1. Introduction
Polycystic ovary syndrome (PCOS) is a common and diverse disorder that affects reproductive, endocrine, and metabolic functions in women [
1]. The prevalence of PCOS among women of reproductive age varies, ranging from 6% to 25% depending on the diagnostic criteria used [
2]. A significant proportion of women with PCOS are overweight or obese, with rates reported up to 61% [
3], and a substantial number also experience insulin resistance, ranging from 44% to 70% [
4]. Insulin resistance is a central factor in the development of hyperandrogenism and chronic inflammation in women with PCOS [
5]. Elevated levels of inflammatory markers such as C-reactive protein (CRP) are associated with an increased risk of cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus (T2DM) in women with PCOS [
6].
CRP is produced by the liver in response to IL-6 and TNF-α [
7], and is considered both an indicator of low-grade chronic inflammation and an active contributor to the development of atherosclerosis [
8]. Adipose tissue releases various bioactive substances known as adipocytokines, including leptin, TNF, IL-6, IL-18, plasminogen activator inhibitor type 1, and adiponectin, which further contribute to inflammation and metabolic disorders [
9,
10]. Adiponectin is thought to have insulin-sensitizing [
9], antiatherogenic, and anti-inflammatory properties [
11], and there is a reverse correlation between adiponectin and CRP levels [
12]. Emerging evidence suggests that novel cardiovascular risk factors are also deregulated in PCOS, with increased CRP [
13,
14], IL-6 [
15,
16], and TNF levels [
17], as well as reduced adiponectin levels observed in both obese and non-obese women with PCOS [
18,
19].
Hormonal manifestations in PCOS involve increased serum concentrations of androgens including testosterone, DHEA, and androstenedione [
20], and reduced sex hormone-binding globulin (SHBG) levels [
21]. Most women with PCOS show elevated luteinizing hormone (LH) and decreased follicle-stimulating hormone (FSH) levels during the follicular phase [
22], contributing to increased androgen concentrations, follicular arrest, and an accumulation of small follicles within the ovary [
23].
The initial approach to treating PCOS involves lifestyle modifications, with an emphasis on weight loss, a key recommendation from the World Health Organization (WHO) in the management of this condition [
24]. Weight management is also recommended for infertile PCOS women undergoing assisted reproductive technology (ART) procedures [
25]. Lifestyle management leads to enhancements in the reproductive, metabolic, endocrine, and psychological aspects of PCOS [
26]. In the broader population, standard guidelines for weight control within lifestyle interventions include a diet that is low in fat (around 30% of total energy intake, with approximately 10% coming from saturated fat and less than 300 mg of daily cholesterol), moderate in protein (about 15%), high in carbohydrates (approximately 55%), and rich in fibre, in conjunction with regular moderate exercise [
27,
28]. Furthermore, previous research has indicated that adopting a nutritious eating pattern could be linked to the metabolic characteristics and levels of inflammatory cytokines in conditions associated with metabolic syndrome [
29,
30].
The effect of diet-induced weight loss on CRP and other inflammatory markers in PCOS women has previously been investigated in several studies with conflicting results. While some studies have reported no differences in CRP levels before and after weight loss in PCOS women [
31,
32], others have found that weight loss led to a reduction in CRP levels in this population [
33,
34]. There have been no previous systematic reviews assessing weight loss through dietary interventions alone on CRP and other inflammatory markers in PCOS women. A systematic review published in 2013 reported that weight loss through dietary intervention resulted in subtle and inconsistent improvements in anthropometric measurements, reproductive health, metabolic factors, and overall quality of life in conjunction with reductions in glycaemic load [
35]. However, it remains unclear whether weight loss has a discernible effect on inflammatory markers in women with PCOS. The purpose of the current meta-analysis was therefore to conduct a comprehensive systematic review of available human studies assessing the effect of weight loss through dietary interventions on inflammatory status and hyperandrogenism in PCOS women.
2. Materials and Methods
This systematic review was prospectively registered with PROSPERO (registration number CRD42023412566) and carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria.
2.1. Eligibility Criteria for Study Selection
We considered all studies including clinical trials and cohort studies comparing the serum levels of CRP, other inflammatory markers (e.g. IL-6, IL-B, TNF-α), androgens and LH in women with PCOS undergoing weight loss through dietary interventions. Only studies that matched and/or adjusted for age and BMI and measured circulating CRP, TNF, IL-16 and/or testosterone were included. The review only included English-language human studies with drug-naïve, nonpregnant women aged 16-39 years who had no prior medical history of conditions that could have affected their inflammation markers or reproductive endocrine profile.
2.2. Outcome Measures
2.2.1. Primary Outcomes
Serum concentrations of CRP, IL-6, TNF, and/or IL-1β in PCOS women before and after weight loss through dietary intervention.
2.2.2. Secondary Outcome Measures
Serum levels of the anti-inflammatory factor adiponectin, androgens (SHBG, testosterone, DHEAS, androstenedione) and LH in PCOS women before and after diet-induced weight loss.
2.3. Search Strategy
EMBASE (Ovid); Medline (Ovid); CENTRAL (
www.thecochranelibrary.com) accessed on 23 October 2023; Clinicaltrials.gov; the EU Clinical Trials Register; PubMed; and the World Health Organisation International Clinical Trials Register were systematically searched starting from 1946 to October 2023 for relevant studies. A combination of the following search terms was used: “Weight Loss” OR “Weight management” OR “diets intake” OR “Energy Intake” OR “LOW-CARB DIET” OR “Caloric Restriction” AND “Polycystic Ovary Syndrome” AND “inflammatory markers” OR “C-reactive protein” OR “Interleukin-6” OR “TNF-ALPHA” OR “IL-1BETA” OR “Adiponectin”. The keywords were combined using Boolean operators for each database, as appropriate.
2.4. Screening and Selection of Retrieved Studies
The titles and abstracts of studies retrieved by the electronic search were independently screened by two authors (SAA and AA) for relevance. Full texts of the pertinent articles were further evaluated, and eligible studies were selected according to the inclusion and exclusion criteria. A third author (STA) adjudicated any discrepancies between authors.
2.5. Assessment of Quality and Risk of Bias
The Cochrane Collaboration’s tool [
36] was used to evaluate the quality of the included randomised controlled trials (RCTs) (
Table 1). The generation of random sequences, allocation concealment, blinding of outcome assessments, incomplete outcome data, selective reporting, and other biases were among the methodological domains that were taken into consideration. However, blinding of participants could not be achieved in our research, as individuals undergoing a diet intervention cannot be effectively blinded. Each assessed item received one of three scores: low, unclear, or high for bias. For this evaluation, we referred to the quality assessment table found in (part 2, Chapter 8.5) of the Cochrane Handbook of Systematic Reviews of Interventions. The quality and risk of bias of the observational studies were evaluated using the Newcastle-Ottawa scale (NOS) for the assessment of cohort studies, based on the recommendation of the Cochrane Collaboration [
36,
37]. The original Newcastle-Ottawa scale for nonrandomized studies assesses three main categories including selection, comparability and outcomes giving a maximum of four, two and three stars for each category, respectively [
37]. This scale was modified to suit the nature of this study giving a maximum of three stars for selection (representativeness of the exposed cohort, ascertainment of exposure, and demonstration that outcome of interest was not present at the start of the study), four for comparability (studies including PCOS women with age ≤40 yr, BMI >25, using low caloric diet interventions, and studies employing the Rotterdam criteria for the diagnosis of PCOS) and two for outcome criteria (assessment of outcome and follow-up long enough for outcomes to occur) [
38,
39] (
Table 2). Studies with six or more stars were classified as being of good quality [
40]. The quality assessment was conducted by two authors (S.A.A. and A.A.), and every disagreement was resolved by a third reviewer (N.E.).
2.6. Data Extraction and Analysis
Data (mean ± SD) were extracted from the individual articles including demographics (age and BMI), inflammatory markers (CRP, TNF, IL-6, IL-1β), adiponectin, androgens (testosterone, SHBG, DHEAS, androstenedione) and LH. These data were uploaded into RevMan software, version 5.9 (The Nordic Cochrane Centre, Copenhagen, Denmark; The Cochrane Collaboration, 2011) for meta-analysis. The standardized mean difference (SMD) between baseline and post-weight loss data and 95% confidence interval (CI) were calculated for inflammatory markers and hormones. For RCTs, we only included baseline and post-weight loss data from the arm including PCOS women undergoing the dietary intervention. The SDM model was used in this meta-analysis due to the differences in CRP measurements among the included studies [
41]. The SMD has been shown to be more generalizable and an easier way to assess the degree of variation between groups, in addition to being independent of the unit of measurement [
42]. According to the general rule described by Cohen et al., a difference that is considered “small” is represented by an SMD of 0.2, “medium” by an SMD of 0.5, and “large” by an SMD of 0.8 [
43].
To assess the statistical heterogeneity between studies, the chi-square test and I-squared (I2) statistics were utilised. I2 ≥50% or chi-square analyses higher than its degree of freedom indicated high heterogeneity. An initial overall meta-analysis for CRP was performed for all included investigations. Further subgrouping analysis of CRP data was conducted with a diet period more or less than 8 weeks.
3. Results
3.1. Search results
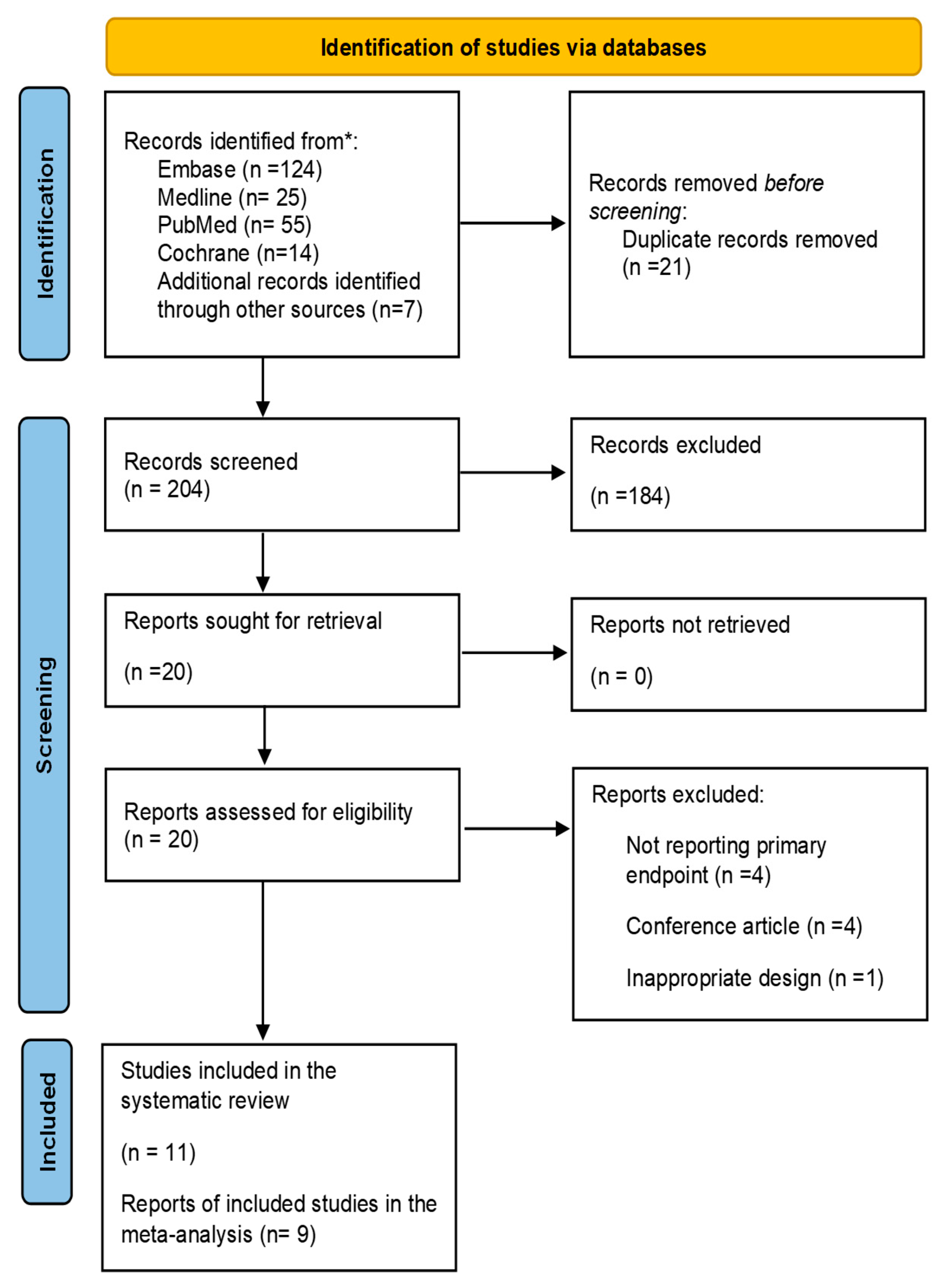
The initial electronic database search identified 225 articles, which were reduced to 204 after removing duplicates. During the screening of the title and abstract, 184 irrelevant articles were excluded. After a thorough review of the full text of the remaining 20 papers, an additional nine did not meet the eligibility criteria and were consequently excluded as illustrated in
Figure 1. The remaining 11 studies fulfilled the eligibility criteria and were included in this review [
26,
31,
32,
33,
34,
44,
45,
46,
47,
48,
49].
3.2. Risk of Bias and Quality Assessment of Selected Studies
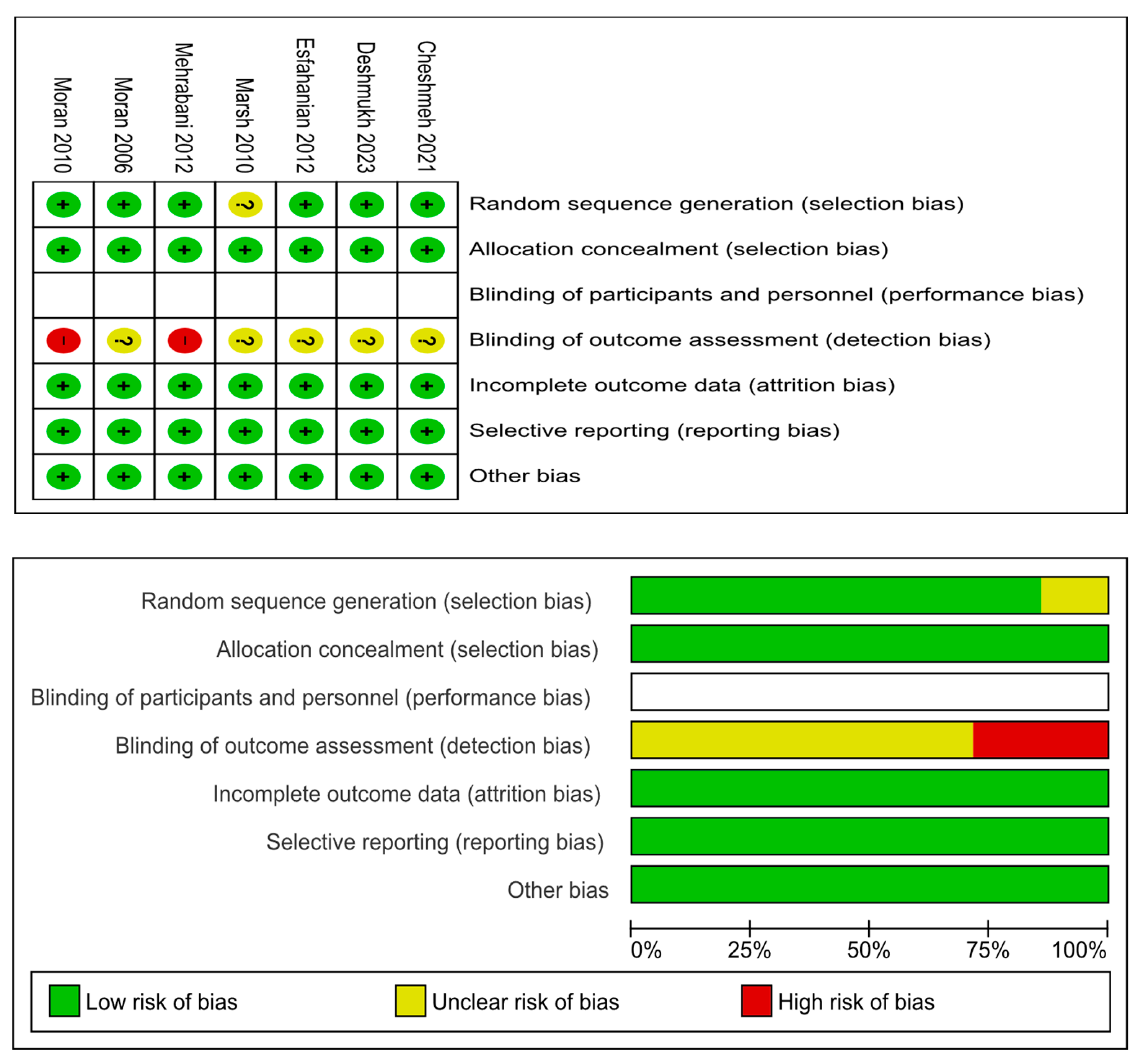
The details of risks of bias assessments within the seven included RCTs are presented in
Figure 2. Six RCTs reported adequate methods of random sequence generation while the remaining RCT [
44] was not clear on this. All seven RCTs reported adequate methods of allocation concealment. None of the seven RCTs blinded their participants or personnel due to the nature of diet intervention. Given that all outcomes of interest were objective outcomes, it is unlikely that non-blinding would introduce any bias. All seven RCTs had low risk of attrition and reporting bias.
Table 1 summarises the quality scores of the four cohort studies included in the review. All studies scored between 6 and 9 on the modified Newcastle–Ottawa scale.
Table 1.
The Newcastle–Ottawa Scale (NOS) [
37] was used for assessing the cohort studies.
Table 1.
The Newcastle–Ottawa Scale (NOS) [
37] was used for assessing the cohort studies.
| 1st Author, Year |
Selection |
Comparability |
Outcome |
Overall |
|
Szczuko, 2018 [45] |
** |
*** |
** |
7 |
|
Moran, 2007 [49] |
*** |
**** |
** |
9 |
|
Asemi, 2015 [46] |
** |
** |
** |
6 |
|
Olszanecka-Glinianowicz, 2008 [48] |
*** |
** |
** |
7 |
The star scoring system was redistributed to have a maximum of three stars for selection, four stars for comparability, and two stars for outcome criteria.
3.3. Included Studies
The review included 11 eligible studies (n = 323) investigating changes in circulating inflammatory markers and androgens after diet induced weight loss in women with PCOS.
3.3.1. Study designs
Seven of the 11 included studies (n=247) were RCTs, while four (n=76) were cohort studies. For the RCTs, only the weight loss intervention arm was included in the meta-analysis.
3.3.2. Study characteristics
The characteristics of all the studies are summarised in
Table 3. In 10 studies, PCOS participants underwent a hypocaloric diet to reduce weight, while the remaining study utilised a 16.5-hour fasting diet style.
3.3.2. Study participants
A total of 323 overweight/obese women with PCOS were enrolled in all 11 included studies. An appropriate participants’ selection was used in all articles, which met our inclusion criteria. The diagnosis of PCOS in all 11 studies adhered to the Rotterdam ESHRE/ASRM criteria. Participants across these studies were within the childbearing age range of 15 to 38 years, had no endocrinological diseases, were not pregnant at the time of participation, and had not been on any medication that could impact the levels of inflammatory markers in the preceding three months.
3.4. Outcome data
Of the 11 included studies, CRP was reported in nine [
26,
31,
32,
33,
34,
44,
46,
47,
49], TNF in four [
31,
32,
45,
48], IL-6 in three [
31,
32,
48], adiponectin in one study [
31] (
Table 2). However, only CRP, IL-6 and TNF-α reported data suitable for the meta-analysis. There was no sufficient data to include in a meta-analysis for adiponectin or IL-1β, which were only included in the systematic review.
Among the 11 articles, SHBG in six (n=223) [
31,
32,
33,
44,
45,
48], DHEAS in six [
31,
32,
34,
44,
45,
48], testosterone was reported in six [
32,
33,
34,
44,
45,
48] and androstenedione in four studies (n=147) [
32,
44,
45,
48] (
Table 3). LH was reported in five studies (n=197) [
32,
33,
44,
45,
48] (
Table 2).
Table 2.
Characteristics of the 11 included studies investigating changes in circulating inflammatory markers and androgens in PCOS women undergoing diet-induced weight loss.
Table 2.
Characteristics of the 11 included studies investigating changes in circulating inflammatory markers and androgens in PCOS women undergoing diet-induced weight loss.
| First Author, Year |
Country |
Study Design |
n |
Age
(Y) |
Diet intervention |
BMI (Kg/m2) - [Weight (Kg)] |
Outcomes measured |
| Type |
Duration
[weeks] |
Before |
After |
p |
Inflammatory markers |
Androgens |
| Moran, 2006 [26] |
Australia |
RCT |
34 |
32.1±5.2 |
energy-restricted diet |
8 |
34.9±7.0
[96.0 ± 3.3] |
-
[90.3±3.3] |
- |
CRP |
– |
| Mehrabani, 2012 [31] |
Iran |
RCT |
26 |
28.5±5.2 |
low-calorie diet |
12 |
31.1±4.6
[78.9 ± 12.4] |
–
[74.8±0.5] |
- |
CRP, TNF-α, IL-6 |
SHBG |
| Cheshmeh, 2021 [32] |
Iran |
RCT |
99 |
33.8±5.4 |
low-calorie diet |
16 |
35.18±5.16 |
32.86±5.95 |
<0.001 |
CRP, TNF-α, IL-6 |
Testosterone, SHBG, DHEAS, Androstenedione |
| Marsh, 2010 [33] |
Australia |
RCT |
50 |
29.3±0.8 |
low-glycaemic index diet |
Up to 48 |
34.7 ± 0.9 |
33.2 ±0.6 |
- |
CRP |
Testosterone, SHBG, |
| Esfahanian, 2012 [34] |
Iran |
RCT |
13 |
20.0± 4.6 |
low-calorie diet |
12 |
34.1 ± 5.4 |
30.1± 5.5 |
<0.001 |
CRP |
Testosterone, DHEAS |
| Deshmukh, 2023 [44] |
UK |
RCT |
11 |
27.7±3.8 |
Very low-calorie diet |
8 |
37.8±3.9 |
33.7±3.9 |
<0.0001 |
CRP |
Testosterone, SHBG, DHEAS, Androstenedione |
| Szczuko, 2018 [45] |
Poland |
Cohort |
22 |
26.6±4.2 |
low-glycaemic index diet |
12 |
28.38
[79.13±14.58] |
26.1
[73.01±10.18] |
< 0.05 |
TNF-α |
Testosterone, SHBG, DHEAS, Androstenedione |
| Asemi, 2015 [46] |
Iran |
Cohort |
27 |
27.5±3.6 |
Fasting [16.5/day] |
4 |
28.6±3.9 |
28.4±3.9 |
0.64 |
CRP |
– |
| Moran, 2010 [47] |
Australia |
RCT |
14 |
32.8±4.5 |
energy-restricted diet |
16 |
37.6 ± 7.1 |
34.9 ± 6.5 |
- |
CRP |
– |
| Olszanecka-Glinianowicz, 2008 [48] |
Poland |
Cohort |
15 |
28.5±7.7 |
low-calorie diet |
ND |
36.1±6.6 |
31.6±5.8 |
<.00001 |
TNF-α, IL-6 |
Testosterone, SHBG, DHEAS, Androstenedione |
| Moran, 2007 [49] |
Australia |
Cohort |
12 |
31.7±6.2 |
Energy-restricted diet |
8 |
35.7±5.8
[95.1±19.3] |
–
[91.2±15.7] |
- |
CRP |
– |
3.4. Systematic Review
3.4.1. Inflammatory markers
Serum
CRP levels were measured before and after diet-induced weight loss in PCOS women in nine studies, n=286, of which three (n=61) reported a significant decrease [
26,
34,
47], while the remaining six (n=225) showed no statistically significant change in circulating CRP after the weight loss [
31,
32,
33,
44,
46,
49] (
Table 4).
Circulating
IL-6 levels were compared before and after diet-induced weight loss in individuals with PCOS in three studies (n=140), with no significant difference reported [
31,
32,
48] (
Table 3).
Serum
TNF-α concentrations were measured in four studies (n=162), of which three (n=136) showed no change [
32,
45,
48], while one (n=26) reported a significant decrease after the diet-induced weight loss [
31] (
Table 3).
2.4.2. Anti-inflammatory marker
One study (n=26) assessed circulating a
diponectin showing significantly increased levels after diet-induced weight loss compared to baseline levels [
31] (
Table 3).
3.4.2. Androgens and LH
Two studies (n=35) reported a significant reduction in serum
testosterone levels in PCOS women after diet-induced weight loss [
34,
45], while four studies (n=175) showed no significant change compared to baseline levels before the dietary interventions [
32,
33,
44,
45,
48] (
Table 4).
Two studies (n=37) measured
SHBG showed a significant increase after diet-induced weight loss in PCOS women [
31,
44], while four studies (n=186) showed no significant change compared to baseline levels before the dietary interventions [
32,
33,
45,
48] (
Table 4).
Circulating
androstenedione was evaluated before and after diet-induced weight loss in four studies (n=147), with two (n=121) reporting a significant reduction [
32,
45] and two (n=26) showing no significant change after the weight loss compared to baseline levels [
44,
48] (
Table 4).
Six articles (n=186) assessed circulating
DHEAS levels before and after diet-induced weight loss. Five studies (n=160) reported no change [
32,
34,
44,
45,
48], while one study (n=26) showed a significant increase in post-weight loss serum DHEAS levels compared to baseline levels before dietary intervention [
31] (
Table 4).
Circulating
LH was measured in five studies (n=197), with four (n=175) reporting no significant difference [
32,
33,
44,
48], one (n=22) showing no significant change after the weight loss compared to baseline levels [
45] (
Table 4).
Table 3.
Changes in circulating CRP and other inflammatory markers after diet-induced weight loss in PCOS women.
Table 3.
Changes in circulating CRP and other inflammatory markers after diet-induced weight loss in PCOS women.
| First Author, Year |
n |
CRP |
IL-6 (pg/mL) |
TNF-α (pg/mL) |
Adiponectin (ng/mL) |
| Before |
After |
p |
Before |
After |
p |
Before |
After |
p |
Before |
After |
p |
| Moran, 2006 [26] |
34 |
3.30 ± 0.40* |
2.80±0.30* |
<0.05 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Mehrabani, 2012 [31] |
26 |
2.70±0.60** |
2.6±00.60 ** |
- |
1.1±0.06 |
1.0±0.06 |
- |
6.3±0.9 |
3.8±0.8 |
<0.005 |
59.6±4.4 |
67.7±4.6 |
<0.005 |
| Cheshmeh, 2021 [32] |
99 |
1.50±0.18* |
1.50±0.18* |
0.1 |
2.28±1.41 |
2.17±1.41 |
0.76 |
6.62±0.51 |
6.58±1.58 |
0.45 |
- |
- |
- |
| Marsh, 2010 [33] |
50 |
5.30±0.80** |
4.10±0.13 ** ◊◊ |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Esfahanian, 2012 [34] |
13 |
60.00±21.00** |
42.00±16.00** |
0.04 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Deshmukh, 2023 [44] |
11 |
7.00±5.70** |
6.30±5.50 ** |
0.7 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Szczuko, 2018 [45] |
22 |
- |
- |
- |
- |
- |
- |
59.7±18.2 |
57.6±12.3 |
- |
- |
- |
- |
| Asemi, 2015 [46] |
27 |
2.96± 2.84** |
2.0 ± 1.68 ** |
0.07 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Moran, 2010 [47] |
14 |
9.0 ± 6.0** |
7.10±5.0 ** |
0.003 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Olszanecka-Glinianowicz, 2008 [48] |
15 |
- |
- |
|
6.0±2.0 |
4.7±2.1 |
>.05 |
6.6±3.0 |
6.1±3.6 |
>.05 |
- |
- |
- |
| Moran, 2007 [49] |
12 |
5.50±3.10** |
5.90±3.30** |
0.066 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Table 4.
Changes in circulating androgens and LH after diet-induced weight loss in PCOS women.
3.5. Meta-Analysis
3.5.1. Inflammatory Markers
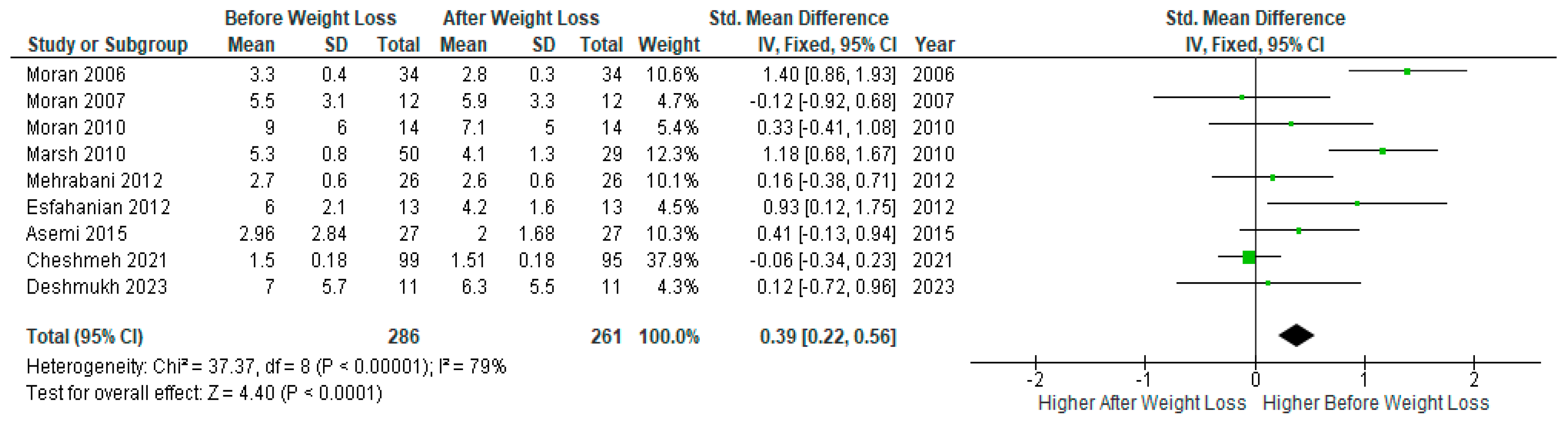
A pooled analysis of nine studies (n=286) showed significantly lower serum
CRP levels after diet-induced weight loss compared to baseline levels before weight loss in PCOS women (SMD 0.39, 95% Cl, 0.22, 0.56; z= 4.40; p <0.0001; I
2=79%). The heterogeneity between studies was high (
Figure 3).
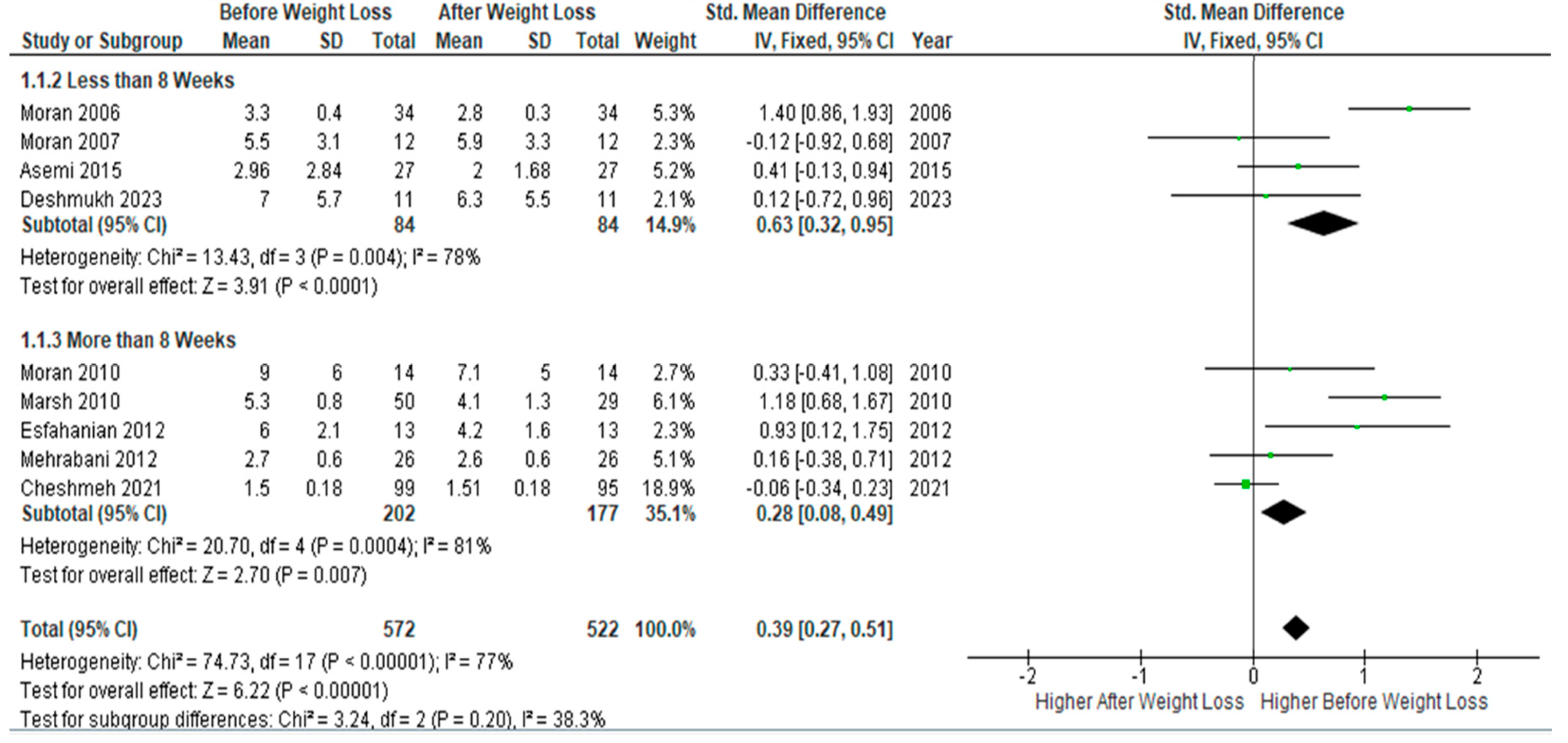
Subgroup meta-analysis of four studies (n= 84) with follow-up periods of ≤8 weeks of dietary interventions showed a statically significant decrease in serum
CRP levels after weight loss compared to baseline levels (SMD 0.63, 95% Cl, 0.32, 0.95; z= 3.91; p <0.0001; I
2=78%). Similarly, but to a less extent, pooled analysis of five studies (n=177) with follow-up periods > 8 weeks showed a significant drop in circulating CRP at follow-up assessment, but with a smaller SMD (SMD 0.28, 95% Cl, 0.08, 0.49; z= 2.70; p =0.007; I
2=81%). Heterogeneity between studies was low (
Figure 4).
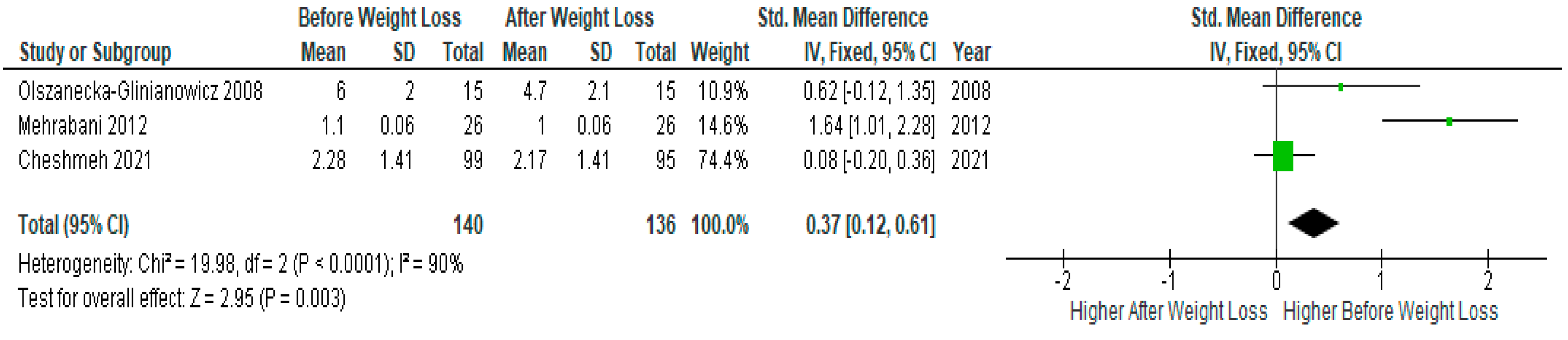
Three studies presented IL-6 data suitable for meta-analysis (n=140) showed significantly lower serum IL-6 levels after diet-induced weight loss compared to baseline levels before weight loss in PCOS women (SMD 0.37, 95% Cl, 0.12, 0.61; z= 2.95; p =0.003; I2=90%). The heterogeneity between studies was high (
Figure 5).
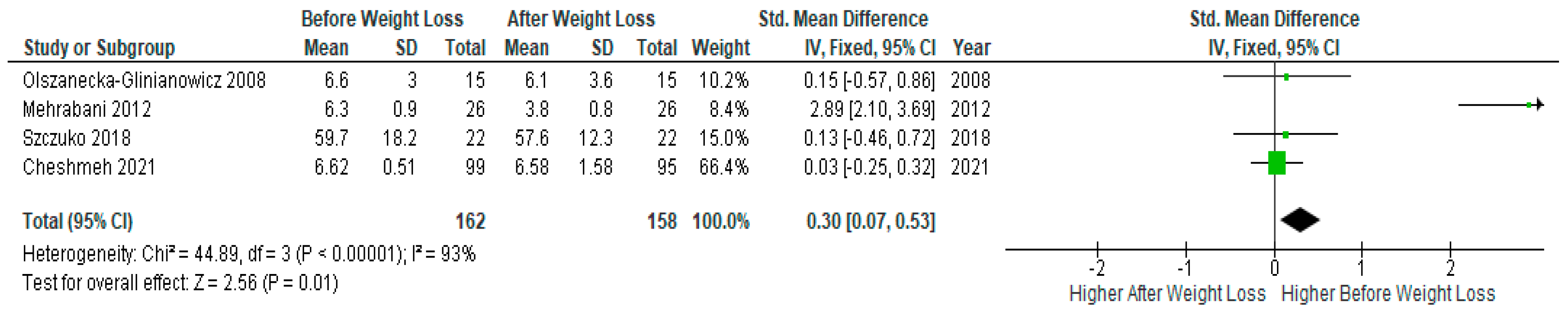
Pooled data of four studies (n=162) revealed significantly lower serum TNF-α levels after diet-induced weight loss compared to baseline levels before weight loss in PCOS women (SMD 0.30, 95% Cl, 0.07, 0.53; z= 2.56; p =0.01; I2=93%). The heterogeneity between studies was high (
Figure 6).
3.5.2. Androgens and LH
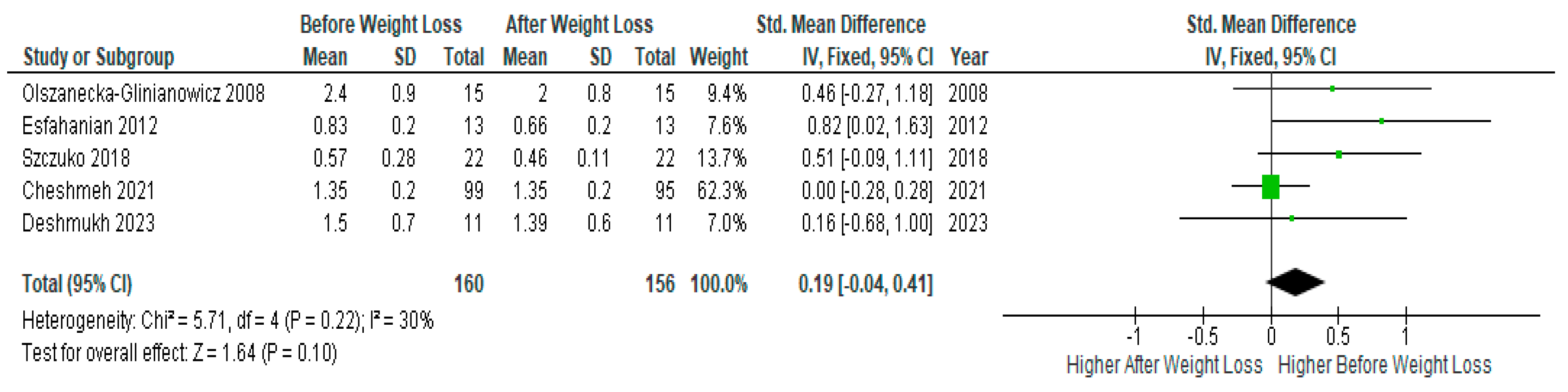
Pooled analysis of five studies (n=160) with relevant data showed no significant difference in serum
testosterone levels before and after diet-induced weight loss in PCOS women (SMD 0.19, 95% Cl, -0.04, 0.41; z= 1.64; p=0.10; I
2=30%). Heterogeneity between studies was moderate (
Figure 7).
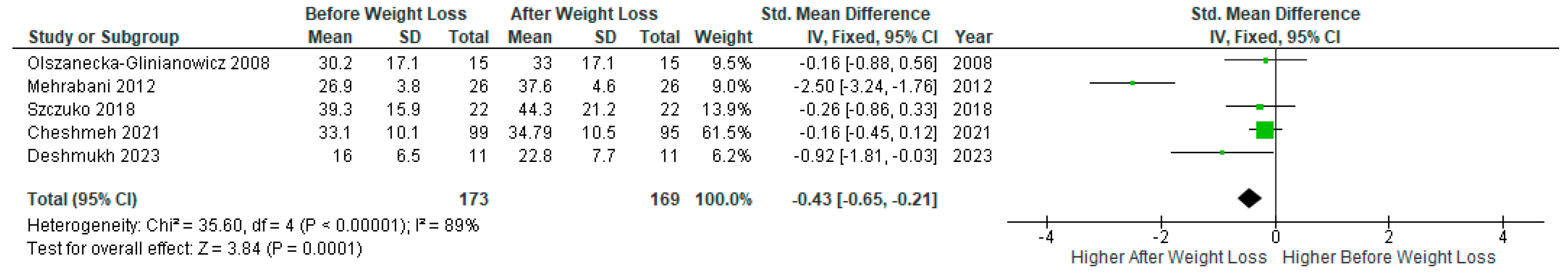
A meta-analysis of five studies (n=173) with relevant data showed a significant increase in SHBG levels after diet-induced weight loss compared to baseline levels in PCOS women (SMD -0.43, 95% Cl, -0.65, -0.21; z= 3.84; p =0.0001; I
2=89%). The heterogeneity between studies was high (
Figure 8).
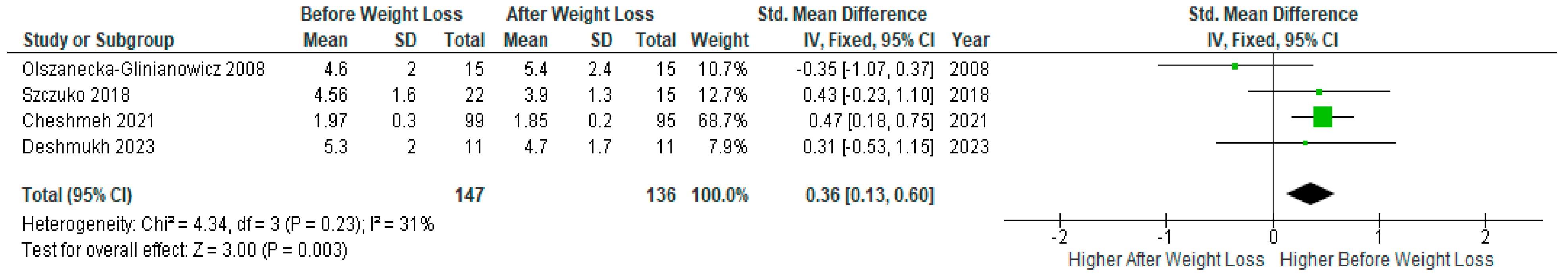
A meta-analysis of four studies (n=147) with relevant data showed a statistically significant decrease in androstenedione levels after weight loss compared to baseline levels before the diet intervention in PCOS women (SMD 0.36, 95% Cl, 0.13, 0.60; z= 3.00; p=0.003; I2=31%). Heterogeneity between studies was low (
Figure 9).
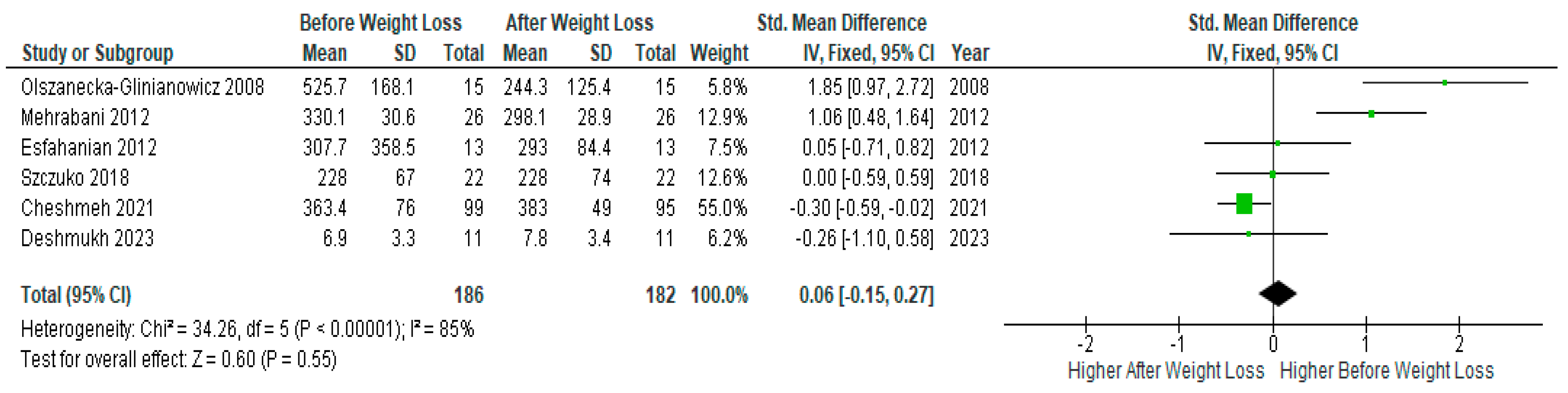
Pooled analysis of six studies (n=186) with relevant data showed no significant difference before and after diet-induced weight loss (SMD 0.06, 95% Cl, -0.15, 0.27; z= 0.60 p=0.55; I2=85%). The heterogeneity between studies was high (
Figure 10).
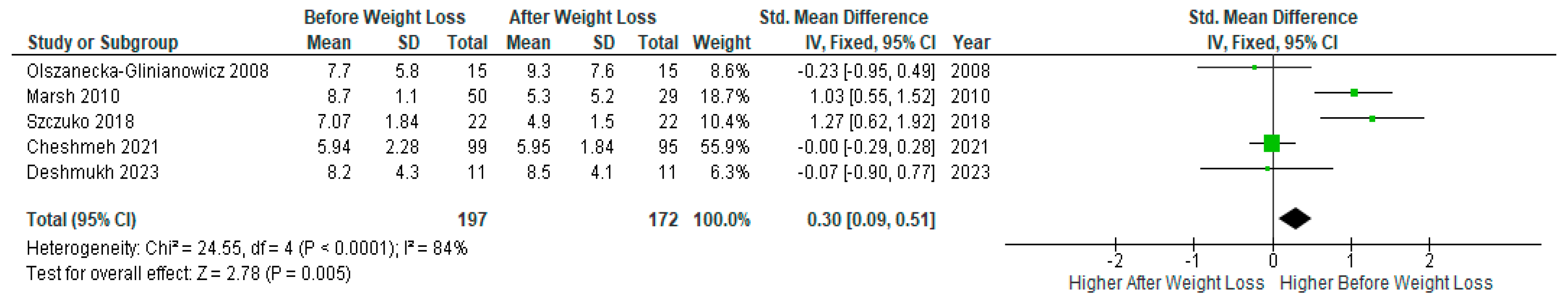
Meta-analysis of five studies (n=197) showed a significant decrease in serum
LH levels after weight loss compared to baseline levels before the diet intervention in PCOS women (SMD 0.30, 95% Cl, 0.09, 0.51; z= 2.78; p=0.005; I2=84%). Heterogeneity between studies was high (
Figure 11).
4. Discussion
To the best of our knowledge, this is the first meta-analysis that investigates the effect of weight loss through dietary intervention alone on circulating levels of inflammatory markers and reproductive endocrine hormones in PCOS women. A total of 11 studies (n=298) were included in the systematic review, of which nine (n=261) were included in the meta-analysis showed a significant reduction in serum CRP after diet-induced weight loss in PCOS women. Also, CRP sub-analysis according to the period of weight loss (≤ 8 weeks or > 8 weeks) showed decreased levels of serum CRP in PCOS women. Also, data analysis of serum IL-6 (three studies) and TNF-α (four studies) showed a significant reduction after diet-induced weight loss in women with PCOS.
In addition, this meta-analysis evaluated the reproductive endocrine profile before and after diet-induced weight loss. Most included studies demonstrated decreased levels of androstenedione (P= 0.003) and LH (P = 0.0001) and increased levels of SHBG (p <0.0001) after diet-induced weight loss in women with PCOS. However, the meta-results did not demonstrate a statistically significant reduction in serum testosterone or DHEAS levels after diet-induced weight loss; although there was a trend toward lower post-weight decrease in testosterone levels, which did not reach statistical significance, possibly due to the limited sample size.
4.1. Comparison with previous studies
The results of our meta-analysis are consistent with several previous studies reporting a decline in inflammatory markers after weight loss through dietary interventions. These studies have demonstrated that a reduction in BMI could result in decreased levels of serum CRP and other inflammatory markers [
32,
34,
44,
47]. Additionally, an early study investigating the association between overweight/obesity and low-grade systemic inflammation, as measured by serum CRP levels, found a correlation between higher BMI and elevated CRP concentrations [
52]. Our research group provide strong evidence for a moderate increase in circulating CRP in PCOS women [
53].
Our results are consistent with a previous systematic review published in 2022 by Moori et al., which investigated the effects of exercise-induced weight loss on circulating CRP and other inflammatory markers. This review reported that exercise could significantly lower serum CRP levels [
54]. Our results are also in agreement with a systematic review published in 2014 that assessed the effectiveness of lifestyle interventions including exercise with or without diet intervention on the endocrine profile in PCOS individuals [
55].
4.2. Limitations and Strengths
The main limitations of this meta-analysis are the small size of many of the included studies and the high heterogeneity between studies. Another limitation is the insufficient data for IL-1 and adiponectin, leading to their inclusion in the systematic review only but not rather in the meta-analysis. Additionally, it is important to acknowledge that one of the criteria in the Cochrane Risk of Bias questionnaire, regarding the blinding of participants, could not be fulfilled in our review as blinding was not possible for the included trials due to the nature of the intervention.
4.3. Interpretation of the results
Possible mechanisms explaining the impact of weight loss through dietary intervention on inflammation involve the reduction of adipose tissue, resulting in decreased production of inflammatory substances [
56]. Another possible mechanism is through elimination of insulin resistance, which is strongly implicated in chronic inflammation [
57]. Additionally, weight loss could increase the production of adipokine, which has anti-inflammatory effects [
58].
Interestingly, this meta-analysis presents possible evidence indicating a positive association between chronic inflammation and reproductive endocrine hormones (SHBG, androstenedione, and LH) in PCOS women. However, the causative nature of this association remains to be evaluated through further research.
4.4. Research Implications of the Findings
Given the above-mentioned limitations of this review, further adequately designed and sufficiently powered studies are needed to further assess the impact of diet-induced weight loss on PCOS-related chronic inflammation and hyperandrogenism. These investigations should also include PCOS women with both high and normal BMI levels to elucidate the effects of weight loss interventions and determine the optimal percentage reduction in BMI that has a more significant impact on their inflammatory markers and endocrine hormones. In addition, future research could also explore possible effect of weight loss intervention on emerging inflammatory pathways including the NLRP3 inflammasome and its related components (such as Casp-1, ASC, and IL-1β) in PCOS patients.
4.5. Clinical Implications of the Findings
Weight loss interventions present a range of positive outcomes for women with PCOS, with minimal side effects. This lifestyle adjustment holds promise in enhancing metabolic health by addressing insulin resistance and metabolic disruptions. Moreover, it shows potential for positively influencing fertility through beneficial changes in reproductive endocrine hormones. The impact extends to cardiovascular health, as reducing inflammatory markers may reduce the elevated cardiovascular risk associated with PCOS. Improvements in lipid profiles and insulin sensitivity contribute to a decreased risk of cardiovascular events. Furthermore, the intervention enhances overall quality of life, encompassing improvements in inflammatory markers and reproductive hormones. This can translate into better mood, reduced hirsutism, and improved menstrual regularity.
While exercise or medical interventions may not be possible or appropriate in some cases of PCOS and PCOS-related morbidities, weight loss through dietary intervention remains a suitable and effective choice.
5. Conclusions
Findings of this meta-analysis suggest that diet-induced weight loss seems to have a positive impact on PCOS-related chronic inflammation and hyperandrogenism. Whether there is any mechanistic correlation between the improvement in inflammation and hyperandrogenism remains to be further investigated. Given the limited number and the small size of the reviewed studies, the results of this review should be interpreted with caution. Further research with a larger sample size and adequate design is needed to further validate the evidence generated in the review.
Author Contributions
Conceptualization, S.A. (Saad Amer), S.A.A. (Salih Atalah Alenezi) and R.K.; methodology, S.A.A., N.E., A.A and S.T.A. (Sulaiman T. Alanazi); software, S.A.A.; validation, S.A. (Saad Amer) and R.K.; formal analysis, S.A.A.; investigation, S.A.A.; resources, S.A.A.; writing—original draft preparation, S.A.A.; writing—review and editing, S.A. (Saad Amer), S.A.A. and R.K.; supervision, S.A. (Saad Amer) and R.K.; funding acquisition, S.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
obtained from the Saudi Arabia Cultural Bureau in London.
Acknowledgments
The research team is grateful to the Saudi Arabia Cultural Bureau in London for their funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clark, Nina M, Amanda J Podolski, Eric D Brooks, Donna R Chizen, Roger A Pierson, Denis C Lehotay, and Marla E Lujan. “Prevalence of Polycystic Ovary Syndrome Phenotypes Using Updated Criteria for Polycystic Ovarian Morphology: An Assessment of over 100 Consecutive Women Self-Reporting Features of Polycystic Ovary Syndrome.” Reproductive Sciences 21, no. 8 (2014): 1034-43. [CrossRef]
- Setji, Tracy L, and Ann J Brown. “Polycystic Ovary Syndrome: Update on Diagnosis and Treatment.” The American journal of medicine 127, no. 10 (2014): 912-19. [CrossRef]
- Lim, Siew S, MJ Davies, Robert J Norman, and LJ Moran. “Overweight, Obesity and Central Obesity in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis.” Human reproduction update 18, no. 6 (2012): 618-37. [CrossRef]
- Diamanti-Kandarakis, Evanthia, and Andrea Dunaif. “Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications.” Endocrine reviews 33, no. 6 (2012): 981-1030. [CrossRef]
- Cho, Li Wei, Harpal S Randeva, and Stephen L Atkin. “Cardiometabolic Aspects of Polycystic Ovarian Syndrome.” Vascular health and risk management 3, no. 1 (2007): 55-63.
- Keskin Kurt, Raziye, Ayşe Güler Okyay, Ali Ulvi Hakverdi, Arif Gungoren, Kenan Serdar Dolapcioglu, Atilla Karateke, and Mustafa Ozcil Dogan. “The Effect of Obesity on Inflammatory Markers in Patients with Pcos: A Bmi-Matched Case–Control Study.” Archives of gynecology and obstetrics 290 (2014): 315-19. [CrossRef]
- Clearfield, Michael B. “C-Reactive Protein: A New Risk Assessment Tool for Cardiovascular Disease.” Journal of Osteopathic Medicine 105, no. 9 (2005): 409-16.
- Jialal, Ishwarlal, Sridevi Devaraj, and Senthil K Venugopal. “C-Reactive Protein: Risk Marker or Mediator in Atherothrombosis?” Hypertension 44, no. 1 (2004): 6-11.
- Yamauchi, Toshimasa, J Kamon, H Waki, Y Terauchi, N Kubota, K Hara, Y Mori, T Ide, K Murakami, and N Tsuboyama-Kasaoka. “The Fat-Derived Hormone Adiponectin Reverses Insulin Resistance Associated with Both Lipoatrophy and Obesity.” Nature medicine 7, no. 8 (2001): 941-46. [CrossRef]
- Alenezi, Salih Atalah, Raheela Khan, Lindsay Snell, Shaimaa Aboeldalyl, and Saad Amer. “The Role of Nlrp3 Inflammasome in Obesity and Pcos—a Systematic Review and Meta-Analysis.” International Journal of Molecular Sciences 24, no. 13 (2023): 10976. [CrossRef]
- Ouchi, Noriyuki, Shinji Kihara, Yukio Arita, Makoto Nishida, Akifumi Matsuyama, Yoshihisa Okamoto, Masato Ishigami, Hiroshi Kuriyama, Ken Kishida, and Hitoshi Nishizawa. “Adipocyte-Derived Plasma Protein, Adiponectin, Suppresses Lipid Accumulation and Class a Scavenger Receptor Expression in Human Monocyte-Derived Macrophages.” Circulation 103, no. 8 (2001): 1057-63. [CrossRef]
- Yuan, Guoyue, Libin Zhou, Jinfeng Tang, Ying Yang, Weiqiong Gu, Fengying Li, Jie Hong, Yanyun Gu, Xiaoying Li, and Guang Ning. “Serum Crp Levels Are Equally Elevated in Newly Diagnosed Type 2 Diabetes and Impaired Glucose Tolerance and Related to Adiponectin Levels and Insulin Sensitivity.” Diabetes research and clinical practice 72, no. 3 (2006): 244-50. [CrossRef]
- Talbott, EO, JV Zborowski, JR Rager, MY Boudreaux, DA Edmundowicz, and DS Guzick. “Evidence for an Association between Metabolic Cardiovascular Syndrome and Coronary and Aortic Calcification among Women with Polycystic Ovary Syndrome.” The Journal of Clinical Endocrinology & Metabolism 89, no. 11 (2004): 5454-61.
- Tarkun, Ilhan, Berrı̇n Ç Arslan, Zeynep Cantürk, Erdem Turemen, Tayfun Şahı̇n, and Can Duman. “Endothelial Dysfunction in Young Women with Polycystic Ovary Syndrome: Relationship with Insulin Resistance and Low-Grade Chronic Inflammation.” The Journal of Clinical Endocrinology & Metabolism 89, no. 11 (2004): 5592-96. [CrossRef]
- Amato, Giovanni, Marisa Conte, Gherardo Mazziotti, Eleonora Lalli, Gabriella Vitolo, Arthur T Tucker, Antonio Bellastella, Carlo Carella, and Alfredo Izzo. “Serum and Follicular Fluid Cytokines in Polycystic Ovary Syndrome During Stimulated Cycles.” Obstetrics & Gynecology 101, no. 6 (2003): 1177-82.
- Vgontzas, Alexandros N, Georgia Trakada, Edward O Bixler, Hung-Mo Lin, Slobodanka Pejovic, Emmanuel Zoumakis, George P Chrousos, and Richard S Legro. “Plasma Interleukin 6 Levels Are Elevated in Polycystic Ovary Syndrome Independently of Obesity or Sleep Apnea.” Metabolism 55, no. 8 (2006): 1076-82. [CrossRef]
- Puder, Jardena J, Sabina Varga, Marius Kraenzlin, Christian De Geyter, Ulrich Keller, and Beat Müller. “Central Fat Excess in Polycystic Ovary Syndrome: Relation to Low-Grade Inflammation and Insulin Resistance.” The Journal of Clinical Endocrinology & Metabolism 90, no. 11 (2005): 6014-21. [CrossRef]
- Carmina, E, Francesco Orio, Stefano Palomba, RA Longo, Teresa Cascella, Annamaria Colao, Gaetano Lombardi, GB Rini, and Rogerio A Lobo. “Endothelial Dysfunction in Pcos: Role of Obesity and Adipose Hormones.” The American journal of medicine 119, no. 4 (2006): 356. e1-56. e6. [CrossRef]
- Sieminska, L, B Marek, B Kos-Kudla, D Niedziolka, D Kajdaniuk, M Nowak, and J Glogowska-Szelag. “Serum Adiponectin in Women with Polycystic Ovarian Syndrome and Its Relation to Clinical, Metabolic and Endocrine Parameters.” Journal of endocrinological investigation 27 (2004): 528-34.
- Rachoń, D. “Differential Diagnosis of Hyperandrogenism in Women with Polycystic Ovary Syndrome.” Experimental and clinical endocrinology & diabetes (2012): 205-09. [CrossRef]
- Martínez-García, M Ángeles, Alessandra Gambineri, Macarena Alpanes, Raul Sanchon, Renato Pasquali, and Hector F Escobar-Morreale. “Common Variants in the Sex Hormone-Binding Globulin Gene (Shbg) and Polycystic Ovary Syndrome (Pcos) in Mediterranean Women.” Human reproduction 27, no. 12 (2012): 3569-76. [CrossRef]
- Erickson, Gregory F, and Douglas R Danforth. “Ovarian Control of Follicle Development.” American journal of obstetrics and gynecology 172, no. 2 (1995): 736-47.
- Franks, Stephen, Jaroslav Stark, and Kate Hardy. “Follicle Dynamics and Anovulation in Polycystic Ovary Syndrome.” Human reproduction update 14, no. 4 (2008): 367-78.
- Balen, Adam H, Lara C Morley, Marie Misso, Stephen Franks, Richard S Legro, Chandrika N Wijeyaratne, Elisabet Stener-Victorin, Bart CJM Fauser, Robert J Norman, and Helena Teede. “The Management of Anovulatory Infertility in Women with Polycystic Ovary Syndrome: An Analysis of the Evidence to Support the Development of Global Who Guidance.” Human reproduction update 22, no. 6 (2016): 687-708. [CrossRef]
- Alenezi, Salih Atalah, Raheela Khan, and Saad Amer. “The Impact of High Bmi on Pregnancy Outcomes and Complications in Women with Pcos Undergoing Ivf—a Systematic Review and Meta-Analysis.” Journal of Clinical Medicine 13, no. 6 (2024): 1578. [CrossRef]
- Moran, Lisa J, Manny Noakes, Peter M Clifton, Gary A Wittert, Gemma Williams, and Robert J Norman. “Short-Term Meal Replacements Followed by Dietary Macronutrient Restriction Enhance Weight Loss in Polycystic Ovary Syndrome.” The American journal of clinical nutrition 84, no. 1 (2006): 77-87. [CrossRef]
- Identification, Expert Panel on the, Treatment of Overweight, Obesity in Adults, National Heart, Lung, Blood Institute, National Institute of Diabetes, Digestive, and Kidney Diseases. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report: National Institutes of Health, National Heart, Lung, and Blood Institute, 1998.
- Krauss, Ronald M, Robert H Eckel, Barbara Howard, Lawrence J Appel, Stephen R Daniels, Richard J Deckelbaum, John W Erdman Jr, Penny Kris-Etherton, Ira J Goldberg, and Theodore A Kotchen. “Aha Dietary Guidelines: Revision 2000: A Statement for Healthcare Professionals from the Nutrition Committee of the American Heart Association.” Circulation 102, no. 18 (2000): 2284-99.
- Bhupathiraju, Shilpa N, and Katherine L Tucker. “Greater Variety in Fruit and Vegetable Intake Is Associated with Lower Inflammation in Puerto Rican Adults.” The American journal of clinical nutrition 93, no. 1 (2011): 37-46. [CrossRef]
- Li, Xinli, Younan Chen, Jingping Liu, Guang Yang, Jiuming Zhao, Guangneng Liao, Meimei Shi, Yujia Yuan, Sirong He, and Yanrong Lu. “Serum Metabolic Variables Associated with Impaired Glucose Tolerance Induced by High-Fat-High-Cholesterol Diet in Macaca Mulatta.” Experimental biology and medicine 237, no. 11 (2012): 1310-21. [CrossRef]
- Mehrabani, Homeira Hamayeli, Saghar Salehpour, Zohreh Amiri, Sara Jalali Farahani, Barbara J Meyer, and Farideh Tahbaz. “Beneficial Effects of a High-Protein, Low-Glycemic-Load Hypocaloric Diet in Overweight and Obese Women with Polycystic Ovary Syndrome: A Randomized Controlled Intervention Study.” Journal of the American College of Nutrition 31, no. 2 (2012): 117-25. [CrossRef]
- Cheshmeh, Sahar, Maysa Ghayyem, Firoozeh Khamooshi, Neda Heidarzadeh-Esfahani, Negin Rahmani, Niloofar Hojati, Elaheh Mosaieby, Shima Moradi, and Yahya Pasdar. “Green Cardamom Plus Low-Calorie Diet Can Decrease the Expression of Inflammatory Genes among Obese Women with Polycystic Ovary Syndrome: A Double-Blind Randomized Clinical Trial.” Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity 27, no. 2 (2021): 821-30. [CrossRef]
- Marsh, Kate A, Katharine S Steinbeck, Fiona S Atkinson, Peter Petocz, and Jennie C Brand-Miller. “Effect of a Low Glycemic Index Compared with a Conventional Healthy Diet on Polycystic Ovary Syndrome.” The American journal of clinical nutrition 92, no. 1 (2010): 83-92. [CrossRef]
- Esfahanian, Fatemeh, Mohammad Mahdi Zamani, Ramin Heshmat, and Fatemeh Moini nia. “Effect of M Etformin Compared with Hypocaloric Diet on Serum C--Reactive Protein Level and Insulin Resistance in Obese and Overweight Women with Polycystic Ovary Syndrome.” Journal of Obstetrics and Gynaecology Research 39, no. 4 (2012): 806-13.
- Moran, Lisa J, Henry Ko, Marie Misso, Kate Marsh, Manny Noakes, Mac Talbot, Meredith Frearson, Mala Thondan, Nigel Stepto, and Helena J Teede. “Dietary Composition in the Treatment of Polycystic Ovary Syndrome: A Systematic Review to Inform Evidence-Based Guidelines.” Journal of the Academy of Nutrition and Dietetics 113, no. 4 (2013): 520-45. [CrossRef]
- Higgins, Julian PT, Douglas G Altman, Peter C Gøtzsche, Peter Jüni, David Moher, Andrew D Oxman, Jelena Savović, Kenneth F Schulz, Laura Weeks, and Jonathan AC Sterne. “The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials.” Bmj 343 (2011). [CrossRef]
- Wells, George A, Beverley Shea, Dianne O’Connell, Joan Peterson, Vivian Welch, Michelle Losos, and Peter Tugwell. “The Newcastle-Ottawa Scale (Nos) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses.” (2000).
- Raffi, Francesca, Mostafa Metwally, and Saad Amer. “The Impact of Excision of Ovarian Endometrioma on Ovarian Reserve: A Systematic Review and Meta-Analysis.” The Journal of Clinical Endocrinology & Metabolism 97, no. 9 (2012): 3146-54. [CrossRef]
- El Shamy, Tarek, Saad AK Amer, Ahmed A Mohamed, Cathryn James, and Kannamannadiar Jayaprakasan. “The Impact of Uterine Artery Embolization on Ovarian Reserve: A Systematic Review and Meta--Analysis.” Acta obstetricia et gynecologica Scandinavica 99, no. 1 (2020): 16-23. [CrossRef]
- Ebada, Mahmoud Ahmed, Abdelmagid M Elmatboly, Ahmed Said Ali, Ahmed Mohamed Ibrahim, Notila Fayed, Ahmed Faisal Faisal, and Souad Alkanj. “An Updated Systematic Review and Meta-Analysis About the Safety and Efficacy of Infliximab Biosimilar, Ct-P13, for Patients with Inflammatory Bowel Disease.” International Journal of Colorectal Disease 34 (2019): 1633-52.
- Tian, Lili. “Inferences on Standardized Mean Difference: The Generalized Variable Approach.” Statistics in medicine 26, no. 5 (2007): 945-53. [CrossRef]
- Takeshima, Nozomi, Takashi Sozu, Aran Tajika, Yusuke Ogawa, Yu Hayasaka, and Toshiaki A Furukawa. “Which Is More Generalizable, Powerful and Interpretable in Meta-Analyses, Mean Difference or Standardized Mean Difference?” BMC medical research methodology 14, no. 1 (2014): 1-7.
- Cohen, Jacob. Statistical Power Analysis for the Behavioral Sciences: Academic press, 2013.
- Deshmukh, Harshal, Maria Papageorgiou, Liz Wells, Shahzad Akbar, Thomas Strudwick, Ketki Deshmukh, Salvatore Giovanni Vitale, Alan Rigby, Rebecca V Vince, and Marie Reid. “The Effect of a Very-Low-Calorie Diet (Vlcd) Vs. A Moderate Energy Deficit Diet in Obese Women with Polycystic Ovary Syndrome (Pcos)—a Randomised Controlled Trial.” Nutrients 15, no. 18 (2023): 3872.
- Szczuko, Małgorzata, Marta Zapałowska-Chwyć, Arleta Drozd, Dominika Maciejewska, Andrzej Starczewski, Paweł Wysokiński, and Ewa Stachowska. “Changes in the Igf-1 and Tnf-A Synthesis Pathways before and after Three-Month Reduction Diet with Low Glicemic Index in Women with Pcos.” Ginekologia polska 89, no. 6 (2018): 295-303. [CrossRef]
- Asemi, Zatollah, Mansooreh Samimi, Mohsen Taghizadeh, and Ahmad Esmaillzadeh. “Effects of Ramadan Fasting on Glucose Homeostasis, Lipid Profiles, Inflammation and Oxidative Stress in Women with Polycystic Ovary Syndrome in Kashan, Iran.” Archives of Iranian medicine 18, no. 12 (2015): 0-0.
- Moran, Lisa J, Manny Noakes, Peter M Clifton, and Robert J Norman. “The Effect of Modifying Dietary Protein and Carbohydrate in Weight Loss on Arterial Compliance and Postprandial Lipidemia in Overweight Women with Polycystic Ovary Syndrome.” Fertility and sterility 94, no. 6 (2010): 2451-54. [CrossRef]
- Olszanecka-Glinianowicz, Magdalena, Barbara Zahorska-Markiewicz, Piotr Kocełak, Joanna Janowska, and Elżbieta Semik-Grabarczyk. “The Effect of Weight Loss on Inflammation in Obese Women with Polycystic Ovary Syndrome.” Endokrynologia Polska 59, no. 1 (2008): 13-17.
- Moran, Lisa J, Manny Noakes, Peter M Clifton, Gary A Wittert, Damien P Belobrajdic, and Robert J Norman. “C-Reactive Protein before and after Weight Loss in Overweight Women with and without Polycystic Ovary Syndrome.” The Journal of Clinical Endocrinology & Metabolism 92, no. 8 (2007): 2944-51. [CrossRef]
- Cumpston, Miranda, Tianjing Li, Matthew J Page, Jacqueline Chandler, Vivian A Welch, Julian PT Higgins, and James Thomas. “Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions.” The Cochrane database of systematic reviews 2019, no. 10 (2019).
- Thomson, Katie, Stephen Rice, Oluwatomi Arisa, Eugenie Johnson, Louise Tanner, Christopher Marshall, Tumi Sotire, Catherine Richmond, Hannah O’Keefe, and Wael Mohammed. “Oral Nutritional Interventions in Frail Older People Who Are Malnourished or at Risk of Malnutrition: A Systematic Review.” Health Technology Assessment (Winchester, England) 26, no. 51 (2022): 1. [CrossRef]
- Visser, Marjolein, Lex M Bouter, Geraldine M McQuillan, Mark H Wener, and Tamara B Harris. “Elevated C-Reactive Protein Levels in Overweight and Obese Adults.” Jama 282, no. 22 (1999): 2131-35. [CrossRef]
- Aboeldalyl, Shaimaa, Cathryn James, Emaduldin Seyam, Emad Moussa Ibrahim, Hossam El-Din Shawki, and Saad Amer. “The Role of Chronic Inflammation in Polycystic Ovarian Syndrome—a Systematic Review and Meta-Analysis.” International Journal of Molecular Sciences 22, no. 5 (2021): 2734. [CrossRef]
- Hafizi Moori, Mozhgan, Saeed Nosratabadi, Naghmeh Yazdi, Razieh Kasraei, Zeinab Abbasi Senjedary, and Razieh Hatami. “The Effect of Exercise on Inflammatory Markers in Pcos Women: A Systematic Review and Meta-Analysis of Randomized Trials.” International Journal of Clinical Practice 2023 (2023). [CrossRef]
- Haqq, Liza, James McFarlane, Gudrun Dieberg, and Neil Smart. “Effect of Lifestyle Intervention on the Reproductive Endocrine Profile in Women with Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis.” Endocrine connections 3, no. 1 (2014): 36-46. [CrossRef]
- Berg, Anders H, and Philipp E Scherer. “Adipose Tissue, Inflammation, and Cardiovascular Disease.” Circulation research 96, no. 9 (2005): 939-49.
- Uusitupa, Matti, Kjeld Hermansen, Markku Juhani Savolainen, U Schwab, Marjukka Kolehmainen, L Brader, Lene Sundahl Mortensen, Lieselotte Cloetens, Anna Johansson--Persson, and Gunilla Önning. “Effects of an Isocaloric Healthy N Ordic Diet on Insulin Sensitivity, Lipid Profile and Inflammation Markers in Metabolic Syndrome–a Randomized Study (Sysdiet).” Journal of internal medicine 274, no. 1 (2013): 52-66.
- Rolland, Catherine, Michelle Hession, and Iain Broom. “Effect of Weight Loss on Adipokine Levels in Obese Patients.” Diabetes, metabolic syndrome and obesity: targets and therapy (2011): 315-23.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).