Submitted:
25 July 2024
Posted:
26 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Water Sampling Campaign
2.2. Strains Isolation, Quantification, Identification and Antimicrobial Susceptibility Profiles
2.3. Characterization of Genotypic Resistance Profiles
2.4. Whole Genome Sequencing (WGS) and Bioinformatic Analyses of Clinical and Aquatic A. baumannii Isolates
2.5. Metagenomic Analysis of Surface Water Samples and Fish Microbiota, to Highlight the Connection between the Environment and Fish Microbiota
3. Results
3.1. Phenotypic and Genotypic AR Profiles of A. baumannii Isolates
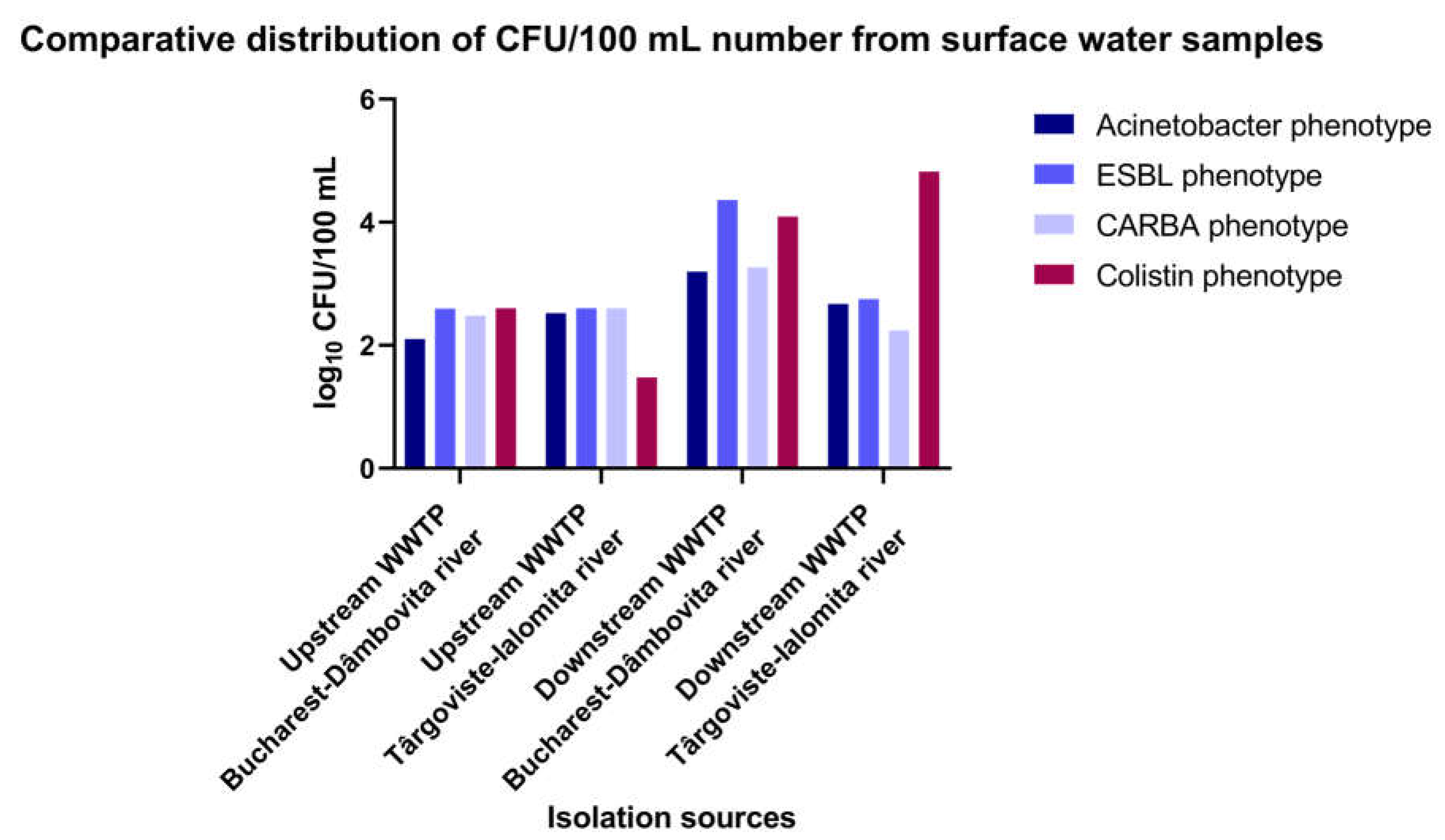
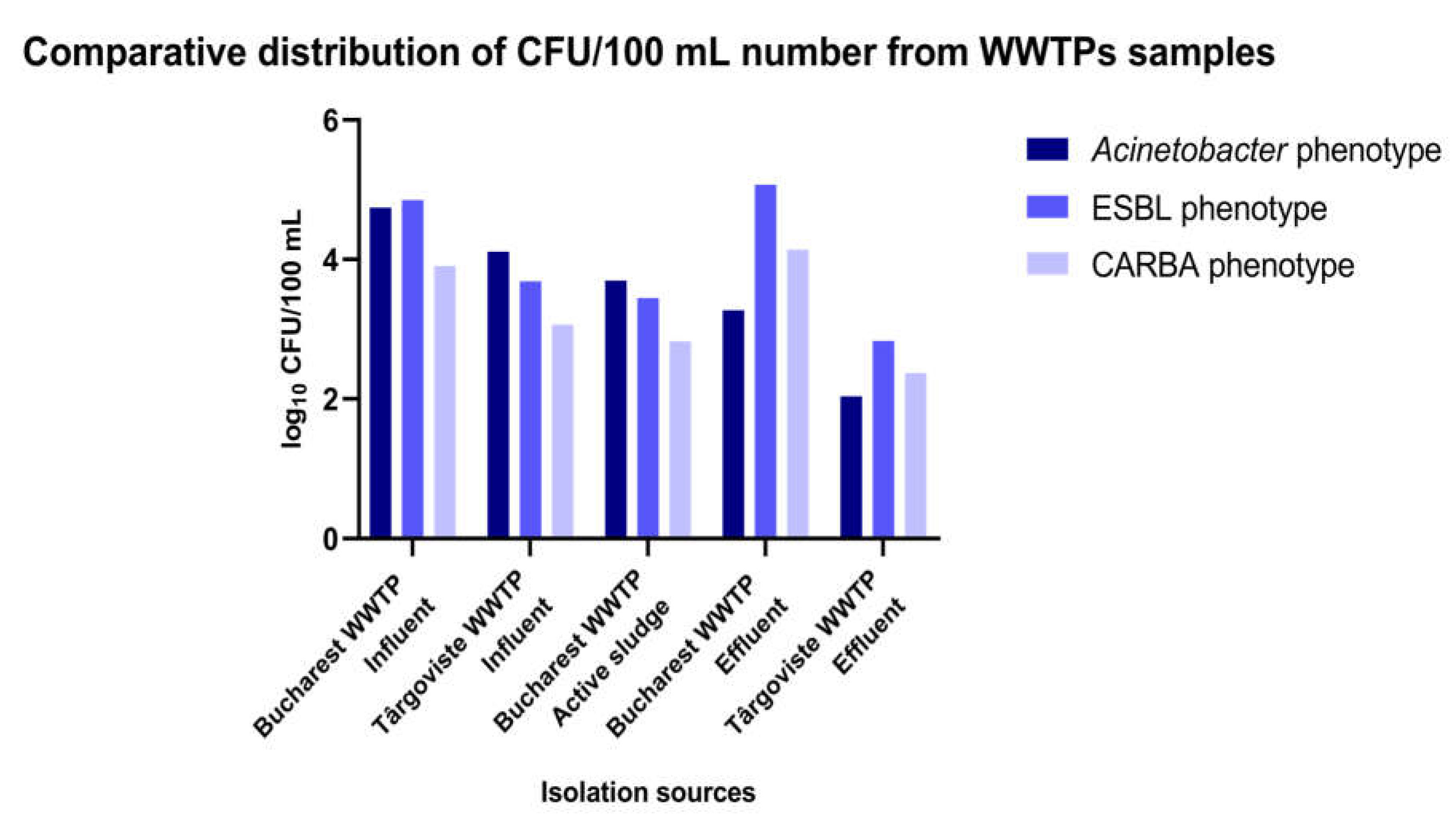
3.1.1. Isolation and Quantification of A. baumannii from Romanian Wastewater and Surface Water Samples
- CARBA phenotype: DO Glina, Bucharest > UP Targoviste > UP Glina, Bucharest > DO Targoviste;
- Colistin phenotype: DO Glina, Bucharest > DO Targoviste > UP Glina, Bucharest > UP Targoviste;
- ESBL and total Acinetobacter phenotype: DO Glina, Bucharest > DO Targoviste > UP; (Figure 1 and Supplementary Table S3).
- 4)
- CARBA and ESBL phenotype: EF Glina, Bucharest > IN Glina, Bucharest > IN Targoviste >AS Glina, Bucharest > EF Targoviste;
- 5)
- total Acinetobacter phenotype: IN Glina, Bucharest > IN Targoviste > AS Glina, Bucharest > EF Glina, Bucharest > EF Targoviste; (Figure 2 and Supplementary Table S4).
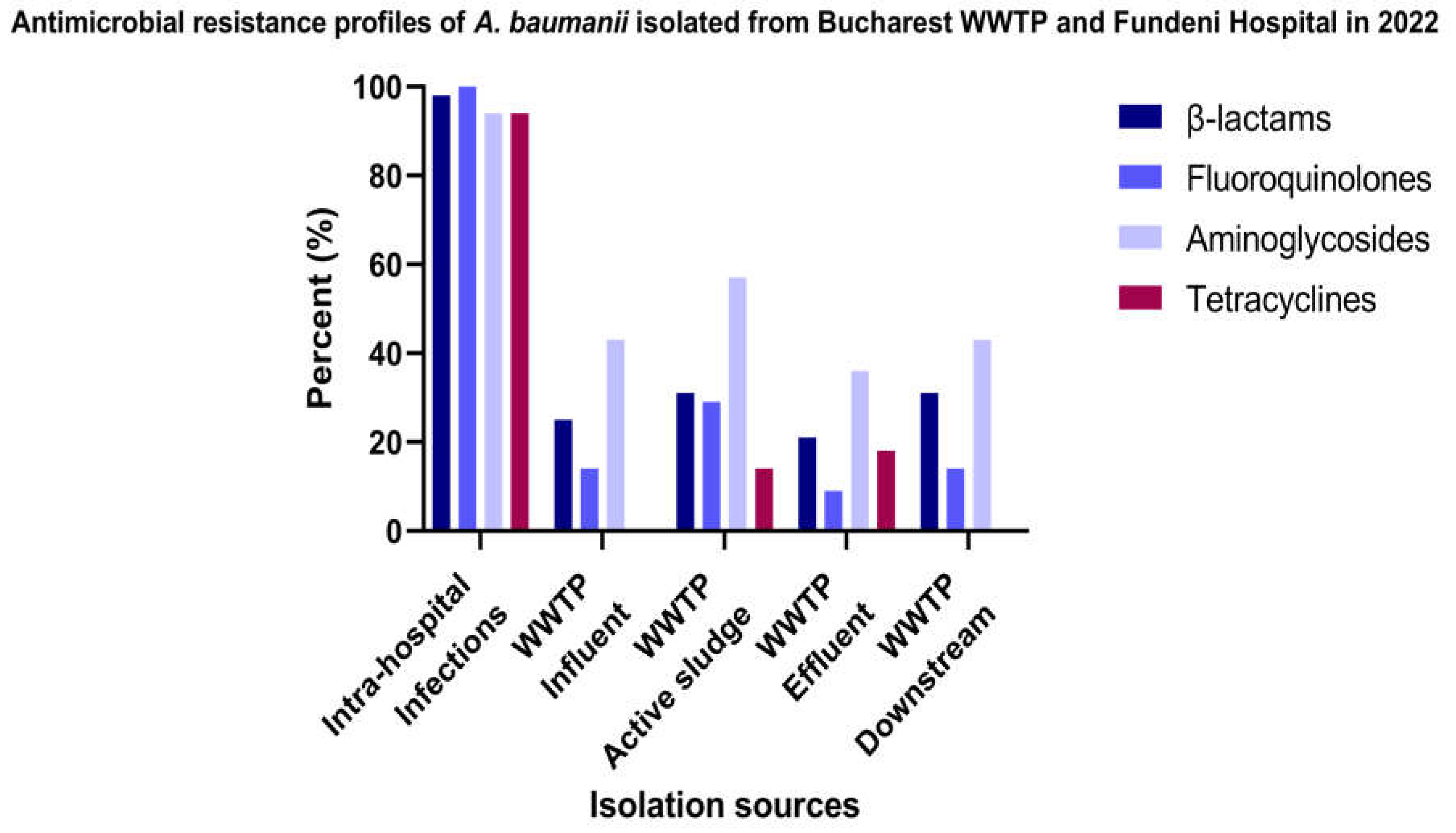
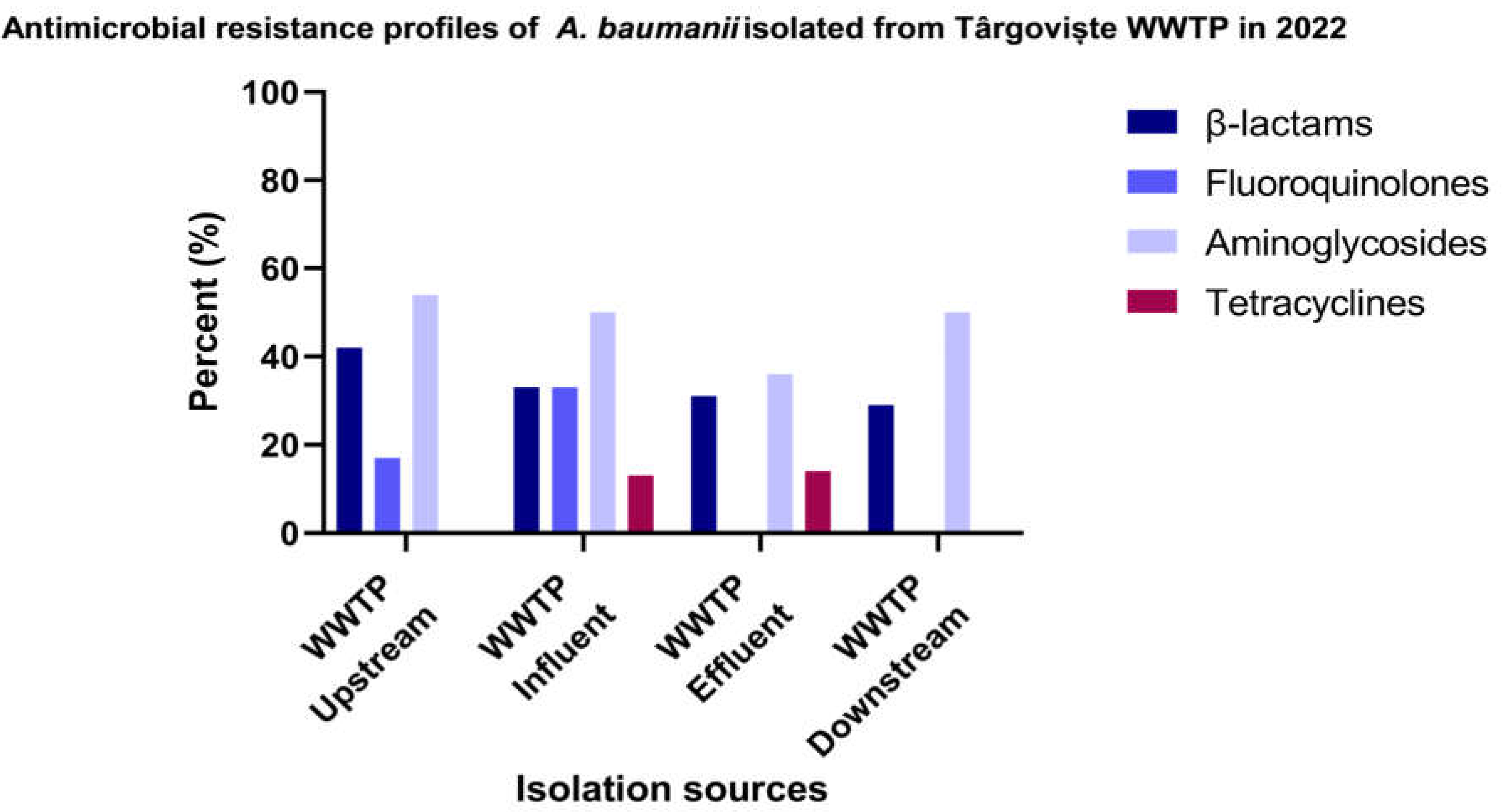
3.1.2. Antimicrobial Susceptibilities Profiles of A. baumannii Isolates from Different Isolation Sources and by Geographical Location
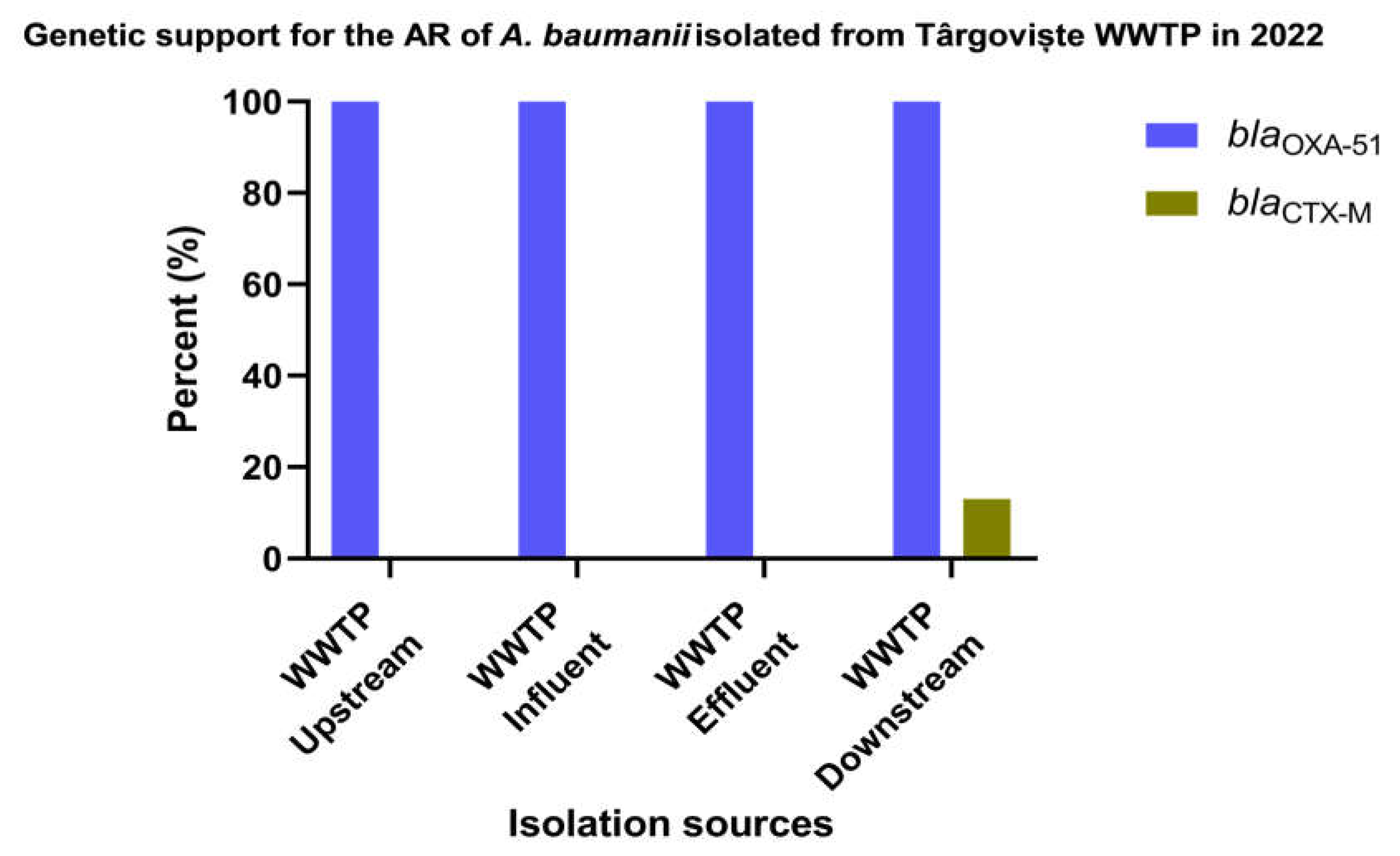
3.1.3. Genotypic Characterization of β-Lactam Resistance in Clinical, Wastewater and Surface Water A. baumannii Isolates
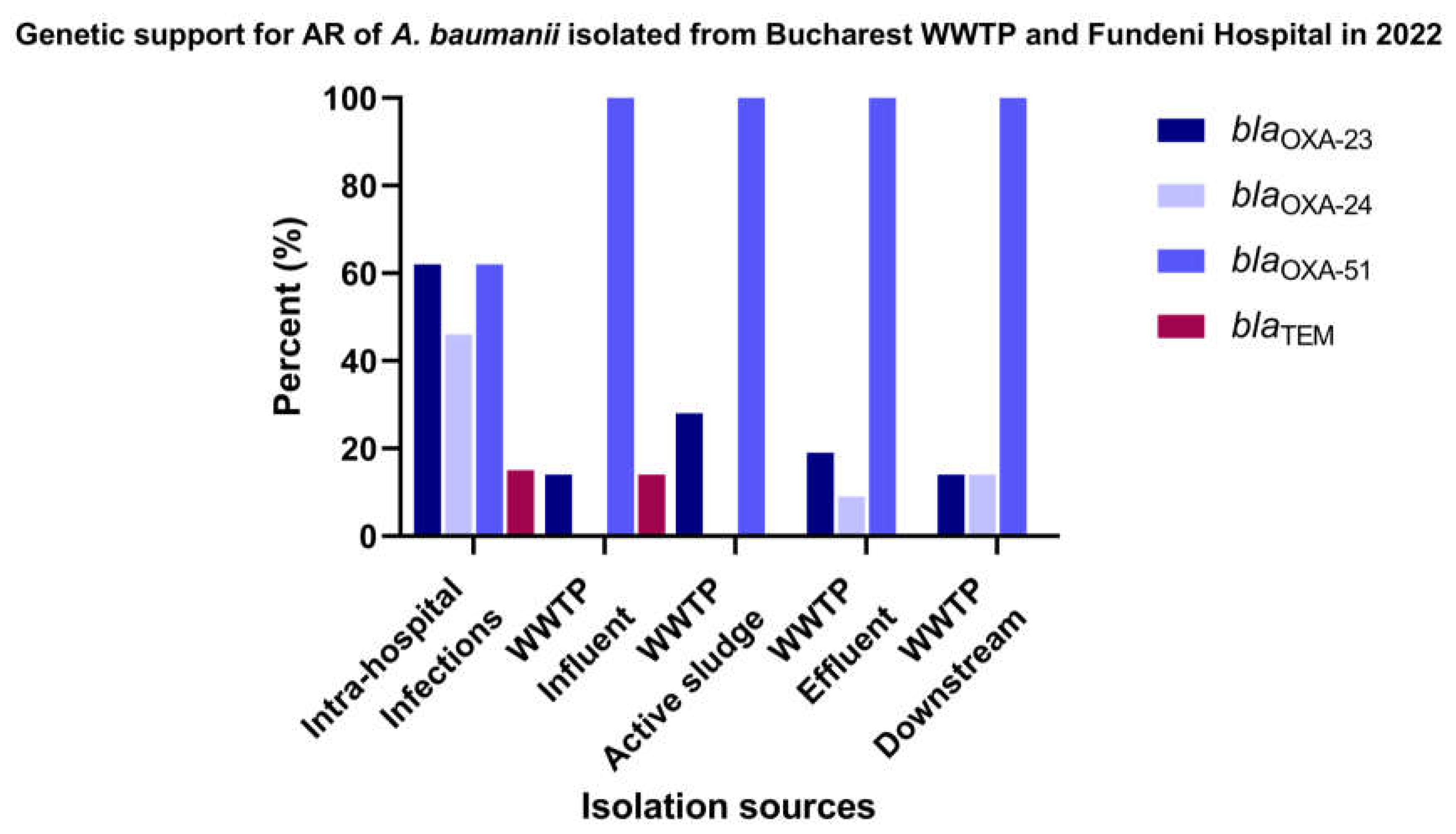
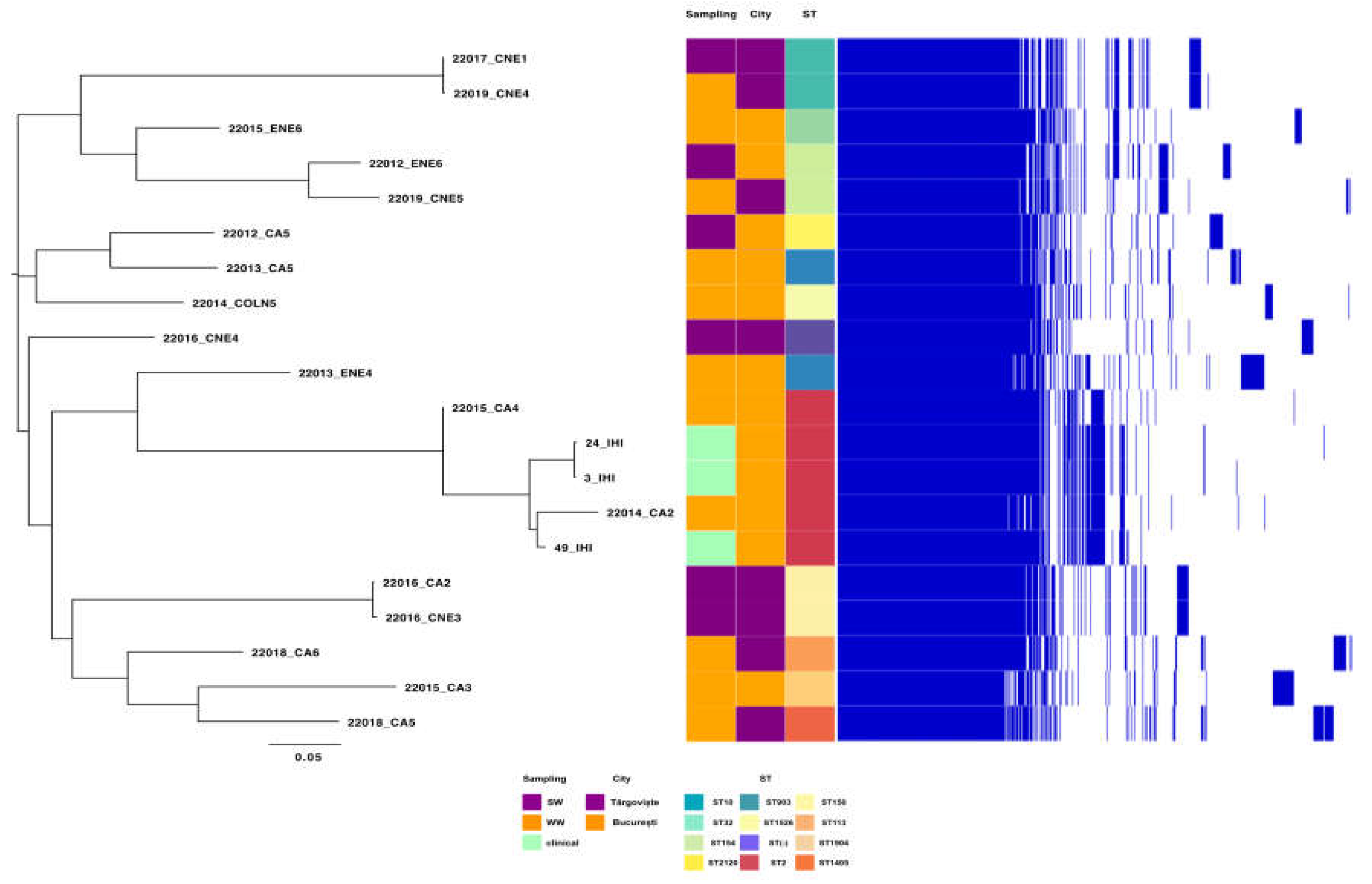
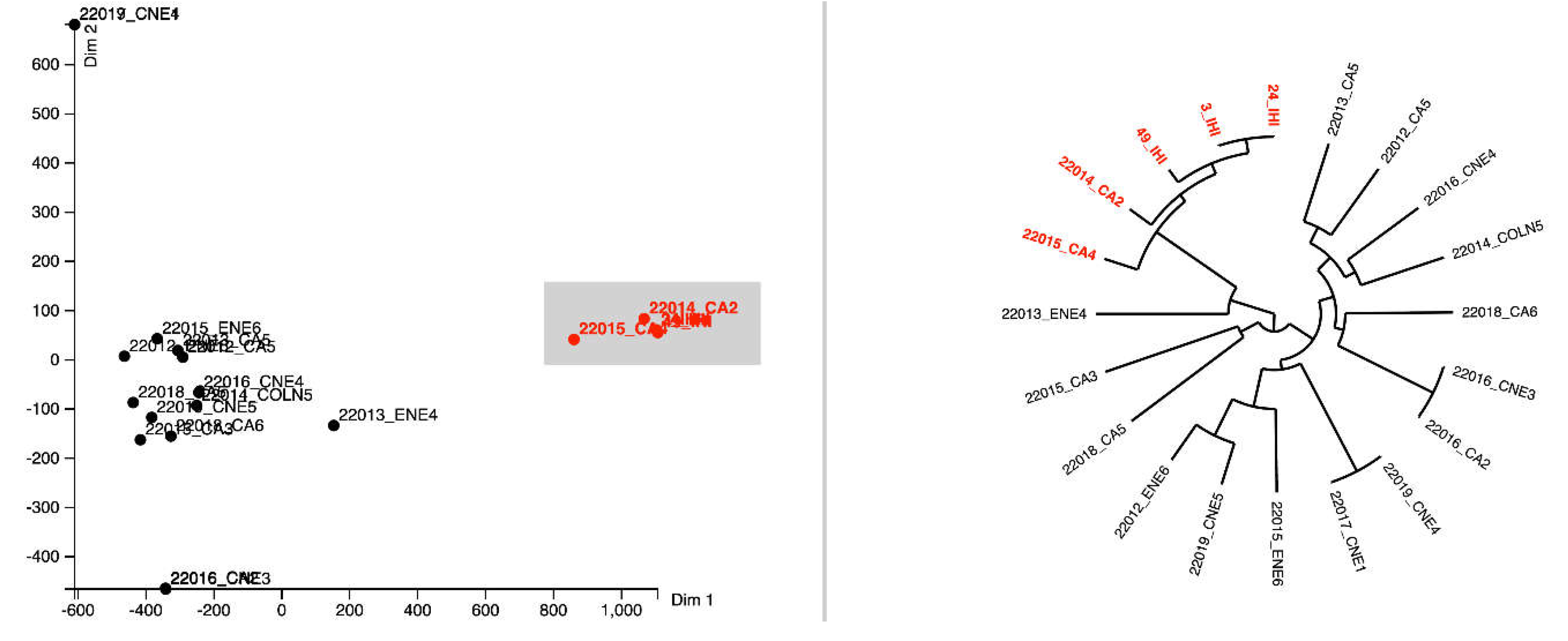
3.1.4. WGS Analysis in Clinical, Wastewater and Surface Water A. baumannii Isolates
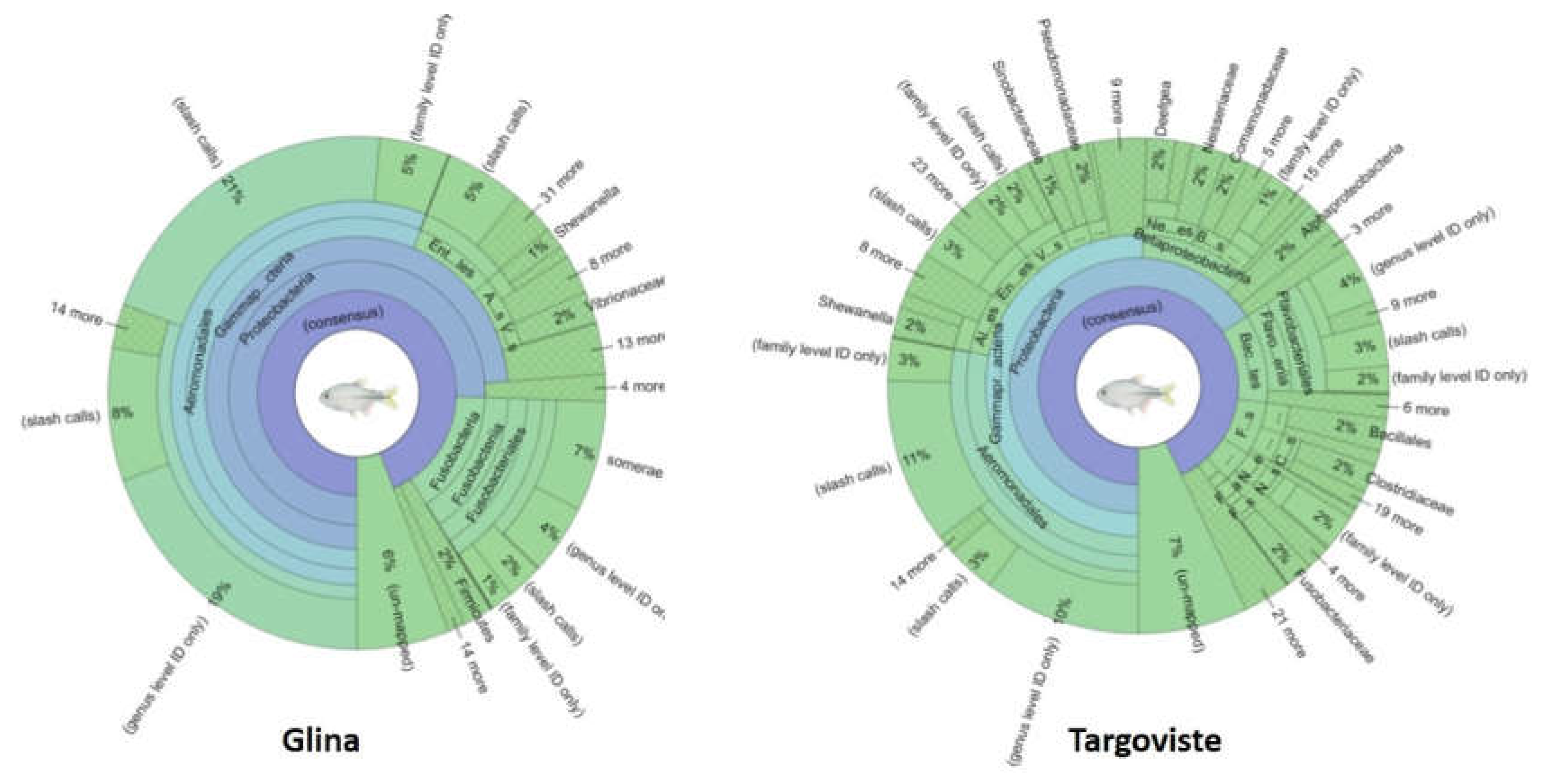
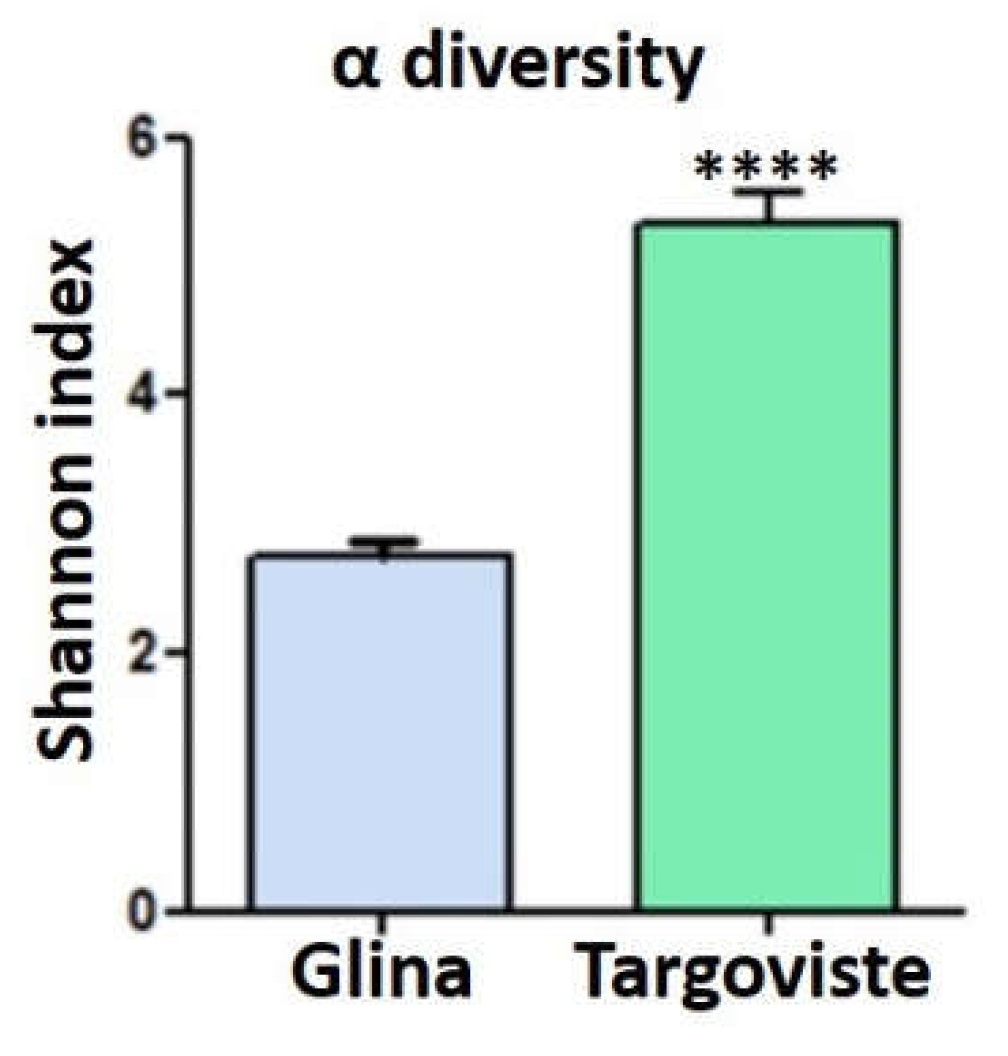
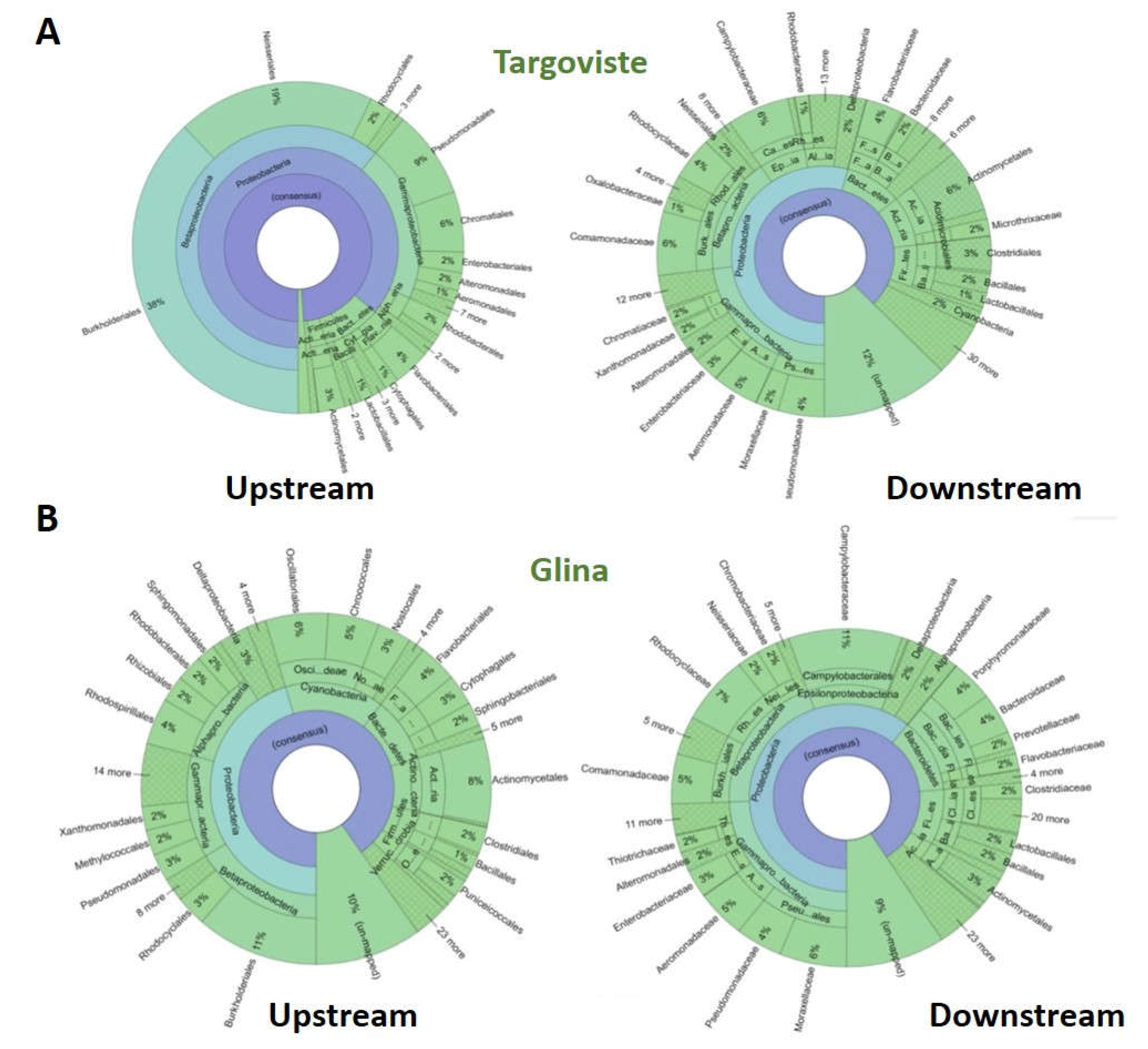
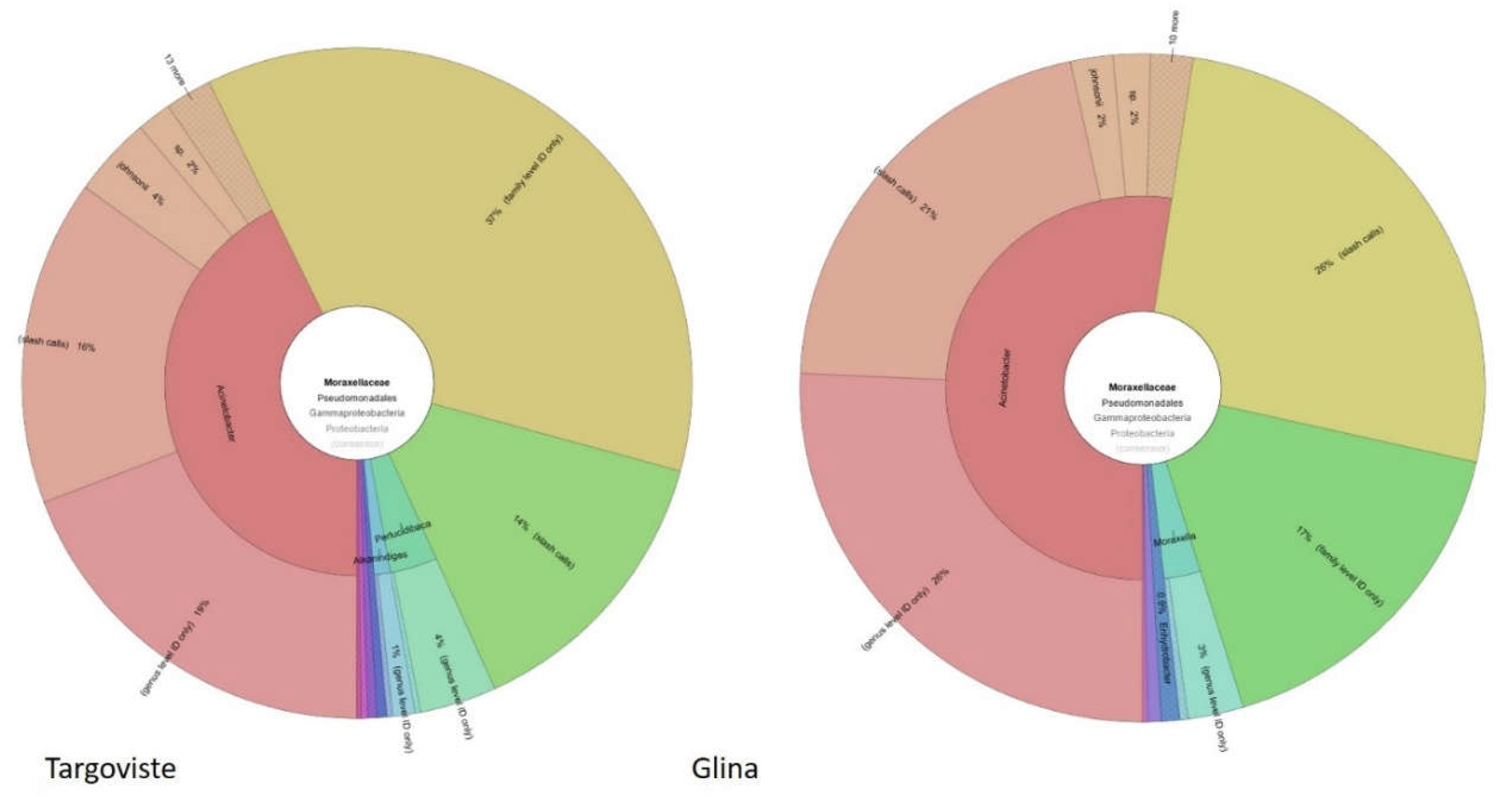
3.2. Metagenomic Analysis of Surface Water Samples and Fish Microbiota, to Highlight the Connection between the Environment and Fish Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Drane, K.; Sheehan, M.; Whelan, A.; Ariel, E.; Kinobe, R. The Role of Wastewater Treatment Plants in Dissemination of Antibiotic Resistance: Source, Measurement, Removal and Risk Assessment. Antibiotics 2024, 13, 668. [Google Scholar] [CrossRef] [PubMed]
- Calistri, P.; Iannetti, S.L.; Danzetta, M.; Narcisi, V.; Cito, F.; Di Sabatino, D.; Bruno, R.; Sauro, F.; Atzeni, M.; Carvelli, A.; Giovannini, A. The Components of “One World - One Health” Approach. Transboundary and Emerging Diseases. November 2013, pp 4–1. [CrossRef]
- Amos, G.C.A.; Ploumakis, S.; Zhang, L.; Hawkey, P.M.; Gaze, W.H.; Wellington, E.M.H. The Widespread Dissemination of Integrons throughout Bacterial Communities in a Riverine System. ISME Journal 2018, 12, 681–691. [Google Scholar] [CrossRef]
- Pérez-Valera, E.; de Melo Rangel, W.; Elhottová, D. Cattle Manure Application Triggers Short-Term Dominance of Acinetobacter in Soil Microbial Communities. Applied Soil Ecology 2022, 176, 104466. [Google Scholar] [CrossRef]
- Poirel, L.; Naas, T.; Nordmann, P. Diversity, Epidemiology, and Genetics of Class D β-Lactamases. Antimicrob Agents Chemother 2010, 54, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Blanco, N.; Harris, A.D.; Rock, C.; Johnson, J.K.; Pineles, L.; Bonomo, R.A.; Srinivasan, A.; Pettigrew, M.M.; Thom, K.A. Risk Factors and Outcomes Associated with Multidrug-Resistant Acinetobacter baumannii upon Intensive Care Unit Admission. Antimicrob Agents Chemother 2018, 62, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, I.; Cristea, V.C.; Marutescu, L.; Popa, M.; Murariu, C.; Trusca, B.S.; Borcan, E.; Ghita, C.; Lazar, V.; Chifiriuc, M.C. Resistance and Virulence Features in Carbapenem-Resistant Acinetobacter baumannii Community Acquired and Nosocomial Isolates in Romania. [CrossRef]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J Mol Biol 2019, 431, 3472–3500. [Google Scholar] [CrossRef] [PubMed]
- Nigro, S.J.; Hall, R.M. Structure and Context of Acinetobacter Transposons Carrying the Oxa23 Carbapenemase Gene. Journal of Antimicrobial Chemotherapy 2016, 71, 1135–1147. [Google Scholar] [CrossRef]
- Philippon, A.; Slama, P.; Dény, P.; Labia, R. A Structure-Based Classification of Class A β-Lactamases, a Broadly Diverse Family of Enzymes. Clin Microbiol Rev 2016, 29, 29–57. [Google Scholar] [CrossRef]
- Bouza, A.A.; Swanson, H.C.; Smolen, K.A.; VanDine, A.L.; Taracila, M.A.; Romagnoli, C.; Caselli, E.; Prati, F.; Bonomo, R.A.; Powers, R.A.; Wallar, B.J. Structure-Based Analysis of Boronic Acids as Inhibitors of Acinetobacter-Derived Cephalosporinase-7, a Unique Class C β-Lactamase. ACS Infect Dis 2018, 4, 325–336. [Google Scholar] [CrossRef]
- Vrancianu, C.O.; Pelcaru, C.F.; Alistar, A.; Gheorghe, I.; Marutescu, L.; Popa, M.; Czobor, I.; Gradisteanu, G.; Dobre, E.G.; Chifiriuc, M.C. Escaping from ESKAPE. Clinical Significance and Antibiotic Resistance Mechanisms in Acinetobacter baumannii: A Review. Biointerface Research in Applied Chemistry. AMG Transcend Association January 1, 2021, pp 8190–8203. [CrossRef]
- Chen, Y.; Gao, J.; Zhang, H.; Ying, C. Spread of the blaOXA-23-Containing Tn2008 in Carbapenem-Resistant Acinetobacter baumannii Isolates Grouped in CC92 from China. Front Microbiol 2017, 8. [Google Scholar] [CrossRef]
- Naderi, G.; Talebi, M.; Gheybizadeh, R.; Seifi, A.; Ghourchian, S.; Rahbar, M.; Abdollahi, A.; Naseri, A.; Eslami, P.; Douraghi, M. Mobile Genetic Elements Carrying Aminoglycoside Resistance Genes in Acinetobacter baumannii Isolates Belonging to Global Clone 2. Front Microbiol 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.-E.; Vallenet, D.; Barbe, V.; Audic, S.; Ogata, H.; Poirel, L.; Richet, H.; Robert, C.; Mangenot, S.; Abergel, C.; Nordmann, P.; Weissenbach, J.; Raoult, D.; Claverie, J.-M. Comparative Genomics of Multidrug Resistance in Acinetobacter baumannii. PLoS Genet 2006, 2, e7. [Google Scholar] [CrossRef] [PubMed]
- Barbu, I.C.; Gheorghe-Barbu, I.; Grigore, G.A.; Vrancianu, C.O.; Chifiriuc, M.C. Antimicrobial Resistance in Romania: Updates on Gram-Negative ESCAPE Pathogens in the Clinical, Veterinary, and Aquatic Sectors. Int J Mol Sci 2023, 24, 7892. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg Goldstein, R.E.; Micallef, S.A.; Gibbs, S.G.; George, A.; Claye, E.; Sapkota, A.; Joseph, S.W.; Sapkota, A.R. Detection of Vancomycin-Resistant Enterococci (VRE) at Four U.S. Wastewater Treatment Plants That Provide Effluent for Reuse. Science of The Total Environment 2014, 466–467, 404–411. [Google Scholar] [CrossRef] [PubMed]
- https://atlas.ecdc.europa.eu/public/index.aspx (Accessed 15 July 2024).
- CLSI, Performance Standards for Antimicrobial Susceptibility Testing. 32nd Ed. CLSI Supplement M100, Clinical Laboratory Standard Institute, 2022.
- Gheorghe-Barbu, I.; Corbu, V.M.; Vrancianu, C.O.; Marinas, I.C.; Popa, M.; Dumbravă, A. Ștefania; Niță-Lazăr, M.; Pecete, I.; Muntean, A.A.; Popa, M.I.; Marinescu, L.; Ficai, D.; Ficai, A.; Czobor Barbu, I. Phenotypic and Genotypic Characterization of Recently Isolated Multidrug-Resistant Acinetobacter baumannii Clinical and Aquatic Isolates and Demonstration of Silver Nanoparticle Potency. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- https://github.com/tseemann/shovill (Accessed 2 July 2024).
- https://github.com/tseemann/abricate (Accessed 2 July 2024).
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and Refined Dataset for Big Data Analysis—10 Years On. Nucleic Acids Res 2016, 44, D694–D697. [Google Scholar] [CrossRef]
- Seemann, T. Mlst (v2.19.0), 2024. Github. https://github.com/tseemann/mlst, (Accessed 1 July 2024).
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid Large-Scale Prokaryote Pan Genome Analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, J.; Croucher, N.J.; Goater, R.J.; Abudahab, K.; Aanensen, D.M.; Harris, S.R. Phandango: An Interactive Viewer for Bacterial Population Genomics. Bioinformatics 2018, 34, 292–293. [Google Scholar] [CrossRef]
- https://drpowell.github.io/FriPan/ (Accessed 12 July 2024).
- https://github.com/kwongj/roary2fripan (Accessed 12 July 2024).
- https://github.com/SethCommichaux/Heap_Law_for_Roary (Accessed 3 July 2024).
- Novović, K.; Jovčić, B. Colistin Resistance in Acinetobacter baumannii: Molecular Mechanisms and Epidemiology. Antibiotics. MDPI March 1, 2023. [CrossRef]
- Jovcic, B.; Novovic, K.; Dekic, S.; Hrenovic, J. Colistin Resistance in Environmental Isolates of Acinetobacter baumannii. Microbial Drug Resistance 2021, 27, 328–336. [Google Scholar] [CrossRef]
- Shelenkov, A.; Akimkin, V.; Mikhaylova, Y. International Clones of High Risk of Acinetobacter Baumannii—Definitions, History, Properties and Perspectives. Microorganisms 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- http://www.mgc.ac.cn/cgi-bin/VFs/genus.cgi?Genus=Acinetobacter. (Accessed 1 July 2024).
- Zaharia, L.; Ioana-toroimac, G.; Cocoş, O.; Ghiţă, F.A.; Mailat, E. Urbanization Effects on the River Systems in the Bucharest City Region (Romania). Ecosystem Health and Sustainability 2016, 2, e01247. [Google Scholar] [CrossRef]
- Manoiu, V.-M. Water Quality Changes in Ialomiţa River under the Influence of Human Settlements and Activities. In Aerul şi Apa: Componente ale Mediului; 2017, 2017, 317–324. [CrossRef]
- Ušjak, D.; Novović, K.; Filipić, B.; Kojić, M.; Filipović, N.; Stevanović, M.M.; Milenković, M.T. In Vitro Colistin Susceptibility of Pandrug-resistant Acinetobacter baumannii Is Restored in the Presence of Selenium Nanoparticles. J Appl Microbiol 2022, 133, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Cafiso, V.; Stracquadanio, S.; Lo Verde, F.; Gabriele, G.; Mezzatesta, M.L.; Caio, C.; Pigola, G.; Ferro, A.; Stefani, S. Colistin Resistant Acinetobacter baumannii: Genomic and Transcriptomic Traits Acquired Under Colistin Therapy. Front Microbiol 2019, 9. [Google Scholar] [CrossRef]
- Kabic, J.; Novovic, K.; Kekic, D.; Trudic, A.; Opavski, N.; Dimkic, I.; Jovcic, B.; Gajic, I. Comparative Genomics and Molecular Epidemiology of Colistin-Resistant Acinetobacter baumannii. Comput Struct Biotechnol J 2023, 21, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, M.M.; Li, B.; Pacey, M.P.; Mettus, R.T.; McElheny, C.L.; Marshall, C.W.; Ernst, R.K.; Cooper, V.S.; Doi, Y. Phylogenomics of Colistin-Susceptible and Resistant XDR Acinetobacter baumannii. Journal of Antimicrobial Chemotherapy 2018, 73, 2952–2959. [Google Scholar] [CrossRef] [PubMed]
- Trebosc, V.; Gartenmann, S.; Tötzl, M.; Lucchini, V.; Schellhorn, B.; Pieren, M.; Lociuro, S.; Gitzinger, M.; Tigges, M.; Bumann, D.; Kemmer, C. Dissecting Colistin Resistance Mechanisms in Extensively Drug-Resistant Acinetobacter baumannii Clinical Isolates. mBio 2019, 10, 10–1128. [Google Scholar] [CrossRef]
- Fam, N.S.; Gamal, D.; Mohamed, S.H.; Wasfy, R.M.; Soliman, M.S.; El-Kholy, A.A.; Higgins, P.G. Molecular Characterization of Carbapenem/ Colistin-Resistant Acinetobacter baumannii Clinical Isolates from Egypt by Whole-Genome Sequencing. Infect Drug Resist 2020, 13, 4487–4493. [Google Scholar] [CrossRef]
- Palmieri, M.; D’Andrea, M.M.; Pelegrin, A.C.; Perrot, N.; Mirande, C.; Blanc, B.; Legakis, N.; Goossens, H.; Rossolini, G.M.; van Belkum, A. Abundance of Colistin-Resistant, OXA-23- and ArmA-Producing Acinetobacter baumannii Belonging to International Clone 2 in Greece. Front Microbiol 2020, 11. [Google Scholar] [CrossRef]
- Thadtapong, N.; Chaturongakul, S.; Soodvilai, S.; Dubbs, P. Colistin and Carbapenem-Resistant Acinetobacter baumannii Aci46 in Thailand: Genome Analysis and Antibiotic Resistance Profiling. Antibiotics 2021, 10, 1054. [Google Scholar] [CrossRef]
- Pournaras, S.; Poulou, A.; Dafopoulou, K.; Chabane, Y.N.; Kristo, I.; Makris, D.; Hardouin, J.; Cosette, P.; Tsakris, A.; Dé, E. Growth Retardation, Reduced Invasiveness, and Impaired Colistin-Mediated Cell Death Associated with Colistin Resistance Development in Acinetobacter baumannii. Antimicrob Agents Chemother 2014, 58, 828–832. [Google Scholar] [CrossRef]
- Agodi, A.; Voulgari, E.; Barchitta, M.; Quattrocchi, A.; Bellocchi, P.; Poulou, A.; Santangelo, C.; Castiglione, G.; Giaquinta, L.; Romeo, M.A.; Vrioni, G.; Tsakris, A. Spread of a Carbapenem- and Colistin-Resistant Acinetobacter baumannii ST2 Clonal Isolate Causing Outbreaks in Two Sicilian Hospitals. Journal of Hospital Infection 2014, 86, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.; Singh-Moodley, A.; Ismail, H.; Thomas, T.; Chibabhai, V.; Nana, T.; Lowman, W.; Ismail, A.; Chan, W.Y.; Perovic, O. Molecular Characterisation of Acinetobacter baumannii Isolates from Bloodstream Infections in a Tertiary-Level Hospital in South Africa. Front Microbiol 2022, 13. [Google Scholar] [CrossRef]
- Nogbou, N.-D.; Ramashia, M.; Nkawane, G.M.; Allam, M.; Obi, C.L.; Musyoki, A.M. Whole-Genome Sequencing of a Colistin-Resistant Acinetobacter baumannii Isolate Isolated at a Tertiary Health Facility in Pretoria, South Africa. Antibiotics 2022, 11, 594. [Google Scholar] [CrossRef]
- Blejan, I.E.; Diaconu, C.E.; Arsene, A.L.; Udeanu, D.I.; Ghica, M.; Drăgănescu, D.; Dragomiroiu, G.T.A.B.; Rădulescu, M.; Maltezou, H.C.; Tsatsakis, A.M.; Papasavva, M.; Drakoulis, N.; Popa, D.E. Antibiotic Resistance in Community-Acquired Pneumonia. A Romanian Perspective. Farmacia 2020, 68, 512–520. [Google Scholar] [CrossRef]
- Goic-Barisic, I.; Hrenovic, J.; Kovacic, A.; Musić, M.Š. Emergence of Oxacillinases in Environmental Carbapenem-Resistant Acinetobacter baumannii Associated with Clinical Isolates. Microbial Drug Resistance 2016, 22, 559–563. [Google Scholar] [CrossRef]
- Pulami, D.; Kämpfer, P.; Glaeser, S.P. High Diversity of the Emerging Pathogen Acinetobacter Baumannii and Other Acinetobacter Spp. in Raw Manure, Biogas Plants Digestates, and Rural and Urban Wastewater Treatment Plants with System Specific Antimicrobial Resistance Profiles. Science of The Total Environment 2023, 859, 160182. [Google Scholar] [CrossRef]
- Higgins, P.G.; Hrenovic, J.; Seifert, H.; Dekic, S. Characterization of Acinetobacter baumannii from Water and Sludge Line of Secondary Wastewater Treatment Plant. Water Res 2018, 140, 261–267. [Google Scholar] [CrossRef]
- Jones, C.L.; Clancy, M.; Honnold, C.; Singh, S.; Snesrud, E.; Onmus-Leone, F.; McGann, P.; Ong, A.C.; Kwak, Y.; Waterman, P.; Zurawski, D.V.; Clifford, R.J.; Lesho, E. Fatal Outbreak of an Emerging Clone of Extensively Drug-Resistant Acinetobacter baumannii With Enhanced Virulence. Clinical Infectious Diseases 2015, 61, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.B.; Pham Thanh, D.; Tran Do Hoan, N.; Wick, R.R.; Ingle, D.J.; Hawkey, J.; Edwards, D.J.; Kenyon, J.J.; Phu Huong Lan, N.; Campbell, J.I.; Thwaites, G.; Thi Khanh Nhu, N.; Hall, R.M.; Fournier-Level, A.; Baker, S.; Holt, K.E. Repeated Local Emergence of Carbapenem-Resistant Acinetobacter baumannii in a Single Hospital Ward. Microb Genom 2016, 2. [Google Scholar] [CrossRef]
- Abhari, S.S.; Badmasti, F.; Modiri, L.; Aslani, M.M.; Asmar, M. Circulation of Imipenem-Resistant Acinetobacter baumannii ST10, ST2 and ST3 in a University Teaching Hospital from Tehran, Iran. J Med Microbiol 2019, 68, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Meumann, E.M.; Anstey, N.M.; Currie, B.J.; Piera, K.A.; Kenyon, J.J.; Hall, R.M.; Davis, J.S.; Sarovich, D.S. Genomic Epidemiology of Severe Community-Onset Acinetobacter baumannii Infection. Microb Genom 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Valcek, A.; Nesporova, K.; Whiteway, C.; De Pooter, T.; Coster, W. De; Strazisar, M.; der Henst, C. Van. Genomic Analysis of a Isolate Collection Containing Multidrug-, Extensively Drug-, Pandrug-, and Carbapenem-Resistant Modern Clinical Isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 2022, 66, e00892–22. [Google Scholar] [CrossRef] [PubMed]
- Savin, M.; Sib, E.; Heinemann, C.; Eichel, V.M.; Nurjadi, D.; Klose, M.; Andre Hammerl, J.; Binsker, U.; Mutters, N.T. Tracing Clinically-Relevant Antimicrobial Resistances in Acinetobacter baumannii -calcoaceticus Complex across Diverse Environments: A Study Spanning Clinical, Livestock, and Wastewater Treatment Settings. Environ Int 2024, 186, 108603. [Google Scholar] [CrossRef]
- Park, S.C.; Lee, K.; Kim, Y.O.; Won, S.; Chun, J. Large-Scale Genomics Reveals the Genetic Characteristics of Seven Species and Importance of Phylogenetic Distance for Estimating Pan-Genome Size. Front Microbiol 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe-Barbu, I.; Surleac, M.; Barbu, I.C.; Paraschiv, S.; Bănică, L.M.; Rotaru, L.I.; Vrâncianu, C.O.; Niță Lazăr, M.; Oțelea, D.; Chifiriuc, M.C. Decoding the Resistome, Virulome and Mobilome of Clinical versus Aquatic Acinetobacter baumannii in Southern Romania. Heliyon 2024, 10. [Google Scholar] [CrossRef]
- Sehnal, L.; Brammer-Robbins, E.; Wormington, A.M.; Blaha, L.; Bisesi, J.; Larkin, I.; Martyniuk, C.J.; Simonin, M.; Adamovsky, O. Microbiome Composition and Function in Aquatic Vertebrates: Small Organisms Making Big Impacts on Aquatic Animal Health. Front Microbiol 2021, 12. [Google Scholar] [CrossRef]
- Shayo, G.M.; Elimbinzi, E.; Shao, G.N.; Fabian, C. Severity of Waterborne Diseases in Developing Countries and the Effectiveness of Ceramic Filters for Improving Water Quality. Bull Natl Res Cent 2023, 47, 113. [Google Scholar] [CrossRef]
| ANTIBIOTIC/ ISOLATE | 22012-CA5 | 22012-ENE6 | 22013-CA5 | 22013-ENE4 | 22014-CA2 | 22014-COLN5 | 22015-CA3 | 22015-CA4 | 22015-ENE6 | 22016-CA2 | 22016-CNE3 | 22016-CNE4 | 22017-CNE1 | 22018-CA5 | 22018-CA6 | 22019-CNE4 | 22019-CNE5 | 24-IHI | 3-IHI | 49-IHI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COLISTIN (µG/ML) | 0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | 1 | <0.25 | <0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





