumihiro Okumura, Hitoshi Sano, Hirotaka Ohara, Takashi Joh. Comparison of intraductal ultrasonography findings between primary sclerosing cholangitis and IgG4-related sclerosing cholangitis. J Gastroenterol Hepatol. 2015, 30: 1104-1109.
- Itaru Naitoh, Takahiro Nakazawa. Classification and Diagnostic Criteria for IgG4-Related Sclerosing Cholangitis. Gut Liver. 2022, 15; 16(1): 28-36.
- Shoko Noda-Narita, Suguru Mizuno, Satoshi Noguchi, Kousuke Watanabe, Yousuke Nakai, Kazuhiko Koike, Hidenori Kage, Takahide Nagase. Development of mild drug-induced sclerosing cholangitis after discontinuation of nivolumab. Eur J Cancer. 2019, 107: 93-6.
- Masashi Kono, Toshiharu Sakurai, Kazuki Okamoto, Shou Masaki, Tomoyuki Nagai, Yoriaki Komeda, Ken Kamata, Kosuke Minaga, Kentarou Yamao, Mamoru Takenaka, Tomohiro Watanabe, Naoshi Nishida, Masatoshi Kudo. Efficacy and Safety of Chemotherapy Following Anti-PD-1 Antibody Therapy for Gastric Cancer: A Case of Sclerosing Cholangitis. Intern Med. 2019, 58: 1263-6.
- Kohei Ogawa, Kenya Kamimura, Shuji Terai. Antiprogrammed Cell Death-1 Immunotherapy-Related Secondary Sclerosing Cholangitis. Hepatology. 2019, 69: 914-6.
- Yudai Koya, Michihiko Shibata, Nobuhiko Shinohara, Satoru Nebuya, Shinji Oe, Yuichi Honma, Michio Senju, Naoko Sato, Masaru Harada. Secondary sclerosing cholangitis with hemobilia induced by pembrolizumab: Case report and review of published work. Hepatol Res. 2019, 49: 950-6.
- Emrah Alper, Mahmut Arabul, Zafer Buyrac, Behlül Baydar, Yucel Ustundag, Mustafa Celik, Belkis Ünsal. The use of radial endosonography findings in the prediction of cholangiocarcinoma in cases with distal bile duct obstructions. Hepatogastroenterology. 2013, 60(124): 678-683.
- Tobias Meister, Hauke S Heinzow, Carina Woestmeyer, Philipp Lenz, Josef Menzel, Torsten Kucharzik, Wolfram Domschke, Dirk Domagk. Intraductal ultrasound substantiates diagnostics of bile duct strictures of uncertain etiology. World J Gastroenterol. 2013, 19(6): 874-81.
- Hidekazu Mukai, Masatsugu Nakajima, Kenjiro Yasuda, Eisai Cho, Yoshihiro Mizuma, Takanobu Hayakumo, Tooru Ashihara, Shigeto Mizuno, Hajime Murakita, Seiichi Hirano, Makoto Hayashi, Etsuko Ikeda, Genichi Kato, Takayoshi Matsui, Masao Kobayashi. Evaluation of endoscopic ultrasonography (EUS) in the diagnosis of cancer of the extrahepatic bile duct. Gastroenterol Endosc. 1990, 32(8): 1893-1902.
- Fumio Arimura, Jun Matumoto, Kazuaki Nakasio, Junichi Yoshikawa, Yasuaki Suekawa, Tuyoshi Sakamoto, Keizou Tanaka, Terukatu Arima, Atumasa Yamaguti, Akirou Sakoda, Sadao Tanaka, Tadashi Sibue, Toshikazu Osame. Gastroenterol Endosc. Endoscopic ultrasonography (EUS) in assessment of spread from extrahepatic bile duct cancer. 1992, 34(8); 1863-70.
- K. Tamada, K. Ido, N. Ueno, M. Ichiyama, T. Tomiyama, T. Nishizono, S. Wada, T. Noda, S. Tano, T. Aizawa, T. Ueno, K. Kimura. Assessment of portal vein invasion by bile duct cancer using intraductal ultrasonography. Endoscopy. 1995, 27(8): 573-8.
- M Kuroiwa 1, Y Tsukamoto, Y Naitoh, Y Hirooka, T Furukawa, T Katou. New technique using intraductal ultrasonography for the diagnosis of bile duct cancer. J Ultrasound Med. 1994, 13(3);189-95.
- J Menzel, C Poremba, K H Dietl, W Domschke. Preoperative diagnosis of bile duct strictures--comparison of intraductal ultrasonography with conventional endosonography. Scand J Gastro. 2000, 35(1); 77-82.
- Yasuo Otsuka, Ken Kamata, Tomoko Hyodo, Takaaki Chikugo, Akane Hara, Hidekazu Tanaka, Tomoe Yoshikawa, Rei Ishikawa, Ayana Okamoto, Tomohiro Yamazaki, Atsushi Nakai, Shunsuke Omoto, Kosuke Minaga, Kentaro Yamao, Mamoru Takenaka, Yasutaka Chiba, Tomohiro Watanabe, Takuya Nakai, Ippei Matsumoto, Yoshifumi Takeyama, Masatoshi Kudo. Utility of contrast-enhanced harmonic endoscopic ultrasonography for T-staging of patients with extrahepatic bile duct cancer. Surg Endosc. 2022, 36(5); 3254-3260.
- Hiroo Imazu, Yujiro Uchiyama, Kazuhiro Matsunaga, Kei-ichi Ikeda, Hiroshi Kakutani, Yoshihiro Sasaki, Kazuki Sumiyama, Tiing Leong Ang, Salem Omar, Hisao Tajiri. Contrast-enhanced harmonic EUS with novel ultrasonographic contrast (Sonazoid) in the preoperative T-staging for pancreaticobiliary malignancies. Scand J Gastroenterol. 2010, 45(6): 732-8.
- E Ryoung Choi, Yun Hee Chung, Jong Kyun Lee, Kyu Taek Lee, Kwang Hyuck Lee, Dong Wook Choi, Seong Ho Choi, Jin Seok Heo, Kee-Taek Jang, Sang Mo Park, Jae Hoon Lim. Preoperative evaluation of the longitudinal extent of borderline resectable hilar cholangiocarcinoma by intraductal ultrasonography. J Gastroenterol Hepatol. 2011, 26(12): 1804-10.
- Hee Man Kim, Jeong Youp Park, Kyung Sik Kim, Mi-Suk Park, Myeong-Jin Kim, Young Nyun Park, Seungmin Bang, Si Young Song, Jae Bock Chung, Seung Woo Park. Intraductal ultrasonography combined with percutaneous transhepatic cholangioscopy for the preoperative evaluation of longitudinal tumor extent in hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2010, 25(2): 286-92.
- Isamu Hosokawa, Tsukasa Takayashiki, Satoshi Kuboki, Shigetsugu Takano, Kentaro Togasaki, Masaru Miyazaki, Masayuki Ohtsuka. Surgery. 2023, 174(1): 11-20.
- Thomas Malikowski, Michael J Levy, Ferga C Gleeson, Andrew C Storm, Eric J Vargas, Mark D Topazian, Barham K Abu Dayyeh, Prasad G Iyer, Elizabeth Rajan, Gregory J Gores, Lewis R Roberts, Vinay Chandrasekhara. Hepatology. 2020, 72(3): 940-948.
- Takeshi Miyata, Masayuki Kitano, Shunsuke Omoto, Kumpei Kadosaka, Ken Kamata, Hajime Imai, Hiroki Sakamoto, Naoshi Nisida, Yogesh Harwani, Takamichi Murakami, Yoshifumi Takeyama, Yasutaka Chiba, Masatoshi Kudo. Contrast-enhanced harmonic endoscopic ultrasonography for assessment of lymph node metastases in pancreatobiliary carcinoma. World J Gastroenterol. 2016, 22(12): 3381-91.
- Anahita Sadeghi, Mehdi Mohamadnejad, Farhad Islami, Abbas Keshtkar, Mohammad Biglari, Reza Malekzadeh, Mohamad A Eloubeidi. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: a systematic review and meta-analysis. Gastrointest Endosc. 2016, 83(2): 290-8.e1.
- Diogo Turiani Hourneaux De Moura, Eduardo Guimarães Hourneaux De Moura, Wanderlei Marques Bernardo, Eduardo Turiani Hourneaux De Moura, Felipe I Baraca, André Kondo, Sérgio Eijii Matuguma, Everson Luis Almeida Artifon. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta-analysis. Endosc Ultrasound. 2018, 7(1): 10-19.
- Jung Hyun Jo, Chang Min Cho, Jae Hyuck Jun, Moon Jae Chung, Tae Hyeon Kim, Dong Wan Seo, Jaihwan Kim, Do Hyun Park. Same-session endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography-based tissue sampling in suspected malignant biliary obstruction: A multicenter experience. J Gastroenterol Hepatol. 2019, 34(4): 799-805.
- Praveen Mathew, Prashant Kanni, Manoj Gowda, Chandrababu Devarapu, Jaseem Ansari, Achal Garg. A Comparative Study of Endoscopic Ultrasound Fine-Needle Aspiration (EUS-FNA) and Endoscopic Retrograde Cholangiopancreatography (ERCP)-Based Brush Cytology for Tissue Diagnosis in Malignant Biliary Obstruction. Cureus. 2022, 14(10): e30291.
- Julie K Heimbach, William Sanchez, Charles B Rosen, Gregory J Gores. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB(Oxford) 2011, 13: 356-360.
- Fujita N, Noda Y, Kobayashi G, et al. Analysis of the layer structure of the gallbladder wall delineated by endoscopic ultrasound using the pinning method. Dig Endosc 1995, 7: 353-356.
- Kazunari Tanaka, Akio Katanuma, Tsuyoshi Hayashi, Toshifumi Kin, Kuniyuki Takahashi. Role of endoscopic ultrasound for gallbladder disease. J Med Ultrason (2001). 2021, 48(2): 187-198.
- Hong Joo Kim, Jung Ho Park, Dong Il Park, Yong Kyun Cho, Chong Il Sohn, Woo Kyu Jeon, Byung Ik Kim, Seon Hyeong Choi. Clinical usefulness of endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig Dis Sci. 2012, 57: 508-15.
- T Ootani, Y Shirai, K Tsukada, T Muto. Relationship between gallbladder carcinoma and the segmental type of adenomyomatosis of the gallbladder. Cancer. 1992, 69(11): 2647-52.
- Pratyaksha Rana, Pankaj Gupta, Daneshwari Kalage, Raghuraman Soundararajan, Praveen Kumar-M, Usha Dutta. Grayscale ultrasonography findings for characterization of gallbladder wall thickening in non-acute setting: a systematic review and meta-analysi. Expert Rev Gastroenterol Hepatol. 2022, 16(1): 59-71.
- T Azuma, T Yoshikawa, T Araida, K Takasaki. Differential diagnosis of polypoid lesions of the gallbladder by endoscopic ultrasonography. Am J Surg. 2001, 181(1): 65-70.
- Young Koog Cheon, Won Young Cho, Tae Hee Lee, Young Deok Cho, Jong Ho Moon, Joon Seong Lee, Chan Sup Shim. Endoscopic ultrasonography does not differentiate neoplastic from non-neoplastic small gallbladder polyps. World J Gastroenterol. 2009, 15: 2361-2366.
- Rebecca Wiles, Ruedi F Thoeni, Sorin Traian Barbu, Yogesh K Vashist, Søren Rafael Rafaelsen, Catherine Dewhurst, Marianna Arvanitakis, Max Lahaye, Marek Soltes, Julie Perinel, Stuart Ashley Roberts. Management and follow-up of gallbladder polyps: Joint guidelines between the European Society of Gastrointestinal and Abdominal Radiology (ESGAR), European Association for Endoscopic Surgery and other Interventional Techniques (EAES), International Society of Digestive Surgery - European Federation (EFISDS) and European Society of Gastrointestinal Endoscopy (ESGE). Eur Radiol. 2017, 27(9): 3856-66.
- Iván Roa, Xabier de Aretxabala, René Morgan, Ricardo Molina, Juan C Araya, Juan Roa, Gilda Ibacahe. [Clinicopathological features of gallbladder polyps and adenomas]. Rev Med Chile. 2004, 132(6): 673-9.
- Tae Won Choi, Jung Hoon Kim, Mi Hye Yu, Sang Joon Park, Joon Koo Han. Pancreatic neuroendocrine tumor: prediction of the tumor grade using CT findings and computerized texture analysis. Acta Radiol. 2018, 59(4): 383-92.
- W B Choi, S K Lee, M H Kim, D W Seo, H J Kim, D I Kim, E T Park, K S Yoo, B C Lim, S J Myung, H J Park, Y I Min. A new strategy to predict the neoplastic polyps of the gallbladder based on a scoring system using EUS. Gastrointest Endosc. 2000, 52(3): 372-9.
- Jun-Ho Choi, Dong-Wan Seo, Joon Hyuk Choi, Do Hyun Park, Sang Soo Lee, Sung Koo Lee, Myung-Hwan Kim. Utility of contrast-enhanced harmonic EUS in the diagnosis of malignant gallbladder polyps (with videos). Gastrointest Endosc. 2013, 78(3): 484-93.
- Yasunobu Yamashita, Reiko Ashida, Takaaki Tamura, Toshio Shimokawa, Hirofumi Yamazaki, Yuki Kawaji, Takashi Tamura, Keiichi Hatamaru, Masahiro Itonaga, Masayuki Kitano. Novel Technique of Endoscopic Ultrasonography for the Differential Diagnosis of Gallbladder Lesions and Intraductal Papillary Mucinous Neoplasms: A Single-Center Prospective Study. Diagnostics (Basel). 2023, 13(13): 2132.
- Mitsuru Sugimoto, Hiroki Irie, Mika Takasumi, Minami Hashimoto, Yuka Oka, Tadayuki Takagi, Rei Suzuki, Naoki Konno, Hiroyuki Asama, Yuki Sato, Jun Nakamura, Tsunetaka Kato, Ryoichiro Kobashi, Yuko Hashimoto, Shigeru Marubashi, Takuto Hikichi, Hiromasa Ohira. A simple method for diagnosing gallbladder malignant tumors with subserosa invasion by endoscopic ultrasonography. BMC Cancer. 2021, 21(1): 288.
- Hiromichi Ito, Kaori Ito, Michael D’Angelica, Mithat Gonen, David Klimstra, Peter Allen, Ronald P DeMatteo, Yuman Fong, Leslie H Blumgart, William R Jarnagin. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg. 2011, 254(2): 320-5.
- M Mitake, S Nakazawa, Y Naitoh, E Kimoto, Y Tsukamoto, T Asai, K Yamao, K Inui, K Morita, Y Hayashi. Endoscopic ultrasonography in diagnosis of the extent of gallbladder carcinoma. Gastrointest Endosc. 1990, 36(6): 562-6.
- Suprabhat Giri, Sumaswi Angadi, Shivaraj Afzalpurkar, Sidharth Harindranath, Jijo Varghese, Sridhar Sundaram. Diagnostic performance and safety of endoscopic ultrasound-guided tissue acquisition of gallbladder lesions: A systematic review with meta-analysis. Indian J Gastroenterol. 2023, 42(4): 467-474.
- Koji Hirata, Masaki Kuwatani, Goki Suda, Marin Ishikawa, Ryo Sugiura, Shin Kato, Kazumichi Kawakubo, Naoya Sakamoto. A Novel Approach for the Genetic Analysis of Biliary Tract Cancer Specimens Obtained Through Endoscopic Ultrasound-Guided Fine Needle Aspiration Using Targeted Amplicon Sequencing. Clin Transl Gastroenterol. 2019, 10(3): e00022.
- Yugo Kai, Kenji Ikezawa, Ryoji Takada, Kazuma Daiku, Shingo Maeda, Yutaro Abe, Takuo Yamai, Nobuyasu Fukutake, Tasuku Nakabori, Hiroyuki Uehara, Shigenori Nagata, Hiroshi Wada, Kazuyoshi Ohkawa. Success rate of microsatellite instability examination and complete response with pembrolizumab in biliary tract cancer. JGH Open. 2021, 5(6): 712-716.
- Seung Bae Yoon, Sung-Hoon Moon, Sung Woo Ko, Hyun Lim, Ho Suk Kang, Jong Hyeok Kim. Brush Cytology, Forceps Biopsy, or Endoscopic Ultrasound-Guided Sampling for Diagnosis of Bile Duct Cancer: A Meta-Analysis. Dig Dis Sci. 2022, 67(7): 3284-3297.
- Takafumi Yanaidani, Kazuo Hara, Nozomi Okuno, Shin Haba, Takamichi Kuwahara, Yasuhiro Kuraishi, Nobumasa Mizuno, Sho Ishikawa, Masanori Yamada, Tsukasa Yasuda. Clinical utility of endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling of patients with biliary tract cancer, especially with intrahepatic cholangiocarcinoma. Clin Endosc. 2024, 57(3): 384-392.
- M Mitake, S Nakazawa, Y Naitoh, E Kimoto, Y Tsukamoto, K Yamao, K Inui. Value of endoscopic ultrasonography in the detection of anomalous connections of the pancreatobiliary duct. Endoscopy. 1991, 23(3): 117-120.
- M Sugiyama, Y Atomi. Endoscopic ultrasonography for diagnosing anomalous pancreaticobiliary junction. Gastrointest Endosc. 1997, 45(3): 261-7.
- Tony E Yusuf, Manoop S Bhutani. Role of endoscopic ultrasonography in diseases of the extrahepatic biliary system. J Gastroenterol Hepatol. 2004, 19(3): 243-50.
- Terumi Kamisawa, Kensuke Takuma, Takao Itoi. Endoscopic diagnosis of pancreaticobiliary maljunction. Gastroenterological Endoscopy. 2010, 52(6): 1511-21.
- Terumi Kamisawa, Kensuke Takuma, Fumihide Itokawa, Takao Itoi. Endoscopic diagnosis of pancreaticobiliary maljunction. World J Gastrointest Endosc. 2011, 3(1): 1-5.
- Moritz N Wente, Johannes A Veit, Claudio Bassi, Christos Dervenis, Abe Fingerhut, Dirk J Gouma, Jakob R Izbicki, John P Neoptolemos, Robert T Padbury, Michael G Sarr, Charles J Yeo, Markus W Büchler. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007, 142(1): 20-5.
- Gastroenterological Endoscopy. 2021, 63(4): 451-480.
- Xiaohua Ye, Lei Wang, Zhendong Jin. Diagnostic accuracy of endoscopic ultrasound and intraductal ultrasonography for assessment of ampullary tumors: a meta-analysis. Scand J Gastroenterol. 2022, 57(10): 1158-1168.
- Chad Defrain, Cindy Y Chang, Wichit Srikureja, Phuong T Nguyen, Mai Gu. Cytologic features and diagnostic pitfalls of primary ampullary tumors by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2005, 105(5): 289-97.
- K J Chang. State of the art lecture: endoscopic ultrasound (EUS) and FNA in pancreatico-biliary tumors. Endoscopy. 2006, 38 Suppl 1: S56-60.
- Takeshi Ogura, Kazuo Hara, Susumu Hijioka, Nobumasa Mizuno, Hiroshi Imaoka, Yasumasa Niwa, Masahiro Tajika, Shinya Kondo, Tsutomu Tanaka, Yasuhiro Shimizu, Waki Hosoda, Yasushi Yatabe, Vikram Bhatia, Kazuhide Higuchi, Kenji Yamao. Can endoscopic ultrasound-guided fine needle aspiration offer clinical benefit for tumors of the ampulla of vater? -an initial study. Endosc Ultrasound. 2012, 1(2): 84-9.
- Suprabhat Giri, Babu P Mohan, Vaneet Jearth, Aditya Kale, Sumaswi Angadi, Shivaraj Afzalpurkar, Sidharth Harindranath, Sridhar Sundaram. Adverse events with EUS-guided biliary drainage: a systematic review and meta-analysis. Gastrointest Endosc. 2023, 98(4): 515-523.e18.
- Reem Z Sharaiha, Muhammad Ali Khan, Faisal Kamal, Amy Tyberg, Claudio R Tombazzi, Bilal Ali, Claudio Tombazzi, Michel Kahaleh. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and meta-analysis. Gastrointest Endosc. 2017, 85(5):904-914.
- Harsha Moole, Matthew L Bechtold, David Forcione, Srinivas R Puli. A meta-analysis and systematic review: Success of endoscopic ultrasound guided biliary stenting in patients with inoperable malignant biliary strictures and a failed ERCP. Medicine (Baltimore). 2017, 96(3):e5154.
- Corey S Miller, Alan N Barkun, Myriam Martel, Yen-I Chen. Endoscopic ultrasound-guided biliary drainage for distal malignant obstruction: a systematic review and meta-analysis of randomized trials. Endosc Int Open. 2019, 7(11): E1563-E1573.
- Suprabhat Giri, Vishal Seth, Shivaraj Afzalpurkar, Sumaswi Angadi, Vaneet Jearth, Sridhar Sundaram. Endoscopic Ultrasound-guided Versus Percutaneous Transhepatic Biliary Drainage After Failed ERCP: A Systematic Review and Meta-analysis. Surg Laparosc Endosc Percutan Tech. 2023, 33(4): 411-419.
- Ji Young Bang, Udayakumar Navaneethan, Muhammad Hasan, Robert Hawes, Shyam Varadarajulu. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos). Gatrointestinal Endosc. 2018, 88(1): 9-17.
- Joo Kyung Park, Young Sik Woo, Dong Hyo Noh, Ju-Il Yang, So Young Bae, Hwan Sic Yun, Jong Kyun Lee, Kyu Taek Lee, Kwang Hyuck Lee. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: prospective randomized controlled study. Gatrointestinal Endosc. 2018, 88(2): 277-282.
- Woo Hyun Paik, Tae Hoon Lee, Do Hyun Park, Jun-Ho Choi, Seon-Ok Kim, Sunguk Jang, Dong Uk Kim, Ju Hyun Shim, Tae Jun Song, Sang Soo Lee, Dong-Wan Seo, Sung Koo Lee, Myung-Hwan Kim. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am J Gastroenterol. 2018, 113(7): 987-997.
- Yunxiao Lyu, Ting Li, Yunxiao Cheng, Bin Wang, Yuchen Cao, Yuan Wang. Endoscopic ultrasound-guided vs ERCP-guided biliary drainage for malignant biliary obstruction: A up-to-date meta-analysis and systematic review. Dig Liver Dis. 2021, 53(10): 1247-1253.
- Gaurav Kakked, Habeeb Salameh, Antonio R Cheesman, Nikhil A Kumta, Satish Nagula, Christopher J DiMaio. Primary EUS-guided biliary drainage versus ERCP drainage for the management of malignant biliary obstruction: A systematic review and meta-analysis. Endosc Ultrasound. 2020, 9(5):298-307.
- Ricardo S Uemura, Muhammad Ali Khan, José P Otoch, Michel Kahaleh, Edna F Montero, Everson L A Artifon. EUS-guided Choledochoduodenostomy Versus Hepaticogastrostomy: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2018, 52(2): 123-130.
- Kejie Mao, Binbin Hu, Feng Sun, Kaiming WanSurg. Choledochoduodenostomy Versus Hepaticogastrostomy in Endoscopic Ultrasound-guided Drainage for Malignant Biliary Obstruction: A Meta-analysis and Systematic Review. Laparosc Endosc Percutan Tech. 2021, 32(1): 124-132.
- Hirofumi Yamazaki, Yasunobu Yamashita, Toshio Shimokawa, Kosuke Minaga, Takeshi Ogura, and Masayuki Kitano. Endoscopic ultrasound-guided hepaticogastrostomy versus choledochoduodenostomy for malignant biliary obstruction: A meta-analysis. DEN Open. 2023, 4(1): e274.
- Fumihiko Miura, Tadahiro Takada, Steven M Strasberg, Joseph S Solomkin, Henry A Pitt, Dirk J Gouma, O James Garden, Markus W Büchler, Masahiro Yoshida, Toshihiko Mayumi, Kohji Okamoto, Harumi Gomi, Shinya Kusachi, Seiki Kiriyama, Masamichi Yokoe, Yasutoshi Kimura, Ryota Higuchi, Yuichi Yamashita, John A Windsor, Toshio Tsuyuguchi, Toshifumi Gabata, Takao Itoi, Jiro Hata, Kui-Hin Liau. TG13 flowchart for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013, 20(1): 47-54.
- Rajesh Krishnamoorthi, Mahendran Jayaraj, Viveksandeep Thoguluva Chandrasekar, Dhruv Singh, Joanna Law, Michael Larsen, Andrew Ross, Richard Kozarek, Shayan Irani. EUS-guided versus endoscopic transpapillary gallbladder drainage in high-risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Surg Endosc. 2020, 34(5): 1904-1913.
- Matheus Candido Hemerly, Diogo Turiani Hourneaux de Moura, Epifanio Silvino do Monte Junior, Igor Mendonça Proença, Igor Braga Ribeiro, Erika Yuki Yvamoto, Pedro Henrique Boraschi Vieira Ribas, Sergio A Sánchez-Luna, Wanderley Marques Bernardo, Eduardo Guimarães Hourneaux de Moura. Endoscopic ultrasound (EUS)-guided cholecystostomy versus percutaneous cholecystostomy (PTC) in the management of acute cholecystitis in patients unfit for surgery: a systematic review and meta-analysis. Surg Endosc. 2023, 37(4): 2421-2438.
Figure 1.
Images and schema of the two types of EUS. (a) Convex-type EUS (GF-UCT260, Olympus, Japan), (b) Radial type EUS (GF-UE290, Olympus, Japan), (c) Scheme of convex-type EUS, (d) Scheme of radial type EUS, (e) Ultrasound view of convex-type EUS, (f) Ultrasound view of radial type EUS.
Figure 1.
Images and schema of the two types of EUS. (a) Convex-type EUS (GF-UCT260, Olympus, Japan), (b) Radial type EUS (GF-UE290, Olympus, Japan), (c) Scheme of convex-type EUS, (d) Scheme of radial type EUS, (e) Ultrasound view of convex-type EUS, (f) Ultrasound view of radial type EUS.
Figure 2.
These are images of intraductal ultrasound probes: (a) (b) The ultrasound probe is attached at the point indicated by the red arrow. (UM-DG20-31R, Olympus, Japan).
Figure 2.
These are images of intraductal ultrasound probes: (a) (b) The ultrasound probe is attached at the point indicated by the red arrow. (UM-DG20-31R, Olympus, Japan).
Figure 3.
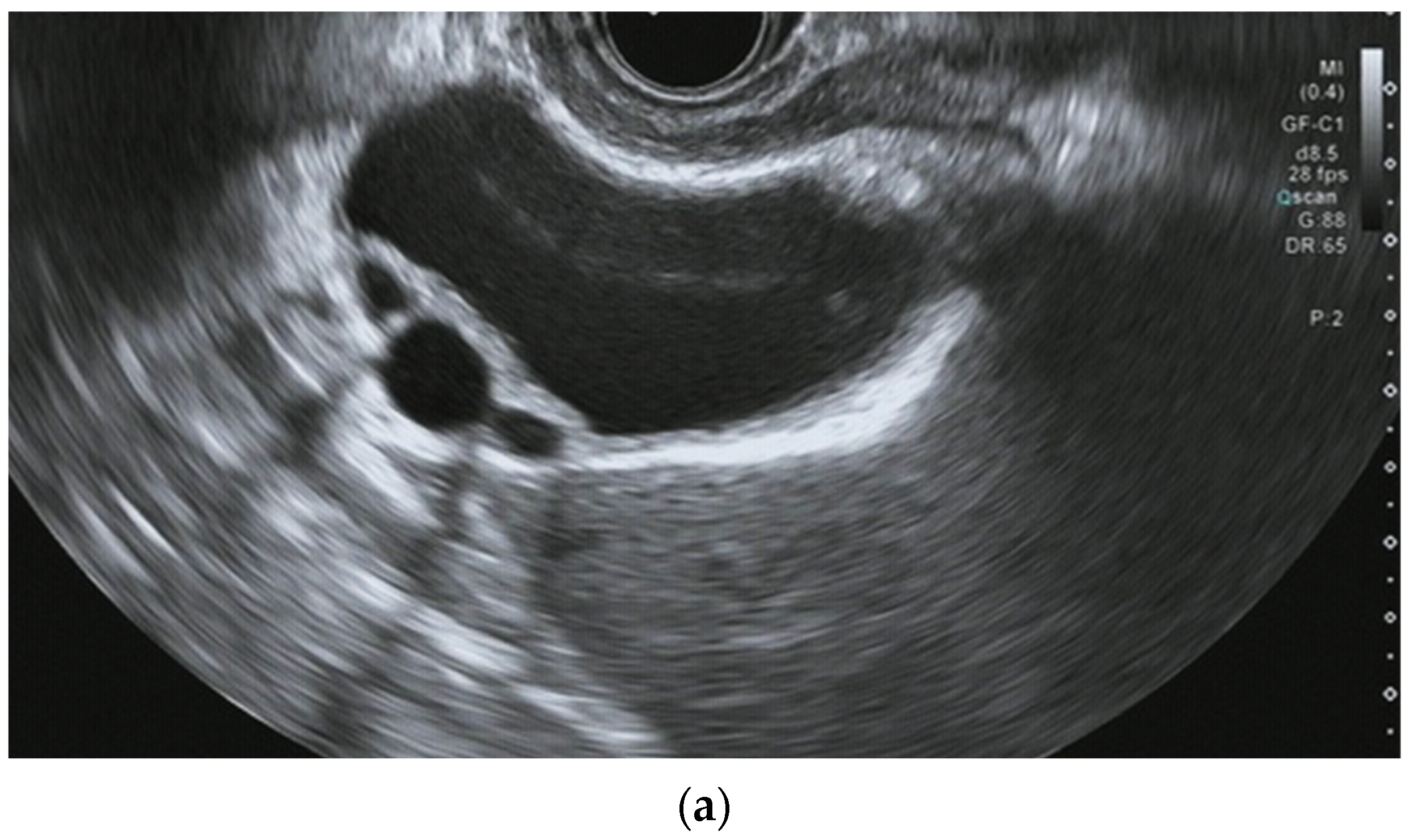
Detection of small choledocholithiasis by intraductal ultrasound (IDUS) and endoscopic ultrasound (EUS): (a) IDUS image of choledocholithiasis (arrow, 5 mm); (b) EUS image of choledocholithiasis (arrow head, 5 mm).
Figure 3.
Detection of small choledocholithiasis by intraductal ultrasound (IDUS) and endoscopic ultrasound (EUS): (a) IDUS image of choledocholithiasis (arrow, 5 mm); (b) EUS image of choledocholithiasis (arrow head, 5 mm).
Figure 4.
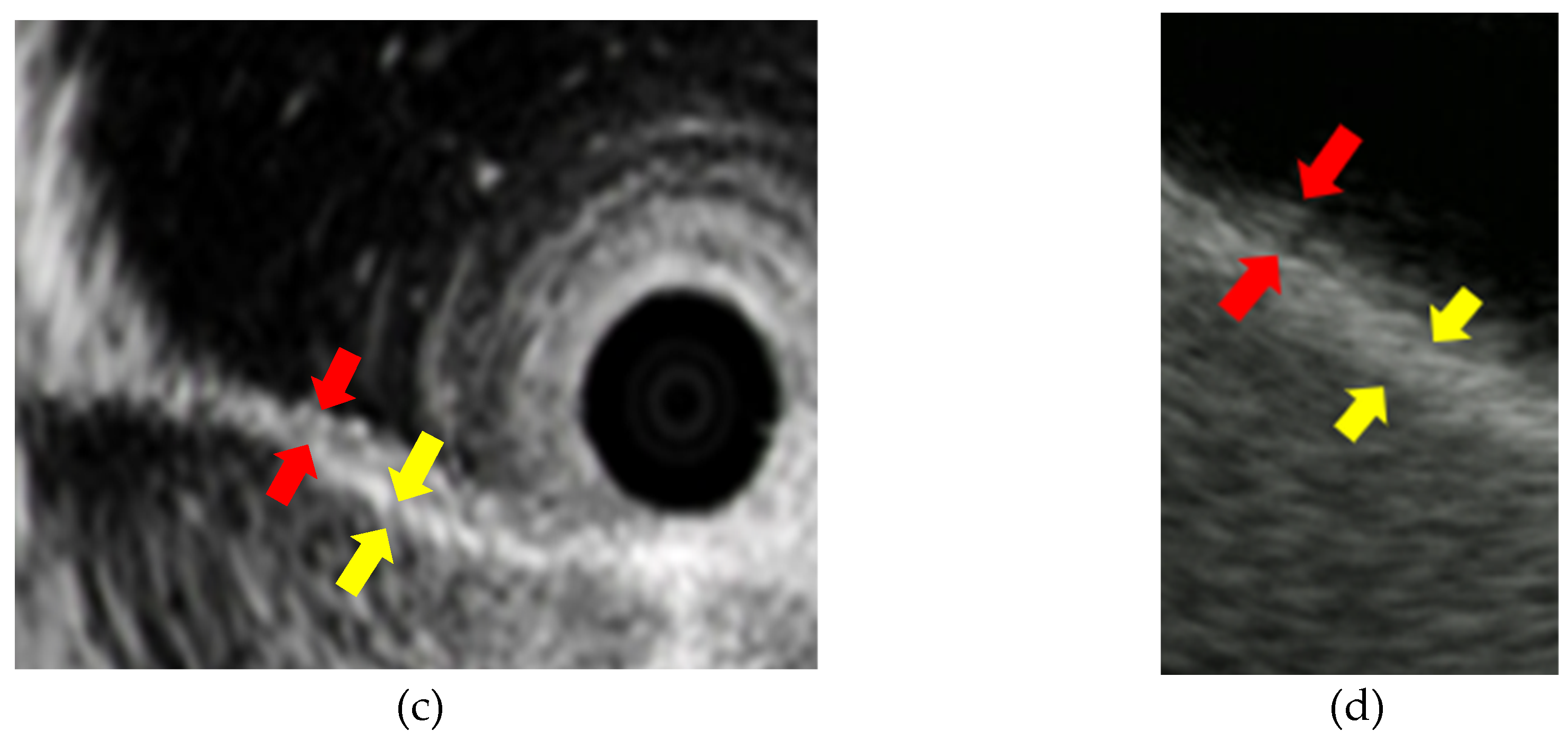
Image of the normal structure of the biliary duct wall and gallbladder wall: (a) (b) Image of the biliary duct wall on intraductal ultrasound and the gallbaldder wall on endoscopic ultrasound; (c) (d) Red arrows show inner hypoechoic layer corresponding to mucosa, muscularis propria, and a part of the subserosa. Yellow arrows show outer hyperechoic layer corresponding to a part of the subserosa and the serosa.
Figure 4.
Image of the normal structure of the biliary duct wall and gallbladder wall: (a) (b) Image of the biliary duct wall on intraductal ultrasound and the gallbaldder wall on endoscopic ultrasound; (c) (d) Red arrows show inner hypoechoic layer corresponding to mucosa, muscularis propria, and a part of the subserosa. Yellow arrows show outer hyperechoic layer corresponding to a part of the subserosa and the serosa.
Figure 5.
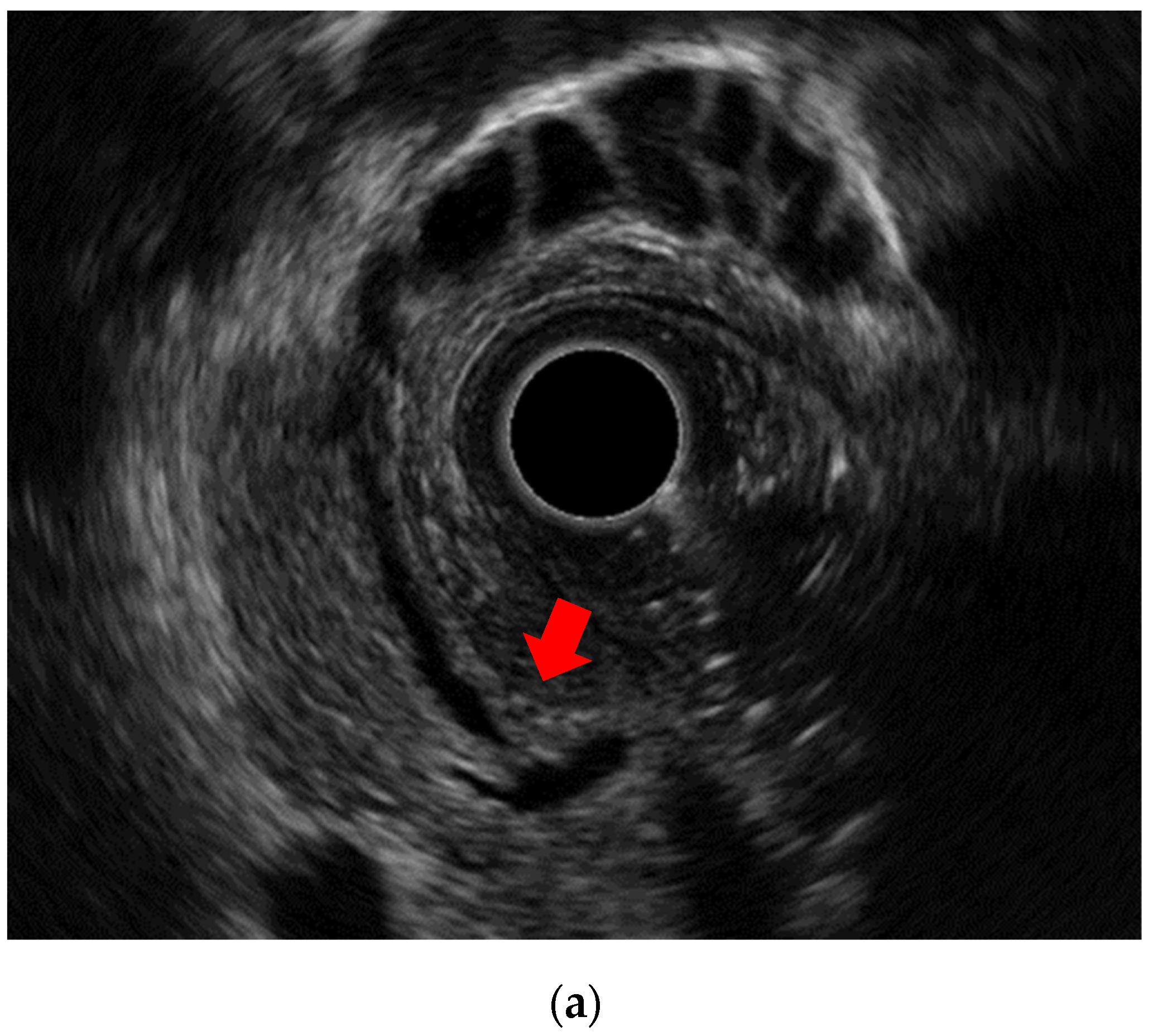
Endoscopic ultrasound image of adenomyomatosis: (a) Thickened wall and cystic anechoic spots are visible. The cystic spots are showing Rokitansky-Aschoff sinuses.
Figure 5.
Endoscopic ultrasound image of adenomyomatosis: (a) Thickened wall and cystic anechoic spots are visible. The cystic spots are showing Rokitansky-Aschoff sinuses.
Figure 6.
Pancreaticobiliary maljunction: (a) An endoscopic ultrasound image showing the pancreatic duct and bile duct converging outside the duodenal wall.
Figure 6.
Pancreaticobiliary maljunction: (a) An endoscopic ultrasound image showing the pancreatic duct and bile duct converging outside the duodenal wall.
Figure 7.
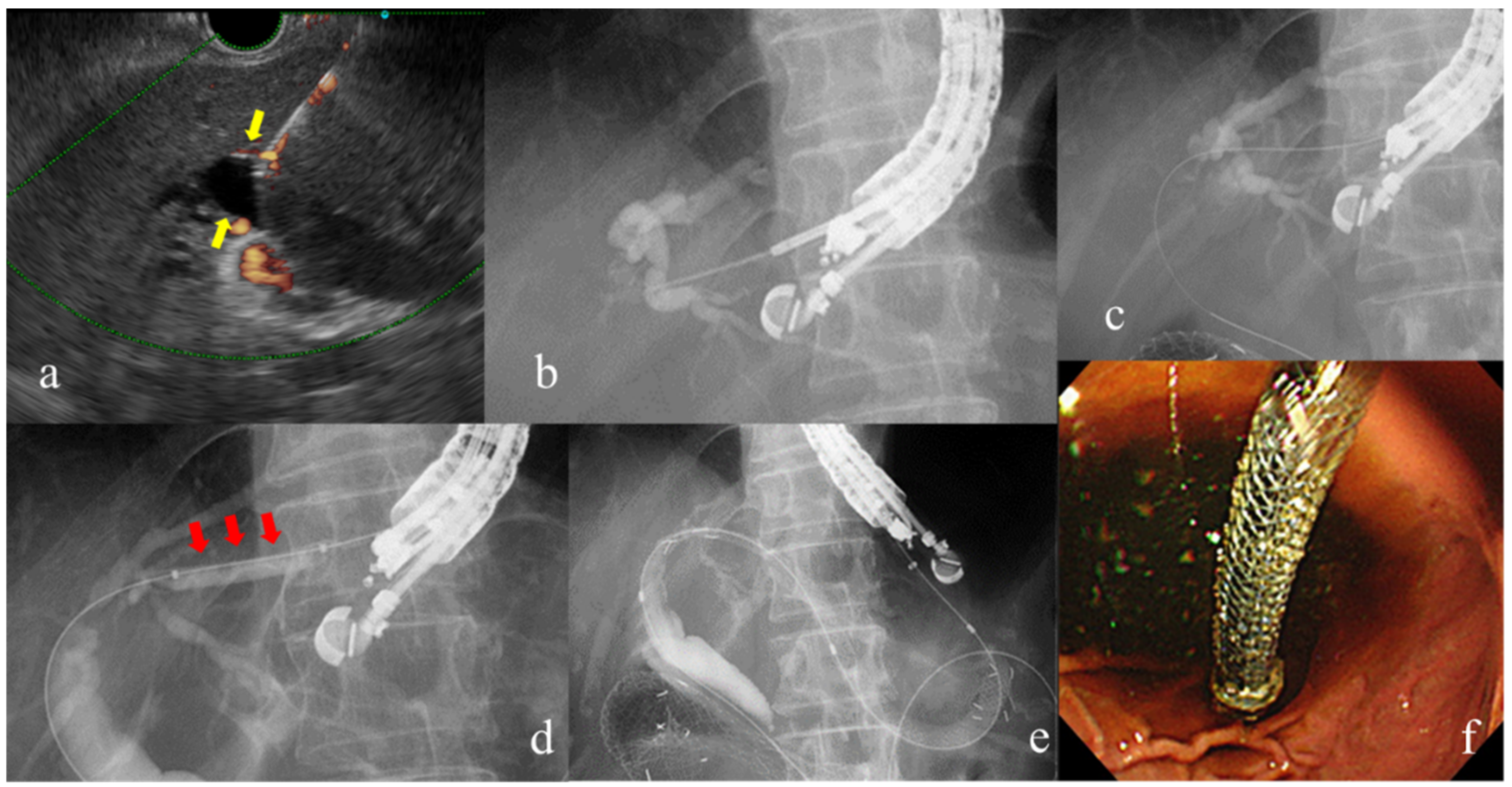
Procedure for EUS-guided hepaticogastrostomy: (a) The dilated intrahepatic bile duct depicted by EUS (yellow arrow shows). (b)Puncture by EUS-FNA needle and cholangiography of the intrahepatic bile ducts using contrast agent. (c)Insertion of the guidewire through the needle. (d) Dilation of the puncture line. (e, f) Placement of the self-expandable metal stent.
Figure 7.
Procedure for EUS-guided hepaticogastrostomy: (a) The dilated intrahepatic bile duct depicted by EUS (yellow arrow shows). (b)Puncture by EUS-FNA needle and cholangiography of the intrahepatic bile ducts using contrast agent. (c)Insertion of the guidewire through the needle. (d) Dilation of the puncture line. (e, f) Placement of the self-expandable metal stent.
Table 1.
Differences in IDUS findings of biliary stricture between IgG4-SC, PSC, and cholangiocarcinoma [
21,
22,
23].
Table 1.
Differences in IDUS findings of biliary stricture between IgG4-SC, PSC, and cholangiocarcinoma [
21,
22,
23].
| |
IgG4-SC |
PSC |
Cholangiocarcinoma |
| Wall thickness |
circular-symmetric |
circular-asymmetric |
asymmetric |
| Three-layer structure |
preservation |
disappearance |
disappearance |
| Internal echo |
homogeneous |
heterogeneous |
heterogeneous |
| Inner margin |
smooth |
irregular |
irregular |
| Outer margin |
smooth |
unclear |
irregular, interruption |
Table 2.
EUS-TA for malignant biliary stricture.
Table 2.
EUS-TA for malignant biliary stricture.
| Author |
Number of patients |
Sensitivity (%) |
Specificity (%) |
Accuracy (%) |
Adverse events (%) |
| Anahita Sadeghi et al. [42] |
957 |
80 |
97 |
93 |
1
(severe: 0.3%) |
| De Moura et al. [43] |
294 |
75 |
100 |
79 |
- |
| Jo et al. [44] |
263 |
73.6 |
100 |
76.1 |
0.7 |
| Praveen Mathew et al. [45] |
77 |
91 |
100 |
93 |
- |
Table 3.
EUS gallbladder wall findings suggestive of malignancy.
Table 3.
EUS gallbladder wall findings suggestive of malignancy.
| |
Benign |
Malignant |
| wall thickening |
< 10 mm |
≥ 10 mm |
| hypoechoic internal echogenicity |
absent |
present |
| internal echo pattern |
homogeneous |
heterogenous |
| wall layer |
present |
disrupted |
Table 4.
Scoring system for EUS findings of gallbladder polypoid lesions (presented with some modifications).
Table 4.
Scoring system for EUS findings of gallbladder polypoid lesions (presented with some modifications).
| |
Score |
| Layer structure |
|
| preserved |
0 |
| lost |
6 |
| Echo pattern |
|
| hyperechoic spots |
0 |
| hyperechoic homogeneous |
1 |
| isoechoic homogeneous |
2 |
| isoechoic heterogeneous |
5 |
| Margin of polyp |
|
| not lobulated |
0 |
| lobulated |
4 |
| Stalk |
|
| pedunculated |
0 |
| sessile |
3 |
| Number of polyps |
|
| multiple |
0 |
| single |
2 |
Table 5.
EUS-TA for ampullary tumors.
Table 5.
EUS-TA for ampullary tumors.
| Author |
Number of patients |
Sensitivity(%) |
Specificity(%) |
Accuracy(%) |
Complications |
| Defrain C et al. [76] |
35 |
82 |
100 |
89 |
no data |
| Chang et al. [77] |
20 |
no data |
no data |
35 |
no data |
| Ogura et al. [78] |
10 |
100 |
100 |
100 |
0 |
Table 6.
Comparison of EUS-BD and PTBD.
Table 6.
Comparison of EUS-BD and PTBD.
| Author |
Technical success (OR) |
Clinical success (OR) |
Adverse events (OR) |
Reintervention rates (OR) |
| Sharaiha et al. [80] |
1.78 (95% CI, .69–4.59) |
0.45(95% CI, 0.23–0.89) |
0.23 (95% CI, 0.12–0.47) |
0.13 (95% CI, 0.07–0.24) |
| Moole et al. [81] |
3.06 (95% CI, 1.11–8.43) |
No data |
-0.21 (95% CI, -0.35–-0.06) |
No data |
| Miller et al. [82] |
1.01 (95% Cl, 0.92–1.11) |
0.99 (95% Cl, 0.88–1.12) |
0.59 (95 % CI, 0.39–0.87) |
0.37 (95 %CI, 0.22–0.61) |
| Giri et al. [83] |
1.12 (95% CI, 0.67–1.88) |
2.55 (95% CI, 1.63–4.56) |
0.41 (95% CI, 0.29–0.59) |
0.20 (95% CI, 0.10–0.38) |
Table 7.
Comparison between EUS-CDS and EUS-HGS.
Table 7.
Comparison between EUS-CDS and EUS-HGS.
| Author |
Technical successs (OR) |
Clinical success (OR) |
Adverse events (OR) |
Reintervention rates (OR) |
| Uemura et al. [89] |
0.96 (95% CI, 0.39–2.33) |
0.76 (95% CI, 0.42–1.35) |
0.97 (95% CI, 0.60–1.56) |
No data |
| Mao et al. [90] |
0.95 (95% CI, 0.51–1.74) |
1.13 (95% CI, 0.66–1.94) |
1.00 (95% CI, 0.70–1.43) |
0.31 (95% CI, 0.16–0.63) |
| Yamazaki et al. [91] |
1.04 (95% CI, 0.62–1.73) |
0.66 (95% CI, 0.43–1.04) |
1.39 (95% CI, 1.00–1.93) |
2.95 (95% CI, 1.54–5.6) (HGS>CDS) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).