1. Introduction
Bisphenol A (BPA) is the most used bisphenol worldwide, with a production still growing [
1]. BPA is mainly used in the polycarbonate plastic production [
2]. However, the increasing evidence on the estrogenic activity of BPA to aquatic species [
3] has led to some limitations in its uses. Consequently, the replacement of BPA with other similar compounds - named BPA analogues - has begun. BPA analogues belong to a chemical group composed of 17 different native bisphenols, from which other 148 chemicals are derived.
Three of the most known BPA analogues are BPAF, BPF and BPS, being largely used in the production of polycarbonate copolymers, epoxy resins, liners, water pipes, toys, adhesives, food packaging and thermal paper manufacture [
4]. The increasing use of such BPA analogues led to a consequent release of detectable concentrations into ecosystems, including freshwater and seawater. Generally, environmental concentrations of BPA analogues range from few ng/L up to hundreds of ng/L in both freshwater and seawater [
5]. For instance, BPAF was recorded at a mean concentration of 140 ng/L in Taihu Lake in China [
6], while BPF reached 2850 ng/L in the Tamagawa River in Japan [
7]. High levels of BPS (up to 65,600 ng/L) were recorded in rivers of China [
8]. As for marine coastal environment, the concentrations of BPA analogues are generally lower than those recorded in freshwater [
5]. However, high concentrations of BPF up to 282 ng/L and 1470 ng/L were detected in seawater in South China and in the Tokyo Bay, respectively [
7,
9]. Furthermore, in marine sediments from coastal areas of Zhejiang in East China, BPA was detected at higher concentrations (mean 13 ng/g dw, dry weight) than BPF (1.6 ng/g dw), BPAF (0.53 ng/g dw) and BPS (0.69 ng/g dw) [
10]. In that study, all seawater samples contained measurable concentrations of BPA (mean 23 ng/L, range 2.7–52 ng/L), BPS (2.2 ng/L, 0.15–12 ng/L), and BPAF (0.34 ng/L, 0.12–0.91 ng/L), while BPF was only detectable in some seawater samples at concentrations lower than 1.0 ng/L [
10]. BPA was predominant also in surface seawater and sediment samples from the Beibu Gulf, South China Sea, with concentrations ranging from 5.26 to 12.04 ng/L in seawater and from 0.56 to 5.22 ng/g dw in sediment samples, followed by BPAF (0.44–0.60 ng/L in seawater and 0.08–0.66 ng/g dw in sediments, respectively) and BPS (0.07–0.63 ng/L in seawater and up to 0.19 ng/g dw in sediments, respectively) [
11]. The predicted no-effect concentrations (PNEC) in freshwater and seawater for the three compounds are respectively 1.02 µg/L and 100 ng/L for BPAF, 5.44 µg/L and 540 ng/L for BPF, and 12.9 µg/L and 27 µg/L for BPS [
12]. However, the only adopted PNEC with a legislative relevance is the BPA PNEC which was settled at 1500 ng/L for freshwater and 150 ng/L in seawater by the EU [
13]. In a recent study, we demonstrated that exposure to BPAF, BPF and BPS induced oxidative stress and ultrastructural changes in the microalgae
Phaeodactylum tricornutum [
14]. In that study, bioaccumulation of the three BPA analogues in microalgae was also recorded. As a result of those findings, in the present study we evaluated for the first time the effects of food-borne exposure to BPAF, BPF, BPS - alone or as a mixture - on some important biomarkers in the clam
Ruditapes philippinarum. The hypothesis we tested was that the ingestion of BPA analogues-contaminated microalgae can induce alterations in clam biomarker responses similar to what was observed in a recent study in which bivalves were exposed to the same contaminants dissolved in seawater [
15].
2. Materials and Methods
2.1. Microalgae Culture and Exposure
P. tricornutum was purchased from the Culture Collection of Algae at Göttingen University (SAG). Microalgae were grown for 10 days in F/2 medium [
16] prepared in 0.45 μm-filtered seawater at 16 °C, with an adopted light intensity of 40.5 µmol photons m
−2s
−1, and a photoperiod of 12:12 light/dark. BPAF and BPF stock solutions (1 mg/L) were prepared in methanol, while BPS (1 mg/L) was dissolved in distilled water. Five experimental conditions (control, BPAF, BPF, BPS and MIX) were prepared in Erlenmeyer flasks with a F/2 volume of 600 mL with an initial concentration of microalgae of 5*10
5 cells/mL (
inoculum), BPA analogues were added in the corresponding experiments at a final concentration of 300 ng/L when a single bisphenol was tested and 100 ng/L of each of them in the mixture treatment.
Microalgae were treated for 7 days to allow them to bioaccumulate BPA analogues [
14]. A solvent control was omitted because in our previous study, we observed that methanol does not cause negative effects on microalgae [
14]. In addition, it has been demonstrated that methanol can exert acute and chronic effects at very high concentrations (tens and hundreds of mg/L) in aquatic species, including marine microalgae [
17,
18].
2.2. Clam Acclimation and Treatment
R. philippinarum specimens were sampled in February 2023 from a licensed fishing area in the Lagoon of Venice (Italy) and acclimated for 7 days in large aquaria filled with aerated seawater (salinity of 35 ± 1, temperature of 11 ± 0.5 °C) and a sandy bottom. After acclimation, 80 clams (mean length: 36,7 mm) were randomly divided in 10 experimental tanks without sand (30 litres capacity, 2 tanks per experimental condition, 40 clams per tanks). Every two days, seawater was renewed, and 200 mL of control or contaminated microalgae suspensions were added. To allow clams to take up contaminants only from the microalgae and not from the medium in which they grew, all microalgae suspensions (both control and treated groups) were centrifuged at 4000 rpm at room temperature for 10 minutes using an ultracentrifuge Avant-J-25. The supernatant (=BPA analogues-contaminated medium) was discharged, and microalgae were then carefully re-suspended in 0.45 mm-filtered seawater. Clam tissues were collected after 7 and 14 days of diet with control or contaminated microalgae.
2.3. Tissue Collection
Haemolymph was collected from the anterior adductor muscle using a 1-mL syringe and stored on ice. At each sampling time (7 and 14 days), 5 pools of haemolymph (from six clams each) for each experimental condition were prepared. After sampling, total haemocyte count (THC), haemocyte diameter and volume, lactate dehydrogenase (LDH) activity and haemocyte proliferation (XTT assay) were measured. The remaining pooled haemolymph was then centrifuged at 780×g for 10 min and the pellets (=haemocytes) were resuspended in distilled water to obtain haemocyte lysate (HL), which was frozen in liquid nitrogen and stored at −80 °C until analyses. After haemolymph sampling, gill and digestive gland were excised and pooled (five pools of six clams each). Aliquots of each pooled tissues were then frozen in liquid nitrogen and stored at −80 °C until analyses.
2.4. Haemolymph and Haemocyte Biomarkers
THC, as well as haemocyte diameter and volume, were determined using a Scepter™ 2.0 Automated Cell Counter (Millipore, FL, USA). In detail, 20 μL of haemolymph were diluted into 2 mL of Coulter Isoton II diluent. THC was expressed as the number of haemocytes (106)/mL of haemolymph, while haemocyte diameter and volume were expressed in μm and picolitres (pL), respectively.
Cell-free haemolymph (CFH) LDH activity was measured using the commercial kit Cytotoxicity Detection Kit (Roche). Briefly, haemolymph was centrifuged at 780×g for 10 min, and 500 μL of supernatant (=CFH) was then collected for the assay following the manufacturer's instructions. The results were expressed as optical density (OD) at 490 nm.
Haemocyte proliferation was evaluated using the Cell proliferation Kit II. A volume of 200 μL of the reagent mixture (provided with the kit) was added to 400 μL of pooled haemolymph and incubated for 4 h at room temperature. The absorbance was then recorded at 450 nm and results were normalized to THC values of each experimental groups and expressed as optical density (OD) at 450 nm.
Lysozyme activity was measured in haemocyte lysate (HL). Briefly, 50 μL of HL was added to 950 μL of a 0.15% suspension of Micrococcus lysodeikticus (Sigma) phosphate buffer (pH 6.2). The absorbance decrease was continuously recorded at 450 nm for 3 min at room temperature. Results were expressed as μg lysozyme/mg of protein.
The arylsulfatase activity was measured in HL samples measuring the production of p-nitrocatechol after 1 h at 515 nm [
19] and then calculated using the formula proposed by Baum et al. [
20]. Results are expressed as μg of p-nitrocatechol produced per hour/mg of protein.
The acid phosphatase and alkaline phosphatase activity were measured both in HL and CFH. The acid phosphatase hydrolyses the substrate 4-nitrophenyl phosphate during the incubation at 37 °C and the absorbance was measured at 405 nm using a microplate reader. Results were expressed as U/mg of protein. Similarly, the alkaline phosphatase hydrolyses the same substrate in an alkaline buffer and after the incubation at 30 °C the absorbance was recorded at 405 nm [
21].
Lastly, total antioxidant capacity of haemolymph was assessed following the CUPRAC method [
22]. In detail, the reaction produced cupric ions that reacted with a specific colorimetric indicator creating a complex that was measured at 450 nm using a microplate reader. The results are expressed as mM of Trolox equivalents.
2.5. Gill and Digestive Gland Biomarkers
Gills and digestive gland samples were homogenised for 5 min and 50 oscillations per second at 4 °C using TissueLyser LT (Quiagen) in four volumes of 10 mM Tris-HCl buffer, pH 7.5, containing 0.15 M KCl, 0.5 M sucrose, 1 mM EDTA and protease inhibitor cocktail (1:10 v/v) (Sigma-Aldrich). After centrifugation at 12,000 g for 30 min at 4 °C, supernatants (SN) were collected for analyses.
Like haemolymph, CUPRAC assay was performed in SN of both gills and digestive gland according to the CUPRAC method [
22]. The results were expressed as mM of Trolox equivalents.
Total SOD activity was measured in both gills and digestive gland SN in triplicate following the xanthine oxidase/cytochrome
c method proposed by Crapo et al. [
23]. Enzyme activity was expressed as U/mg protein, one unit of SOD has been defined as the amount of sample causing 50% inhibition under the assay conditions.
CAT activity was measured in gills and digestive gland SN in triplicate at 240 nm and expressed as U/mg protein [
24]. One unit of CAT was defined as the amount of enzyme that catalysed the dismutation of 1 μmol of H
2O
2/min.
Acetylcholinesterase (AChE) activity was measured only in gills following the colorimetric reaction between acetylthiocholine and the reagent dithiobisnitrobenzoate [
25]. Absorbance was recorded at 405 nm for 5 min on a microplate reader at room temperature. Results are expressed as nmol/min/mg of protein. Similarly, the butyrylcholinesterase (BChE) activity was measured using butyrylthiocholine as substrate and the absorbance was quantified at 405 nm [
26]. Results are expressed as nmol/min/mg protein.
Glutathione reductase (GR) activity was measured in both gills and digestive gland SN according to Smith et al. [
27], by measuring the (5-thio (2-nitrobenzoic acid)) TNB production at 412 nm. Enzyme activity was expressed as U/mg protein.
Glutathione S-transferase (GST) activity was measured only in digestive gland SN using 1-chloro-2,4-dinitrobenzene (CDNB) and reduced glutathione (GSH) as substrates [
28]. GST activity was expressed as nmol/min/mg protein.
Protein carbonyl content (PCC) and lipid peroxidation (LPO) were measured as oxidative damage biomarkers. Briefly, PCC were measured in duplicate using the method of Mecocci et al. [
29] following the reaction with 2,4-dinitrophenylhydrazide (DNPH). Results were expressed as nmol carbonyl group/mg protein. LPO was quantified using the malondialdehyde (MDA) assay, according to the method of [
30]. Absorbance was measured spectrophotometrically at 532 nm and the results were expressed as nmoles of thiobarbituric reactive substances (TBARS)/mg protein. TBARS, considered as ‘‘MDA-like peroxide products’’, were quantified by reference to MDA absorbance (ε = 156 × 10
3 M
−1 cm
−1) [
31].
Total protein concentration in SN samples was quantified according to [
32].
2.6. Epigenetic Biomarkers
The histone N-terminal acetyltransferases (HAT) and histone deacetylases activities (HDAC) were evaluated in gills and digestive glands. The HAT activity was measured at 412 nm [
33]. In detail, SN samples were prepared as described above, followed by a sonication step. Then, we used the histone extracted from the calf thymus (Sigma Aldrich, Milan) as an acetyl acceptor and acetyl-CoA as an acetyl group donator. The resulting free thiol group was quantified using 5,5′-dithiobis-(2-nitrobenzoic acid) at 412 nm in a microplate. Results are expressed as μM of 5-thio-2-nitrobenzoic acid (TNB
-)/mg proteins. The HDAC activity of class I and II was quantified following the spectrophotometric method proposed by Yuan et al. [
34]. Briefly, SN samples reacted with the synthetic substrate Boc-Lys(Ac)-pNA removing the acetyl group from the lysine. This reaction led to the formation of a chromogen compound that was quantified at 405 nm in a microplate. Results are expressed as OD at 405 nm/mg proteins.
2.7. Bioaccumulation
Methanol, acetonitrile, ammonium acetate, bisphenol F (BPF), bisphenol AF (BPAF), bisphenol S (BPS) and bisphenol A d-16, used as internal standard, were purchased from Merck (Milan, Italy). Ultrapure-grade water was produced with a Pure-Lab Option Q apparatus (Elga Lab Water, High Wycombe, UK). Bioaccumulation of BPs was evaluated in five organisms collected after 7 and 14 days of diet with BPA analogues-contaminated microalgae. Each sample was accurately weighed and homogenized (Homogeniser SHM1, Avantor, VWR International Srl, Milano, Italia) after the addition of 1 mL of ultrapure water. The homogenate was treated with 7 mL of cold acetonitrile containing the internal standard at 500 µg/L, vortexed for 3 minutes, and centrifuged for 5 minutes at 5000 rpm. After a further centrifugation step (13000 rpm, 10 minutes, 4°C), 20 µL of the supernatant were analysed by UHPLC-HRMS. The system was equipped with an Agilent 1260 Infinity II LC chromatographer coupled to an Agilent 6545 LC/Q-TOF mass analyser (Agilent Technologies, Palo Alto, Santa Clara, CA, USA). The analytical column was a Kinetex 2.6 µm C18 Polar, 100 A, 100 x 2.1 mm (Phenomenex, Bologna, Italy), at 25°C. Mobile phase A and B were water and acetonitrile, respectively, both containing 10 mM ammonium acetate and the eluent flow rate was 0.30 mL/min. The mobile phase gradient profile was as follows (t in min): t0–4 0% B; t4–22 0–100% B, t22–25 100% B; t25–32 0% B. The MS conditions were: electrospray (ESI) ionization in negative mode, gas temperature 320°C, drying gas 12 L/min, nebulizer 35 psi, sheath gas temperature 350 °C, sheath gas flow 11 L/min, VCap 5000 V, nozzle voltage 0 V, fragmentor 150 V. Centroid full scan mass spectra were recorded in the range 100–1000 m/z with a scan rate of 2 spectrum/s. The QTOF calibration was performed daily with the manufacturer’s solution in this mass range. The MS were analysed by the Mass Hunter Qualitative Analysis software (Agilent Technologies, Palo Alto, Santa Clara,CA, USA).
Homogenates from no-treated organisms were used to build a matrix-matched seven points external calibration curve, in the range 0.1-100 µg/L (corresponding to 0.8–800 ng/g in the initial animal tissues). Linearity was evaluated by the least squares regression and R2>0.998 was obtained for all the analytes. LODs were 40 ng/g for BPF, 2 ng/g for BPS and 1 ng/g for BPAF. Each treated organism was analysed separately, and results are reported as mean and standard deviation.
2.8. Statistical Analysis
The normal distribution of data (Shapiro-Wilk's test) and the homogeneity of the variances (Bartlett's test) were assessed. The results obtained were compared by means of the two-way ANOVA analysis, using “exposure time”, “treatment” (=diet) and “exposure time-treatment interaction” as independent factors. Pairwise comparisons among experimental conditions were performed using the Fisher’s LSD post-hoc test. Significant difference was set at p<0.05. All results are expressed as means ± standard deviation (SD), n = 5. The software package Origin 2023 (Origin lab) was used for statistical analyses.
4. Discussion
To the best of our knowledge, this is the first study demonstrating the effects of a diet of BPA analogues-contaminated microalgae in the clam R. philippinarum. Consequently, the comparison of our results with those of the literature is limited and often relates to data obtained in aquatic organisms directly exposed to BPA and BPA analogues-contaminated water and not fed by contaminated food.
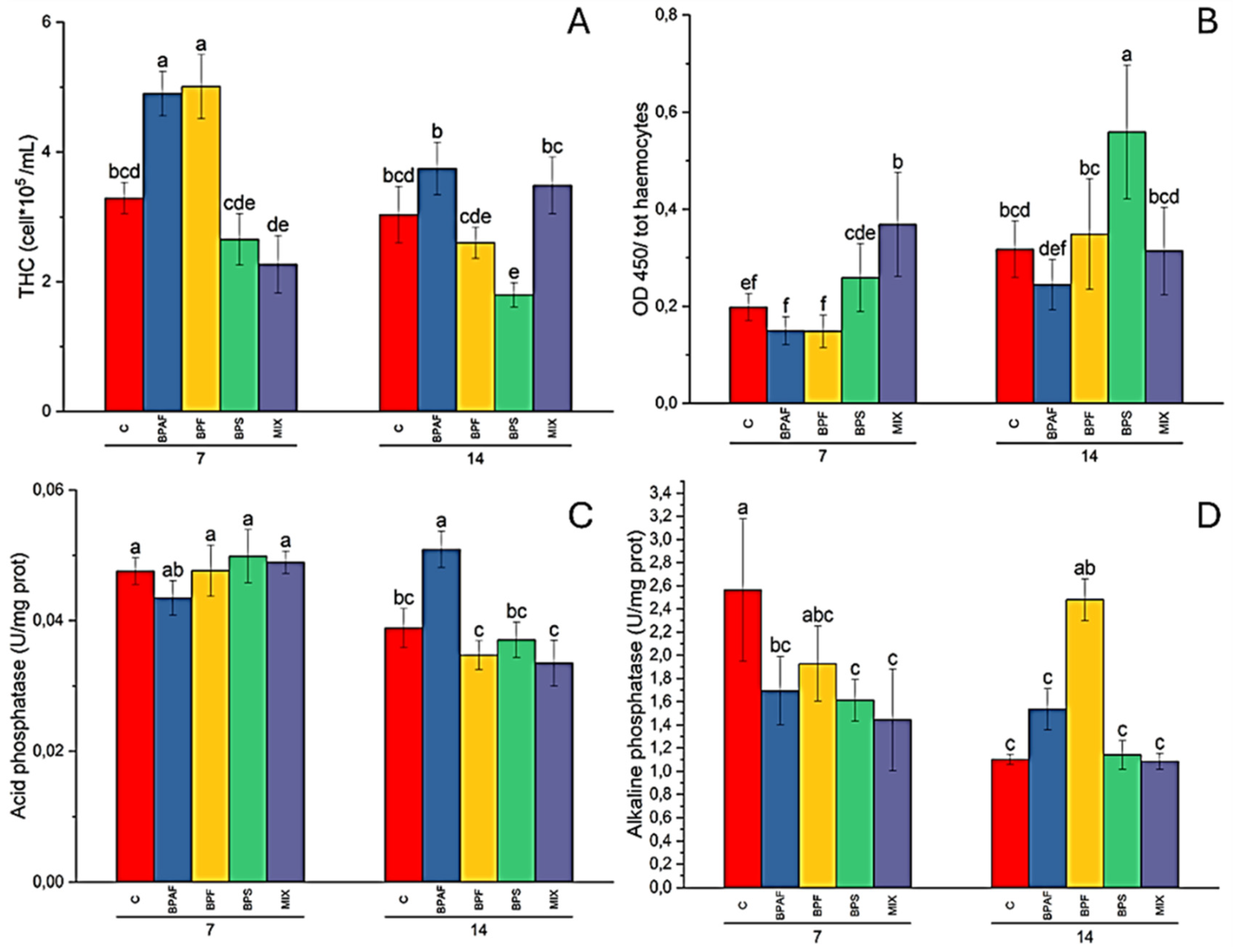
At the cellular level, we observed increased THC values in clams fed for 7 days with BPAF- and BPF-exposed microalgae, whereas THC was reduced in clams fed for 14 days with BPS-exposed microalgae. Cell proliferation significantly increased in clams fed for 7 days with MIX-exposed microalgae and in those fed for 14 with BPS-exposed microalgae. Our results highlighted a negative relationship between THC and cell proliferation, both after 7 and 14 days of clam diet (Pearson correlation coefficient: -0.808, p<0.001), increased THC values generally corresponding to reduced haemocyte proliferation, and vice versa. We hypothesised that the increase in cell proliferation was, at least in part, an attempt of clams to cope with the reduction in THC values, as in the cases of clams fed for 7 days with MIX-contaminated microalgae and in those fed for 14 days with BPS-treated microalgae. In contrast, there was no increase in cell proliferation in clams where there were high levels of THC, as in the case of clams fed with BPAF- and BPF-treated microalgae. In our previous study, no significant alterations of THC were observed in clams exposed to the three bisphenols dissolved in water at the same concentrations used in this study, whereas there was a general reduction in both diameter and volume of haemocytes [
15]. In that study, a significant increase in cell proliferation was recorded in clams exposed for 7 and 14 days to bisphenol mixture. An impairment of THC was also reported by Tang et al. [
35] in the clam
Tegillarca granosa exposed to BPA. Indeed, the authors reported that THC was reduced after 2 weeks of exposure to 10 and 100 ng/L of BPA, with a decreased percentage of red granulocytes and an increased percentage of both basophil granulocytes and hyalinocytes [
35]. BPA was able to reduce THC values also in the crab
Charybdis japonica exposed for 1, 3, and 6 days to 1 mg/L of BPA [
36]. In a recent study, the marine bivalve
Lithophaga lithophaga was exposed for 28 days to 0.25, 1, 2, and 5 µg/L BPA [
37]. In that study, the authors observed an increase in THC value in mussels exposed to 0.25, 2 and 5 µg/L. Interestingly, they also observed a reduction in both mean haemocyte diameter and haemocyte nucleus diameter in all the treatments and all the haemocyte cell types (agranulocytes, hyalinocytes, and granulocytes) [
37].
Our findings indicate that BPA analogues can affect THC in clams fed with contaminated microalgae, similarly to what was observed for BPA in different model species and experimental designs.
Based on results of LDH assay, in the present study we can state that BPA analogues were not able to cause cytotoxic effects in clams fed with contaminated microalgae, similarly to what was observed in our previous survey with clams exposed via seawater to the same contaminants [
15]. However, it has been demonstrated that higher concentrations of both BPF and BPS than those tested in our studies (0, 15.63, 31.25, 62.50, 125, 250, and 500 μM) can cause cytotoxic effects in a concentration-dependent manner in hepatocytes of the rainbow trout
Oncorhyncus mykiss treated for 24 h [
38,
39]. As for hydrolytic enzymes, CFH acid phosphatase activity was significantly increased in clams feed for 14 days with BPAF-contaminated microalgae, while HL alkaline phosphatase was reduced in clams feed for 7 days with BPAF, BPS and MIX-contaminated microalgae and increased in clams after 14-days diet with BPF-contaminated microalgae. These results contrast with the findings obtained in clams exposed to BPA analogues-contaminated seawater. Indeed, in that case, acid phosphatase activity decreased significantly in CFH after 7 days of exposure of clams to BPAF, BPF and BPS, and after 14 days in BPF-, BPS- and MIX-exposed clams [
15]. Moreover, it has recently been demonstrated that BPA can alter both acid phosphatase and lysozyme activity in the marine worm
Urechis unicinctus exposed for 15 days to 0.07, 7 and 700 μg/L [
40]. In detail, that study reported that the acid phosphatase activity of the experimental group exposed to the highest concentration initially increased and then decreased. Moreover, the acid phosphatase activity of BPA-exposed groups was significantly higher than that of the control group on days 5 and 15. Regarding the lysozyme activity, it was significantly decreased in the worms exposed to 0.07 μg/L after 0,5; 1; 3 and 5 days, while it was significantly increased after 10 and 15 days [
40]. On the contrary, the exposure to both 7 and 700 μg/L caused a significant decrease of lysozyme activity in all the sampling times [
40].
Overall, it seems that BPA analogues can exert different effects on clam haemocytes, depending on exposure modality, via seawater or contaminated diet. However, it is difficult to state which of the two modalities is more dangerous for
R. philippinarum haemocytes because both (the one adopted in this study and that of the study by Fabrello et al. [
15] caused effects on haemocyte parameters.
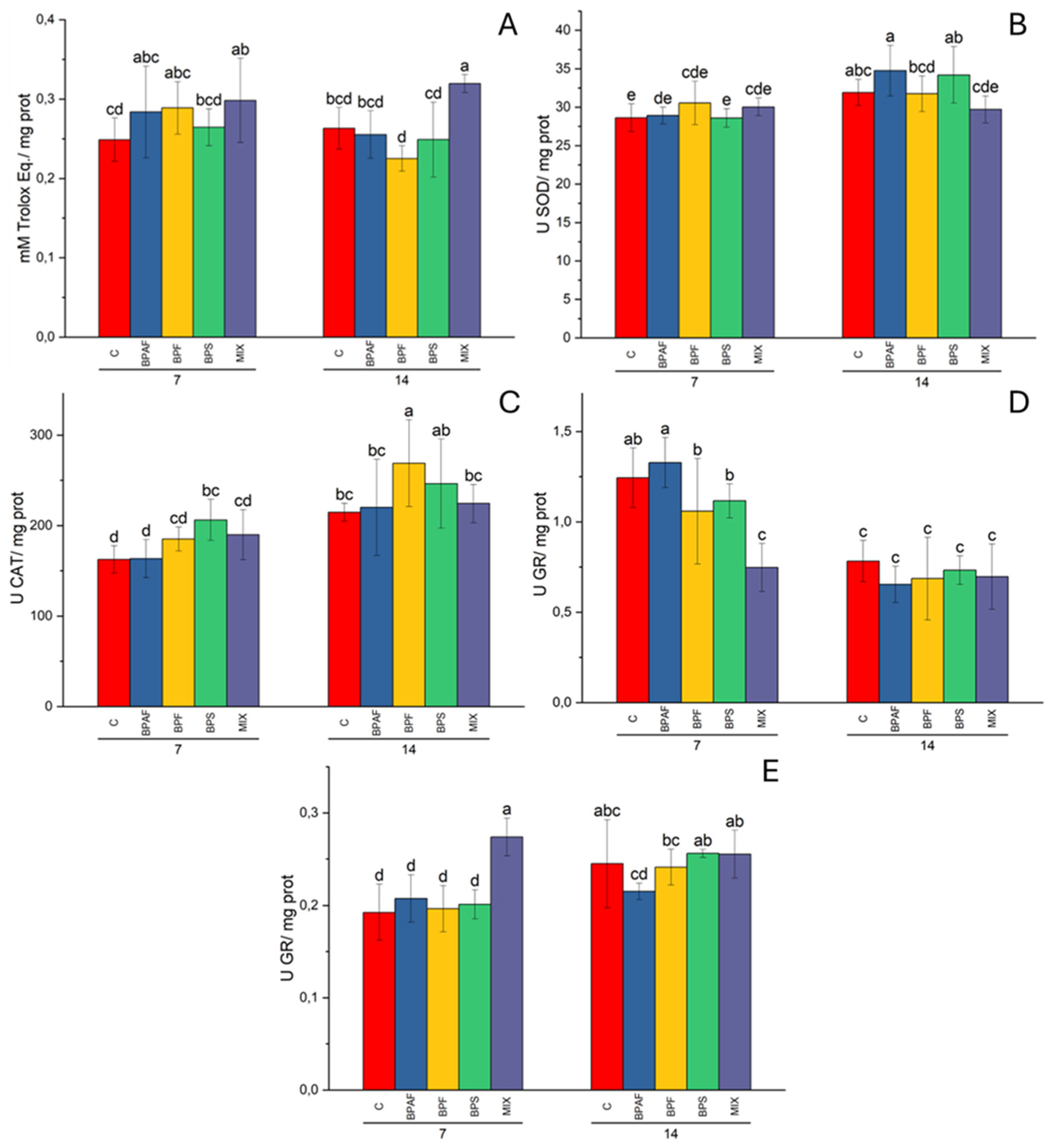
BPA analogues-contaminated diet was able to alter the total antioxidant capacity in clam gills, where CUPRAC levels increased in bivalves fed for 7 and 14 days with MIX-contaminated microalgae. Moreover, gill CAT activity significantly increased in clams fed for 7 and 14 days with microalgae exposed to BPS and BPF. Interestingly, no significant alterations of the cupric reducing antioxidant capacity (=CUPRAC) were recorded in digestive gland, suggesting that the main diet-mediated toxic effects occurred in gills during the first part of the feeding process. This evidence is in accordance with our previous results obtained in clams exposed to contaminated seawater [
15]. Indeed, in that study, no significant alterations of CUPRAC levels were observed in digestive glans, whereas there was a significant reduction of the total antioxidant capability in gills from clams exposed for 14 days to BPS and MIX. In addition, gill SOD activity increased significantly in animals exposed to BPS (after 14 days) and MIX (after 7 and 14 days), while CAT activity increased following exposure for 7 and 14 days to MIX [
15].
The glutathione cycle plays a pivotal role in both restoring the oxidative status inside the cells and detoxifying xenobiotics. Two of the main glutathione cycle-involved enzymes are GR and GST. In the present study, the first one was significantly affected by ingestion of contaminated microalgae in both gills and digestive gland of clams. Indeed, GR activity was significantly increased in gills of clams fed for 7 days with MIX-exposed microalgae, while a reduction in GR activity was found in digestive gland. Recently, the effects of BPA on a simplified food chain were investigated by Esperanza et al. [
41] in which the clams
Corbicula fluminea were exposed for 30 days to BPA-contaminated microalgae, BPA-contaminated water or BPA in both microalgae and water. For the preparation of BPA-contaminated microalgae, they exposed
Chlamydomonas reinhardtii cultures for 24 hours at 30 mg/L of BPA, while the tested BPA concentration in water was 7.5 mg/L. Like our study, Esperanza et al. [
41] measured several biomarkers in clams. CAT, selenium dependent-GPX and total GPX activities were significantly increased in the whole tissues, whereas GR activity increased at all the exposure conditions, even if the exposure to only contaminated microalgae caused the lowest GR increase. Contrary to what was observed in our study concerning GST results, which did not reveal any alteration, Esperanza et al. [
41] observed a significant inhibition of GST activity after exposure to both BPA-contaminated water and microalgae. Regarding BPA analogues very few studies have been conducted on a simplified marine food chain. In
Chlamys farreri, the effects of exposure via microalgae alone or microalgae + water contaminated with the BPA analogue tetrabromobisphenol A (TBBPA) were assessed [
42]. Firstly, the authors exposed the microalgae
Nitzschia closterium f. minutissima to 400 μg/L of TBBPA for 24 h and then they provided the microalgae to scallops for 10 days. After 0.5, 1, 3, 6 and 10 days of diet, GST activity, as well as GSH levels, was significantly increased by experimental conditions in both gills and digestive gland. The authors reported that TBBPA also increased SOD activity at almost all the conditions tested concluding that TBBPA was able to cause oxidative stress in clams [
42]. They also reported a significant reduction of microsomal cytochrome P450 in the gills and digestive gland. Similarly, cytochrome b5 values were significantly reduced by all treatments, even if 3 days of the water+food-borne exposure caused a significant increase in gills [
42].
As for results of previous studies on the effects of food contaminated by other contaminants, Iummato et al. [
43] analysed the biochemical alterations in the golden mussel
Limnoperna fortunei under dietary glyphosate exposure. Mussels were fed for 4 weeks with the green algae
Scenedesmus vacuolatus previously exposed to a commercial formulation of glyphosate (6 mg/L active principle) with the addition of alkyl aryl polyglycol ether surfactant. Then, the authors measured the activity of SOD, CAT, GST, and alkaline phosphatase, as well as the glutathione (GSH) content after 1, 7, 14, 21 and 28 days of dietary exposure of mussels. They found that mussels fed on glyphosate-exposed microalgae for 28 days showed an increased GST activity, whereas alkaline phosphatase activity was significantly increased at 21 and 28 days of dietary exposure. On the contrary, GSH content, CAT and SOD activities did not show significant differences between treated and untreated bivalves [
43]. A similar experimental plan was adopted to assess the effect and transfer of other compounds, such as heavy metals, nanoparticles and hydrocarbons [
44,
45,
46,
47]. For instance, the effects of benzo(α)pyrene and 7,12-dimethyl benz(α)anthracene) on a marine food chain were evaluated at a concentration of 5 ng/L each on the mussels
Mytilus galloprovincialis that were directly exposed to contaminated seawater and in fishes
Dicentrarchus labrax that were exposed to contaminated seawater or fed with contaminated mussels for 75 days. Benzo(α)pyrene-monooxygenase activity increased in treated shellfish, while ethoxyresorufin-O-deethylase (EROD) activity increased after 20 days in fishes exposed to contaminated seawater or fed with contaminated mussels [
44]. More recently, Wang et al. [
47] assessed the trophic transfer and effects of titanium dioxide nanoparticles (TiO
2 NPs) from the marine microalga
Nitzschia closterium to the scallop
Chlamys farreri. In detail, they exposed the scallop through aqueous exposure or dietary exposure, and they found an increased lysosomal membrane permeability, DNA damage, and histopathological effects induced by TiO
2 NPs mainly in scallops after aqueous exposure rather than dietary exposure [
47]. In another study, effects of silver nanoparticles (Ag NPs) (soluble or as lactate Ag NPs) at low concentrations (10 μg/L) were evaluated in the bivalve
Scrobicularia plana exposed for 14 days directly (water) or via the diet (microalgae) [
48]. Interestingly, the authors highlighted that the response of oxidative stress biomarkers (CAT, GST, SOD) in the whole soft tissues of bivalves was more important after dietary than waterborne exposure to Ag. In detail, CAT activity significantly increased by both water and dietary Ag, whereas Ag-contaminated diet caused a significant increase activity of both SOD and GST activity [
48].
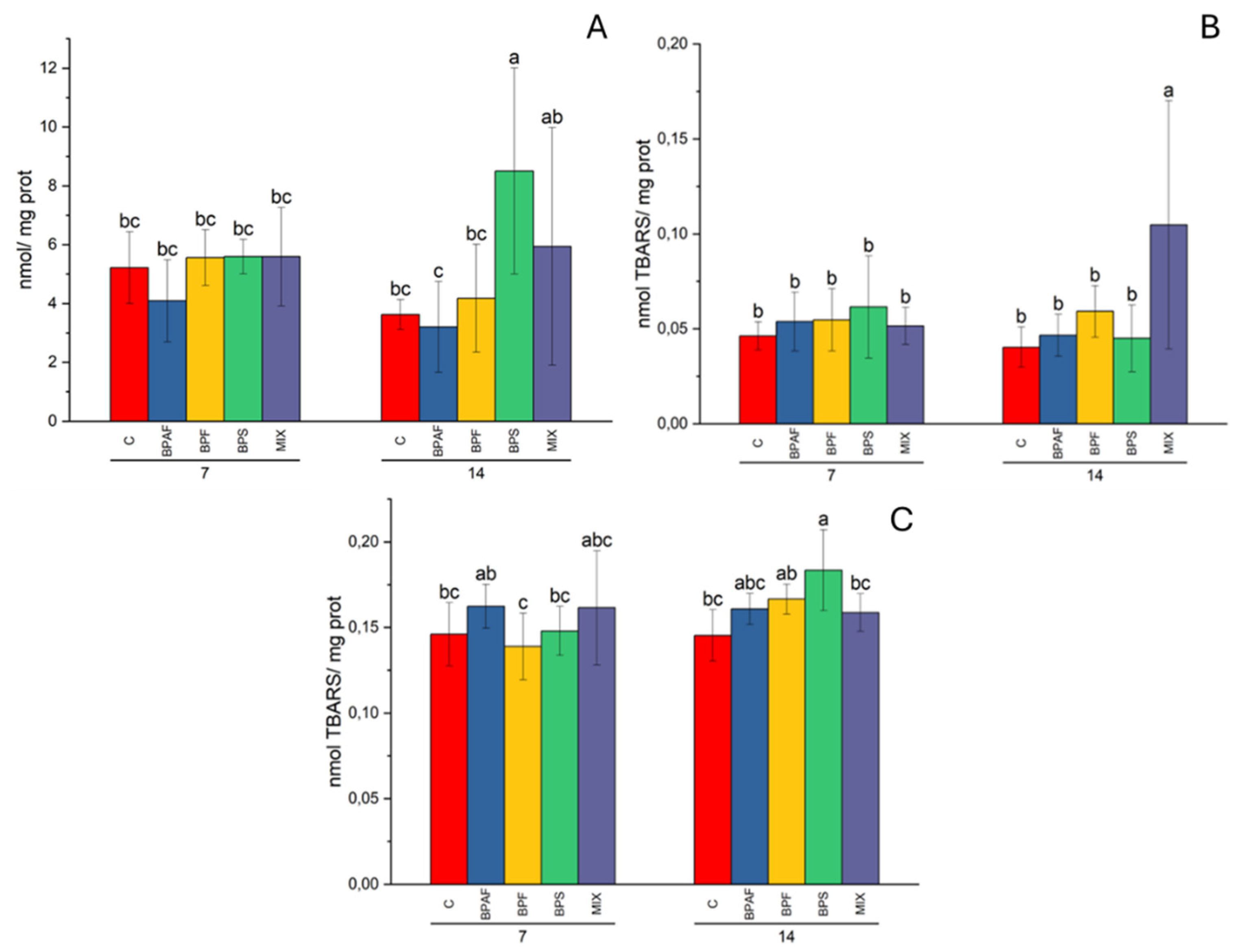
We have also evaluated oxidative damage to both lipids and proteins in clams fed with BPA analogues-contaminated microalgae. As a result, oxidative damage to proteins (PCC levels) increased significantly only in the digestive gland of clams fed for 14 days with BPS-exposed microalgae. Moreover, LPO increased in clam gills following a diet of 14 days with BPS-exposed microalgae, while in the digestive gland LPO levels increased significantly after 14 days in clams fed with MIX-contaminated microalgae. Esperanza et al. [
41] reported an increased LPO level in the clams
Corbicula fluminea exposed for 30 days to BPA-contaminated water or BPA-contaminated microalgae and water. Overall, results obtained in this study indicated that BPA analogues can alter the antioxidant system and cause oxidative damage in clams.
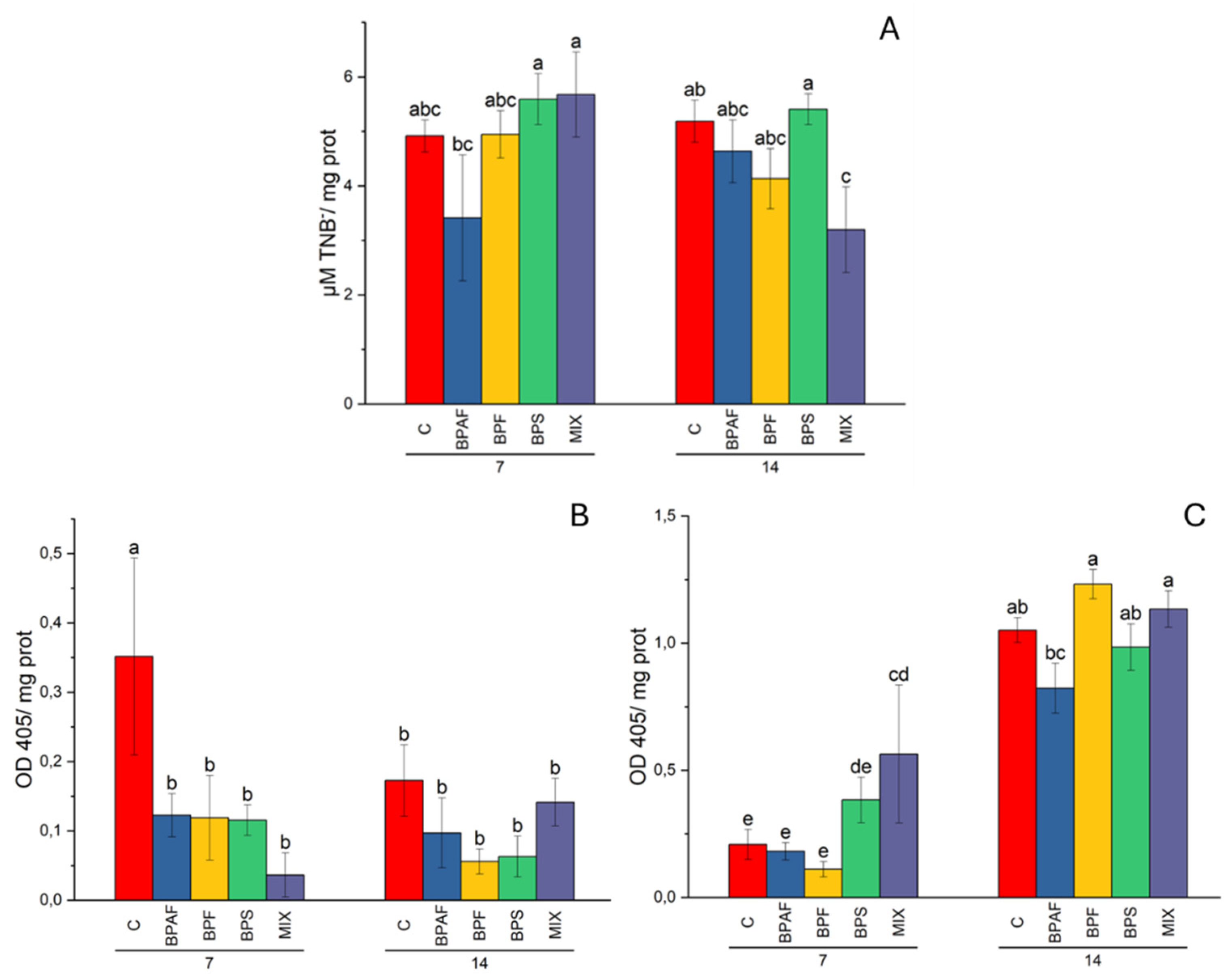
Previous studies indicated that bisphenols can cause neurotoxic effects [
49,
50]. However, we did not observe neurotoxicity in both the present and the previous study [
15]. Therefore, we can exclude that BPA analogues are neurotoxic to clams, under the experimental conditions tested at least.
The effects of a contaminated diet on activity of enzymes involved in epigenetic mechanisms were also evaluated for the first time in clams. We measured the activity of enzymes involved in the addition and remotion of acetyl groups from the histones, which is a well-known histone posttranslational modification that can change the regulation of gene expression [
51]. Regarding ecotoxicological studies, it has been demonstrated that exposure to chemical compounds can alter epigenetic mechanisms, as reported in zebrafish exposed to several compounds, such as benzo-α pyrene, heavy metals, PFASs and BPA [
52,
53,
54,
55,
56]. In particular, it has been demonstrated that exposure to BPA induced global transcriptomic changes in zebrafish embryos and larvae with an alteration in the gene expression of histone deacetylases and DNA methyltransferases [
57]. Similarly, Gonzalez-Rojo et al. [
58] reported that BPA significantly altered the gene expression of histone acetylation-related genes. In details, they observed that zebrafish males exposed to 2 mg/L of BPA showed alterations of expression of two histone deacetylase genes in testes after 21 days of exposure. There was a decrease in gene expression of the
kat6a gene and at the same time an increase in the
hdac4 gene expression level. Interestingly, they also observed that the global H3 histone acetylation in testes increased after the exposure to both 0,1 mg/L and 2 mg/L, while HAT activity in testes nuclear extracts significantly increased after exposure for 21 days to 2 mg/L of BPA. Our results indicated that BPA analogues provided through food can also alter the enzyme activity of both HAT and HDAC. In particular, clams fed for 14 days with MIX-treated microalgae had a significantly reduced HAT activity in digestive gland. In the same tissue, HDAC activity was significantly increased by the same treatment, but after 7 days of diet. Altered enzyme activities could have caused a reduction of the global histone acetylation level in the digestive gland of clams. On the contrary, gills HDAC activity significantly decreased after 7 days at all the treatments, in comparison to the related control, suggesting that an increased histone acetylation level. However, the global histone acetylation level was not evaluated in this study.
Regarding bioaccumulation, no detectable concentrations of bisphenols were found in clams after ingestion of contaminated microalgae. However, it is reported that diet can be a vehicle for bisphenols between different food chain levels. Indeed, Hu et al. [
42] reported that TBBPA was significantly bioaccumulated after both food-borne and water+food-borne exposure of the mollusc
Clamys farreri. Interestingly, bioaccumulation was observed in gills, digestive gland, muscle and soft tissues after 0.5, 1, 3 and 6 days of exposure. However, the dietary uptake was lower than the direct TBBPA uptake from water [
42]. A similar result was reported in
Scrobicularia plana for both soluble and lactate Ag NPs, when bioaccumulation was higher following 14 days of waterborne than dietary exposure [
48]. Similar conclusions (greater bioaccumulation in clams exposed to seawater compared to those fed contaminated food) can be formulated for this study, because in the previous one we demonstrated that clams exposed to bisphenols through seawater can accumulate all three contaminants [
15].
In conclusion, our study demonstrated that a diet of BPA analogues-contaminated microalgae can affect important biomarkers measured in different clam tissues. To the best of our knowledge, this is the first study demonstrating the effects of ingestion of contaminated food in the clam R. philippinarum, suggesting a potential ecotoxicological risk for the marine food chain, at least at the first levels.