1. Introduction
Breast cancer is a rising health problem worldwide; this neoplasia originates in the mammary gland and then develops metastasis. Breast cancer can be classified upon the over-expression of three receptors: The estrogen receptor alpha (ER-α), the progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2). The luminal A subtype is characterized by the over-expression of ER-α responding to 17β-estradiol (E2), promoting proliferation; it presents a high incidence worldwide and has reduced aggressiveness due to its epithelial phenotype [
1,
2]. On the contrary, the triple-negative subtype lacks of the three receptors over-expression and has a lower incidence than the luminal type; however, it presents a higher aggressiveness due to its mesenchymal phenotype [
3]. The characteristics of the luminal subtype have allowed the development of therapies against specific targets such as ER-α [
7]; however, the acquisition of resistance to drugs such as tamoxifen (TMX, which binds to ER-a) [
4,
5] and the development of metastatic properties such as migration and invasion have been reported [
6].
The tumor microenvironment plays a crucial role in cancer development because it modifies the properties of cancer cells; this is integrated by immune and connective cells that secrete cytokines, such as IL-6. This cytokine induces the EMT, favoring metastasis development in luminal breast cancer cells [
7,
8]. Also, IL-6 has been related to TMX resistance through a decrease in ER-α levels [
9,
10]; however, the cancer cells continue expressing genes under E2 regulation [
11], suggesting the participation of another receptor for this hormone. The GPR30/GPER is an alternative E2 receptor that can regulate the expression of ER-α target genes [
12]. This receptor is expressed in luminal and triple-negative breast cancer cells [
13] and has been related to TMX resistance [
14] and cancer progression [
15,
16]. The GPR30 and IL-6 have been involved in metastasis, but an interplay between them has not been reported.
The metastasis is the main difficulty of the present therapy against cancer because the dispersion and heterogeneity of affected cells complicate their elimination [
17]. Despite the successful treatment for luminal cancer, about 40% of the patients develop resistance to the drugs, and cancer becomes more aggressive [
18]; for this reason, it is important to understand the mechanisms through which the luminal breast cancer cells develop metastatic proprieties to find potential targets and strategies to control and eradicate this pathology. This work aims to determine the GPR30 role in the metastatic properties induced by IL-6 in luminal breast cancer cells.
2. Results
2.1. GPR30 Regulates the Proliferative Effects of E2 and IL-6 in Breast Cancer Cells
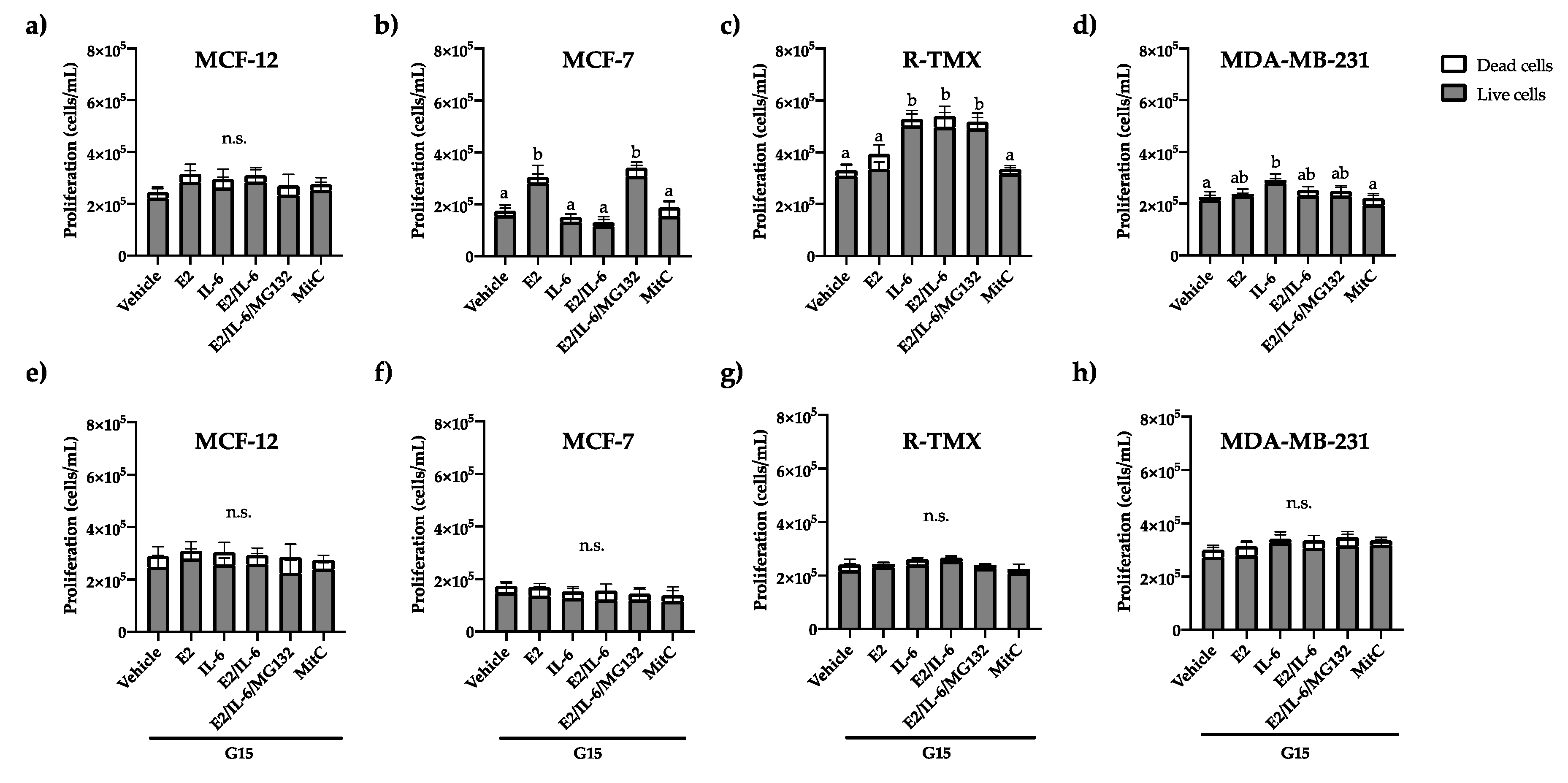
One of the initial hallmarks of cancer is sustained proliferation, which is responsible for the neoplasia generation [
19]. Based on this, we evaluate the effect of E2 and IL-6 on breast cancer cell proliferation by trypan blue exclusion assay. The proliferation of MCF-12 (cells from healthy mammary glands that do not overexpress receptors such as ER-α) was not affected by E2 nor IL-6 (
Figure 1a). Also, MCF-7 cells stimulated their proliferation in the presence of E2, which was inhibited by IL-6; however, this effect was reverted by the proteasomal inhibitor MG132 (
Figure 1b), indicating that IL-6 regulates proteasomally the ER-α function. Besides, the proliferation of MCF-7 tamoxifen-resistant (R-TMX) was induced only by IL-6 and was independent of E2 (
Figure 1c). As expected, the proliferation of triple-negative breast cancer cells MDA-MB-231 was not affected by E2, but IL-6 stimulated it (
Figure 1d). All the proliferative effects were inhibited by the GPR30 antagonist G15 (
Figure 1e-h) or PTX (
Figure S1), suggesting the GPR30 participation in the proliferation stimulated by E2 and IL-6 in breast cancer cells.
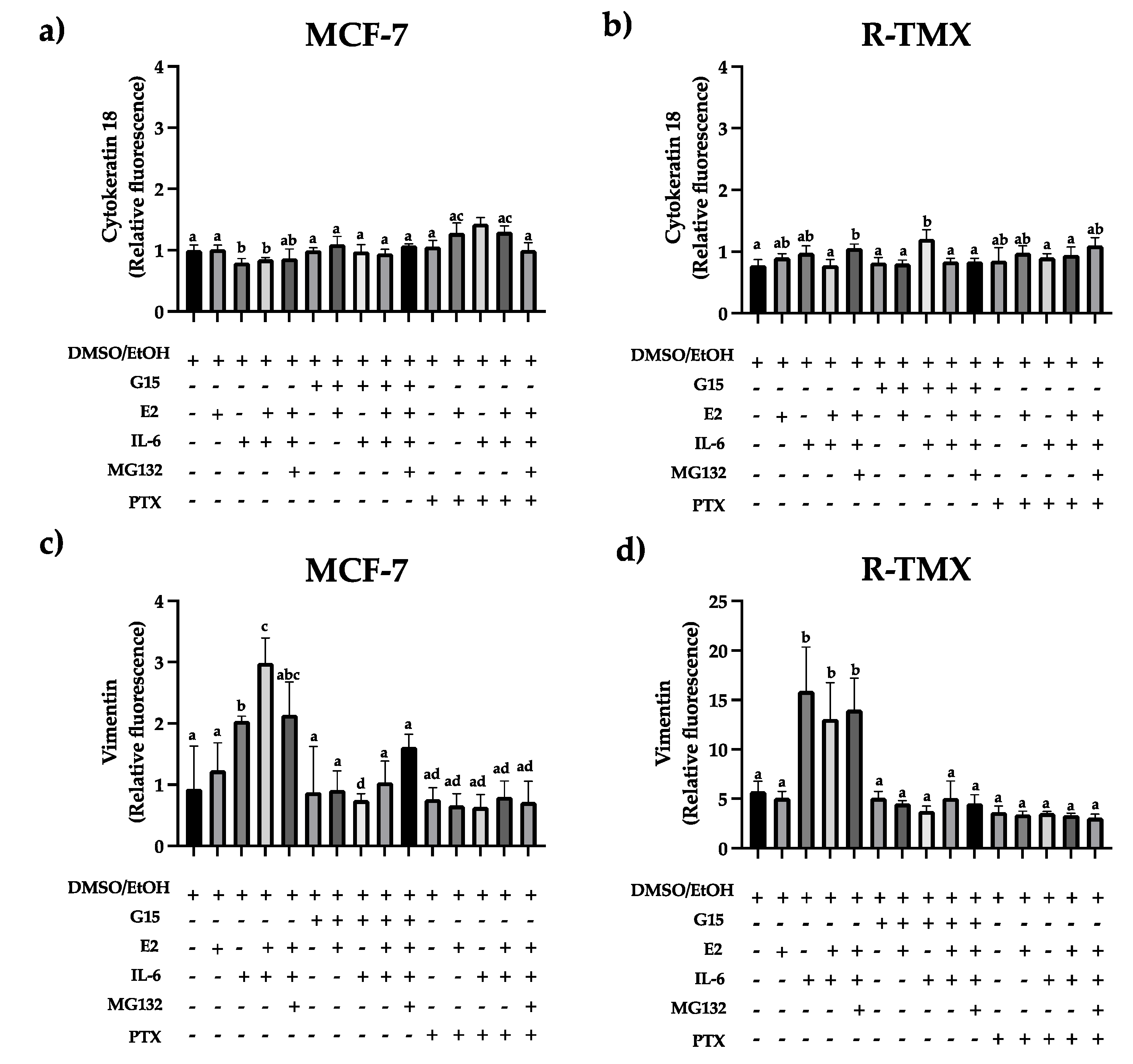
2.2. IL-6 Promotes the Expression of Vimentin through GPR30
Tumor formation is insufficient to classify neoplasia as cancer; metastasis development is compulsory [
1]. The EMT implies the loss of cell junctions, such as desmosomes, by decreasing adherent protein and increasing motility characteristics [
20]. Cytokeratin 18 is an intermediate filament expressed in epithelial carcinoma and is diminished in basal subtypes, contrary to vimentin, which is implicated in the migration mechanism [
21]. We evaluate the cytokeratin 18 and vimentin expression in MCF-7 and R-TMX cells. Results showed a slight decrease in cytokeratin 18 levels in MCF-7 cells stimulated with IL-6, which was reverted when cells were treated with the GPR30 antagonist G15 or PTX (
Figure 2a). An opposite behavior was observed with vimentin (
Figure 2c), suggesting that IL-6 promotes EMT protein expression through GPR30. Also, vimentin levels were strongly increased in R-TMX cells by IL-6 ( ~15 fold), which was reverted by G15 or PTX (
Figure 2d). Despite this mesenchymal behavior, cytokeratin 18 levels were similar in both cellular types (
Figure 2b).
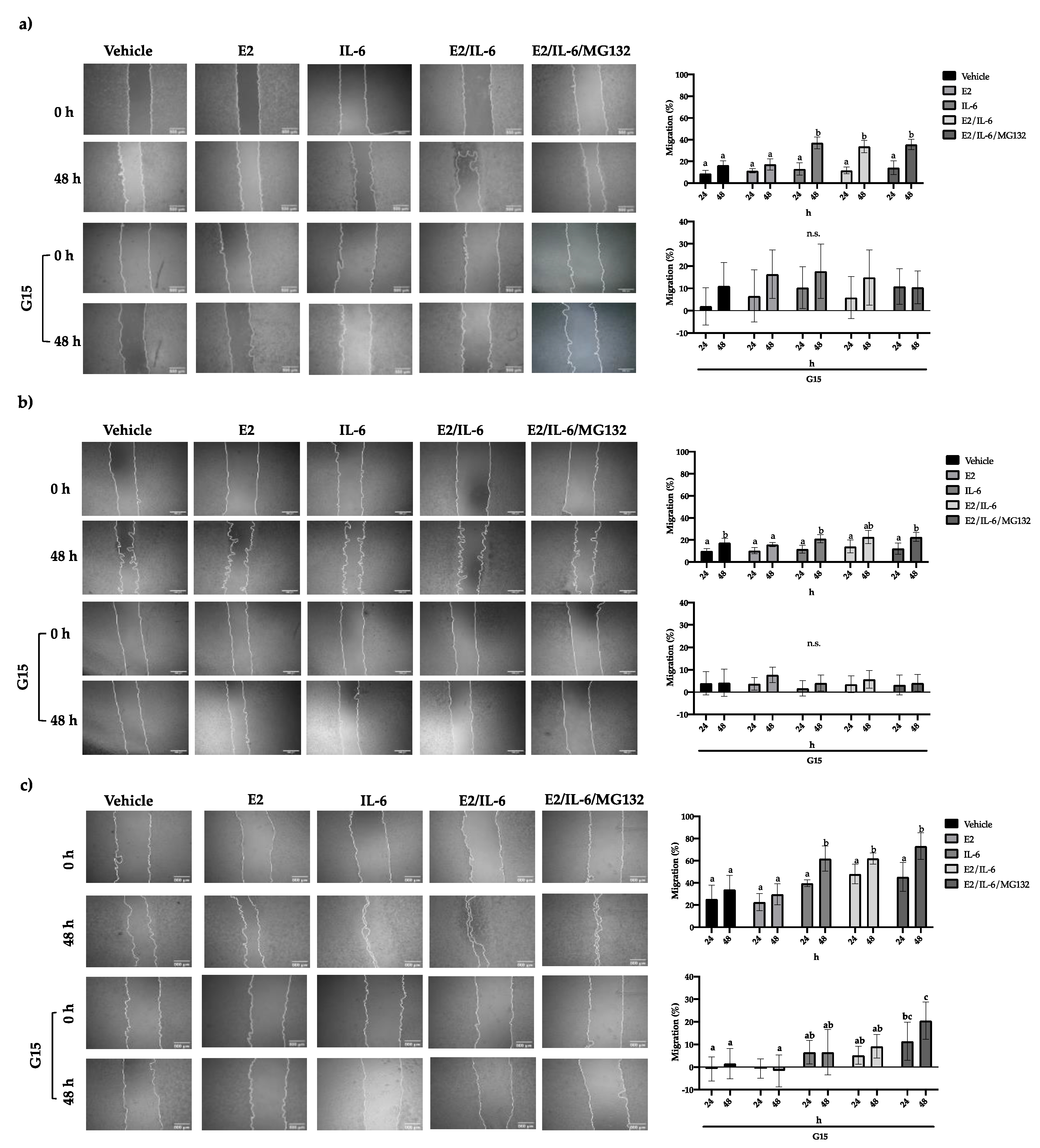
2.3. GPR30 Regulates Basal and IL-6-Induced Migration in Breast Cancer Cells
The increase in vimentin levels is related to a directed migration [
22]. This process includes detachment and mobilization of the cells. For this, we evaluate the migratory capability of breast cancer cells by wound healing assay. MCF-12 cell migration was not affected by E2 or IL-6 (
Figure S2). Also, the MCF-7 cells did not show any changes in their migration rate in response to E2; however, the IL-6 increased it ~20% at 48 h (
Figure 3a), which was inhibited by the GPR30 antagonist G15 (
Figure 3a) or PTX (
Figure S3). A similar behavior was observed in R-TMX cells (
Figure 3b). Noteworthy, the MDA-MB-231 cell line showed a basal migration of 35%, which was enhanced to 60% by IL-6; however, G15 inhibited both the basal and stimulated migration (
Figure 3c). These results suggest that GPR30 is involved in the migration of breast cancer cells.
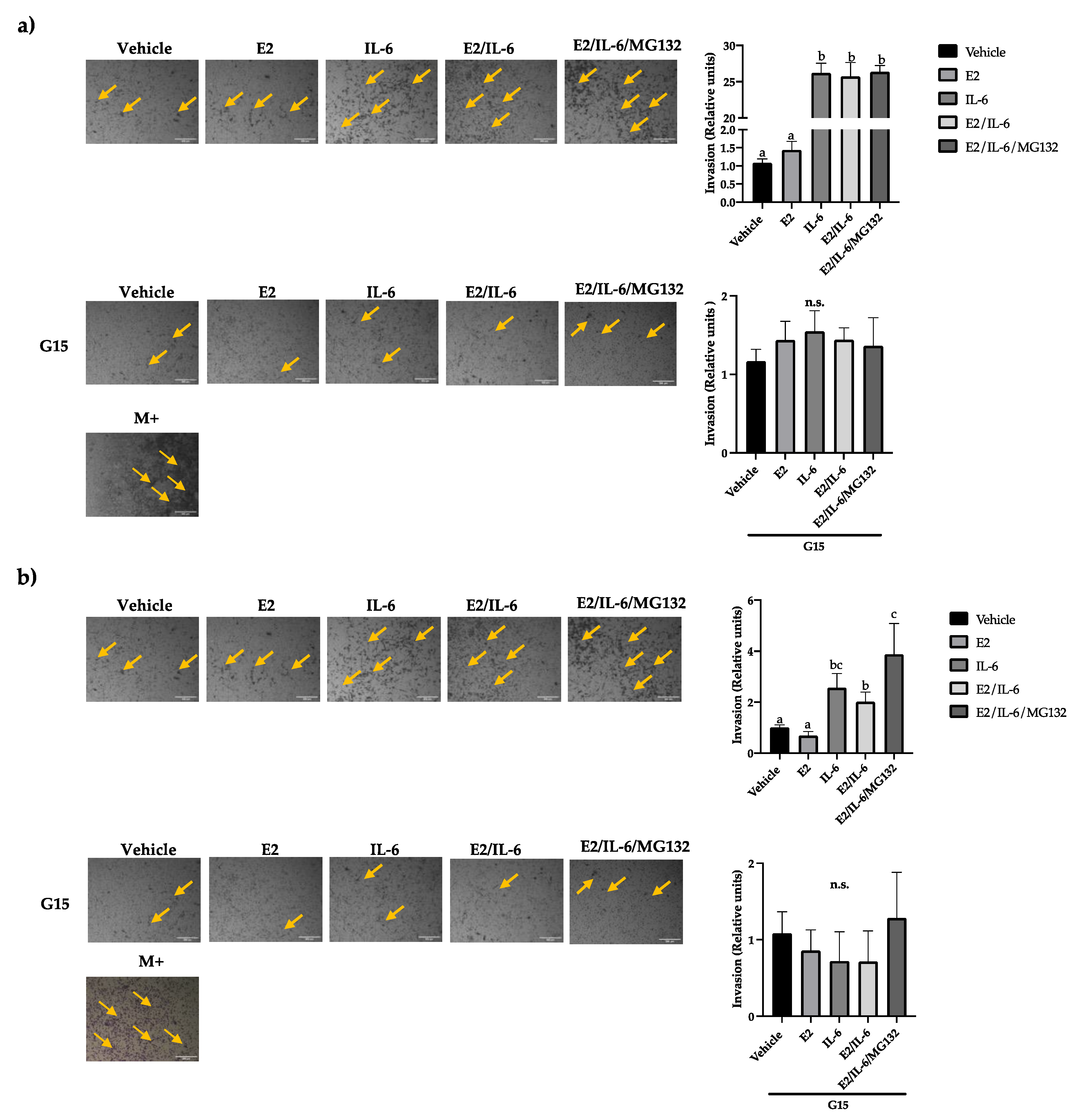
2.4. IL-6 Promotes Breast Cancer Cell Invasion through the GPR30 Receptor
Once the malignant cells arrive at secondary organs, they invade them to develop metastatic tumors. This process involves a directed migration through the extracellular matrix [
1,
23]. We evaluate the IL-6-induced invasion of breast cancer cells by Transwell assay. The MCF-12 cells did not show invasiveness even though they were stimulated with E2 or IL-6 (
Figure S2). Besides, the MCF-7 cells did not increase their invasion capability in response to E2 (
Figure 4a). However, the MCF-7 invasiveness was induced by IL-6 (~25 fold), which was reversed by the GPR30 antagonist G15 (
Figure 4a) or PTX (
Figure S3). Similar results were observed in R-TMX cells, but the IL-6 induction was lower (~2 fold) (
Figure 4b). Also, the invasion of MDA-MB-231 cells was enhanced by IL-6 ~3 fold (
Figure 4c); interestingly, the basal and induced invasion was inhibited by G15, highlighting the role of the GPR30 in the cell invasion of breast cancer cells.
2.5. GPR30 Is Also Involved in the IL-6-Induced TMX Resistance in Luminal Breast Cancer
One of the main problems of current therapies against cancer is the development of resistance to the drugs. TMX resistance is the most common in breast cancer. We assessed if IL-6 induces TMX resistance in MCF-7 cells. Interestingly, cells treated with IL-6 showed an increased time-dependent TMX resistance (
Figure S4,
Table 1). Noteworthy, the GPR30 antagonist G15 diminished the TMX IC
50 values in cells treated with IL-6, suggesting a re-sensibilization to TMX (
Figure S4, table 1). Also, the G15 significantly reduced the IC
50 of TMX in R-TMX cells.
The TMX resistance and metastasis development have been related to increased GPR30 levels; this receptor is located in membranous organelles and can be mobilized in cancer [
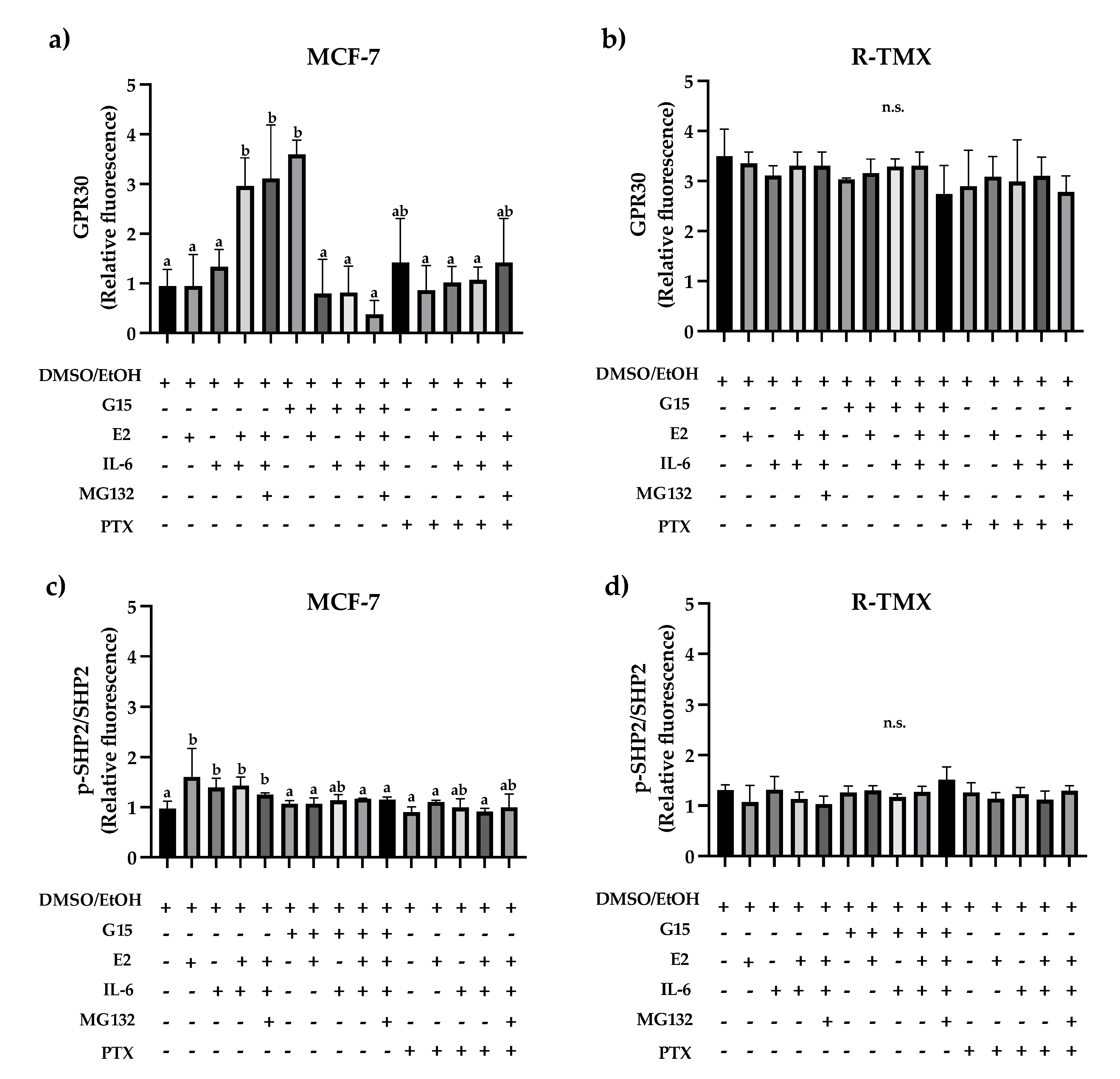
14]. Based on this, we evaluated the intracellular GPR30 levels. IL-6 increased GPR30 levels (3-fold) in MCF-7 cells (
Figure 5a), which was unaffected by E2. Noteworthy, the antagonist G15 or PTX reverted the IL-6 induction. Also, R-TMX cells showed increased GPR30 levels concerning MCF-7 cells; however, all treatments did not modify them (
Figure 5b).
On the other hand, the tyrosine phosphatase SHP-2 has a relevant role in the metastasis development of luminal breast cancer [
24] and is involved in IL-6 signaling [
25]. We evaluated the activated protein levels. The E2 and IL-6 induced similar SHP2 phosphorylation in MCF-7 cells (
Figure 5c). Noteworthy, the antagonist G15 or PTX diminished this effect, supporting the role of GPR30 in the activation of SHP-2 (
Figure 5c). In contrast, in R-TMX cells, the phosphorylation levels were not modified (
Figure 5d).
3. Discussion
Cancer is a disease characterized by the proliferative and metastatic capacity of the cancerous cells, and the tumor microenvironment can influence these properties. In the present work, we evaluated the effect of IL-6 cytokine on breast cancer cell proliferation, migration, and invasion properties, which are relevant for EMT. We showed evidence that GPR30, an alternative non-canonical E2 receptor, regulates the EMT induced by IL-6 in MCF-7 cells.
Luminal breast cancer is epithelial, which means that it has accentuated neoplastic properties and requires external stimulation to acquire a metastatic phenotype [
26]. MCF-7 cells belong to this subtype, and their stimulation with E2 promotes their proliferation; however, IL-6 inhibits the effect of the hormone because it induces the proteasomal degradation of ER-α [
27]. This report agrees with our results because the IL-6 effect on MCF-7 proliferation was reverted by the proteasomal inhibitor MG132 (
Figure 1). Also, the tamoxifen-resistant variant and the triple-negative variant did not respond to the E2 stimulus (
Figure 1), which was associated with a decrease in ER-α levels, as reported [
28]. Besides, we observed an IL-6-induced proliferation in R-TMX cells, which has been reported in cancer cells that do not respond to estrogen; this induction could be regulated through the epidermal growth factor receptor (EGFR) [
29]. Noteworthy, both proliferative effects were inhibited by the GPR30 antagonist G15 or the
Pertussis toxin (PTX); the last suggests that GPR30 activity is associated with an inhibitory alpha subunit (Gαi) in these models. Also, it has been reported that ER-α and GPR30 receptors may interact physically and that inhibition of one of these decreases the proliferative effect of E2 [
30,
31], which agrees with the results of this work. In addition, the inhibition of the effect of IL-6 by the antagonist G15 could be due to the cross-linking of the signaling pathways of EGFR and GPR30 since the latter can transactivate the former. However, further experiments are necessary to clarify it.
On the other hand, the epithelial-mesenchymal transition (EMT) is a prerequisite for metastasis development. It can be detected by a decrease in cytokeratin 18 (CTK 18) levels and an increase in vimentin (Vim) levels [
32]. We showed that E2 did not affect the levels of these proteins in MCF-7 and R-TMX cells, but IL-6 induced the pattern mentioned above (
Figure 2). Likewise, the antagonist G15 or PTX inhibited the IL-6 effect, allowing the cells to recover their basal levels of CTK 18 and Vim, suggesting that GPR30 receptor is involved in the EMT induced by IL-6. In support of the above, the migration and invasion properties of MCF-7 and R-TMX cells induced by IL-6 were inhibited by the antagonist G15 or PTX (
Figure 3 and 4). Interestingly, in MB-MDA-231 cells, we observed a similar behavior (
Figure 4). These results suggest that GPR30 has a relevant role in the EMT in the different phases of breast cancer cells, and it could be a target for future drugs.
GPR30 is located in the endoplasmic reticulum of luminal and triple-negative breast cancer cells [
33], and an increase in this receptor has been related to the aggressiveness of the pathology [
33,
34]. In agreement, we showed an intracellular rise in GPR30 receptor levels in MCF-7 cells induced with IL-6 (
Figure 5a). Also, the intracellular levels of GPR30 in R-TMX were similar in all treatments. In this sense, TMX resistance has been related to the membrane localization of GPR30, which may favor EGFR transactivation. The latter is overactivated in drug-resistant breast cells and regulates the expression of GPR30 [
34,
35]. However, further studies are required to evaluate the membrane abundance of GPR30 in R-TMX cells.
Likewise, the metastatic properties mentioned above are related to SHP-2 [
24], a protein under the regulation of ER-α, so we consider that GPR30 could regulate it. We observed an increase in the phosphorylation of this protein in response to E2 and IL-6 in MCF-7 cells, which agrees with the increase in the intracellular GPR30. Also, R-TMX cells did not present significant changes in protein levels or activation, suggesting an alternative pathway regulating these processes. Previous reports indicate a regulation of SHP2-independent GPR30/EGFR [
34,
35]. Further experiments are necessary to test it in our model. In summary, the results support that the metastatic properties of MCF-7 cells regulated by IL-6 involve the participation of the receptor GPR30, which could be evaluated as a target for future drugs.
4. Materials and Methods
4.1. MCF-12, MCF-7 and MDA-MB-231 Cell Culture
The breast cancer cell lines MCF-7 and MDA-MB-231 and the healthy breast line MCF-12 were used in this work. MCF-12 and MCF-7 cells were cultured in F12 medium supplemented with 5% fetal bovine serum (FBS) (Biowest cat. S1820-500), 5% calf serum (ST) (Biowest cat. S0400-500), 1% penicillin/streptomycin (Gibco cat. 15140122) and 0.05% amphotericin B (Sigma Aldrich cat. A2942). The culture of MDA-MB-231 cells was carried out with DMEM supplemented with the abovementioned composition, adding L-glutamine (Gibco cat. 20530-081) to a final concentration of 200 mM. The three cell lines were cultured at 37 °C and 5% CO2.
4.2. Treatments
The cell lines were exposed to the vehicle DMSO (Sigma Aldrich cat. D1436) at 0.005% and ethanol (J. Baker cat. 9000-02) at 0.05% as controls. The treatments consisted of an individual or combined exposure to E2 10 nM (Sigma Aldrich E2758), IL-6 50 ng/mL (Peprotech 200-06), G15 0.625 μM (Peprotech cat. 1160560), MG132 0.3 μM (Sigma Aldrich C2211) and PTX 100 ng/mL (Sigma Aldrich P2980).
4.3. Tamoxifen Resistant Line (R-TMX)
10,000 MCF-7 cells were cultured per well in a 96-well plate and exposed to 1 μM tamoxifen (TMX) in supplemented F12 medium in a final volume of 100 μL [
36,
37]. The cells were subcultured every three days, placing the same initial cell quantity and exposed to the same culture medium for 16 generations. Throughout this period, the TMX toxicity in the cells was evaluated biweekly, ultimately determining an IC
50 above 20 μM, thereby considering that the cells had acquired resistance to the drug. These cells were maintained in F12 medium supplemented with FBS (5%), ST (5%), penicillin/streptomycin (1%), amphotericin B (0.05%), and TMX (1 μM) at 37 °C and 5% CO
2.
4.4. Proliferation and TMX Cytotoxicity
10,000 MCF-12, MCF-7, and R-TMX, and 20,000 MDA-MB-231 cells/well were cultured in 96 well culture plates (NEST cat. 701001) in supplemented medium (100 μL/well) for 24 h, after which they were synchronized by starvation for 18 h. Subsequently, treatments were added for 24 and 48 h, and cells were harvested with trypsin. Cell viability was assessed by trypan blue assay in a Bio-Rad automatic cell counter (Model TC20). To evaluate the cytotoxicity of TMX, MCF-7 and R-TMX cells were cultured following the previously described methodology, with 48 h exposure to the following treatments: TMX (1, 2, 3, 4, 5, 10, 15 and 20 μM), IL-6 (50 ng/mL) and G15 (0.625 μM), individually and together.
4.5. Flow Cytometry
80,000 MCF-7 and R-TMX cells were cultured in 24-well plates, starved by 18 h, and then stimulated for 48 h with the treatments. After this time, they were harvested with trypsin and washed with PBS, subsequently fixed with methanol absolute for 15 min and permeabilizated with Tween 20 (0.5%) for 20 min. The cells were incubated with the following antibodies: 1:500 anti-vimentin (SC-6260), anti-cytokeratin 18 (SC-32329), 1:1000 anti-GPR30 (ab39742), anti SHP2 (PRS3901) or anti-p-SHP2 (ab62322) overnight at 4°C. Next, the cells were incubated with anti-mouse IgG Fab2 Alexa Fluor 488 (4408S) or anti-rabbit IgG Fab2 PE (8885S) for 2 h and analyzed in a BD Accuri™ C6 cytometer (BD Biosciences) set to 10, 000 events per assay. The data were analyzed in FlowJo Software and were normalized concerning the vehicle.
4.6. Wound Healing Assay
Cell migration was assessed by wound healing assay. 80,000 cells were cultured per well in 24-well plates (NEST cat. 702001), and proliferation was inhibited with mitomycin C (4 μg/mL, Sigma Aldrich cat. M0503) for 2 h. Then, the wounds were made in a cross shape with a 200 μL tip and a sterile slide. Two washes were then performed with PBS, and the treatments were placed in a final volume of 400 μL per well. Images of the wounds were captured at times of 0, 24, and 48 h at 4X using an inverted microscope. The images were analyzed using ImageJ software; the wound area was measured by the wound healing tool expansion. There were analyzed 4 photographs per treatment per time for each assay, and this was performed three times.
4.7. Evaluation of Cell Invasion by Transwell Chamber
Cell cultures were carried out in 50 mm diameter dishes at confluence; subsequently, they were synchronized by starvation for 18 h. Simultaneously, 100 μL of matrigel (1.25 mg/mL) was placed in the inserts of 8 μm Transwell plates (Corning incorporated-Costar® cat. 3422) and incubated at 37°C for 24 h. After this time, the cells were harvested, and 25,000 cells were placed in a volume of 20 μL on the polymerized matrigel. The corresponding treatments were placed in the 24-well plate in a volume of 400 μL per well, and the Transwell inserts were placed on top of them. They were incubated at 37°C and 5% CO2 for 24 h. After this, the matrigel was removed, and the insert was washed twice with PBS. The cells were then fixed with 4% p-formaldehyde, permeabilized with methanol (J. Baker cat. 9070-03), and finally, dyed with crystal violet (Sigma Aldrich cat. C0775) 1:5. The images were captured on the microscope with the 4X and 10X objective and analyzed with ImageJ software.
4.8. Statistic Analysis
Statistical analyses of the triplicated assays were performed in Prisma Graph Pad software with group testing using two-way ANOVA and post hoc Tukey (α≤ 0.05) for migration evaluation and one-way ANOVA for proliferation, flow cytometry, and invasion assays. The TMX resistance was analyzed by t-student p ≤ 0.05.
5. Conclusions
The results indicate that the GPR30 receptor is involved in IL-6-induced metastatic properties, such as migration and invasion, in MCF-7 luminal breast cancer cells.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1. The PTX inhibits the proliferation of breast cancer cells induced by E2 and IL-6; Figure S2. MCF-12 cell migration and invasion; Figure S3. The PTX inhibits the MCF-7 and R-TMX cell migration and invasion; Figure S4. The GPR30 is involved in the IL-6-induced TMX resistance in luminal breast cancer.
Author Contributions
Conceptualization, A.C.T.-G., A.O.-Z. and J.E.L.-M.; funding acquisition, A.O.-Z. and J.E.L.-M.; Investigation, A.C.T.-G., B.R.-B., J.I.R.-P., A.O.-Z., J.E.L.-M.; project administration, J.E.L.-M.; writing—original draft, A.C.T.-G. and J.E.L.-M.; writing—review and editing, A.C.T.-G., A.O.-Z. and J.E.L.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from CONAHCYT-316003, ICTI-PICIR-2023-064 and CIC 14.5 from Universidad Michoacana de San Nicolás de Hidalgo to J.E.L.-M.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Acknowledgments
A.C.T.G (790382) was supported by a scholarship from CONAHCyT.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morra, A.; Jung, A.Y.; Behrens, S.; Keeman, R.; Ahearn, T. U.; Anton-Culver, H.; Arndt, V.; Augustinsson, A.; Auvinen, P. K.; Beane, L. E.; Becher, H.; Beckman, M. W.; Blomqvist, C.;Bojesen, S. E.; Bolla, M. K.; Brenner, H.; Bicreno, I.; Brucker, S. Y.; Camp, M. J.; Campa, D.; Canzian, F.; Castelao, J. E.; Chanock, S. J.; Choi, J. Y.; Clarke, C. L.; Couch, F. J.; Cox, A.; Cross, S. S.; Czene, K.; Dörk, T.; Dunning, A. M.; Dwek, M.; Easton, D. F.; Eccles, D. M.; Egan, K. M.; Evans, D. G.; Fasching, P.; Flyger, H.; Gago-Dominguez, M.; Gapstur, S. M.; García-Sáenz, J. A.; Gaudet, M. M; Gile, G. G.; Grip, M.; Guénel, P.; Haiman, C. A.; Haiman, C. A.; Hakansson, N.; Hall, P.; Hamann, U.; Han, S. N.; Hart, S. N.; Hartman, M.; Heyworth, J. S.; Hoppe, R.; Hopper, J. L.; Hunter, D. J.; Ito, H.; Jager, A.; Jakimovska, M.; Jakubowska, A.; Janni, W.; Kaaks, R.; Kang, D.; Lindblom, A.; Lubinski, J.; Lush, M.; Mannermaa, A.; Manoochehri, M.; Margolin, S.; Mariapun, S.; Matsuo, D.; Mavroudis, H.; Milne, R. L.; Mauranen, T. A.; Newman, W. G.; Nog, D, Y.; Nordestagaard, B. G.; Obi, B.; Olshan, A. F.; Olsson, H.; Park-Simon, T. W.; Petridis, C.; Pharoah, P.; Plaseska*Karanfilska, D.; Presneau, D.; Rashid, M. U.; Rennert, G.; Rennert, H. S.; Rhenius, V.; Romero, A.; Saloustros, E.; Sawyer, E. J.; Schneeweiss, S.; Schwentner, L.; Scott, C.; Shah, M.; Shen, C.; Shu, X. O.; Southey, M. C.; Stram, D. P.; Tamimi, R. M.; Tapper, W.; Tollenaar, R. A. E. M.; Tomlinson, I.; Torres, D.; Troester, M.A.; Truong, T.; Vachon, C. M.; Wang, Q.; Wang, S. S.; Williamns, J. A.; Winqvist, R.; Wolk, A.; Wu, A. H:; Schmit, J.; Chang-Claude, J.; ABCTB Investigators; NBCS Collaborators. 2021. Breast cancer risk factors and survival by tumor subtype: pooled analyses from the breast cancer association consortium. Cancer Epidemiology, Biomarkers & Prevention. 30(4):623-642. [CrossRef]
- Koch, C.; Kuske, A.; Joosse, A. A.; Yigit, G.; Sflomos, G.; Thaler, S.; Smit, D. J.; Werner, S.; Borgmann, K.; Gärtner, S.; Mossahebi Mohammadi, P.; Battista, L.; Cayrefourcq, L.; Altmüller, J.; Salinas-Riester, G.; Raithatha, K.; Zibat, A.; Goy, Y.; Ott, L.; Bartkowiak, K.; Tm, Z.; Zhou, Q.; Speicher, M. R.; Müller, V.; Gorges, T. M.; Jücker, M.; Thiery, J. P.; Brisken, C.; Riethdorf, C.; Alix-Panabières, C.; Pantel, K. 2020. Characterization of circulating breast cancer cells with tumorigenic and metastatic capacity. EMBO Molecular Medicine, 12(9), e11908. [CrossRef]
- Li, Y.; Li, H.; Zhang, Y.; Merkher, L.; Chen, N.; Liu, S.; Leonov, Y.; Chen. 2022. Recent advances in therapeutic strategies for triple-negative breast cancer. Journal of Hematology & Oncology, 15(1), 121. [CrossRef]
- Szostakowska, M.; Trębińska-Stryjewska, E. A.; Grzybowska, A.; Fabisiewicz, A. 2019. Resistance to endocrine therapy in breast cancer: Molecular mechanisms and future goals. Breast Cancer Research and Treatment, 173(3), 489-497. [CrossRef]
- Wang, Q.; Wang, M.; Gun, X. Y.; Hong. 2019. Induced tamoxifen resistance is mediated by increased methylation of E-cadherin in estrogen receptor-expressing breast cancer cells. Scientific Reports, 9(1), 14140. [CrossRef]
- Mirzaei, M.; Sheikholeslami, S. A.; Jalili, A.; Bereimipour, S.; Sharbati, V.; Kaveh, S.; Salari. 2022. Investigating the molecular mechanisms of tamoxifen on the EMT pathway among patients with breast cancer. Journal of Medicine and Life, 15(6), 835-844. [CrossRef]
- Zhou, X.; Zhang, J.; Lv, W.; Zhao, Y.; Xia, Y.; Wu, Q.; Zhang. 2022. The pleiotropic roles of adipocyte secretome in remodeling breast cancer. Journal of Experimental & Clinical Cancer Research, 41(1), 203. [CrossRef]
- Abaurrea, A. M.; Araujo, M. M.; Caffarel. 2021. The role of the IL-6 cytokine family in epithelial-mesenchymal plasticity in cancer progression. International Journal of Molecular Sciences, 22(15), 8334. [CrossRef]
- Tsoi, H.; Man, E. P. S.; Chau, K. M.; Khoo, U. S. 2021. Targeting the IL-6/STAT3 signalling cascade to reverse tamoxifen resistance in estrogen receptor positive breast cancer. Cancers (Basel), 13(7), 1511. [CrossRef]
- Yamaguchi, N.; Nakayama, Y.; Yamaguchi. 2017. Down-regulation of Forkhead box protein A1 (FOXA1) leads to cancer stem cell-like properties in tamoxifen-resistant breast cancer cells through induction of interleukin-6. Journal of Biological Chemistry, 292(20), 8136-8148. [CrossRef]
- Cheng, R.; Qi, L.; Kong, Z.; Wang, Y.; Fang, J.; Wang. 2020. Identification of the significant genes regulated by estrogen receptor in estrogen receptor-positive breast cancer and their expression pattern changes when tamoxifen or fulvestrant resistance occurs. Frontiers in Genetics, 11. [CrossRef]
- Vivacqua, A.; 2020. GPER1 and microRNA: two players in breast cancer progression. International Journal of Molecular Sciences. 22(1):98. [CrossRef]
- Muhammad, G. E.; Forcados, A. P.; Yusuf, M. B.; Abubakar, I. Z.; Sadiq, I.; Elhussin, M. A. T.; Siddique, S.; Aminu, R. B.; Suleiman, Y. S.; Abubakar, B. S.; Katsayal, C. C.; Yates, S.; Mahavadi. 2022. Comparative G-protein-coupled estrogen receptor (GPER) systems in diabetic and cancer conditions: A Review. Molecules, 27(24), 8943. [CrossRef]
- Mo, Z.; Liu, M.; Yang, H.; Luo, H.; Li, G.; Tu, G.; Yang. 2013. GPR30 as an initiator of tamoxifen resistance in hormone-dependent breast cancer. Breast Cancer Research, 15(6), R114. [CrossRef]
- Xu, C.X.; Xiong, W.; Wang, M.; Yang, J.; Shi, H.; Chen, H.; Gang, N. 2018. Nuclear G Protein-Coupled oestrogen receptor (GPER) predicts poor survival in patients with ovarian cancer. Journal of International Medical Research, 46, 723-731. [CrossRef]
- Xu, S.; Yu, D.; Dong, L.; Lee, T. 2019. G protein-coupled estrogen receptor: a potential therapeutic target in cancer. Frontiers in Endocrinology, 10, 725. [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, M.; Jaymand, S.; Kolahian, T.; Javaheri, P.; Zare. 2020. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Communication and Signaling, 18, 59. [CrossRef]
- Mishra, A.; Srivastava, A.; Pateriya, M. S.; Tomar, A. K.; Mishra, A.; Shrivastava. 2021. Metabolic reprogramming confers tamoxifen resistance in breast cancer. Chemico-Biological Interactions, 347, 109602. [CrossRef]
- Hanahan, D. 2022. Hallmarks of cancer: new dimensions. Cancer Discovery 12(1):31-46.
- Kyuno, D.; Takasawa, A.; Kikuchi, I.; Takemas, M.; Osanai, T.; Kojima, T. 2021. Role of tight junctions in the epithelial-to-mesenchymal transition of cancer cells. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1863(3), 183503. [CrossRef]
- Menz, A.; Weitbrecht, T.; Gorbokon, N.; Büscheck, F.; Luebke, A. M.; Kluth, M.; Hube-Magg, C.; Hinsch, D.; Höflmayer, S.; Weidemann, C.; Fraune, K.; Möller, C.; Bernreuther, P.; Lebok, T.; Clauditz, G.; Sauter, R.; Uhlig, W.; Wilczak, S.; Steurer, S.; Minner, E.; Burandt, R.; Krech, D.; Dum, T.; Krech, A.; Marx, R.; Simon. 2021. Diagnostic and prognostic impact of cytokeratin 18 expression in human tumors: a tissue microarray study on 11,952 Molecular Medicine. Molecular Medicine, 27(1), 16. [CrossRef]
- Battaglia, R. A.; Delic, S.; Herrmann, H. N. T.; Snider. 2018. Vimentin on the move: new developments in cell migration. F1000Research, 7, F1000 Faculty Rev-1796. [CrossRef]
- Krakhmal, N. V.; Zavyalova, M. V.; Denisov, E. V.; Vtorushin, S. V.; Perelmuter. 2015. Cancer invasion: patterns and mechanisms. Acta Naturae, 7(2), 17-28. [PubMed]
- Sun, X.; Zhang, Z.; Wang, W.; Ji, R.; Tian, F.; Zhang, R.; Niu. 2017. Shp2 plays a critical role in IL-6-induced EMT in breast cancer cells. International Journal of Molecular Sciences, 18(2), 395. [CrossRef]
- Fiebelkorn, J.; Guendel, A.; Guendel, B.; Mehwald, N.; Jetka, T.; Komorowski, M.; Waldherr, S.; Schaper, A.; Dittrich. 2021. The tyrosine phosphatase SHP2 increases robustness and information transfer within IL-6-induced JAK/STAT signalling. Cell Communication and Signaling, 19(1), 94. [CrossRef]
- Xie, G.; Ji, A.; Yuan, Q.; Jin, Z.; Yuan, Y.; Ren, C.; Guo, Q.; Yao, K.; Yang, X.; Lin, L.; Chen. 2014. Tumour-initiating capacity is independent of epithelial-mesenchymal transition status in breast cancer cell lines. British Journal of Cancer, 110(10), 2514-2523. [CrossRef]
- Dhimolea, E.; de Matos Simoes, R.; Kansara, D.; Weng, X.; Sharma, S.; Awate. 2020. Pleiotropic mechanisms drive endocrine resistance in the three-dimensional bone microenvironment. Cancer Research, 0571.2020. [CrossRef]
- Yao, J.; Deng, K.; Huang, J.; Zeng, R.; Zuo. 2020. Progress in the understanding of the mechanism of tamoxifen resistance in breast cancer. Frontiers in Pharmacology, 11, 592912. [CrossRef]
- Chen, J.; Wei, Y.; Yang, Q.; Huang, Y.; Chen, K.; Zeng, J.; Chen. 2022. IL-6: The link between inflammation, immunity and breast cancer. Frontiers in Oncology, 12, 903800. [CrossRef]
- Yang, H.; Wang, H.; Liao, Q.; Wang. 2021. Activation of GPER by E2 promotes proliferation, invasion and migration of breast cancer cells by regulating the miR-124/CD151 pathway. Oncology Letters, 21(6), 432. [CrossRef]
- Romano, S. N.; Gorelick, D. A. 2018. Crosstalk between nuclear and G protein-coupled estrogen receptors. General and Comparative Endocrinology, 261, 190-197. [CrossRef]
- Shi., R, Wang., C, Fu., N, Liu., L, Zhu., D, Wei., Z, Zhang., H, Xing., J, Wang., Y. (2019). Downregulation of cytokeratin 18 enhances BCRP-mediated multidrug resistance through induction of epithelial-mesenchymal transition and predicts poor prognosis in breast cancer. Oncology Reports. 41(5):3015-3026. [CrossRef]
- Xu., T, Ma., D, Chen., S, Tang., R, Yang., J, Meng., C, Feng., Y, Liu., L, Wang., J, Luo., H, Yu., K. 2022. High GPER expression in triple-negative breast cancer is linked to pro-metastatic pathways and predicts poor patient outcomes. NPJ Breast Cancer. 8(1):100. [CrossRef]
- Tutzauer., J, Sjöström., M, Bendahl., P.-O, Rydén., L, Fernö., M, Leeb-Lundberg., L. M. F, & Alkner, S. 2020. Plasma membrane expression of G protein-coupled estrogen receptor (GPER)/G protein-coupled receptor 30 (GPR30) is associated with worse outcome in metachronous contralateral breast cancer. PLOS ONE, 15(4), e0231786. [CrossRef]
- Lappano, R.; Pisano, A.; Maggiolini, M. 2014. GPER function in breast cancer: an overview. Frontiers. 6;5:66. [CrossRef]
- Singh, M,; Zhou, X.; Chen, X.; Santos, G. S.; Peuget, S.; Cheng, Q.; Rihani, A.; Arnér, E. S. J.; Hartman, J.; Selivanova1, G. 2020. Identification and targeting of selective vulnerability rendered by tamoxifen resistance. Breast Cancer Research. 22(1):80. [CrossRef]
- Molina, L.; Bustamante, F.; Ortloff, A.; Ramos, I.; Ehrenfeld, P.; Figueroa, C. D. 2020. Continuous exposure of breast cancer cells to tamoxifen upregulates GPER-1 and increases cell proliferation. Frontiers in Endocrinology. 11:563165. [CrossRef]
Figure 1.
The GPR30 antagonist G15 inhibits the proliferation of breast cancer cells induced by E2 and IL-6. (a,e) MCF-12, (b,f) MCF-7, and (c,g) R-TMX cell proliferation was evaluated at 24 h. MDA-MB-231 (d,h) cell proliferation was evaluated at 48 h. Data were obtained by trypan blue exclusion assay. E2 (10 nM), IL-6 (50 ng/mL), MG132 (0.03 μM), and G15 (0.625 μM). Each bar shows the mean of triplicates ± SE of three independent experiments. Different letters represent a statistically significant difference (One-way ANOVA post hoc Tukey, p≤ 0.05). n.s.= not significant.
Figure 1.
The GPR30 antagonist G15 inhibits the proliferation of breast cancer cells induced by E2 and IL-6. (a,e) MCF-12, (b,f) MCF-7, and (c,g) R-TMX cell proliferation was evaluated at 24 h. MDA-MB-231 (d,h) cell proliferation was evaluated at 48 h. Data were obtained by trypan blue exclusion assay. E2 (10 nM), IL-6 (50 ng/mL), MG132 (0.03 μM), and G15 (0.625 μM). Each bar shows the mean of triplicates ± SE of three independent experiments. Different letters represent a statistically significant difference (One-way ANOVA post hoc Tukey, p≤ 0.05). n.s.= not significant.
Figure 2.
IL-6 regulates the mesenchymal protein pattern expression in MCF-7 and R-TMX cells. (a, b) Cytokeratin 18 and (c, d) vimentin expression of MCF-7 and R-TMX cells was assessed at 48 h in the presence of E2 (10 nM), IL-6 (50 ng/mL), MG132 (0.03 μM), G15 (0.625 μM) and PTX (100 ng/mL) by flow cytometry. Each bar shows the mean of triplicates ± SE of three independent experiments. Different letters represent a statistically significant difference (One-way ANOVA post hoc Tukey, p≤ 0.05).
Figure 2.
IL-6 regulates the mesenchymal protein pattern expression in MCF-7 and R-TMX cells. (a, b) Cytokeratin 18 and (c, d) vimentin expression of MCF-7 and R-TMX cells was assessed at 48 h in the presence of E2 (10 nM), IL-6 (50 ng/mL), MG132 (0.03 μM), G15 (0.625 μM) and PTX (100 ng/mL) by flow cytometry. Each bar shows the mean of triplicates ± SE of three independent experiments. Different letters represent a statistically significant difference (One-way ANOVA post hoc Tukey, p≤ 0.05).
Figure 3.
Breast cancer cell migration. (a) MCF-7, (b) R-TMX, and (c) MDA-MB-231 cell migration was evaluated by wound healing assay at 24 and 48 h in the presence of E2 (10 nM), IL-6 (50 ng/mL), MG132 (0.03 μM), and G15 (0.625 μM). The photographs are 4X, and the scale bar represents 500 μm. Each bar shows the mean of triplicates ± SE of three independent experiments. Different letters represent a statistically significant difference (Two-way ANOVA post hoc Tukey, p≤ 0.05).
Figure 3.
Breast cancer cell migration. (a) MCF-7, (b) R-TMX, and (c) MDA-MB-231 cell migration was evaluated by wound healing assay at 24 and 48 h in the presence of E2 (10 nM), IL-6 (50 ng/mL), MG132 (0.03 μM), and G15 (0.625 μM). The photographs are 4X, and the scale bar represents 500 μm. Each bar shows the mean of triplicates ± SE of three independent experiments. Different letters represent a statistically significant difference (Two-way ANOVA post hoc Tukey, p≤ 0.05).
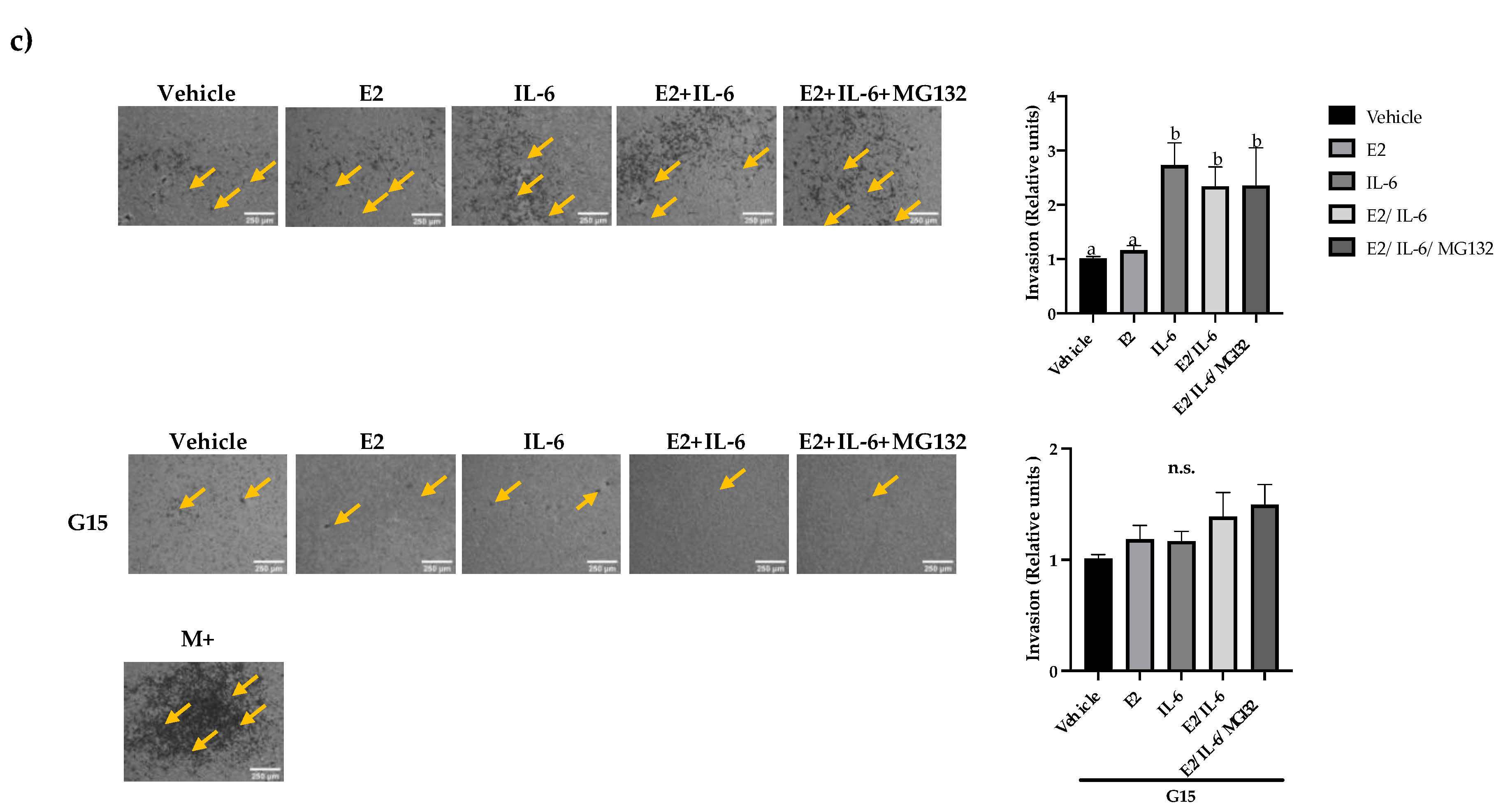
Figure 4.
Breast cancer cell invasion. (a) MCF-7, (b) R-TMX, and (c) MDA-MB-231 cell invasion was assessed by Transwell chamber assay at 24 h of stimulation with E2 (10 nM), IL-6 (50 ng/mL), MG132 (0.03 μM) and G15 (0.625 μM). M+ = Cells cultured in F-12 medium supplemented with FBS 10%. 10X photographs, scale bar represents 250 μm. Each bar shows the mean of triplicates ± SE of three independent experiments. Different letters represent a statistically significant difference (One-way ANOVA post hoc Tukey, p≤ 0.05). n.s.= not significant. Arrows highlight the cell invasion.
Figure 4.
Breast cancer cell invasion. (a) MCF-7, (b) R-TMX, and (c) MDA-MB-231 cell invasion was assessed by Transwell chamber assay at 24 h of stimulation with E2 (10 nM), IL-6 (50 ng/mL), MG132 (0.03 μM) and G15 (0.625 μM). M+ = Cells cultured in F-12 medium supplemented with FBS 10%. 10X photographs, scale bar represents 250 μm. Each bar shows the mean of triplicates ± SE of three independent experiments. Different letters represent a statistically significant difference (One-way ANOVA post hoc Tukey, p≤ 0.05). n.s.= not significant. Arrows highlight the cell invasion.
Figure 5.
GPR30 and SHP2 levels in breast cancer cells. (a) GPR30 and (c) SHP2 levels in MCF-7 cells and (b) GPR30 and (d) SHP2 levels in R-TMX at 48 h in the presence of E2 (10 nM), IL-6 (50 ng/mL), MG132 (0.03 μM), G15 (0.625 μM), and PTX (100 ng/mL). Protein levels were assessed by flow cytometry as described in materials and methods. Each bar shows the mean of triplicates ± SE of three independent experiments. Different letters represent a statistically significant difference (One-way ANOVA post hoc Tukey, p≤ 0.05). n.s.= not significant.
Figure 5.
GPR30 and SHP2 levels in breast cancer cells. (a) GPR30 and (c) SHP2 levels in MCF-7 cells and (b) GPR30 and (d) SHP2 levels in R-TMX at 48 h in the presence of E2 (10 nM), IL-6 (50 ng/mL), MG132 (0.03 μM), G15 (0.625 μM), and PTX (100 ng/mL). Protein levels were assessed by flow cytometry as described in materials and methods. Each bar shows the mean of triplicates ± SE of three independent experiments. Different letters represent a statistically significant difference (One-way ANOVA post hoc Tukey, p≤ 0.05). n.s.= not significant.
Table 1.
Mean lethal concentrations (IC50) of TMX in MCF-7 cells stimulated with IL-6 (50 ng/mL) and G15 (0.625 μM).
Table 1.
Mean lethal concentrations (IC50) of TMX in MCF-7 cells stimulated with IL-6 (50 ng/mL) and G15 (0.625 μM).
| Cell Conditions |
IC50 TMX (μM) |
| MCF-7 |
3.8 |
| MCF-7 + G15 |
4.26 |
| MCF-7 + IL-6 (24 h) |
10.98* |
| MCF-7 + IL-6 (48 h) |
12.69* |
| MCF-7 + IL-6 (24 h) + G15 |
3.87 |
| MCF-7 + IL-6 (48 h) + G15 |
5.08* |
| R-TMX |
>20* |
| R-TMX + G15 |
9.05* |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).