1. Introduction
Surgery remains the gold standard treatment for severe mitral valve regurgitation [
1]. The widespread adoption of minimally invasive, endoscopic and robot-assisted techniques in numerous centers is driven by their feasibility and effectiveness, reduced risk of infection, enhanced patient satisfaction in terms of cosmesis and pain, along with shorter length of hospital stay [
2]. The DeBakey cross-clamp has been the mainstay of aortic occlusion during open cardiac surgery [
3]. Nonetheless, aortic occlusion and myocardial protection strategies underwent further adaptations following the increasing adoption of minimally invasive approaches in mitral valve surgery [
4]. In the context of minimally invasive mitral valve surgery (MIMVS), the transthoracic clamp (TTC) and the endoaortic balloon occlusion (EABO) approaches have been employed as alternative strategies for aortic occlusion and myocardial protection [
5]. TTC incorporates a longer DeBakey-type clamp inserted through the intercostal spaces [
5]. On the other hand, EABO employs a transcatheter intraluminal balloon as an alternative strategy for aortic occlusion and myocardial protection [
4].
To date, there have been two previous meta-analyses on this topic available in the literature [
5,
6]. The first meta-analysis [
6] was associated with limitations related to the lack of a sensitivity analysis regarding the cannulation site in the EABO approach. Moreover, both meta-analyses are associated with two additional limitations: 1) they did not include larger multicentric studies with adjusted outcomes published in the five-year interval since the last meta-analysis [
5], 2) they did not perform sensitivity analyses a) with risk-adjusted populations and b) using the leave-one-out method, and 3) they did not perform subgroup analyses regarding the MIMVS setting (video-assisted or robotic-assisted). The first point is important since there are no available randomized control trials and most of previous studies were small with most surgeons exclusively using one technique or the other, especially given the steep learning curve of the EABO approach, thus posing a certain bias. The second point (sensitivity analysis) is necessary to provide the best up-to-date level of evidence given the increasing popularity of robot-assisted mitral valve surgery. Aiming to address these issues, we performed an updated meta-analysis comparing TTC and EABO as two alternative strategies for aortic occlusion and myocardial protection in the setting of minimally invasive and robot-assisted mitral valve surgery.
2. Materials and Methods
2.1. Search and Articles Selection Strategy
We conducted the present meta-analysis in accordance with the protocol agreed by all authors and following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [
7]. The PRISMA Checklist 2020 is demonstrated in
Table S1. A thorough literature search was performed in Pubmed (Medline), Scopus (ELSEVIER), and Cochrane Central Register of Controlled Studies (CENTRAL) (last search: June 24th, 2024). The following terms were employed in every possible combination: “transthoracic clamp”, “cross-clamp”, “ttc”, “aortic balloon”, “eabo”, “aortic occlusion”, “mitral valve surgery”, “mitral valve replacement”, “mitral valve repair”, “mvr”, “minimally invasive mitral valve surgery”, “mimvs”. Inclusion criteria were (1) original reports with ≥ 10 patients, (2) written in English, (3) published from 2000 to 2024, (4) conducted on human subjects, and (5) reporting comparative outcomes of patients undergoing MIMVS (including robot-assisted) with employment of either TTC or EABO approach for aortic occlusion. Duplicate articles were excluded. The reference lists of all included articles were also reviewed for additional studies. Two independent reviewers (DEM, SS) extracted data from the included studies. Any discrepancies between the investigators were discussed with the senior author (BR) to include articles that best matched the criteria until consensus was reached. The authors had personal equipoise regarding the best intervention.
2.2. Data Extraction and Endpoints
For each eligible study, data was extracted relative to demographics (number of patients, gender, age, type of TTC, type of EABO, previous cerebrovascular events), along with the incidence of all-cause mortality, cerebrovascular accident (CVA), aortic dissection, aortic cross-clamp time, cardiopulmonary bypass (CPB) time, the incidence of conversion to sternotomy, re-exploration, new onset atrial fibrillation (AF), postoperative acute kidney injury (AKI), ventilation time, intensive care unit (ICU) stay, and length of hospital stay (LOS). The incidence of all-cause mortality, CVA, and aortic dissection were the primary endpoints. Aortic cross-clamp and CPB time, along with the incidence of conversion to sternotomy, re-exploration, new onset AF, postoperative AKI, ventilation time, ICU stay, and LOS were the secondary endpoints.
2.3. Sensitivity Analysis on Primary Endpoints
To further validate our outcomes, we performed additional sensitivity analyses regarding both the primary and secondary endpoints. At first, we performed subgroup analyses using the following subgroups: 1) EABO with aortic cannulation, 2) EABO with femoral cannulation, 3) video-assisted approach, 4) robotic-assisted approach, 5) only studies with risk-adjusted patient groups. Secondly, we conducted further sensitivity analyses using the leave-one-out method. The leave-one-out method involves performing a meta-analysis on each subset of the studies obtained by leaving out exactly one study.
2.4. Quality and Publication Bias Assessment
The Newcastle-Ottawa Quality Assessment Scale (NOS) [
8] was used as an assessment tool to evaluate non-Randomized Controlled Trials (RCTs). The scale’s range varies from zero to nine stars, and studies with a score equal to or higher than five were considered to have adequate methodological quality. The Risk of Bias in Non-Randomized Studies of Interventions tool (ROBINS-I) was also systematically used to assess the included studies for risk of bias [
9]. No RCTs were included in the present meta-analysis. Two reviewers (DEM, SS) rated the studies independently and a final decision was reached by consensus. The risk of publication bias was evaluated by the visual inspection of the funnel plots.
2.5. Statistical Analysis
Regarding the categorical outcomes, the Odds Ratio (ORs) and 95% confidence interval (95% CI) were estimated, by means of the Random-Effects model (Mantel-Haenszel statistical method). OR < 1 denoted an outcome that was more frequent in the EABO group. Continuous outcomes were evaluated by means of weighted mean difference (WMD) with its 95 % CI, using Random-Effects (Inverse Variance statistical method) models. In cases where WMD < 0, values in the EABO group were higher. The Random-Effects model was chosen, given that it was not expected that all included studies would share a common effect size. Inter-study heterogeneity was assessed through Cochran
Q statistic and by estimating
I2 [
10]. Forest plots were produced regarding the variables that were analyzed. Data analysis was performed using the Cochrane Collaboration Review Manager version 5.4.1.
3. Results
3.1. Search Strategy and Patient Demographics
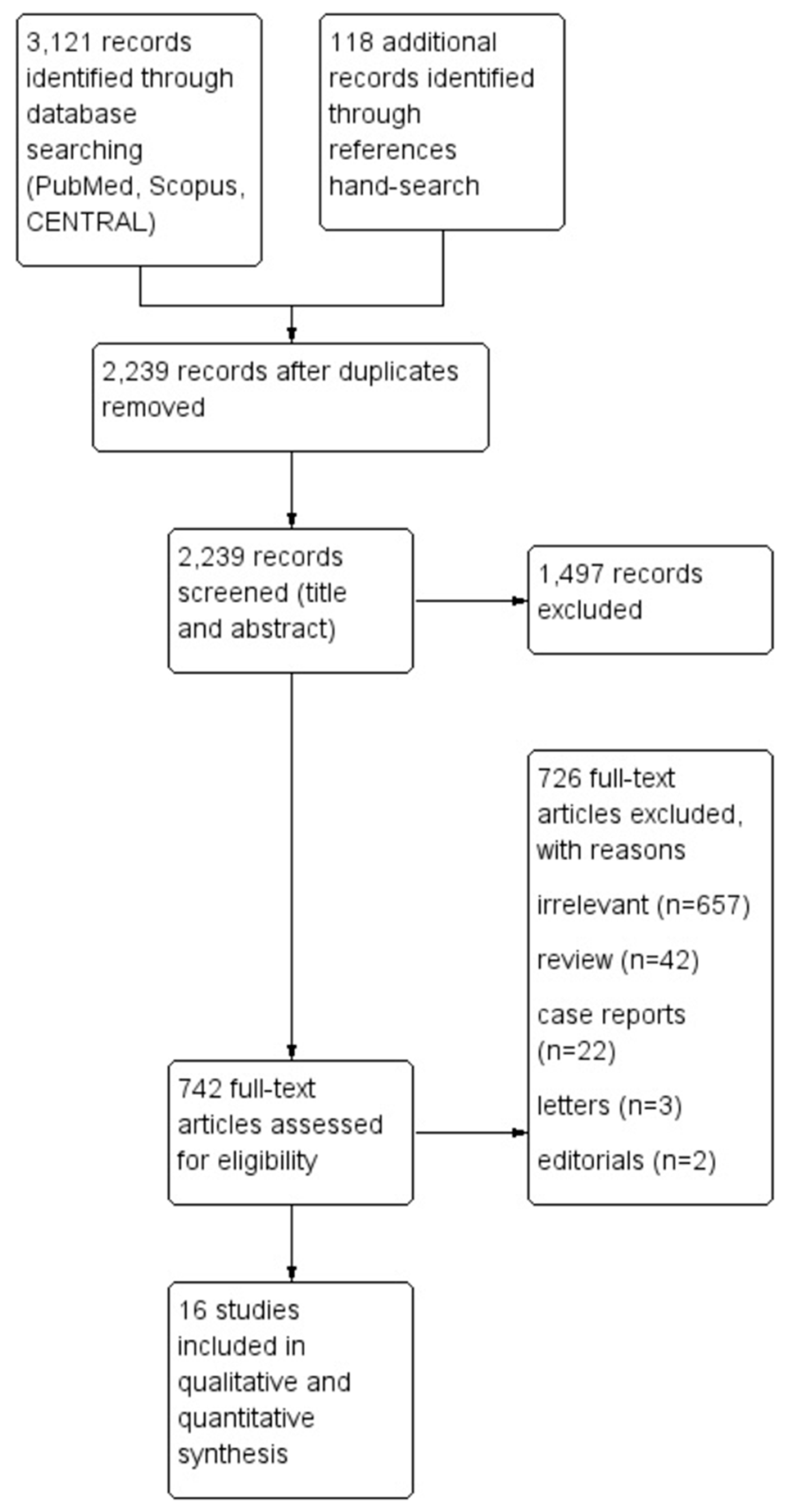
The flow diagram regarding the search strategy is shown in
Figure 1 and the Prisma Checklist 2020 (
Supplementary material). The characteristics of the included studies are summarized in
Table 1. Among the 3,239 articles in Pubmed, Scopus, and CENTRAL that were retrieved, sixteen studies [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26] were included in the qualitative and the quantitative synthesis. The level of agreement between the two reviewers was “almost perfect” (kappa = 0.91; 95% CI: 0.81, 1.00).
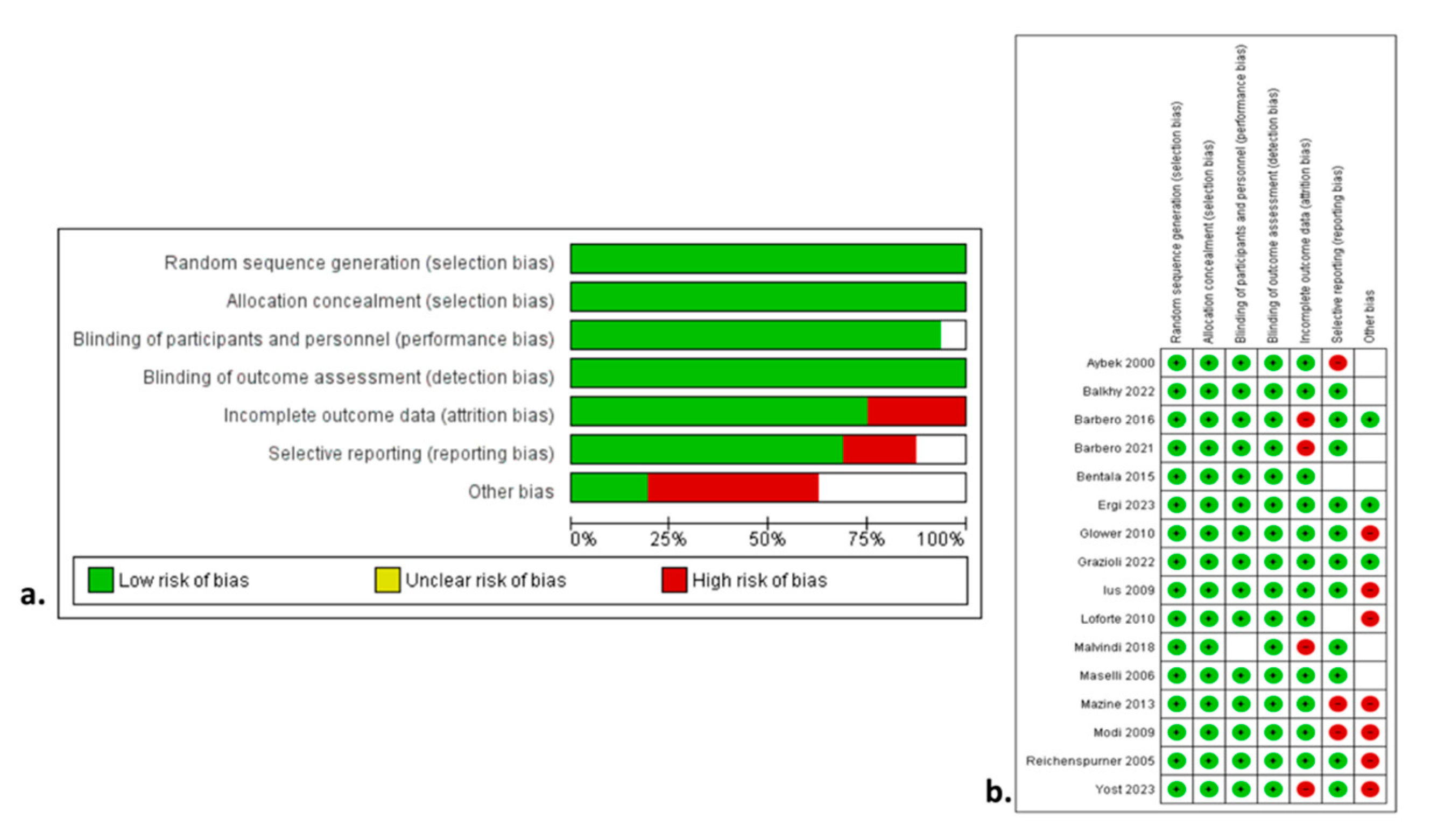
Figure 2a,b show the qualitative assessment with the ROBINS-I tool. The authors’ main concerns were related to biases owing to the selection of participants and performance. The study design was prospective in four studies [
14,
16,
22,
24], and retrospective in twelve studies [
11,
12,
13,
15,
17,
18,
19,
20,
21,
23,
25,
26]. PSM was performed in four studies [
12,
16,
18,
19]. Three studies [
20,
21,
22] were retrospective using a prospectively collected database. No RCTs were included in the current meta-analysis. The included studies were conducted in Germany [
11,
24,
25], USA [
12,
16,
17,
26], Italy [
13,
14,
19,
20,
21,
22], Netherlands [
15], Canada [
23], and one was multinational [
18]. The studies were published between 2000 and 2023. The total sample size was 6,335 patients (TTC: 3,271; EABO: 3,064). The ratio of mitral valve repair (MVR) operations ranged from 9% to 73% with significant heterogeneity among studies. The comparison of the two groups in terms of baseline characteristics is demonstrated in
Table 2. TTC and EABO groups had similar baseline characteristics, except from the previous cardiac surgery variable, with more redo cases incorporated into the EABO group (OR: 0.45; 95% CI: 0.22 - 0.91;
p = 0.03). The primary and secondary endpoints, along with the sensitivity subgroup analyses are demonstrated cumulatively in
Table 3.
3.2. Primary Endpoints: All-Cause Mortality, CVA, and Aortic Dissection
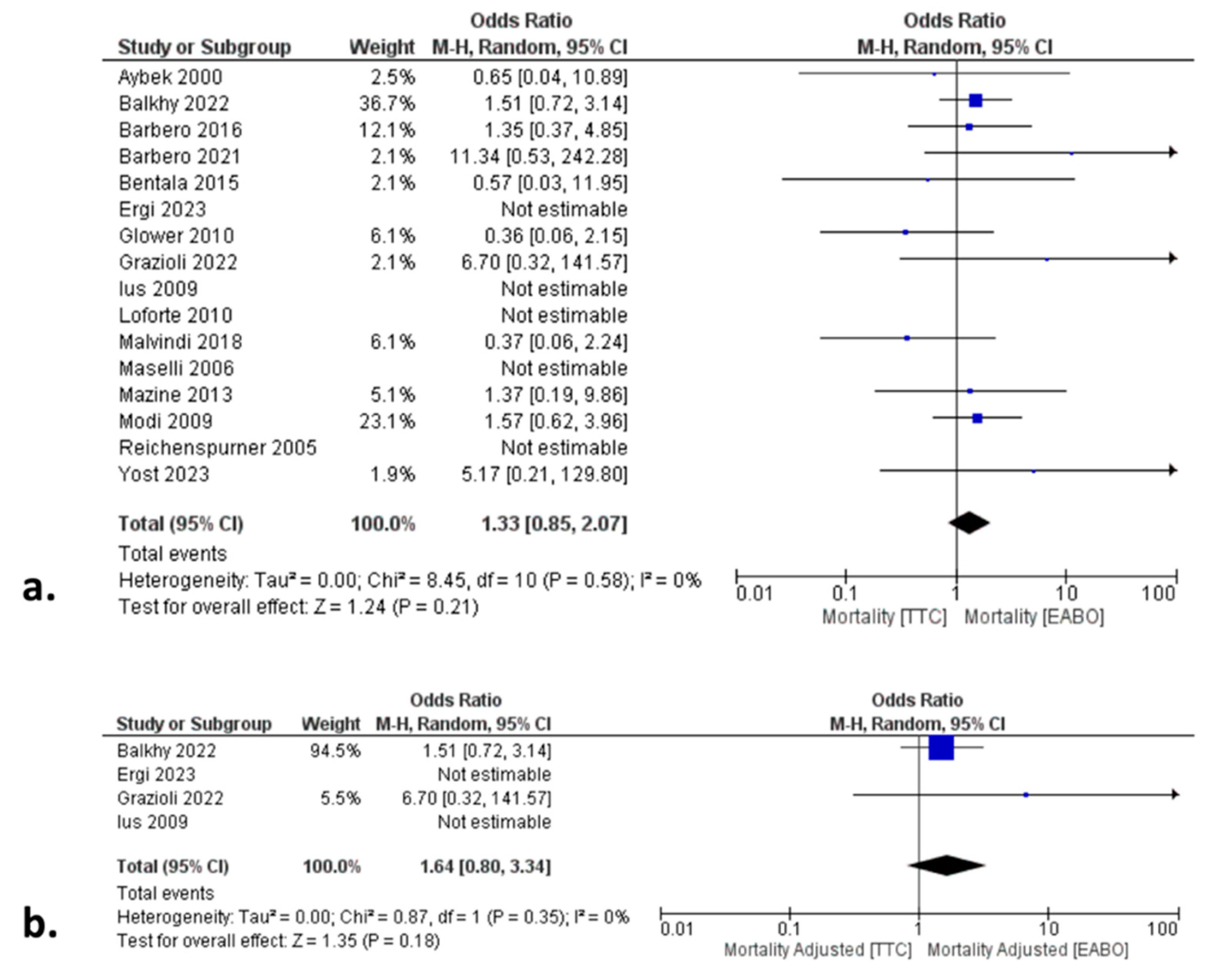
In the total cohort analysis, there was no significant difference between the two groups in terms of all-cause mortality (HR: 1.33; 95% CI:0.85, 2.07;
p=0.21) (
Figure 2a), incidence of CVA (OR: 0.68; 95% CI: 0.44, 1.04;
p=0.07), and aortic dissection (OR: 0.51; 95% CI:0.20, 1.33;
p=0.17) (
Table 3).
3.3. Secondary Endpoints
In the total cohort analysis, both groups demonstrated similar CPB (OR: -1.68; 95% CI: -8.21, 4.85; p=0.61), cross-clamp (OR: -3.27; 95% CI: -7.61, 1.07; p=0.14), and ventilation (OR: -0.03; 95% CI: -0.58, 0.52; p=0.92) time. In addition, there was no significant difference between the two groups regarding the incidence of conversion to sternotomy (OR: 0.51; 95% CI: 0.19, 1.39; p=0.19), re-exploration (OR: 0.90; 95% CI: 0.64, 1.28; p=0.57), new-onset AF (OR: 0.86; 95% CI: 0.61, 1.21; p=0.37), and postoperative AKI (OR: 1.22; 95% CI: 0.91, 1.65; p=0.19). Finally, both groups were similar regarding ICU stay (OR: -0.27; 95% CI: -0.72, 0.19; p=0.25) and LOS (OR: 0.30; 95% CI: -0.60, 1.21; p=0.51).
3.4. Subgroup and Sensitivity Analyses
To further validate our outcomes, we performed subgroup analyses comparing TTC vs EABO in patients a) with femoral cannulation EABO, b) aortic cannulation EABO, c) undergoing video-assisted, and d) robotic-assisted MIMVS. In the femoral EABO subgroup, all outcomes were similar to the TTC group, except from the aortic cross-clamp time that was higher in the EABO group. In contrast, the aortic EABO subgroup demonstrated significantly lower cross-clamp time compared to the TTC group. Consequently, aortic cannulation EABO approach was associated with the shortest cross-clamp time of all three subgroups. In the video-assisted subgroup analysis, EABO was associated with a higher incidence of CVA, conversion to sternotomy, and longer ICU stay compared to the TTC group.
Moreover, the validity of the total cohort analysis outcomes was further affirmed by the risk-adjusted subgroup analyses, in which patients were matched for baseline characteristics to minimize the risk of bias related to cofounders. In fact, outcomes of this subgroup analysis were similar to the total cohort analysis outcomes, with no difference between the two groups in any of the primary or secondary endpoints (
Figure 2b,
Table 3). Finally, no difference was found when we applied the leave-one out sensitivity analysis method, thus further supporting the validity of our outcomes.
3.5. Quality and Publication Bias Assessment
The NOS assessment of quality for all studies is shown in
Table 1. Figure demonstrates the qualitative assessment of the studies according to the ROBINS-I tool.
Figure 3a,b show the qualitative assessment with the ROBINS-I tool. The authors’ main concerns were mainly related to biases associated with the outcome data and selective reporting. The primary endpoints were associated with low heterogeneity. Most of the secondary endpoints were related to low heterogeneity. In contrast, CPB and cross-clamp time, along with the incidence of conversion to sternotomy, ICU stay, and LOS were associated with high heterogeneity. The main factors affecting and increasing heterogeneity in these variables are the level of expertise, the volume of cases, the differences in operation setting and aortic occlusion devices, along with the differences in the perioperative pathway protocols among different institutions. Funnel plots
(Figure S1) seemed asymmetrical, with studies being absent from either top or bottom of the graph, thus suggesting certain publication bias. The relatively small number of the included studies was the main reason for the reported asymmetry.
4. Discussion
The current meta-analysis identified sixteen articles comparing TTC versus EABO as two alternative methods of aortic occlusion for minimally invasive mitral valve surgery and incorporated 6,335 patients. According to our total cohort analysis, TTC and EABO demonstrate comparable outcomes with regards to the primary and secondary outcomes. Although a previous meta-analysis [
5] was conducted in 2019 (study period until 12/2018), numerous newer studies have been published with important characteristics (PSM study design in three of them [
12,
16,
18] and robotic-assisted MIMVS in two studies [
16,
26]), and the sensitivity analyses were limited. Given the lack of a randomized trial, the present meta-analysis provides the best currently available level of evidence on this topic.
All included studies reported postoperative all-cause mortality. According to the whole cohort analysis and all related sensitivity analyses, both techniques were associated with similar all-cause mortality rate. This was an expected outcome given the growing evidence suggesting that baseline characteristics and CPB time, rather than aortic clamping technique, are predictors of mortality [
13,
24]. In fact, in the present study we tried to limit the impact of potential cofounders by assessing the similarity of the baseline characteristics in the total cohort, and by performing a PSM sensitivity analysis. Given the low heterogeneity, the similarity and the replicability of these outcomes in all sensitivity analyses, we suggest that both techniques are equally safe in terms of all-cause mortality and that survival is not influenced by the aortic occlusion technique.
Fifteen studies reported outcomes on postoperative CVA. The overall cohort analysis showed no difference between the two groups in the risk of CVA with either technique. In addition, the incidence of CVA was similar between TTC and EABO in either the femoral or the aortic cannulation EABO subgroup. However, in the video-assisted MIMVS subgroup analysis, the incidence of CVA was higher in the EABO cohort. A potential mechanism is the increased risk of embolus derived from the aortic wall of patients with severe atheromatous disease and porcelain aorta during the manipulation of the balloon catheter and the inflation-deflation-reinflation circles that may occur in cases of balloon migration. Overall, both TTC and EABO are associated with similarly low risk of CVA; however, EABO (aortic) seems the least risk prone for this outcome. Nonetheless, the PSM and leave-one-out sensitivity analyses confirmed the equal outcomes demonstrated by the total cohort analysis. Finally, there was zero heterogeneity in all analyses regarding CVA incidence.
Seventeen studies reported CPB time and sixteen studies reported aortic cross-clamp time. There was no difference between the two groups in terms of CPB and cross-clamp time in the total cohort, PSM, and video-assisted approach analyses. Nonetheless, there was high heterogeneity among the included studies, probably attributed to differences in terms of the level of expertise, the point of standing in the learning curve, the volume of cases, the operation setting, the cross-clamp devices, and the perioperative pathway protocols among different institutions. Outcomes were different in the cannulation approach subgroup analyses. However, there was no difference regarding CPB time in all analyses, and cross-clamp time was higher in the femoral EABO and lower in the aortic EABO group compared to the TTC group. These results are consistent with the previous meta-analysis [
5] regarding cross-clamp time but differ with respect to CPB time. The main reasons for this difference from the previous meta-analysis are the inclusion of six newer studies with a larger number of patients included, and surgeons more experienced in MIMVS. However, the difference between the femoral cannulation approach EABO and the TTC technique remains, mainly due to the more straightforward nature and shorter learning curve of the TTC occlusion maneuver [
27].
Fifteen studies were included in the aortic dissection assessment. According to the total and PSM analyses, both techniques were associated with a similar incidence of aortic dissections. This finding is in contrast to the previous meta-analysis, which reported a higher incidence of aortic dissection for the EABO group. In addition, the cannulation approach (femoral or aortic) did not affect our outcomes. There is evidence demonstrating the correlation between the learning curve and the incidence of iatrogenic aortic dissections [
28]. Because we included newer studies with larger patient volumes, the impact of learning was limited, and the outcomes were similar between the two groups. Furthermore, according to the total cohort and PSM analyses there was no difference between the two groups regarding the perioperative morbidity.
The limitations of the current meta-analysis are relevant to the limitations posed by the included studies. No RCTs were included. Although most studies were retrospective in nature, seven of them provided either risk-adjusted/PSM analyses or used prospectively collected data. In addition, the included studies are related to potential biases regarding the outcome data and selective reporting. Moreover, differences among institutions in selection criteria, surgeon expertise, different occlusion devices, and perioperative management pose certain limitations.
On the other hand, the strengths of this study include a) the clear data-extraction protocol, b) the well-specified inclusion-exclusion criteria, c) the search performed in three different databases, d) the quality assessment of the included studies, e) the detailed presentation of the results of data-extraction and analyses, f) the significantly larger patient sample compared to the previous meta-analyses, g) the groups were similar in almost all baseline characteristics, and h) the thorough sensitivity and subgroups analyses performed.
5. Conclusion
In the context of patients undergoing MIMVS, aortic occlusion with either the TTC or EABO approach is similarly safe and feasible. There was no difference between the two groups regarding the primary endpoints (all-cause mortality, CVA, aortic dissections) between the two groups in the non-adjusted and adjusted total cohort analyses. Furthermore, the aortic cannulation EABO approach was associated with the shortest cross-clamp time. The current study represents the best currently available level of evidence on the topic and should be further supported by a well-designed future RCT.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Dimitrios Magouliotis and Basel Ramlawi; Data curation, Dimitrios Magouliotis, Massimo Baudo, Yoshiyuki Yamashita, Andrew Xanthopoulos, Arian Arjomandi Rad and Thanos Athanasiou; Formal analysis, Dimitrios Magouliotis, Serge Sicouri, Massimo Baudo, Yoshiyuki Yamashita, Arian Arjomandi Rad and Thanos Athanasiou; Funding acquisition, Dimitrios Magouliotis, Serge Sicouri and Basel Ramlawi; Investigation, Dimitrios Magouliotis, Serge Sicouri, Massimo Baudo, Yoshiyuki Yamashita, Andrew Xanthopoulos and Arian Arjomandi Rad; Methodology, Dimitrios Magouliotis, Serge Sicouri, Massimo Baudo, Yoshiyuki Yamashita, Andrew Xanthopoulos, Arian Arjomandi Rad, Thanos Athanasiou and Basel Ramlawi; Project administration, Dimitrios Magouliotis, Serge Sicouri and Basel Ramlawi; Resources, Dimitrios Magouliotis, Serge Sicouri, Andrew Xanthopoulos, Arian Arjomandi Rad and Basel Ramlawi; Software, Dimitrios Magouliotis, Thanos Athanasiou and Basel Ramlawi; Supervision, Dimitrios Magouliotis, Serge Sicouri, Thanos Athanasiou and Basel Ramlawi; Validation, Dimitrios Magouliotis, Massimo Baudo, Andrew Xanthopoulos, Thanos Athanasiou and Basel Ramlawi; Visualization, Dimitrios Magouliotis, Massimo Baudo, Yoshiyuki Yamashita, Andrew Xanthopoulos and Basel Ramlawi; Writing – original draft, Dimitrios Magouliotis; Writing – review & editing, Dimitrios Magouliotis, Serge Sicouri, Massimo Baudo, Yoshiyuki Yamashita, Andrew Xanthopoulos, Arian Arjomandi Rad, Thanos Athanasiou and Basel Ramlawi.
Funding
The participating authors declare no sources of financial report that require acknowledgement.
Acknowledgments
Does not apply.
Conflicts of Interest
The authors declare no conflicts of interest.
Ethical Approval
Does not apply.
References
- Watt TMF, Brescia AA, Murray SL, Burn DA, Wisniewski A, Romano MA, et al. Degenerative Mitral Valve Repair Restores Life Expectancy. Ann Thorac Surg 2020;109:794–801.
- Mihaljevic T, Jarrett CM, Gillinov AM, Williams SJ, DeVilliers PA, Stewart WJ, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72–80.e1–4.
- Bates MJ, Chitwood WR (2021) Minimally invasive and robotic approaches to mitral valve surgery: transthoracic aortic crossclamping is optimal. JTCVS Techniques 10:84–88. https:// doi.org/ 10. 1016/j. xjtc. 2021. 09.
- Mohr FW, Falk V, Diegeler A, et al (1998) Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 115:567–576.
- Rival PM, Moore THM, McAleenan A, Hamilton H, Du Toit Z, Akowuah E et al. Transthoracic clamp versus endoaortic balloon occlusion in minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2019;56:643–53.
- Kowalewski M, Malvindi P, Suwalski P, Raffa G, Pawliszak W, Perlinski D et al. Clinical safety and effectiveness of endoaortic as compared to transthoracic clamp for small thoracotomy mitral valve surgery: metaanalysis of observational studies. Ann Thoracic Surg 2017;103:676–86.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-5. [CrossRef]
- Sterne JA, Hern an MA, Reeves BC, et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016;355:i4919.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011 Available from www.cochrane-handbook.org.
- Aybek T, Dogan S, Wimmer-Greinecker G, Westphal K, Mortiz A. The micro-mitral operation comparing the Port-Access technique and the transthoracic clamp technique. J Card Surg. 2000 Jan-Feb;15(1):76-81. [CrossRef]
- Balkhy HH, Grossi EA, Kiaii B, Murphy D, Geirsson A, Guy S, et al. A Retrospective Evaluation of Endo-Aortic Balloon Occlusion Compared to External Clamping in Minimally Invasive Mitral Valve Surgery. Semin Thorac Cardiovasc Surg. 2024 Spring;36(1):27-36. [CrossRef]
- Barbero C, Marchetto G, Ricci D, El Qarra S, Attisani M, Filippini C, et al. Right Minithoracotomy for Mitral Valve Surgery: Impact of Tailored Strategies on Early Outcome. Ann Thorac Surg. 2016 Dec;102(6):1989-1994. [CrossRef]
- Barbero C, Rinaldi M, Pocar M, Cura Stura E, Calia C, Sebastiano V, et al. Endo-Aortic vs. Trans-Thoracic Clamping in Right Mini-Thoracotomy Mitral Valve Surgery: Outcome on Myocardial Protection. Front Cardiovasc Med. 2021 Sep 9;8:719687. [CrossRef]
- Bentala M, Heuts S, Vos R, Maessen J, Scohy TV, Gerritse BM et al. Comparing the endo-aortic balloon and the external aortic clamp in minimally invasive mitral valve surgery. Interact CardioVasc Thorac Surg 2015;21:359–65.
- Ergi DG, Rowse PG, Daly RC, Crestanello JA, Schaff HV, Dearani JA, et al. Single Center Prospective Study of Cross-Clamp versus Balloon Occlusion in Robotic Mitral Surgery, The Annals of Thoracic Surgery (2024). [CrossRef]
- Glower DD, Desai B. Transaortic endoclamp for mitral valve operation through right minithoracotomy in 369 patients. Innovations (Phila). 2010 Nov;5(6):394-9. [CrossRef]
- Grazioli V, Giroletti L, Graniero A, Albano G, Mazzoni M, Panisi PG, et al. Comparative myocardial protection of endoaortic balloon versus external clamp in minimally invasive mitral valve surgery. J Cardiovasc Med (Hagerstown). 2023 Mar 1;24(3):184-190. [CrossRef]
- Ius F, Mazzaro E, Tursi V, Guzzi G, Spagna E, Vetrugno L, et al. Clinical results of minimally invasive mitral valve surgery: endoaortic clamp versus external aortic clamp techniques. Innovations (Phila). 2009 Nov;4(6):311-8. [CrossRef]
- Loforte A, Luzi G, Montalto A, Ranocchi F, Polizzi V, Sbaraglia F, et al. Video-assisted minimally invasive mitral valve surgery: external aortic clamp versus endoclamp techniques. Innovations (Phila). 2010 Nov;5(6):413-8. [CrossRef]
- Malvindi PG, Margari V, Mastro F, Visicchio G, Kounakis G, Favale A, et al. External aortic cross-clamping and endoaortic balloon occlusion in minimally invasive mitral valve surgery. Ann Cardiothorac Surg. 2018 Nov;7(6):748-754. [CrossRef]
- Maselli D, Pizio R, Borelli G, Musumeci F. Endovascular balloon versus transthoracic aortic clamping for minimally invasive mitral valve surgery: impact on cerebral microemboli. Interact Cardiovasc Thorac Surg. 2006 Apr;5(2):183-6. [CrossRef]
- Mazine A, Pellerin M, Lebon JS, Dionne PO, Jeanmart H, Bouchard D. Minimally invasive mitral valve surgery: influence of aortic clamping technique on early outcomes. Ann Thorac Surg. 2013 Dec;96(6):2116-22. [CrossRef]
- Modi P, Rodriguez E, Hargrove WC 3rd, Hassan A, Szeto WY, Chitwood WR Jr. Minimally invasive video-assisted mitral valve surgery: a 12-year, 2-center experience in 1178 patients. J Thorac Cardiovasc Surg. 2009 Jun;137(6):1481-7. [CrossRef]
- Reichenspurner H, Detter C, Deuse T, Boehm DH, Treede H, Reichart B. Video and robotic-assisted minimally invasive mitral valve surgery: a comparison of the Port-Access and transthoracic clamp techniques. Ann Thorac Surg. 2005 Feb;79(2):485-90; discussion 490-1. [CrossRef]
- Yost CC, Rosen JL, Mandel JL, et al. Endoaortic balloon occlusion versus transthoracic cross-clamp for totally endoscopic robotic mitral valve surgery: a retrospective cohort study. J Robot Surg. 2023 Oct;17(5):2305-2313. [CrossRef]
- Marullo A, Irace F, Vitulli P, Peruzzi M, Rose D, D’Ascoli R. Recent developments in minimally invasive cardiac surgery: evolution or revolution? Biomed Res Int 2015;2015:1–6.
- Atluri P, Goldstone AB, Fox JY, Szeto W, Hargrove WC. Port access cardiac operations can be safely performed with either endoaortic balloon or Chitwood clamp. Ann Thorac Surg 2014;98:1579–84.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).