1. Introduction
The sugar agroindustry has historically been one of the most significant economic activities in the Northwestern region of Argentina. In recent years, sugarcane production in this country has steadily increased; while the 2015 harvest totaled 17.7 million tons, by 2021 this figure rose to nearly 23.5 million tons. All this production is concentrated in the Northwestern region, with 11.80% corresponding to the Province of Salta [
1]. The San Martín del Tabacal Sugar Mill, located in the north of the province, is the most important, accounting for 80% of the provincial production [
2].
Among the by-products of sugar production, bioethanol production from molasses fermentation stands out [
3]. In addition to bioethanol, this process generates a very dark liquid residue called "vinasse", produced in a ratio of 1:12-15 bioethanol:vinasse [
4,
5,
6]. The properties of vinasse can vary, primarily depending on the raw material used, the distillation process, and the treatment carried out to separate the alcohol from the already fermented substrate [
7,
8]. However, in general, this effluent is characterized by being a dark brown liquid with a honey-like smell and malt-like taste [
9]. It is acidic (pH≈4) and has a high content of suspended solids and dissolved salts (including ions such as NH
4+, NO
3-, NO
-, PO
43-, SO
42-, K
+, Ca
2+, Mg
2+) that are reflected in high electrical conductivity and ash percentage values [
10]. Additionally, it contains a very high organic load, making it hundreds of times more polluting than domestic wastewater [
11,
12,
13,
14]. Vinasse chemical composition is remarkably diverse, ranging from relatively simple, low molecular weight compounds like glucose, fructose, and sucrose to a series of complex organic molecules such as melanoidins, humic acids, lignins, metal sulfides, and phenolic compounds like flavonoids, which are responsible for its dark color and recalcitrant nature. Various heavy metals such as cadmium, manganese, iron, zinc, nickel, and lead have also been detected [
15]. The dark pigmentation of vinasse is due to several factors. Firstly, the presence of phenolic components such as tannic and humic acids, which often act as toxic substances. Secondly, products generated during the thermal decomposition of sucrose and reducing sugars like glucose or fructose, and most notably, the presence of melanoidins, high molecular weight compounds that are difficult to degrade, resulting from the Maillard reaction [
6,
16,
17,
18].
Of all the waste generated by alcohol production industries, distillery vinasse stands out as the most harmful to the natural environment. It is produced in large quantities and has high pollution potential, not only due to its high organic load but also because of its dark color [
19]. Contamination of water bodies with vinasse leads to decreased light penetration, thus reducing photosynthetic activity and dissolved oxygen. Additionally, the high nutrient load causes eutrophication of the system, contributing to the increase of insect populations and disease vectors [
12,
20]. Furthermore, the phenolic compounds and melanoidins in vinasse have antioxidant properties that can reduce or inhibit microbial activity, affecting natural biogeochemical cycles such as carbon and nitrogen cycles, which are essential for ecosystems [
21].
Various strategies have been suggested to address the treatment and management of vinasse. These include incineration, anaerobic and aerobic digestion using yeasts, and disposal in evaporation ponds. Among its most notable uses are composting and direct application to agricultural fields as fertilizer [
22]. The fertigation method and the use of evaporation ponds are the most common practices in Northwestern of Argentina. Particularly at the San Martín del Tabacal Sugar Mill, the area of these vinasse evaporation ponds covers about 106 hectares. However, in recent decades, the use of white rot fungi from genera such as Phanerochaete, Trametes, Pleurotus, Pycnoporus, and Schizophyllum has been proposed as an attractive alternative for treating this waste. The growing acceptance of these organisms for the degradation of xenobiotic and recalcitrant compounds is due to the presence of a non-specific extracellular enzymatic battery capable of breaking down many bonds [
23]. Among the exoenzymes that are part of the ligninolytic multi-enzyme complex, we can mention peroxidase systems, such as lignin peroxidases and manganese peroxidases, as well as laccase, cellulase, and hemicellulose systems. Those enzymes work synergistically in breaking down a wide variety of macromolecules [
24]. Several of these projects not only aimed to reduce the contaminant load of the effluent but also sought alternatives for enzyme extraction or fungal biomass production, with the advantage of being edible, as in the case of
Pleurotus sp. [
25,
26]. Other research focused on developing fungi with favorable characteristics in the medical field and obtaining natural pigments, as in the case of
Pycnoporus sanguineus [
27,
28].
In this study, we evaluated the biomass production and bioremediation capacity of a local strain of Pycnoporus sp. on vinasse produced in the region.
2. Materials and Methods
The fruiting bodies of the fungi were collected from decaying trunks and branches located in a natural area in Vaqueros, La Caldera Department, Salta, within the Yungas province or the Tucumano-Oranense jungle 24°40'55.846"S 65°24'52.145"W). These samples were then transported to the laboratory for morphological description and strain isolation.
Sugarcane vinasse was obtained from the Sugar Mill San Martín de El Tabacal, located in the Orán Department, Salta. At the start of the bioassay, the effluent was characterized by measuring pH, electrical conductivity, true color, Chemical Oxygen Demand (COD), inorganic forms of nitrogen, soluble reactive phosphorus, and sulfates, according to APHA techniques [
29].
A laboratory-scale bioassay was conducted, testing three dilutions of vinasse from El Tabacal with distilled water (V5%, V10%, and V25%). Each treatment had 4 replicates, using 250 mL bioreactors containing 100 mL of the respective medium, inoculated with a previously isolated strain of the fungus Pycnoporus sp. Additionally, control for each treatment were established using the same dilutions (C5%, C10%, and C25%), but without the fungus inoculation. These experimental units were randomly placed in an incubator at a temperature of 27 ± 1°C. After 60 days of incubation, biomass production was assessed by calculating its dry weight. The efficiency of Pycnoporus sp. as a bioremediation agent was also evaluated by measuring the removal of COD and True Color (TC).
To compare differences between treatments, ANOVA was performed previous assuring the data met the assumptions of normality and homoscedasticity. When normality was not met, non-parametric Kruskal-Wallis test was used. Contrasts between groups were calculated using Tukey test. Additionally, linear regressions were conducted with fungal biomass production and NH4+ consumption as dependent variables, and vinasse concentration (5%, 10%, and 25%) as the regressor variable using Infostat v. 2018.
3. Results
3.1. Characterization of the Effluent
The effluent was characterized by its dark brown color, high electrical conductivity values, acidic pH, and high levels of organic load and inorganic nutrients (
Table 1).
3.2. Description of the Fungal Strain
The identification and characterization of the strain were conducted through the study of morphological characteristics; based on Robledo et al. (2003) [
29] it is reported as
Pycnoporus aff.
sanguineus.
The sexual fruiting bodies, with a hard to leathery texture, exhibited a fan-shaped (flabelliform) appearance, an intense orange coloration, and were strongly attached to the substrate at the base. Additionally, concentric rings were observed on the upper surface and tiny pores on the lower surface, numbering 5 to 6 per mm (
Figure 1).
3.3. Fungal Biomass Production
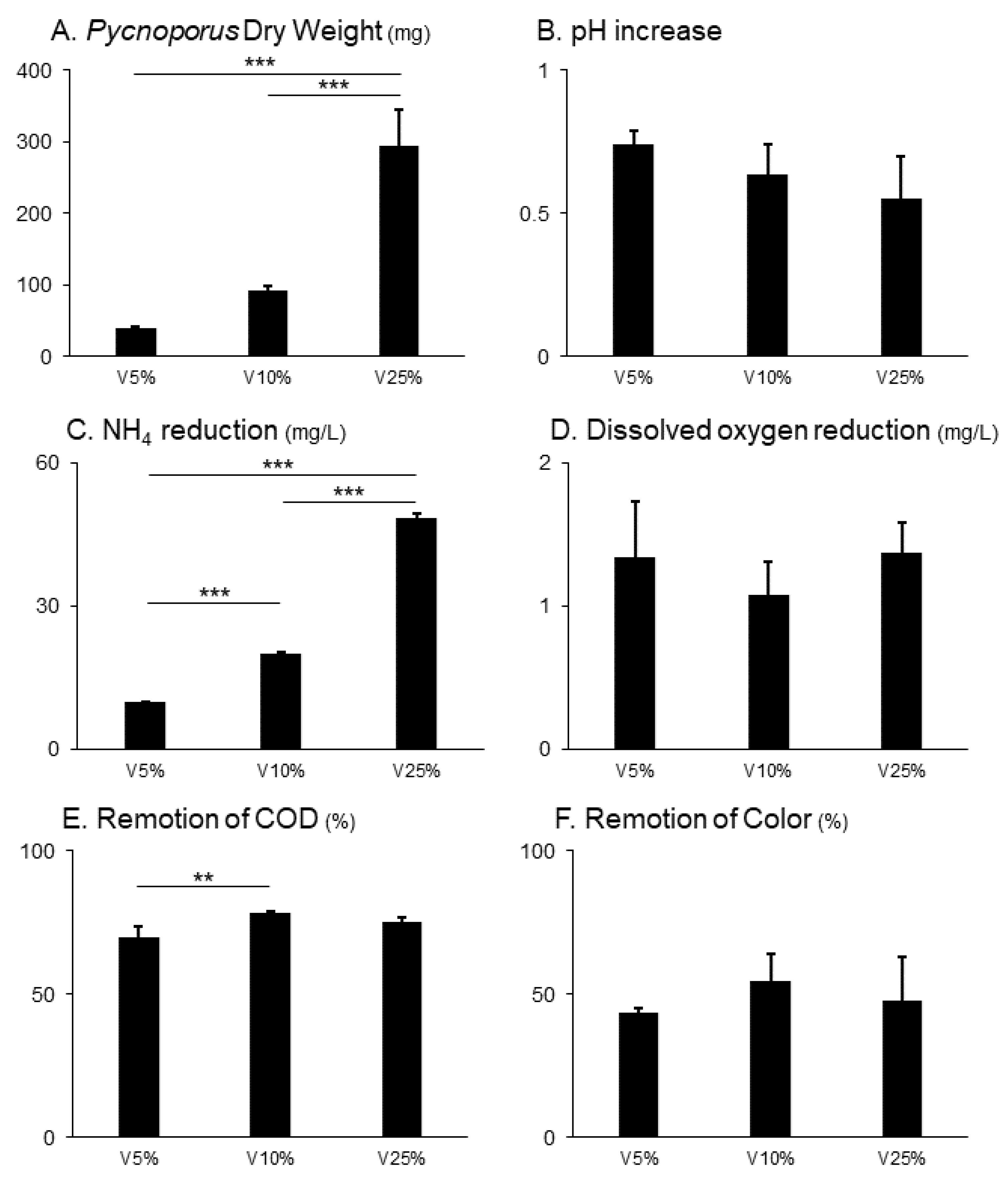
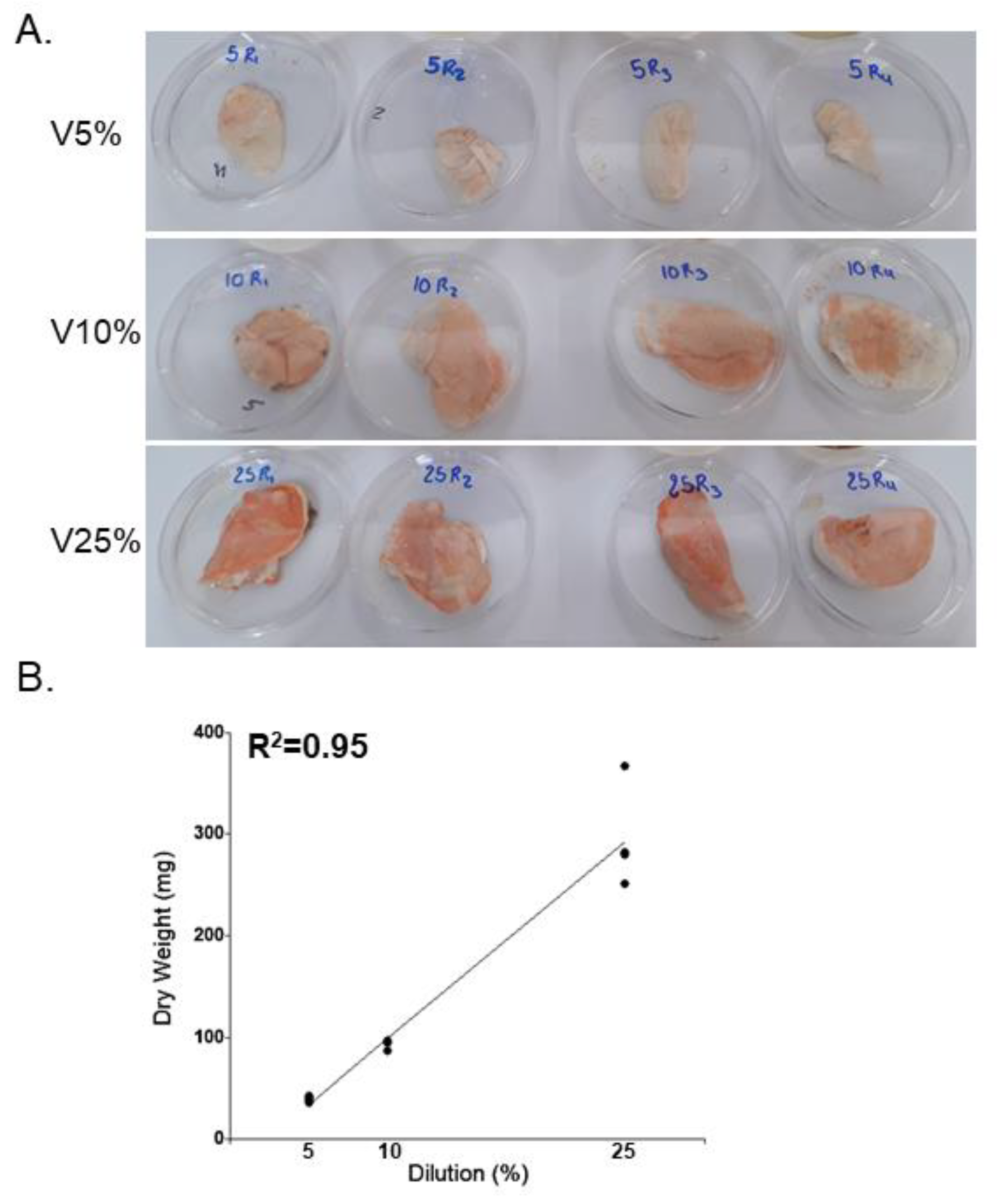
Regarding the DW of the fungus in the experimental units after 60 days of incubation, values ranged from 35.7 to 366.3 mg. Statistically significant differences were found between each of the treatments (p<0.001): V5% = 38.93 ± 2.8 mg; V10% = 92.68 ± 4.79 mg; V25% = 249.48 ± 49.93 mg, with the highest fungal development observed in the V25% treatment (
Figure 2 A). These results were reflected in the linear regression model, which was highly significant between vinasse concentration and biomass production of the native strain of
Pycnoporus aff.
sanguineus (R
2 = 0.95; p<0.0001) (
Figure 3).
3.4. Efficiency of the Vinasse Remediation
In relation to the ammonium reduction the results ranged between 10 and 49 mg/L, and statistically significant differences were observed between each of the treatments, with average values of V5% = 10.05 ± 0.06 mg/L; V10% = 20.13 ± 0.38 mg/L; and V25% = 48.38 ± 0.95 mg/L (
Figure 2 C). The linear regression model significantly fit the relationship between NH
4+ reduction and the different vinasse concentrations (R
2 = 1; p<0.0001).
Regarding the removal of organic matter, measured through COD, the results varied between 65.25% and 78.79%. The treatments carried out at higher vinasse concentrations (V10% and V25%) proved to be the most efficient, with averages of 78.53% ± 0.38 and 75.13% ± 1.78 respectively, and both treatments were not statistically different from each other (
Figure 2 E). Meanwhile, the treatment using the most diluted vinasse (V5%) showed a significantly lower average COD removal efficiency (69.95% ± 3.84). pH increase, dissolved oxygen reduction and remotion of the color were similar across all treatments (
Figure 2 B, D and F).
4. Discussion
The study examined the vinasse from El Tabacal sugar mill, finding its dark brown color, acidic pH, and high values of electrical conductivity, organic load, and inorganic nutrients like those reported in numerous studies. The vinasse exhibited a pH of 4.18, matching reported values and attributed to organic acids produced during fermentation. The electrical conductivity was 9,242 μS/cm, lower than those in other regional studies. The initial COD was 45,320 mg O2/L, comparable to other findings. Nitrate and phosphate levels aligned with previous research, while sulfate levels were lower. The vinasse's nutrient content enhanced fungal biomass production, suggesting potential for bioremediation. The study supports the use of white-rot fungi in bioremediation of sugarcane waste.
The characteristic dark brown color, acidic pH, and high values of electrical conductivity, organic load, and inorganic nutrients present in the vinasse from El Tabacal sugar mill were like those reported for vinasse in numerous studies [
4,
31,
32]. The pH of the sample, 4.18, matched values reported previously since vinasses derived from the fermentation of molasses generally have pH values ranging between 4.2 and 5 [
33]. This is related to the presence of organic acids generated during the fermentation process by yeasts and occasionally by bacteria [
13]. The dissolved salt load, expressed in values of electrical conductivity (9,242 μS/cm), was lower than those obtained in studies conducted on vinasse from other countries in the region such as Mexico: 19,500 μS/cm, Colombia: 17,000 μS/cm [
34]. Regarding COD, the initial value recorded in the vinasse prior to biotreatment was 45,320 mg O
2/L, comparable to those obtained for vinasses by other researchers [
12]. Finally, in terms of nutrient content, we determined that nitrate and phosphate values were like those reported by Gil Rolón (2018) [
35], who also worked with vinasse from the El Tabacal sugar mill, while the sulfate levels obtained in the measurements (700 mg/L) were lower than those reported in the same work (4000 mg/L).
The increase in fungal biomass production observed in this work, as the concentration of vinasse increases, could be explained by the increase in the concentration of nutrients and carbonaceous materials in the medium, resulting in a more enriched environment that stimulates fungal growth. Similar results were reported in works with
Pycnoporus sp. [
36] and
Pleurotus sp., where 25% vinasse showed a stimulating effect on strain growth [
37]. The same was found for vinasse treated by white-rot fungi of other species such as
Phanerochaete chrysosporium,
Ganoderma sp., and
Trichoderma reesei [
38].
On the other hand, both the increase in ammonium (NH
4+) consumption and the increase in pH, as vinasse concentration increases, can be explained by the occurrence of biodegradation processes of organic matter, because of the work of bond-breaking, performed by the fungal exoenzymatic battery [
15]. As complex structures are transformed into simpler molecules through mineralization processes and more specifically in the case of NH
4+, by ammonification processes, the release of ammonium molecules into the medium is triggered, generating an increase in the pH of the effluent. However, simultaneously, the fungus, when feeding, uses this nitrogen from the medium in the manufacture of proteins and other important molecules for its biomass growth. This is consistent with the results obtained in this work, where a higher ammonium consumption is reflected in the V25% treatment, where fungal biomass production was the highest.
The similarity in the difficult biodegradability of substrates such as celluloses, hemicelluloses, lignin, and compounds such as melanoidins and humic acids enables the use of white-rot fungi in bioremediation processes. Numerous studies demonstrate the participation of lignocellulolytic enzymatic systems in the decolorization process of vinasses from alcohol distilleries. Even though no significant differences were found in the percentages of color removal between the different treatments after 60 days of incubation in the present study, we can mention that the average removal values were like those reported by Ahmed (2016) [
36], who working with 10% diluted vinasse and the same fungus species,
Pycnoporus sp., achieved color removals of 51% after 12 days of treatment. These decolorization efficiencies were also like those achieved by other fungi, such as
Ganoderma sp. (65%),
Aspergillus niger (63%), and
Trichoderma reesei (55%). While for other species like
Phanerochaete sp. and
Pleurotus shimeji, higher values (85%) have been reported [
14].
Some of the compounds responsible for the color in vinasses also contribute to the high COD values as they are susceptible to oxidation for their breakdown into smaller molecules. In our study, we observed that treatments with higher vinasse concentrations were the most effective, with average removals exceeding 70%, with the V10% treatment showing the highest percentage (78.53%). These values were somewhat higher than those reported by Ahmed (2016) [
36], who reported removals of 67.83% using 25% diluted vinasse and the fungus
Pycnoporus sp. In other studies, researchers found similar values to ours regarding the reduction of COD in sugarcane vinasse treated by the fungus
Pleurotus sp. [
14,
38]. Gil Rolón (2018) [
35] working with vinasse of the same origin as ours and a strain of the fungus
Pleurotus ostreatus, reported average removals of 76.35 and 70.96%, in vinasse dilutions of 10% and 25%, respectively. Comparatively, our native strain of
Pycnoporus aff.
sanguineus demonstrated greater efficiency in COD removal (78.53%).
Finally,
Pycnoporus is a representative genus of saprophytic homobasidiomycetes that have lignocellulytic potential [
39]. The red or orange pigments characteristic of the fruiting bodies (basidiocarps) of this fungus are compounds derived from cinnabarin [
40]. which also have antibiotic, antiviral, and antitumor activities [
41,
42]. Thus, bioremediation with
Pycnoporus sp. could be very interesting in relation of its derivates.
5. Conclusions
The need to implement clean and environmentally friendly technologies for the treatment of agro-industrial effluents highlights our work as a significant contribution to the study of bioremediation processes for these wastes. The results obtained show the potential of the native strain isolated from Pycnoporus aff. sanguineus to be used in vinasse bioremediation processes, which proved to be an excellent substrate for fungal biomass development and a promising percentage of color and COD removal were achieved.
Author Contributions
Conceptualization, C.F., V.L.L. and L.M.; methodology, C.F., C.N.B., and L.M.; formal analysis, C.F., V.L.L. and L.M.; resources, L.M.; writing—original draft preparation, C.F., V.L.L., C.N.B., A.F., M. C., and L.M.; visualization, C.F., and V.L.L; supervision, L.M..; funding acquisition, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CIUNSa Project name: “Utilización de cepas autóctonas de microorganismos (hongos y microalgas) en el tratamiento de desechos agroindustriales de producción regional” by L.M.
Acknowledgments
We thanks to the Universidad Nacional de Salta for its continuous support on our research.
Conflicts of Interest
The authors declare no conflicts of interest
References
- Estadísticas del Centro Azucarero Argentino (2015 a 2021). https://centroazucarero.com.ar/produccion-de-azucar-2020-2029 (assessed on January 2024).
- Informe productivo provincial de Salta 2021. Secretaría de Política Económica. Subsecretaría de Programación Regional y Sectorial. https://www.argentina.gob.ar/sites/default/files/informe_productivo_salta_oct2021.pdf (assessed on January 2024).
- Nishihara Hun, A.L., Mele, F.D., Pérez, G.A. 2015. Environmental profile of the sugar industry in the province of Tucumán obtained from the Life Cycle Analysis. Industrial Crop Science and Technology Journal, pp. 64-77, Year 5, No. 7.
- Alzate, C.E.A. 2015. Physicochemical characterization of a vinasse resulting from the production of alcohol in a liquor industry, from the use of sugarcane. Ingenierías USBMed, 6(2), 36-41.
- Rulli, M.M., Villegas, L.B., & Colin, V.L. 2020. Treatment of sugarcane vinasse using an autochthonous fungus from the northwest of Argentina and its potential application in fertigation practices. Journal of Environmental Chemical Engineering, 8(5), 104371.
- Ospina León, L.J., Manotas-Duque, D., & Ramírez-Malule, H. 2023. Challenges and opportunities of the sugar cane vinasse. A bibliometric analysis. Ingeniería y Competitividad, 25(1).
- Tapie Canacuan, W. 2016. Evaluación in vitro del tratamiento de la vinaza de caña de azúcar con Pleurotus ostreatus en producción animal. MSc. Thesis, Facultad de Ciencias Agropecuarias, Universidad Nacional de Colombia, Colombia.
- Carbajo, M.S., Ojeda Fermoselle, A.C., Meneguzzi, N., Canteros, B.I., & Rodríguez, G. 2018. Behavior of fungi in media with sugarcane vinasse. II Simposio de Residuos Agropecuarios y Agroindustriales del NOA y Cuyo (p. 47).
- Bermúdez Savon, R.C., Hoyos-Hernández, J.A., & Rodríguez-Pérez, S. 2000. Evaluation of the decrease of the pollutant load of distillery vinasse by anaerobic treatment. International Journal of Environmental Pollution, 16(3), 103-107.
- Ortegón, G.P., Arboleda, F.M., Candela, L., Tamoh, K., & Valdes-Abellan, J. 2016. Vinasse application to sugar cane fields. Effect on the unsaturated zone and groundwater at Valle del Cauca (Colombia). Science of the Total Environment, 539, 410-419.
- Hoarau, J., Caro, Y., Grondin, I., & Petit, T. 2018. Sugarcane vinasse processing: Toward a status shift from waste to valuable resource. A review. Journal of water process engineering, 24, 11-25.
- Zúñiga Cerón, V., & Gandini Ayerbe, M.A. 2013. Environmental characterization of stillage from sugar cane waste from the production of ethanol. Dyna, 80(177), 124-131.
- Ahmed, O., Sulieman, A.M.E., & Elhardallou, S.B. 2013. Physicochemical, chemical and microbiological characteristics of vinasse, A by-product from ethanol industry. American Journal of Biochemistry, 3(3), 80-83.
- Tapie, W.A., Garcia, D.P., & Guerrero, H.S. 2016. Biodegradation of sugarcane vinasses by the white-rot fungi Pleurotus ostreatus in a packed bed reactor. Tropical and Subtropical Agroecosystems, 19(2), 145-150.
- Santal, A.R., & Singh, N. 2013. Biodegradation of melanoidin from distillery effluent: role of microbes and their potential enzymes. Biodegradation of Hazardous and Special Products, 5, 71-100.
- Comparato, C.N., de Araujo, M.N., Sakamoto, I.K., Fuess, L., Damianovic, M.H.R.Z., & da Silva, A.J. 2024. Melanoidin Content Determines the Primary Pathways in Glucose Dark Fermentation: A Preliminary Assessment of Kinetic and Microbial Aspects. Fermentation, 10(6), 272.
- Aguiar, M.M., Wadt, L.C., Vilar, D.S., Hernandez-Macedo, M.L., Kumar, V., Monteiro, R.T. & Ferreira, L. 2023. Vinasse bio-valorization for enhancement of Pleurotus biomass productivity: chemical characterization and carbohydrate analysis. Biomass Conversion and Biorefinery, 13(11), 10031-10040.
- Santal, A.R., & Singh, N. 2013. Biodegradation of melanoidin from distillery effluent: role of microbes and their potential enzymes. Biodegradation of Hazardous and Special Products, 5, 71-100.
- Ferreira, L.F., Aguiar, M., Pompeu, G., Messias, T.G., & Monteiro, R.R. 2010. Selection of vinasse degrading microorganisms. World Journal of Microbiology and Biotechnology, 26, 1613-1621.
- Hoaraua, J., Caro, Y., Grondin, I., & Petit, T. 2018. Sugarcane vinasse processing: Toward a status shift from waste to valuable resource. A review. Journal of water process engineering, 24, 11-25.
- De Chaves, M.G., Silva, G.G.Z., Rossetto, R., Edwards, R.A., Tsai, S.M., & Navarrete, A.A. 2019. Acidobacteria subgroups and their metabolic potential for carbon degradation in sugarcane soil amended with vinasse and nitrogen fertilizers. Frontiers in microbiology, 10, 1680.
- Carpanez, T.G., Moreira, V.R., Assis, I.R., & Amaral, M.C.S. 2022. Sugarcane vinasse as organo-mineral fertilizers feedstock: Opportunities and environmental risks. Science of The Total Environment, 832, 154998.
- Hammel, K.E. 1992. Oxidation of aromatic pollutants by lignin-degrading fungi and their extracellular peroxidases. Metal ions in biological systems, 28, 41-60.
- Montoya, S., Sánchez, O.J., & Levin, L. 2014. Evaluation of endoglucanase, exoglucanase, laccase, and lignin peroxidase activities on ten white-rot fungi. Biotechnology in the Agricultural and Agroindustrial Sector, 12(2), 115-124.
- Velázquez-Cedeño, M.A., Mata, G., & Savoie, J.M. 2002. Waste-reducing cultivation of Pleurotus ostreatus and Pleurotus pulmonarius on coffee pulp: changes in the production of some lignocellulolytic enzymes. World Journal of Microbiology and Biotechnology, 18, 201-207.
- Aguiar, M.M., Wadt, L.C., Vilar, D.S., Hernandez-Macedo, M.L., Kumar, V., Monteiro, R.T. & Ferreira, L. 2023. Vinasse bio-valorization for enhancement of Pleurotus biomass productivity: chemical characterization and carbohydrate analysis. Biomass Conversion and Biorefinery, 13(11), 10031-10040.
- Pineda Insuasti, J.A., Gómez-Andrade, W.E., Duarte-Trujillo, A.S., Soto-Arroyave, C.P., Pineda-Soto, C.A., Fierro-Ramos, F.J., Mora-Muñoz, E.S., & Álvarez-Ramos, S.E. 2017. Production of Pycnoporus spp. and their secondary metabolites: A review. ICIDCA Journal on Sugarcane Derivatives, 51(2), 60-69.
- Rivas Rosero, C.A. 2018. Comparative study of the action of Pycnoporus sanguineus on three lignocellulosic residues for the production of fungal biomass in solid-state fermentation. SATHIRI, (7), 207–215.
- APHA. 2005. Standard Methods for the Examination of Water and Wastewater, 21 ed.
- Robledo, G.L. , Urcelay, C., Rajchenberg, M., & Domínguez, L. 2003. Polypores (Aphyllophorales, Basidiomycota) parasitic and saprophytic on Alnus acuminata in northwestern Argentina. X Bol. Soc. Argent. Bot. 38 (3-4): 207 - 224.
- Torres Gaviria, L.F., Ocampo Vélez, J.C., & Socarrás Cárdenas, A. 2018. Reduction of potassium levels in distillery vinasse using ion exchange resins. Journal of Agricultural and Environmental Research, 10(1), 107–118.
- Caballero, R.E., Jiménez, V., Miranda, M., Rovira, D., González, P., & de Pérez, J.R.C. 2021. Optimization of conditions for the production of laccase by Trametes villosa (Sw.) Kreisel and its application in the biotreatment of sugar cane vinasse.
- Salomon, K.R. & Lora, E.E.S. 2009. Estimate of the electric energy generating potential for different sources of biogas in Brazil. Biomass and Bioenergy, 33(9), pp.1101–1107.
- García, A., & Rojas, C. 2006. Potential uses of vinasse in agriculture according to its mode of action on soils. Tecnicaña, 10(17), 3-13.
- Gil Rolón, M. 2018. Biodepuración de vinazas de caña de azúcar por microorganismos. Profesional Thesis Eng. Natural Resources and Environment, Facultad de Ciencias Naturales. Universidad Nacional de Salta, Argentina.
- Ahmed, P.M. 2016. Biorremediación de vinazas de destilerías de alcohol por microorganismos autóctonos aislados de ambientes contaminados. PhD. Thesis in Biology. Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT), Instituto de Tecnología Agroindustrial del Noroeste Argentino (ITANOA), CONICET, Argentina.
- Rodríguez, S., Fernández, M., Bermúdez, R. C., & Morris, H. 2003. Treatment of colored industrial effluents with Pleurotus spp. Revista Iberoamericana de Micología, 20(4), 164-168.
- Ferreira, L.F., Aguiar, M., Pompeu, G., Messias, T.G., & Monteiro, R.R. 2010. Selection of vinasse degrading microorganisms. World Journal of Microbiology and Biotechnology, 26, 1613-1621.
- Alexopoulos, C.J., Mims, C.W., & Blackwell, M. 1996. Phylum Basidiomycota order Aphyllophorales, polypores, Chantharelles, tooth fungi, coral fungi and corticioids. In: Harris, D. (ed). Introductory Mycology 4th Ed. New York, U. S. A. Wiley and Sons Inc. pp: 563-597.
- Eggert, C., Temp, U. & Eriksson, K.E.L. 1996. The lignolytic system of the white-rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl. Environ. Microbiol. 62: 1151-1158.
- Lomascolo, A., Uzan-Boukhris, E., Herpoël-Gimbert, I., Sigoillot, J.C., & Lesage-Meessen, L. 2011. Peculiarities of Pycnoporus species for applications in biotechnology. Applied microbiology and biotechnology, 92, 1129-1149.
- Pięt, M., Zając, A., Paduch, R., Jaszek, M., Frant, M., Stefaniuk, D., ... & Grzywnowicz, K. 2021. Chemopreventive activity of bioactive fungal fractions isolated from milk-supplemented cultures of Cerrena unicolor and Pycnoporus sanguineus on colon cancer cells. 3 Biotech, 11, 1-13.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).