Submitted:
24 July 2024
Posted:
25 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
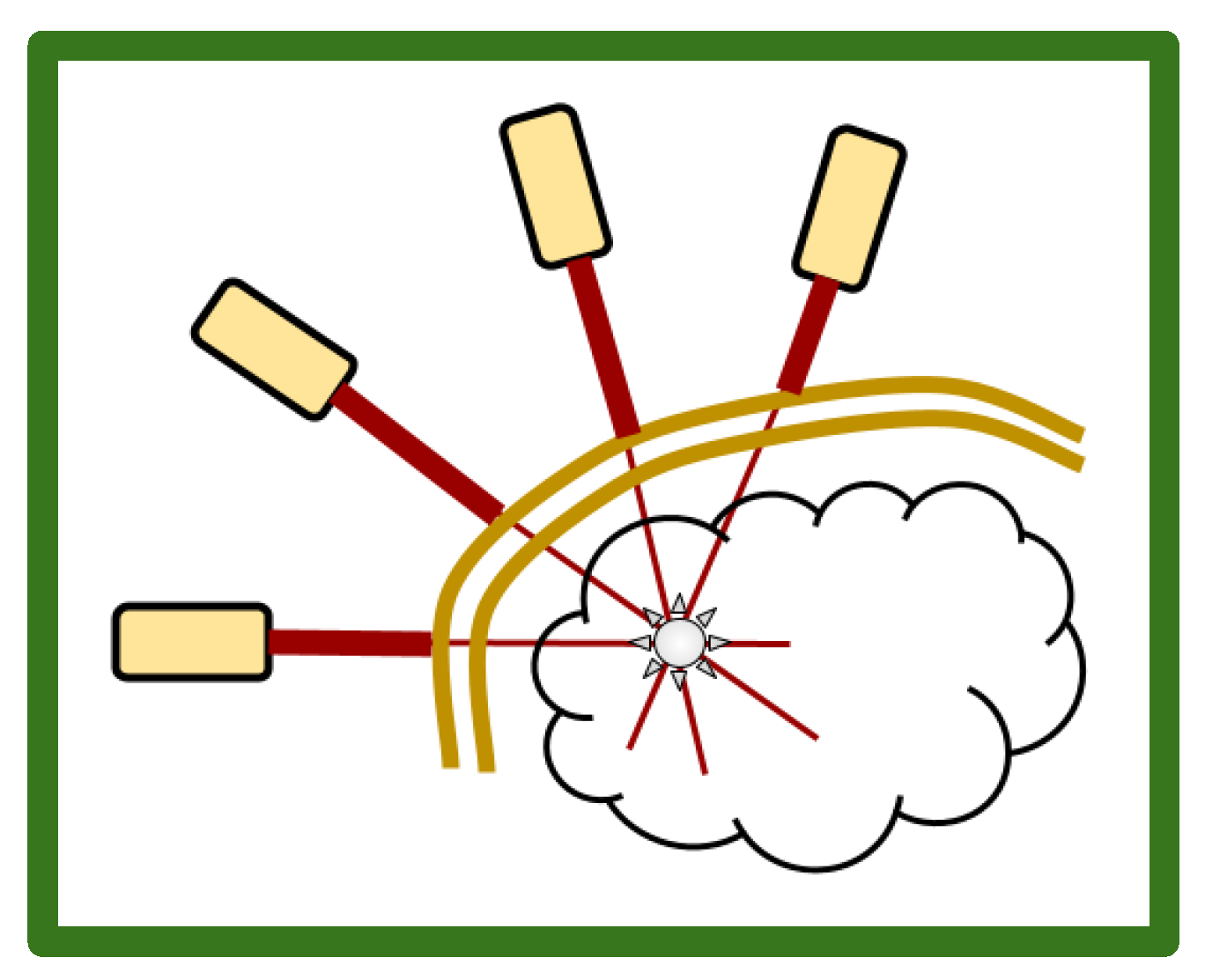
2. Multiple array, Ultra Low Fluence, Transcranial, Repetitive, 5-ALA PDT
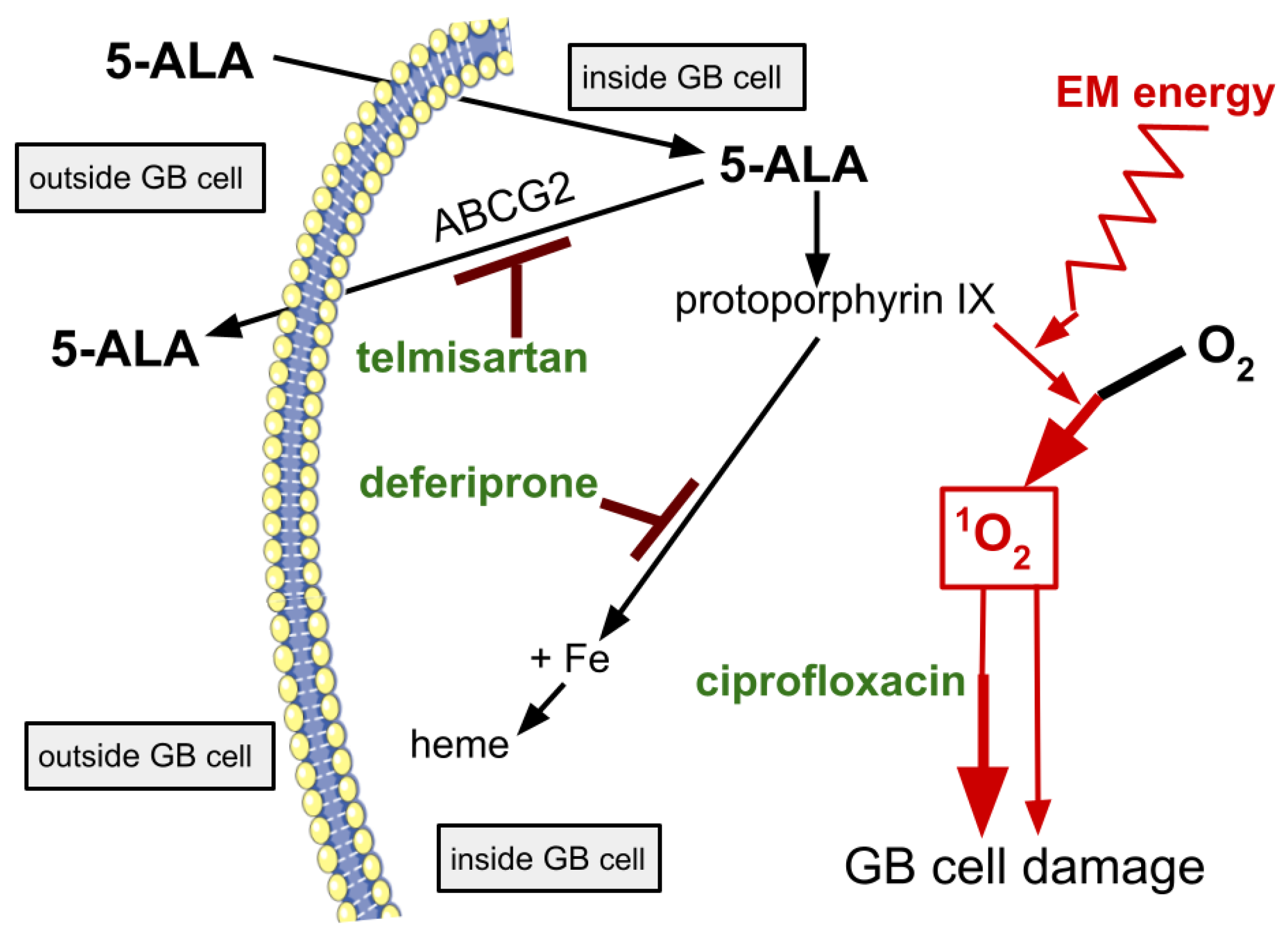
3. The LIT-PDT Drugs
3.1. Ciprofloxacin
3.2. Deferiprone
3.3. ABCG2 and Telmisartan or Ziprasidone
3.3.1. Telmisartan
3.3.2. Ziprasidone
4. LIT-PDT and GB Ecosystems
5. Discussion and Conclusions
References
- Cramer SW, Chen CC. Photodynamic Therapy for the Treatment of Glioblastoma. Front Surg. 2020;6:81. [CrossRef]
- Harada Y, Murayama Y, Takamatsu T, Otsuji E, Tanaka H. 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence Imaging for Tumor Detection: Recent Advances and Challenges. Int J Mol Sci. 2022;23(12):6478. [CrossRef]
- Huis In 't Veld RV, Heuts J, Ma S, Cruz LJ, Ossendorp FA, Jager MJ. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics. 2023;15(2):330. [CrossRef]
- Vivas-Buitrago T, Domingo RA, Tripathi S, De Biase G, Brown D, Akinduro OO, Ramos-Fresnedo A, Sabsevitz DS, Bendok BR, Sherman W, Parney IF, Jentoft ME, Middlebrooks EH, Meyer FB, Chaichana KL, Quinones-Hinojosa A. Influence of supramarginal resection on survival outcomes after gross-total resection of IDH-wild-type glioblastoma. J Neurosurg. 2021;136(1):1-8. [CrossRef]
- Poon MTC, Sudlow CLM, Figueroa JD, Brennan PM. Longer-term (≥ 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis. Sci Rep. 2020;10(1):11622. [CrossRef]
- Seyve A, Lozano-Sanchez F, Thomas A, Mathon B, Tran S, Mokhtari K, Giry M, Marie Y, Capelle L, Peyre M, Carpentier A, Feuvret L, Sanson M, Hoang-Xuan K, Honnorat J, Delattre JY, Ducray F, Idbaih A. Initial surgical resection and long time to occurrence from initial diagnosis are independent prognostic factors in resected recurrent IDH wild-type glioblastoma. Clin Neurol Neurosurg. 2020;196:106006. [CrossRef]
- Birzu C, French P, Caccese M, Cerretti G, Idbaih A, Zagonel V, Lombardi G. Recurrent Glioblastoma: From Molecular Landscape to New Treatment Perspectives. Cancers (Basel). 2020;13(1):47. [CrossRef]
- Szklener K, Bilski M, Nieoczym K, Mańdziuk D, Mańdziuk S. Enhancing glioblastoma treatment through the integration of tumor-treating fields. Front Oncol. 2023;13:1274587. [CrossRef]
- Eatz TA, Eichberg DG, Lu VM, Di L, Komotar RJ, Ivan ME. Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: a systematic review. J Neurooncol. 2022;156(2):233-256. [CrossRef]
- Olar A, Aldape KD. Using the molecular classification of glioblastoma to inform personalized treatment. J Pathol. 2014;232(2):165-77. [CrossRef]
- Gebhardt BJ, Dobelbower MC, Ennis WH, Bag AK, Markert JM, Fiveash JB. Patterns of failure for glioblastoma multiforme following limited-margin radiation and concurrent temozolomide. Radiat Oncol. 2014;9:130. [CrossRef]
- Tedford CE, DeLapp S, Jacques S, Anders J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg Med. 2015;47(4):312-22. [CrossRef]
- Mathews MS, Angell-Petersen E, Sanchez R, Sun CH, Vo V, Hirschberg H, Madsen SJ. The effects of ultra low fluence rate single and repetitive photodynamic therapy on glioma spheroids. Lasers Surg Med. 2009;41(8):578-84. [CrossRef]
- Pignatelli P, Umme S, D'Antonio DL, Piattelli A, Curia MC. Reactive Oxygen Species Produced by 5-Aminolevulinic Acid Photodynamic Therapy in the Treatment of Cancer. Int J Mol Sci. 2023;24(10):8964. [CrossRef]
- Cramer SW, Chen CC. Photodynamic Therapy for the Treatment of Glioblastoma. Front Surg. 2020;6:81. [CrossRef]
- Marcus SL, de Souza MP. Theranostic Uses of the Heme Pathway in Neuro-Oncology: Protoporphyrin IX (PpIX) and Its Journey from Photodynamic Therapy (PDT) through Photodynamic Diagnosis (PDD) to Sonodynamic Therapy (SDT). Cancers (Basel). 2024;16(4):740. [CrossRef]
- Li B, Shen Y, Lin H, Wilson BC. Correlation of in vitro cell viability and cumulative singlet oxygen luminescence from protoporphyrin IX in mitochondria and plasma membrane. Photodiagnosis Photodyn Ther. 2024;46:104080. [CrossRef]
- Stummer W, Müther M, Spille D. Beyond fluorescence-guided resection: 5-ALA-based glioblastoma therapies. Acta Neurochir (Wien). 2024;166(1):163. [CrossRef]
- Peciu-Florianu I, Vannod-Michel Q, Vauleon E, Bonneterre ME, Reyns N. Long term follow-up of patients with newly diagnosed glioblastoma treated by intraoperative photodynamic therapy: an update from the INDYGO trial (NCT03048240). J Neurooncol. 2024;168(3):495-505. [CrossRef]
- Ibarra LE, Vilchez ML, Caverzán MD, Milla Sanabria LN. Understanding the glioblastoma tumor biology to optimize photodynamic therapy: From molecular to cellular events. J Neurosci Res. 2021;99(4):1024-1047. [CrossRef]
- Vermandel M, Dupont C, Lecomte F, Leroy HA, Tuleasca C, Mordon S, Hadjipanayis CG, Reyns N. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: a preliminary analysis of the INDYGO clinical trial. J Neurooncol. 2021;152(3):501-514. [CrossRef]
- da Silva EB Jr, Vasquez MWM, de Almeida Teixeira BC, Neto MC, Sprenger F, Filho JLN, Almeida-Lopes L, Ramina R. Association of 5-aminolevulinic acid fluorescence guided resection with photodynamic therapy in recurrent glioblastoma: a matched cohort study. Acta Neurochir (Wien). 2024;166(1):212. [CrossRef]
- Schipmann S, Müther M, Stögbauer L, Zimmer S, Brokinkel B, Holling M, Grauer O, Suero Molina E, Warneke N, Stummer W. Combination of ALA-induced fluorescence-guided resection and intraoperative open photodynamic therapy for recurrent glioblastoma: case series on a promising dual strategy for local tumor control. J Neurosurg. 2020;134(2):426-436. [CrossRef]
- Bisland SK, Lilge L, Lin A, Rusnov R, Wilson BC. Metronomic photodynamic therapy as a new paradigm for photodynamic therapy: rationale and preclinical evaluation of technical feasibility for treating malignant brain tumors. Photochem Photobiol. 2004;80:22-30. [CrossRef]
- Bogaards A, Varma A, Zhang K, Zach D, Bisland SK, Moriyama EH, Lilge L, Muller PJ, Wilson BC. Fluorescence image-guided brain tumour resection with adjuvant metronomic photodynamic therapy: pre-clinical model and technology development. Photochem Photobiol Sci. 2005;4(5):438-42. [CrossRef]
- Guo HW, Lin LT, Chen PH, Ho MH, Huang WT, Lee YJ, Chiou SH, Hsieh YS, Dong CY, Wang HW. Low-fluence rate, long duration photodynamic therapy in glioma mouse model using organic light emitting diode (OLED). Photodiagnosis Photodyn Ther. 2015;12(3):504-10. [CrossRef]
- Dupont C, Mordon S, Deleporte P, Reyns N, Vermandel M. A novel device for intraoperative photodynamic therapy dedicated to glioblastoma treatment. Future Oncol. 2017;13(27):2441-2454. [CrossRef]
- Eljamel MS, Goodman C, Moseley H. ALA and Photofrin fluorescence guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial. Lasers Med Sci. 2008;23(4):361-7. [CrossRef]
- Bader N, Peschmann C, Kast RE, Heiland T, Merz T, McCook O, Alfieri A, Karpel-Massler G, Capanni F, Halatsch ME. Globus Lucidus: A porcine study of an intracranial implant designed to deliver closed, repetitive photodynamic and photochemical therapy in glioblastoma. Photodiagnosis Photodyn Ther. 2024;46:104059. [CrossRef]
- Andrieux G, Das T, Griffin M, Straehle J, Paine SML, Beck J, Boerries M, Heiland DH, Smith SJ, Rahman R, Chakraborty S. Spatially resolved transcriptomic profiles reveal unique defining molecular features of infiltrative 5-ALA metabolizing cells associated with glioblastoma recurrence. Genome Med. 2023;15(1):48. [CrossRef]
- Yano H, Nakayama N, Ohe N, Miwa K, Shinoda J, Iwama T. Pathological analysis of the surgical margins of resected glioblastomas excised using photodynamic visualization with both 5-aminolevulinic acid and fluorescein sodium. J Neurooncol. 2017;133(2):389-397. [CrossRef]
- Aguilar Cosme JR, Gagui DC, Green NH, Bryant HE, Claeyssens F. In Vitro Low-Fluence Photodynamic Therapy Parameter Screening Using 3D Tumor Spheroids Shows that Fractionated Light Treatments Enhance Phototoxicity. ACS Biomater Sci Eng. 2021;7(11):5078-5089. [CrossRef]
- Madsen SJ, Sun CH, Tromberg BJ, Wallace VP, Hirschberg H. Photodynamic therapy of human glioma spheroids using 5-aminolevulinic acid. Photochem Photobiol. 2000;72(1):128-34. [CrossRef]
- Lapchak PA, Boitano PD. Transcranial Near-Infrared Laser Therapy for Stroke: How to Recover from Futility in the NEST-3 Clinical Trial. Acta Neurochir Suppl. 2016;121:7-12. [CrossRef]
- Lapchak PA, Boitano PD, Butte PV, Fisher DJ, Hölscher T, Ley EJ, Nuño M, Voie AH, Rajput PS. Transcranial Near-Infrared Laser Transmission (NILT) Profiles (800 nm): Systematic Comparison in Four Common Research Species. PLoS One. 2015;10(6):e0127580. [CrossRef]
- Lapchak, PA. Taking a light approach to treating acute ischemic stroke patients: transcranial near-infrared laser therapy translational science. Ann Med. 2010;42(8):576-86. [CrossRef]
- Joshi H, Sinha P, Bowers D, John JP. Dose response of transcranial near infrared light stimulation on brain functional connectivity and cognition in older adults-A randomized comparison. J Biophotonics. 2024;17(2):e202300215. [CrossRef]
- Salehpour F, Cassano P, Rouhi N, Hamblin MR, De Taboada L, Farajdokht F, Mahmoudi J. Penetration Profiles of Visible and Near-Infrared Lasers and Light-Emitting Diode Light Through the Head Tissues in Animal and Human Species: A Review of Literature. Photobiomodul Photomed Laser Surg. 2019;37(10):581-595. [CrossRef]
- Fradkin Y, De Taboada L, Naeser M, Saltmarche A, Snyder W, Steingold E. Transcranial photobiomodulation in children aged 2-6 years: a randomized sham-controlled clinical trial assessing safety, efficacy, and impact on autism spectrum disorder symptoms and brain electrophysiology. Front Neurol. 2024;15:1221193. [CrossRef]
- Shahdadian S, Wang X, Liu H. Directed physiological networks in the human prefrontal cortex at rest and post transcranial photobiomodulation. Sci Rep. 2024;14(1):10242. [CrossRef]
- Huang N, Yao D, Jiang W, Wei C, Li M, Li W, Mu H, Gao M, Ma Z, Lyu J, Tong Z. Safety and Efficacy of 630-nm Red Light on Cognitive Function in Older Adults With Mild to Moderate Alzheimer's Disease: Protocol for a Randomized Controlled Study. Front Aging Neurosci. 2020;12:143. [CrossRef]
- Blivet G, Roman FJ, Lelouvier B, Ribière C, Touchon J. Photobiomodulation Therapy: A Novel Therapeutic Approach to Alzheimer's Disease Made Possible by the Evidence of a Brain-Gut Interconnection. J Integr Neurosci. 2024;23(5):92. [CrossRef]
- Su M, Nizamutdinov D, Liu H, Huang JH. Recent Mechanisms of Neurodegeneration and Photobiomodulation in the Context of Alzheimer's Disease. Int J Mol Sci. 2023;24(11):9272. [CrossRef]
- Gao Y, An R, Huang X, Liu W, Yang C, Wan Q. Effectiveness of photobiomodulation for people with age-related cognitive impairment: a systematic review and meta-analysis. Lasers Med Sci. 2023;38(1):237. [CrossRef]
- Nairuz T, Sangwoo-Cho, Lee JH. Photobiomodulation Therapy on Brain: Pioneering an Innovative Approach to Revolutionize Cognitive Dynamics. Cells. 2024;13(11):966. [CrossRef]
- Bicknell B, Liebert A, Herkes G. Parkinson's Disease and Photobiomodulation: Potential for Treatment. J Pers Med. 2024;14(1):112. [CrossRef]
- Stevens AR, Hadis M, Milward M, Ahmed Z, Belli A, Palin W, Davies DJ. Photobiomodulation in Acute Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J Neurotrauma. 2023;40(3-4):210-227. [CrossRef]
- Lim, L. Traumatic Brain Injury Recovery with Photobiomodulation: Cellular Mechanisms, Clinical Evidence, and Future Potential. Cells. 2024;13(5):385. [CrossRef]
- Ceranoglu TA, Hutt Vater C. Dr. Joseph Biederman's Enduring Legacy: Illuminating the Path to Addressing Autistic Traits in Attention Deficit Hyperactivity Disorder With Transcranial Photobiomodulation. J Atten Disord. 2024;28(5):664-668. [CrossRef]
- Farazi N, Salehi-Pourmehr H, Farajdokht F, Mahmoudi J, Sadigh-Eteghad S. Photobiomodulation combination therapy as a new insight in neurological disorders: a comprehensive systematic review. BMC Neurol. 2024;24(1):101. [CrossRef]
- Ma H, Du Y, Xie D, Wei ZZ, Pan Y, Zhang Y. Recent advances in light energy biotherapeutic strategies with photobiomodulation on central nervous system disorders. Brain Res. 2024;1822:148615. [CrossRef]
- Chamkouri H, Liu Q, Zhang Y, Chen C, Chen L. Brain photobiomodulation therapy on neurological and psychological diseases. J Biophotonics. 2024;17(1):e202300145. [CrossRef]
- Kast RE, Skuli N, Sardi I, Capanni F, Hessling M, Frosina G, Kast AP, Karpel-Massler G, Halatsch ME. Augmentation of 5-Aminolevulinic Acid Treatment of Glioblastoma by Adding Ciprofloxacin, Deferiprone, 5-Fluorouracil and Febuxostat: The CAALA Regimen. Brain Sci. 2018;8(12):203. [CrossRef]
- Alomari S, Zhang I, Hernandez A, Kraft CY, Raj D, Kedda J, Tyler B. Drug Repurposing for Glioblastoma and Current Advances in Drug Delivery-A Comprehensive Review of the Literature. Biomolecules. 2021;11(12):1870. [CrossRef]
- Ferrario N, Marras E, Vivona V, Randisi F, Fallica AN, Marrazzo A, Perletti G, Gariboldi MB. Mechanisms of the Antineoplastic Effects of New Fluoroquinolones in 2D and 3D Human Breast and Bladder Cancer Cell Lines. Cancers (Basel). 2024;16(12):2227. [CrossRef]
- Kloskowski T, Fekner Z, Szeliski K, Paradowska M, Balcerczyk D, Rasmus M, Dąbrowski P, Kaźmierski Ł, Drewa T, Pokrywczyńska M. Effect of four fluoroquinolones on the viability of bladder cancer cells in 2D and 3D cultures. Front Oncol. 2023;13:1222411. [CrossRef]
- Huang CY, Yang JL, Chen JJ, Tai SB, Yeh YH, Liu PF, Lin MW, Chung CL, Chen CL. Fluoroquinolones Suppress TGF-β and PMA-Induced MMP-9 Production in Cancer Cells: Implications in Repurposing Quinolone Antibiotics for Cancer Treatment. Int J Mol Sci. 2021;22(21):11602. [CrossRef]
- Abdel-Aal MAA, Abdel-Aziz SA, Shaykoon MSA, Abuo-Rahma GEA. Towards anticancer fluoroquinolones: A review article. Arch Pharm (Weinheim). 2019;352(7):e1800376. [CrossRef]
- Zandi A, Zanjani TM, Ziai S, Poul YK, Hoseini M. Evaluation of the Cytotoxic Effects of Ciprofloxacin on Human Glioblastoma A-172 Cell Line. Middle East J Cancer. 2017;8(3):119-126.
- Zandi A, Zanjani TM, Ziai S, Poul YK, Hoseini M. The Synergistic Effects of the Combination of Ciprofloxacin and Temozolomide on Human Glioblastoma A-172 Cell Line. Middle East J Cancer. 2017;8(1): 31-38.
- Esmaeilzadeh A., Ebtekar M, Biglari A, Hassan Z. Influence of ciprofloxacin on glioma cell line GL26: A new application for an old antibiotic. African J Microbiol Res. 2012;6(23):4891-4896. [CrossRef]
- Beberok A, Rzepka Z, Respondek M, Rok J, Sierotowicz D, Wrześniok D. GSH depletion, mitochondrial membrane breakdown, caspase-3/7 activation and DNA fragmentation in U87MG glioblastoma cells: New insight into the mechanism of cytotoxicity induced by fluoroquinolones. Euro J Pharmacology. 2018;835:94-107. [CrossRef]
- Ueta K, Yamamoto J, Tanaka T, Nakano Y, Kitagawa T, Nishizawa S. 5-Aminolevulinic acid enhances mitochondrial stress upon ionizing irradiation exposure and increases delayed production of reactive oxygen species and cell death in glioma cells. Int J Mol Med. 2017;39(2):387-398. [CrossRef]
- Gull HH, Karadag C, Senger B, Sorg RV, Möller P, Mellert K, Steiger HJ, Hänggi D, Cornelius JF. Ciprofloxacin enhances phototoxicity of 5-aminolevulinic acid mediated photodynamic treatment for chordoma cell lines. Photodiagnosis Photodyn Ther. 2021;35:102346. [CrossRef]
- Cornelius JF, Slotty PJ, El Khatib M, Giannakis A, Senger B, Steiger HJ. Enhancing the effect of 5-aminolevulinic acid based photodynamic therapy in human meningioma cells. Photodiagnosis Photodyn Ther. 2014;11(1):1-6. [CrossRef]
- Gera K, Cline C, Al-Mansour Z, Medvec A, Lee JH, Galochkina Z, Hsu J, Hiemenz J, Farhadfar N, Dean EA, Wingard JR, Brown R. A phase ib clinical trial of oral ciprofloxacin and etoposide in subjects with resistant acute myeloid leukemia. Leuk Lymphoma. 2024:1-9. [CrossRef]
- Qin J, Zhou C, Zhu M, Shi S, Zhang L, Zhao Y, Li C, Wang Y, Wang Y. Iron chelation promotes 5-aminolaevulinic acid-based photodynamic therapy against oral tongue squamous cell carcinoma. Photodiagnosis Photodyn Ther. 2020;31:101907. [CrossRef]
- Magnussen A, Reburn C, Perry A, Wood M, Curnow A. Experimental investigation of a combinational iron chelating protoporphyrin IX prodrug for fluorescence detection and photodynamic therapy. Lasers Med Sci. 2022;37(2):1155-1166. [CrossRef]
- Howley R, Mansi M, Shinde J, Restrepo J, Chen B. Analysis of Renal Cell Carcinoma Cell Response to the Enhancement of 5-aminolevulinic Acid-mediated Protoporphyrin IX Fluorescence by Iron Chelator Deferoxamine. Photochem Photobiol. 2023;99(2):787-792. [CrossRef]
- Čunderlíková B, Kalafutová A, Babál P, Mlkvý P, Teplický T. Suppression of resistance to aminolevulinic acid-based photodynamic therapy in esophageal cell lines by administration of iron chelators in collagen type I matrices. Int J Radiat Biol. 2023;99(3):474-487. [CrossRef]
- Nomoto T, Komoto K, Nagano T, Ishii T, Guo H, Honda Y, Ogura SI, Ishizuka M, Nishiyama N. Polymeric iron chelators for enhancing 5-aminolevulinic acid-induced photodynamic therapy. Cancer Sci. 2023;114(3):1086-1094. [CrossRef]
- Chen Y, Deng H, Yang L, Guo L, Feng M. Desferrioxamine Enhances 5-Aminolaevulinic Acid- Induced Protoporphyrin IX Accumulation and Therapeutic Efficacy for Hypertrophic Scar. J Pharm Sci. 2023;112(6):1635-1643. [CrossRef]
- Uzungil V, Tran H, Aitken C, Wilson C, Opazo CM, Li S, Payet JM, Mawal CH, Bush AI, Hale MW, Hannan AJ, Renoir T. Novel Antidepressant-Like Properties of the Iron Chelator Deferiprone in a Mouse Model of Depression. Neurotherapeutics. 2022;19(5):1662-1685. [CrossRef]
- Romano N, Baiardi G, Pinto VM, Quintino S, Gianesin B, Sasso R, Diociasi A, Mattioli F, Marchese R, Abbruzzese G, Castaldi A, Forni GL. Long-Term Neuroradiological and Clinical Evaluation of NBIA Patients Treated with a Deferiprone Based Iron-Chelation Therapy. J Clin Med. 2022;11(15):4524. [CrossRef]
- Kontoghiorghes GJ. The Vital Role Played by Deferiprone in the Transition of Thalassaemia from a Fatal to a Chronic Disease and Challenges in Its Repurposing for Use in Non-Iron-Loaded Diseases. Pharmaceuticals (Basel). 2023;16(7):1016. [CrossRef]
- Piffaretti D, Burgio F, Thelen M, Kaelin-Lang A, Paganetti P, Reinert M, D'Angelo ML. Protoporphyrin IX tracer fluorescence modulation for improved brain tumor cell lines visualization. J Photochem Photobiol B. 2019;201:111640. [CrossRef]
- Reburn C, Gawthorpe G, Perry A, Wood M, Curnow A. Novel Iron-Chelating Prodrug Significantly Enhanced Fluorescence-Mediated Detection of Glioma Cells Experimentally In Vitro. Pharmaceutics. 2023;15(12):2668. [CrossRef]
- Teng L, Nakada M, Zhao SG, Endo Y, Furuyama N, Nambu E, Pyko IV, Hayashi Y, Hamada JI. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br J Cancer. 2011;104(5):798-807. [CrossRef]
- de Souza AL, Marra K, Gunn J, Samkoe KS, Kanick SC, Davis SC, Chapman MS, Maytin EV, Hasan T, Pogue BW. Comparing desferrioxamine and light fractionation enhancement of ALA-PpIX photodynamic therapy in skin cancer. Br J Cancer. 2016;115(7):805-13. [CrossRef]
- Kawai N, Hirohashi Y, Ebihara Y, Saito T, Murai A, Saito T, Shirosaki T, Kubo T, Nakatsugawa M, Kanaseki T, Tsukahara T, Shichinohe T, Li L, Hirano S, Torigoe T. ABCG2 expression is related to low 5-ALA photodynamic diagnosis (PDD) efficacy and cancer stem cell phenotype, and suppression of ABCG2 improves the efficacy of PDD. PLoS One. 2019;14(5):e0216503. [CrossRef]
- Howley R, Olsen J, Chen B. Effectiveness of lapatinib for enhancing 5-aminolevulinic acid mediated protoporphyrin IX fluorescence and photodynamic therapy in human cancer cell lines with varied ABCG2 activities. Photochem Photobiol. 2024 Mar 13. [CrossRef]
- Kobuchi H, Moriya K, Ogino T, Fujita H, Inoue K, Shuin T, Yasuda T, Utsumi K, Utsumi T. Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation. PLoS One. 2012;7(11):e50082. [CrossRef]
- Ishikawa T, Takahashi K, Ikeda N, Kajimoto Y, Hagiya Y, Ogura S, Miyatake S, Kuroiwa T. Transporter-Mediated Drug Interaction Strategy for 5-Aminolevulinic Acid (ALA)-Based Photodynamic Diagnosis of Malignant Brain Tumor: Molecular Design of ABCG2 Inhibitors. Pharmaceutics. 2011;3(3):615-35. [CrossRef]
- Mansi M, Howley R, Chandratre S, Chen B. Inhibition of ABCG2 transporter by lapatinib enhances 5-aminolevulinic acid-mediated protoporphyrin IX fluorescence and photodynamic therapy response in human glioma cell lines. Biochem Pharmacol. 2022;200:115031. [CrossRef]
- Müller P, Abdel Gaber SA, Zimmermann W, Wittig R, Stepp H. ABCG2 influence on the efficiency of photodynamic therapy in glioblastoma cells. J Photochem Photobiol B. 2020;210:111963. [CrossRef]
- Chandratre S, Olsen J, Howley R, Chen B. Targeting ABCG2 transporter to enhance 5-aminolevulinic acid for tumor visualization and photodynamic therapy. Biochem Pharmacol. 2023;217:115851. [CrossRef]
- de Gooijer MC, de Vries NA, Buckle T, Buil LCM, Beijnen JH, Boogerd W, van Tellingen O. Improved Brain Penetration and Antitumor Efficacy of Temozolomide by Inhibition of ABCB1 and ABCG2. Neoplasia. 2018;20(7):710-720. [CrossRef]
- Deng F, Sjöstedt N, Santo M, Neuvonen M, Niemi M, Kidron H. Novel inhibitors of breast cancer resistance protein (BCRP, ABCG2) among marketed drugs. Eur J Pharm Sci. 2023;181:106362. [CrossRef]
- Nagasaka Y, Oda K, Iwatsubo T, Kawamura A, Usui T. Effects of aripiprazole and its active metabolite dehydroaripiprazole on the activities of drug efflux transporters expressed both in the intestine and at the blood-brain barrier. Biopharm Drug Dispos. 2012;33(6):304-15. [CrossRef]
- Weiss J, Sauer A, Divac N, Herzog M, Schwedhelm E, Böger RH, Haefeli WE, Benndorf RA. Interaction of angiotensin receptor type 1 blockers with ATP-binding cassette transporters. Biopharm Drug Dispos. 2010;31(2-3):150-61. [CrossRef]
- Miyata H, Takada T, Toyoda Y, Matsuo H, Ichida K, Suzuki H. Identification of Febuxostat as a New Strong ABCG2 Inhibitor: Potential Applications and Risks in Clinical Situations. Front Pharmacol. 2016;7:518. [CrossRef]
- Assandri A, Ratti B, Cristina T. Pharmacokinetics of rifapentine, a new long lasting rifamycin, in the rat, the mouse and the rabbit. J Antibiot (Tokyo). 1984;37(9):1066-75. [CrossRef]
- Rodriguez-Perez AI, Sucunza D, Pedrosa MA, Garrido-Gil P, Kulisevsky J, Lanciego JL, Labandeira-Garcia JL. Angiotensin Type 1 Receptor Antagonists Protect Against Alpha-Synuclein-Induced Neuroinflammation and Dopaminergic Neuron Death. Neurotherapeutics. 2018;15(4):1063-1081. [CrossRef]
- Torika N, Asraf K, Danon A, Apte RN, Fleisher-Berkovich S. Telmisartan Modulates Glial Activation: In Vitro and In Vivo Studies. PLoS One. 2016;11(5):e0155823. [CrossRef]
- Ripperger A, Krischer A, Robaa D, Sippl W, Benndorf RA. Pharmacogenetic Aspects of the Interaction of AT1 Receptor Antagonists With ATP-Binding Cassette Transporter ABCG2. Front Pharmacol. 2018;9:463. [CrossRef]
- Hu M, Lee HK, To KK, Fok BS, Wo SK, Ho CS, Wong CK, Zuo Z, Chan TY, Chan JC, Tomlinson B. Telmisartan increases systemic exposure to rosuvastatin after single and multiple doses, and in vitro studies show telmisartan inhibits ABCG2-mediated transport of rosuvastatin. Eur J Clin Pharmacol. 2016;72(12):1471-1478. [CrossRef]
- Salacz ME, Kast RE, Saki N, Brüning A, Karpel-Massler G, Halatsch ME. Toward a noncytotoxic glioblastoma therapy: blocking MCP-1 with the MTZ Regimen. Onco Targets Ther. 2016;9:2535-45. [CrossRef]
- Quan W, Xu CS, Li XC, Yang C, Lan T, Wang MY, Yu DH, Tang F, Wang ZF, Li ZQ. Telmisartan inhibits microglia-induced neurotoxic A1 astrocyte conversion via PPARγ mediated NF-κB/p65 degradation. Int Immunopharmacol. 2023;123:110761.
- . [CrossRef]
- Chang YL, Chou CH, Li YF, Huang LC, Kao Y, Hueng DY, Tsai CK. Antiproliferative and apoptotic effects of telmisartan in human glioma cells. Cancer Cell Int. 2023;23(1):111. [CrossRef]
- Wang Y, Zhang T, Li C, Guo J, Xu B, Xue L. Telmisartan attenuates human glioblastoma cells proliferation and oncogenicity by inducing the lipid oxidation. Asia Pac J Clin Oncol. 2022;18(3):217-223. [CrossRef]
- Paul SK, Guendouzi A, Banerjee A, Guendouzi A, Haldar R. Identification of approved drugs with ALDH1A1 inhibitory potential aimed at enhancing chemotherapy sensitivity in cancer cells: an in-silico drug repurposing approach. J Biomol Struct Dyn. 2024:1-15. [CrossRef]
- Kumar U, Aich J, Devarajan S. Exploring the repurposing potential of telmisartan drug in breast cancer: an in-silico and in-vitro approach. Anticancer Drugs. 2023;34(10):1094-1103. [CrossRef]
- Yamana Y, Fujihara S, Kobara H, Oura K, Samukawa E, Chiyo T, Okamura M, Yamana H, Tadokoro T, Fujita K, Morishita A, Iwama H, Masaki T. MicroRNA profiles following telmisartan treatment in pancreatic ductal adenocarcinoma cells. J Cancer Res Ther. 2022;18(Supplement):S305-S312. [CrossRef]
- Khorsand M, Mostafavi-Pour Z, Razban V, Khajeh S, Zare R. Combinatorial effects of telmisartan and docetaxel on cell viability and metastatic gene expression in human prostate and breast cancer cells. Mol Biol Res Commun. 2022;11(1):11-20. [CrossRef]
- Khorsand M, Khajeh S, Eslami M, Nezafat N, Ghasemi Y, Razban V, Mostafavi-Pour Z. Telmisartan anti-cancer activities mechanism through targeting N-cadherin by mimicking ADH-1 function. J Cell Mol Med. 2022;26(8):2392-2403. [CrossRef]
- Tsujiya Y, Hasegawa A, Yamamori M, Okamura N. Telmisartan-Induced Cytotoxicity via G2/M Phase Arrest in Renal Cell Carcinoma Cell Lines. Biol Pharm Bull. 2021;44(12):1878-1885. [CrossRef]
- Tsujiya Y, Yamamori M, Hasegawa AI, Yamamoto Y, Yashiro M, Okamura N. Telmisartan Exerts Cytotoxicity in Scirrhous Gastric Cancer Cells by Inducing G0/G1 Cell Cycle Arrest. Anticancer Res. 2021;41(11):5461-5468. [CrossRef]
- Kast RE, Ellingson BM, Marosi C, Halatsch ME. Glioblastoma treatment using perphenazine to block the subventricular zone's tumor trophic functions. J Neurooncol. 2014;116(2):207-12. [CrossRef]
- Kast RE. Adding perphenazine to increase effectiveness of standard glioblastoma chemoirradiation. J BUON. 2020;25(4):1676-1686.
- Jeon HM, Oh YT, Shin YJ, Chang N, Kim D, Woo D, Yeup Y, Joo KM, Jo H, Yang H, Lee JK, Kang W, Sa J, Lee WJ, Hale J, Lathia JD, Purow B, Park MJ, Park JB, Nam DH, Lee J. Dopamine receptor D2 regulates glioblastoma survival and death through MET and death receptor 4/5. Neoplasia. 2023;39:100894. [CrossRef]
- Wang Y, Wang X, Wang K, Qi J, Zhang Y, Wang X, Zhang L, Zhou Y, Gu L, Yu R, Zhou X. Chronic stress accelerates glioblastoma progression via DRD2/ERK/β-catenin axis and Dopamine/ERK/TH positive feedback loop. J Exp Clin Cancer Res. 2023;42(1):161. [CrossRef]
- Weissenrieder JS, Reed JL, Green MV, Moldovan GL, Koubek EJ, Neighbors JD, Hohl RJ. The Dopamine D2 Receptor Contributes to the Spheroid Formation Behavior of U87 Glioblastoma Cells. Pharmacology. 2020;105(1-2):19-27. [CrossRef]
- He Y, Li J, Koga T, Ma J, Dhawan S, Suzuki Y, Furnari F, Prabhu VV, Allen JE, Chen CC. Epidermal growth factor receptor as a molecular determinant of glioblastoma response to dopamine receptor D2 inhibitors. Neuro Oncol. 2021;23(3):400-411. [CrossRef]
- Liu Z, Jiang X, Gao L, Liu X, Li J, Huang X, Zeng T. Synergistic Suppression of Glioblastoma Cell Growth by Combined Application of Temozolomide and Dopamine D2 Receptor Antagonists. World Neurosurg. 2019;128:e468-e477. [CrossRef]
- Shi L, Chen H, Chen K, Zhong C, Song C, Huang Y, Wang T, Chen L, Li C, Huang A, Qi S, Li H, Lu Y. The DRD2 Antagonist Haloperidol Mediates Autophagy-Induced Ferroptosis to Increase Temozolomide Sensitivity by Promoting Endoplasmic Reticulum Stress in Glioblastoma. Clin Cancer Res. 2023;29(16):3172-3188.
- . [CrossRef]
- Awuah WA, Kalmanovich J, Mehta A, Huang H, Abdul-Rahman T, Cheng Ng J, Yarlagadda R, Kamanousa K, Kundu M, Nansubuga EP, Hasan MM, Lyndin M, Isik A, Sikora V, Alexiou A. Multilevel Pharmacological Effects of Antipsychotics in Potential Glioblastoma Treatment. Curr Top Med Chem. 2023;23(5):389-402. [CrossRef]
- Caragher SP, Shireman JM, Huang M, Miska J, Atashi F, Baisiwala S, Hong Park C, Saathoff MR, Warnke L, Xiao T, Lesniak MS, James CD, Meltzer H, Tryba AK, Ahmed AU. Activation of Dopamine Receptor 2 Prompts Transcriptomic and Metabolic Plasticity in Glioblastoma. J Neurosci. 2019;39(11):1982-1993. [CrossRef]
- Abbruzzese C, Matteoni S, Persico M, Villani V, Paggi MG. Repurposing chlorpromazine in the treatment of glioblastoma multiforme: analysis of literature and forthcoming steps. J Exp Clin Cancer Res. 2020;39(1):26. [CrossRef]
- Pinheiro T, Otrocka M, Seashore-Ludlow B, Rraklli V, Holmberg J, Forsberg-Nilsson K, Simon A, Kirkham M. A chemical screen identifies trifluoperazine as an inhibitor of glioblastoma growth. Biochem Biophys Res Commun. 2017;494(3-4):477-483. [CrossRef]
- Salvalaggio A, Pini L, Bertoldo A, Corbetta M. Glioblastoma and brain connectivity: the need for a paradigm shift. Lancet Neurol. 2024;23(7):740-748. [CrossRef]
- Broekman ML, Maas SLN, Abels ER, Mempel TR, Krichevsky AM, Breakefield XO. Multidimensional communication in the microenvirons of glioblastoma. Nat Rev Neurol. 2018;14(8):482-495. [CrossRef]
- White J, White MPJ, Wickremesekera A, Peng L, Gray C. The tumour microenvironment, treatment resistance and recurrence in glioblastoma. J Transl Med. 2024;22(1):540. [CrossRef]
- Kast RE, Alfieri A, Assi HI, Burns TC, Elyamany AM, Gonzalez-Cao M, Karpel-Massler G, Marosi C, Salacz ME, Sardi I, Van Vlierberghe P, Zaghloul MS, Halatsch ME. MDACT: A New Principle of Adjunctive Cancer Treatment Using Combinations of Multiple Repurposed Drugs, with an Example Regimen. Cancers (Basel). 2022;14(10):2563. [CrossRef]
- Rumie Vittar NB, Lamberti MJ, Pansa MF, Vera RE, Rodriguez ME, Cogno IS, Milla Sanabria LN, Rivarola VA. Ecological photodynamic therapy: new trend to disrupt the intricate networks within tumor ecosystem. Biochim Biophys Acta. 2013;1835(1):86-99. [CrossRef]
- Duenas-Gonzalez A, Gonzalez-Fierro A, Bornstein-Quevedo L, Gutierrez-Delgado F, Kast RE, Chavez-Blanco A, Dominguez-Gomez G, Candelaria M, Romo-Pérez A, Correa-Basurto J, Lizano M, Perez-de la Cruz V, Robles-Bañuelos B, Nuñez-Corona D, Martinez-Perez E, Verastegui E. Multitargeted polypharmacotherapy for cancer treatment. theoretical concepts and proposals. Expert Rev Anticancer Ther. 2024:1-13. [CrossRef]
- Prager BC, Bhargava S, Mahadev V, Hubert CG, Rich JN. Glioblastoma Stem Cells: Driving Resilience through Chaos. Trends Cancer. 2020;6(3):223-235. [CrossRef]
- Gimple RC, Bhargava S, Dixit D, Rich JN. Glioblastoma stem cells: lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 2019;33(11-12):591-609. [CrossRef]
- Vilchez ML, Rodríguez LB, Palacios RE, Prucca CG, Caverzán MD, Caputto BL, Rivarola VA, Milla Sanabria LN. Isolation and initial characterization of human glioblastoma cells resistant to photodynamic therapy. Photodiagnosis Photodyn Ther. 2021;33:102097. [CrossRef]
- Rodríguez Aguilar L, Vilchez ML, Milla Sanabria LN. Targeting glioblastoma stem cells: The first step of photodynamic therapy. Photodiagnosis Photodyn Ther. 2021;36:102585. [CrossRef]
- Omura N, Nonoguchi N, Fujishiro T, Park Y, Ikeda N, Kajimoto Y, Hosomi R, Yagi R, Hiramatsu R, Furuse M, Kawabata S, Fukunaga K, Kuroiwa T, Nakano I, Wanibuchi M. Ablation efficacy of 5-aminolevulinic acid-mediated photodynamic therapy on human glioma stem cells. Photodiagnosis Photodyn Ther. 2023;41:103119. [CrossRef]
- Schimanski A, Ebbert L, Sabel MC, Finocchiaro G, Lamszus K, Ewelt C, Etminan N, Fischer JC, Sorg RV. Human glioblastoma stem-like cells accumulate protoporphyrin IX when subjected to exogenous 5-aminolaevulinic acid, rendering them sensitive to photodynamic treatment. J Photochem Photobiol B. 2016;163:203-10. [CrossRef]
- Kawai N, Hirohashi Y, Ebihara Y, Saito T, Murai A, Saito T, Shirosaki T, Kubo T, Nakatsugawa M, Kanaseki T, Tsukahara T, Shichinohe T, Li L, Hirano S, Torigoe T. ABCG2 expression is related to low 5-ALA photodynamic diagnosis (PDD) efficacy and cancer stem cell phenotype, and suppression of ABCG2 improves the efficacy of PDD. PLoS One. 2019;14(5):e0216503. [CrossRef]
- Fujishiro T, Nonoguchi N, Pavliukov M, Ohmura N, Kawabata S, Park Y, Kajimoto Y, Ishikawa T, Nakano I, Kuroiwa T. 5-Aminolevulinic acid-mediated photodynamic therapy can target human glioma stem-like cells refractory to antineoplastic agents. Photodiagnosis Photodyn Ther. 2018;24:58-68. [CrossRef]
- Spring BQ, Watanabe K, Ichikawa M, Mallidi S, Matsudaira T, Timerman D, Swain JWR, Mai Z, Wakimoto H, Hasan T. Red light-activated depletion of drug-refractory glioblastoma stem cells and chemosensitization of an acquired-resistant mesenchymal phenotype. Photochem Photobiol. 2024 Jun 23. [CrossRef]
- Rogers, GS. Continuous low-irradiance photodynamic therapy: a new therapeutic paradigm. J Natl Compr Canc Netw. 2012;10 Suppl 2:S14-7. [CrossRef]
- Nairuz T, Sangwoo-Cho, Lee JH. Photobiomodulation Therapy on Brain: Pioneering an Innovative Approach to Revolutionize Cognitive Dynamics. Cells. 2024;13(11):966. [CrossRef]
- Johnson PK, Fino PC, Wilde EA, Hovenden ES, Russell HA, Velez C, Pelo R, Morris AJ, Kreter N, Read EN, Keleher F, Esopenko C, Lindsey HM, Newsome MR, Thayn D, McCabe C, Mullen CM, Davidson LE, Liebel SW, Carr L, Tate DF. The Effect of Intranasal Plus Transcranial Photobiomodulation on Neuromuscular Control in Individuals with Repetitive Head Acceleration Events. Photobiomodul Photomed Laser Surg. 2024;42(6):404-413. [CrossRef]
- Chan ST, Mercaldo N, Figueiro Longo MG, Welt J, Avesta A, Lee J, Lev MH, Ratai EM, Wenke MR, Parry BA, Drake L, Anderson RR, Rauch T, Diaz-Arrastia R, Kwong KK, Hamblin M, Vakoc BJ, Gupta R. Effects of Low-Level Light Therapy on Resting-State Connectivity Following Moderate Traumatic Brain Injury: Secondary Analyses of a Double-blinded Placebo-controlled Study. Radiology. 2024;311(2):e230999. [CrossRef]
- Shahdadian S, Wang X, Liu H. Directed physiological networks in the human prefrontal cortex at rest and post transcranial photobiomodulation. Sci Rep. 2024;14(1):10242. [CrossRef]
- Kim JH, Son HS, Yu DA, Choe YB, Lee YW. Assessment of Effects of Low-Level Light Therapy on Scalp Condition and Hair Growth. Indian J Dermatol. 2023;68(4):487. [CrossRef]
- Estrada-Rojas K, Cedeño Ortiz NP. Increased Improvement in Speech-Language Skills After Transcranial Photobiomodulation Plus Speech-Language Therapy, Compared to Speech-Language Therapy Alone: Case Report with Aphasia. Photobiomodul Photomed Laser Surg. 2023;41(5):234-240. [CrossRef]
- Frost GA, Halliday GM, Damian DL. Photodynamic therapy-induced immunosuppression in humans is prevented by reducing the rate of light delivery. J Invest Dermatol. 2011;131(4):962-8. [CrossRef]
| O2 | ground state triplet oxygen molecule, 3O2 stable, atmospheric oxygen |
| 3O2 | another designation of O2, the common form of atmospheric oxygen. |
| •OH | hydroxyl radical, a neutral, highly reactive ROS. |
| 1O2 | singlet oxygen molecule, half-life of microseconds, an ROS. |
| O2 •− | superoxide anion radical, an oxygen molecule with one unpaired outer shell electron, negative charge −1, an ROS. |
| O3 | ozone, trioxygen molecule, an ROS. |
| H2O2 | hydrogen peroxide, a non radical, non ionic, ROS. |
| term | definition | units |
| fluence | the energy delivered to a given area | J / cm2 |
| flux | the momentary power received by a given area | W / cm2 |
| irradiance | the momentary power received by a given area | W / cm2 |
| light dose | J/cm2 delivered over a specified time interval | J / cm2 |
| GB tissue depth |
external flux required to achieve 17 µW / cm2 |
|---|---|
| at 3 cm ➞ | 1.4 mW / cm2 |
| at 5 cm ➞ | 3.3 mW / cm2 |
| at 7 cm ➞ | 7.5 mW / cm2 |
| at 9 cm ➞ | 17 mW / cm2 |
| drug | general medicine use | use in LIT-PDT |
|---|---|---|
| ciprofloxacin | antibiotic | increased protoporphyrin |
| deferiprone | iron chelation | iron chelation |
| telmisartan | hypertension | ABCG2 inhibition |
| ziprasidone | psychosis | D2 blocking |
| time | 5-ALA dose | illumination |
|---|---|---|
| - 36 hr | 1st 20 mg/kg p.o. | off |
| -33 hr | none | on |
| -28 hr | 2nd 20 mg/kg p.o | on |
| -9 hr | 3rd 20 mg/kg p.o. | on |
| -1 hr | none | off |
| 0 hr - surgery | none | off |
| + 4 hr, H&E, etc | none | off |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





