Submitted:
26 July 2024
Posted:
29 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and experimental procedure
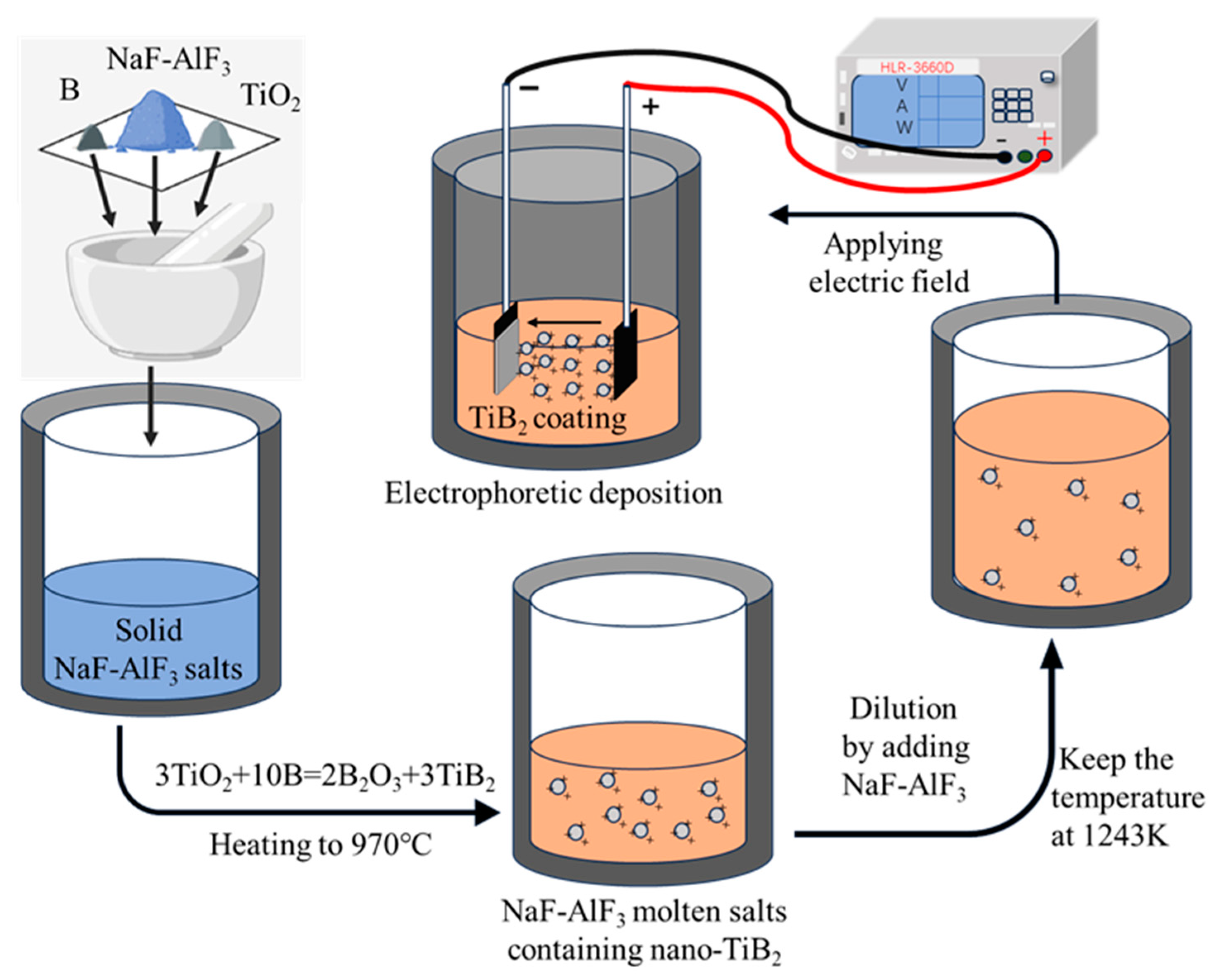
2.1. EPD of TiB2 coatings in NaF-AlF3 molten salts
- (1)
- Synthesis of nano-TiB2 via borothermal reduction in molten salts
- (2)
- EPD of TiB2 coatings
2.2. Corrosion resistance test of TiB2 coatings in molten zinc
2.3. Characterization
3. Results and discussion
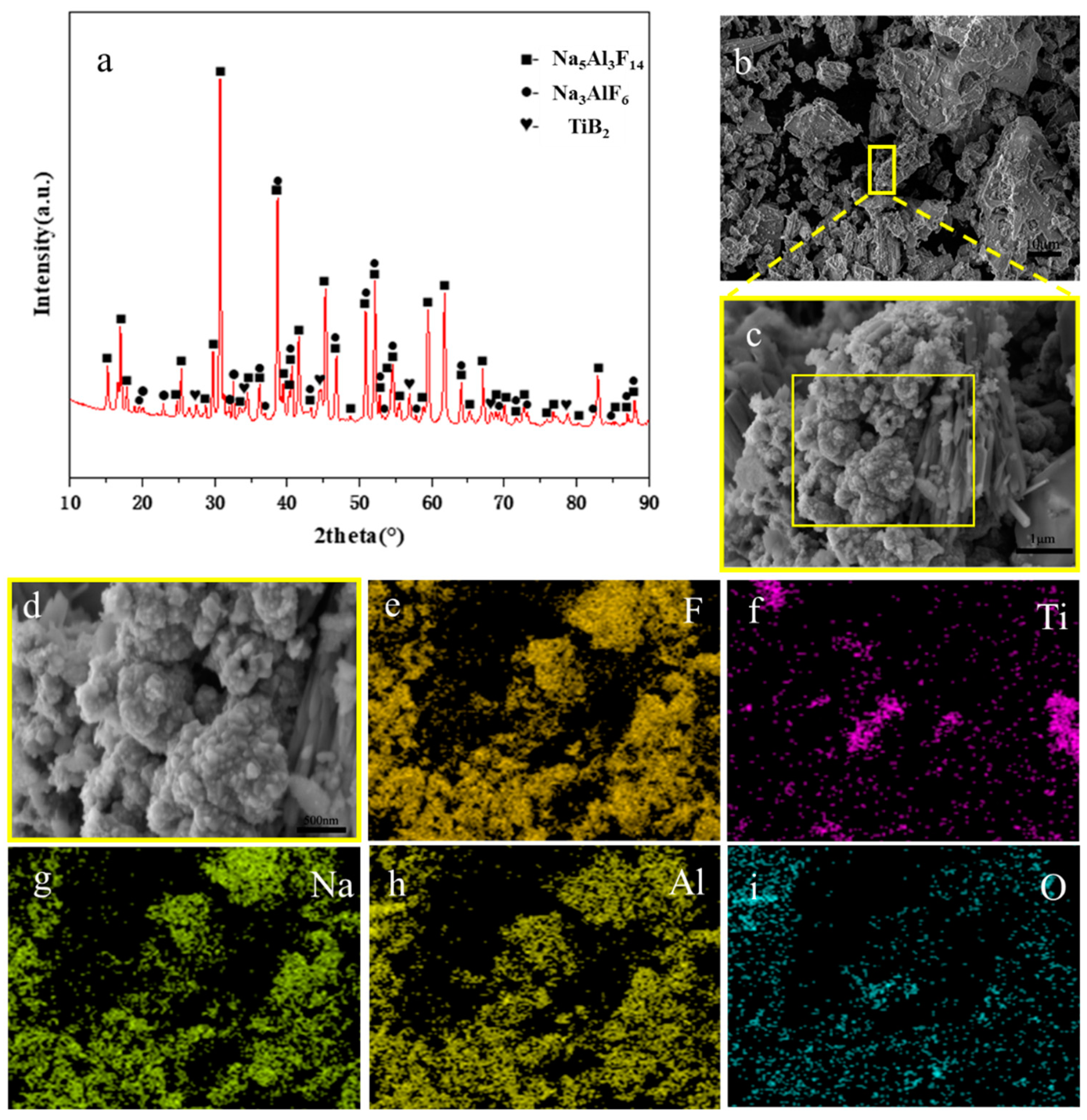
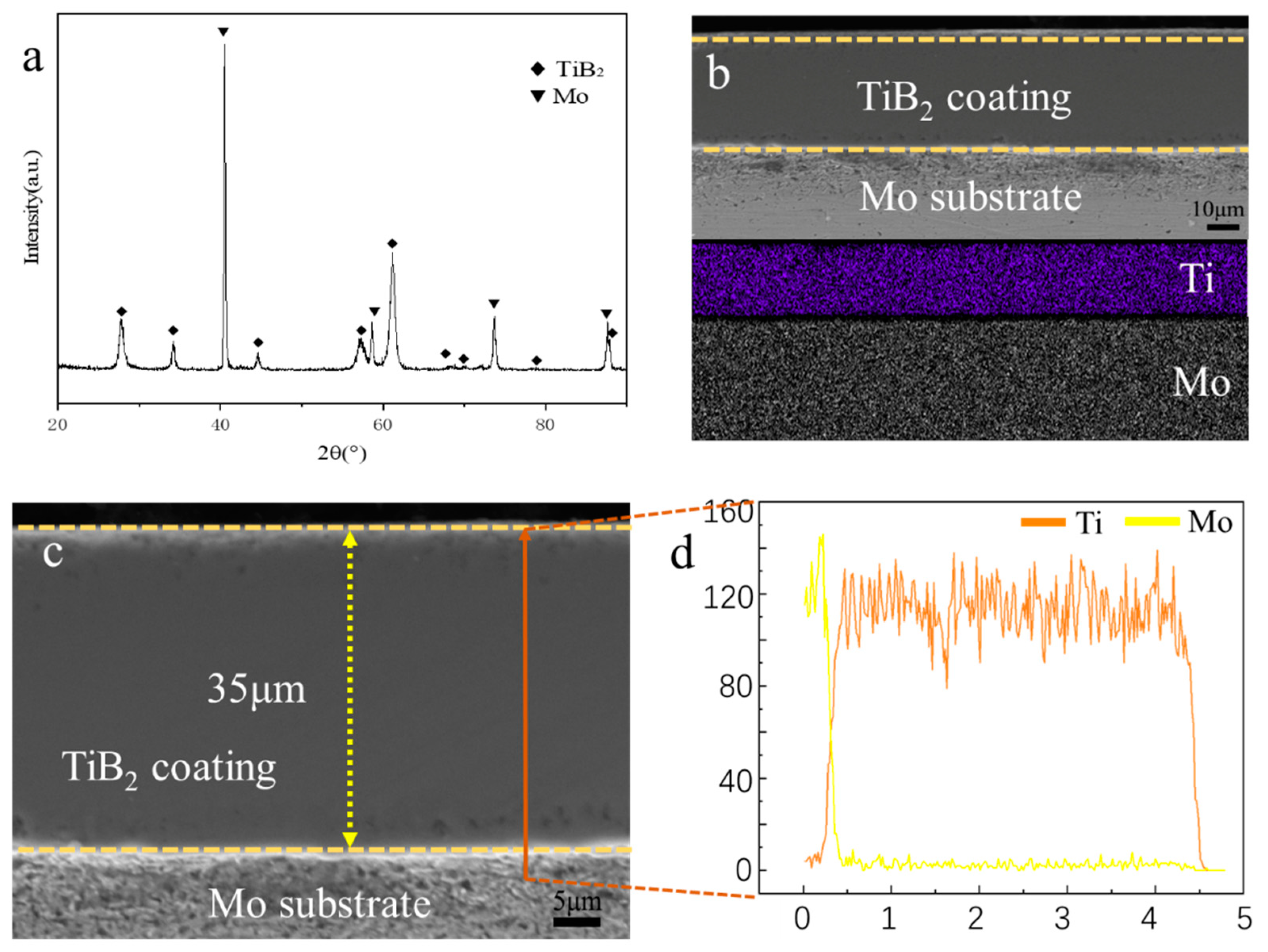
3.1. Synthesis of TiB2 NPs and EPD of TiB2 coatings in NaF-AlF3 molten salts
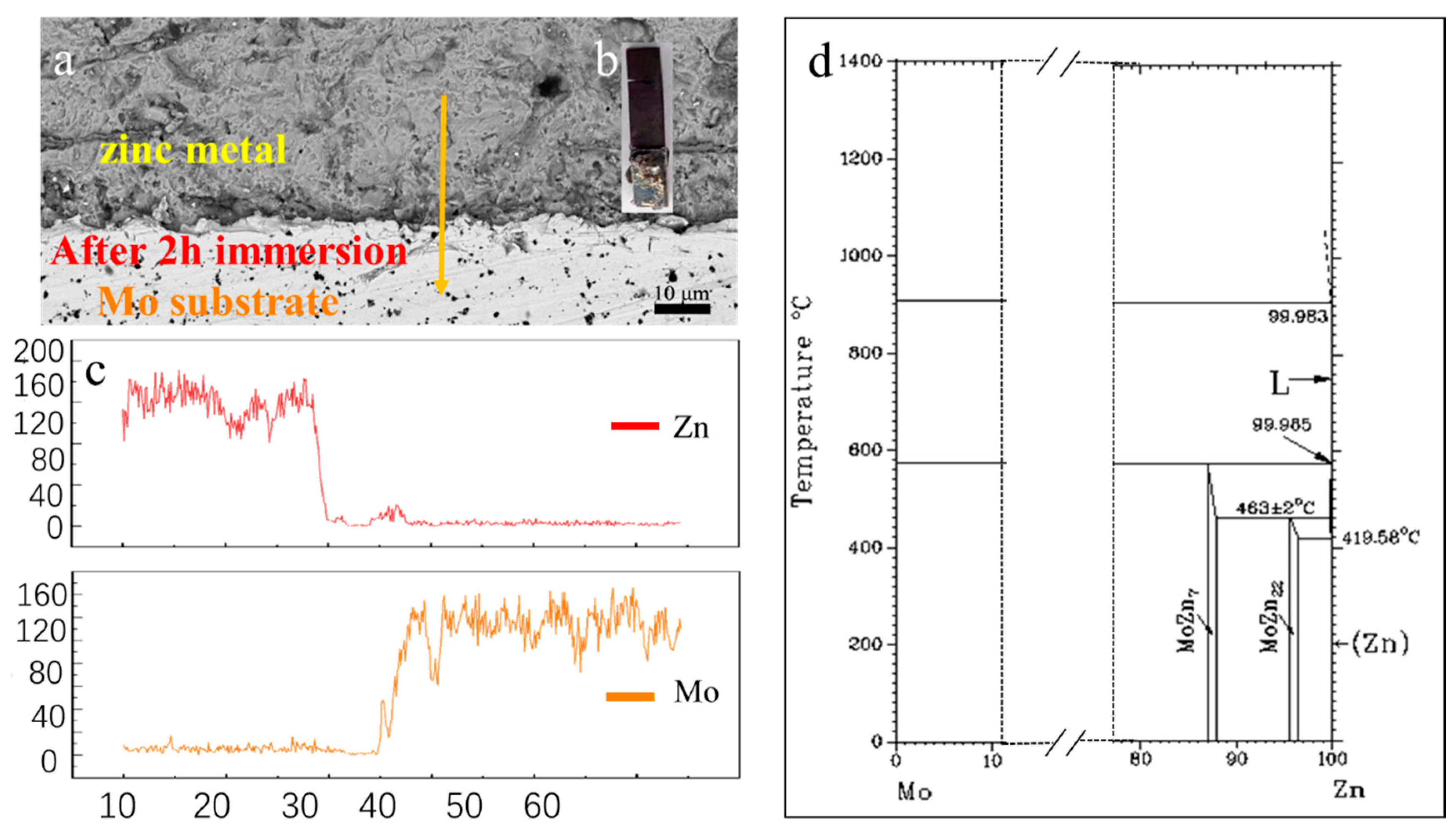
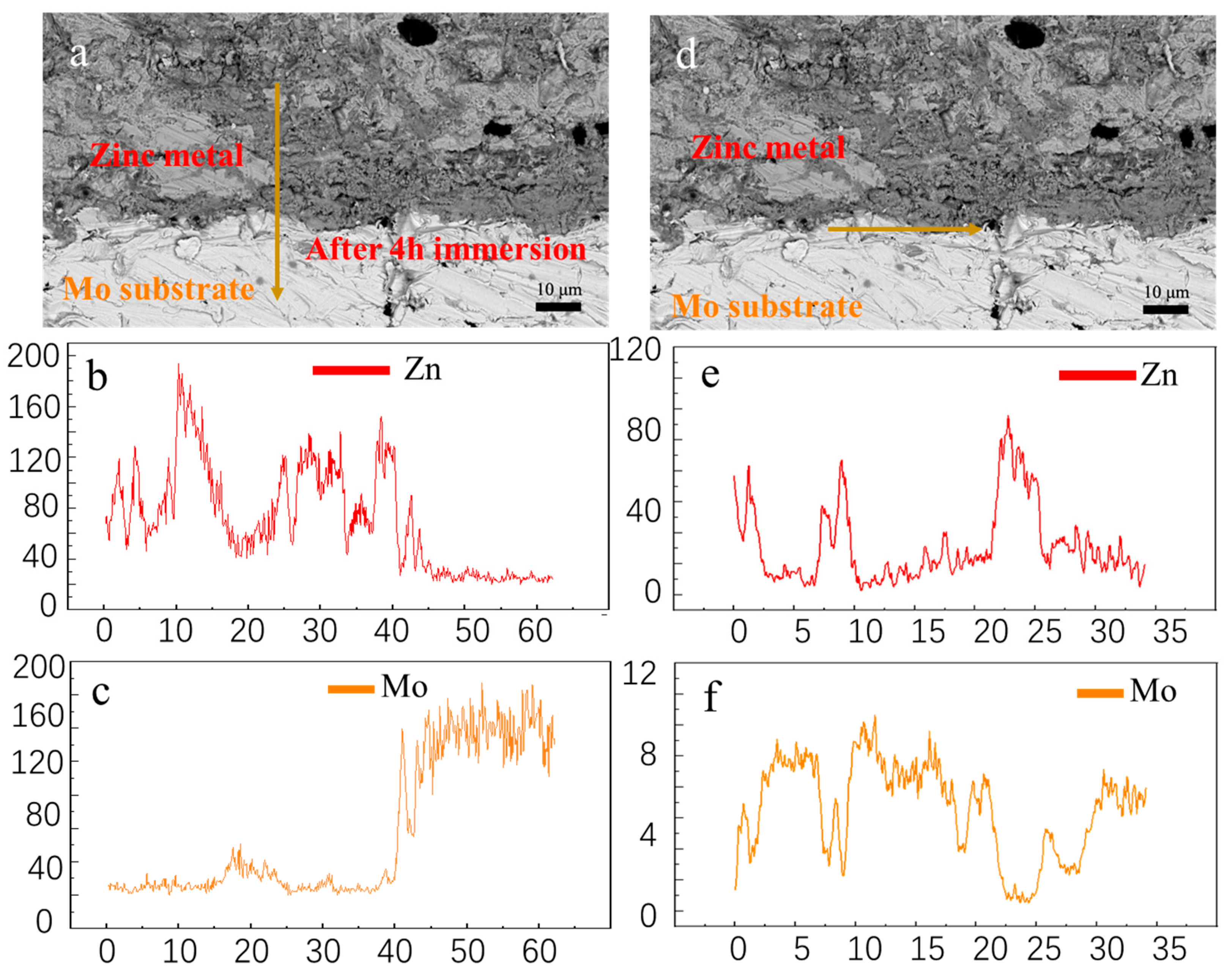
3.2. Corrosion behavior of uncoated molybdenum in molten zinc
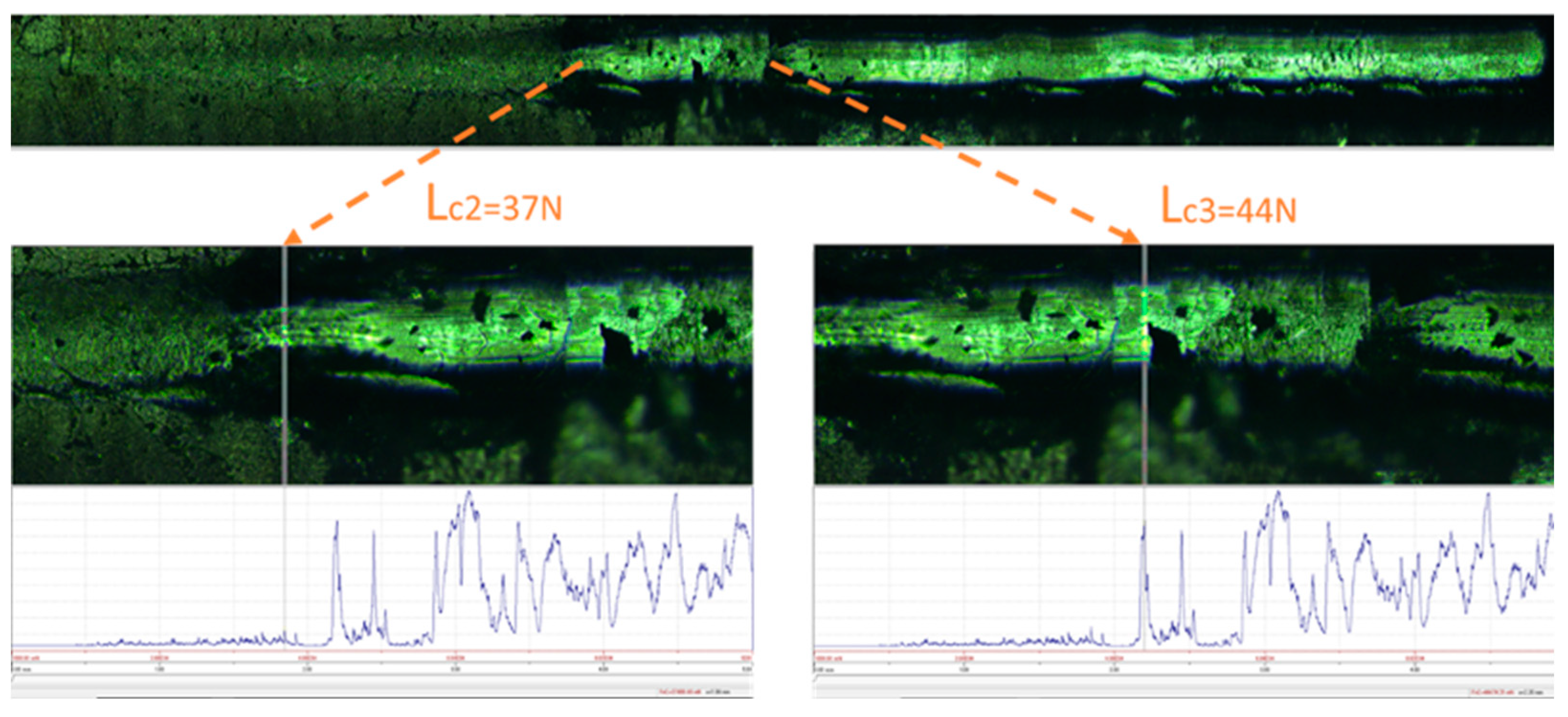
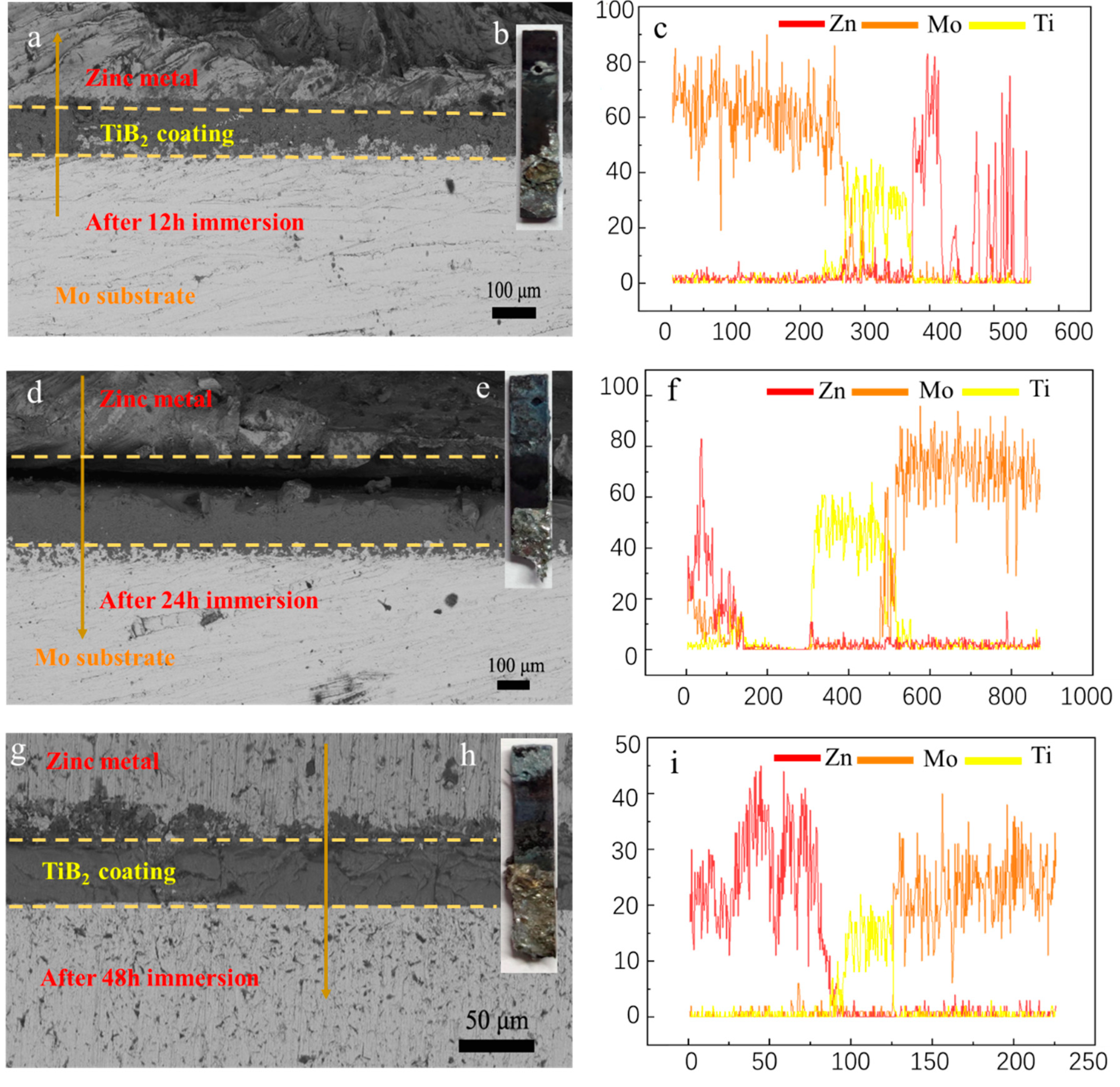
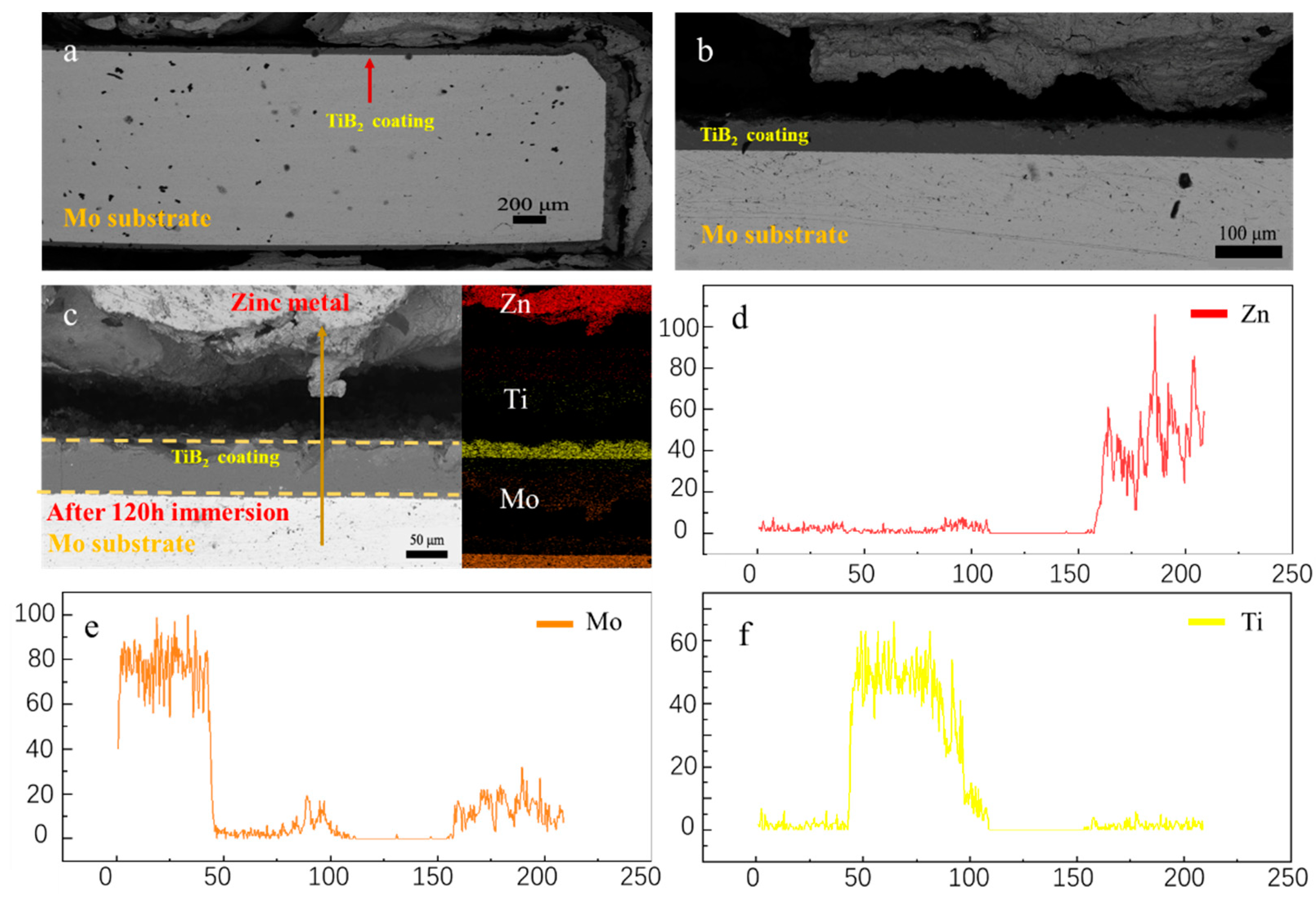
3.3. Corrosion behavior of molybdenum with TiB2 coatings in molten zinc
4. Conclusion
Funding
Conflicts of Interest
References
- Lyon, S.B. Corrosion of Molybdenum and Its Alloys. Shreir's Corrosion. 2010, 3, 2157–2167. [Google Scholar]
- Zhou, Y.H.; Gao, Y.M.; Wei, S.Z.; Pan, K.M.; Hu, Y.J. Preparation and Characterization of Mo/Al2O3 Composites. INT J REFRACT MET H. 2016, 54, 186–195. [Google Scholar] [CrossRef]
- Wang, B.; Yang, D.; Zhou, Y.C.; Xu, L.J.; Li, Y.P.; Li, X.Q.; Wei, S.Z. Mechanical Properties and Corrosion Behavior in Molten Zinc of Mo–ZrO2 Alloys. J MATER RES TECHNOL. 2023, 25, 4942–4959. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, Y.C.; Yang, D.; Xu, L.J.; Li, X.Q.; Wei, S.Z. Effect of Al2O3 Particles on the Corrosion Behavior of Molybdenum Alloys in Molten Zinc. CORROS SCI. 2023, 220, 111266. [Google Scholar] [CrossRef]
- Chakraborty, S.P.; Banerjee, S.; Singh, K.; Sharma, I.G.; Grover, A.K.; Suri, A.K. Studies on the Development of Protective Coating on TZM Alloy and Its Subsequent Characterization. J MATER PROCESS TECH. 2008, 207, 240–247. [Google Scholar] [CrossRef]
- Seong, B.G.; Hwang, S.Y.; Kim, M.C.; Kim, K.Y. Reaction of WC–Co Coating with Molten Zinc in a Zinc Pot of a Continuous Galvanizing Line. SURF COAT TECH. 2001, 138, 101–110. [Google Scholar] [CrossRef]
- Yong, Z.; Wu, Q.; Ma, H.L.; Wu, Y.D.; Akbar, M.; Zhao, X.R.; Yang, N. Effect of Zinc Oxide on Corrosion Resistance of Magnesium Ammonium Phosphate Cement-Based Coating. CONSTR BUILD MATER. 2023, 398, 132473. [Google Scholar]
- Wande, C.; Li, T.S.; Xue, D.Z.; Yang, H.J.; Cheng, P.M.; Chen, C. ; Sun, Y, J. ; Zeng, Y.; Ding, X.D.; Sun, J. Enhancement of the Corrosion Resistance of Molybdenum by La2O3 Dispersion. CORROS SCI. 2021, 186, 109469. [Google Scholar]
- Ma, S.Q.; Xing, J.D.; Fu, H.G.; He, Y.L.; Bai, Y.; Li, Y.F.; Bai, Y.P. Interface Characteristics and Corrosion Behavior of Oriented Bulk Fe2B Alloy in Liquid Zinc. CORROS SCI. 2014, 78, 71–80. [Google Scholar] [CrossRef]
- Wang, M.M.; Gao, R.; Gao, H.Y.; Zhou, Y.; Fan, Y.Y.; Zhao, Y.Y.; Ju, J.; Liu, Y.H.; Kang, M.D.; Wang, J. Improved Corrosion Resistance of Ni-Modified Fe-Cr-B Steel in Molten Zinc Via Phase Transformation and Microstructure Control. SURF COAT TECH. 2019, 374, 975–986. [Google Scholar] [CrossRef]
- Yu, Z.D.; Chen, M.H.; Wang, J.L.; Li, F.J.; Zhu, S.L.; Wang, F.H. Enamel Coating for Protection of the 316 Stainless Steel Against Tribo-Corrosion in Molten Zinc Alloy at 460 °C. J MATER SCI TECHNOL. 2021, 65, 126–136. [Google Scholar] [CrossRef]
- Yadav, K.K.; Guchhait, S.K.; Sunaina, S.; Ankush, C.M.; Hussain, A.K.; Ganguli, M. Jha. Synthesis of Zirconium Diboride and Its Application in the Protection of Stainless steel Surface in Harsh Environment. J SOLID STATE ELECTR. 2019, 23, 1–11. [Google Scholar]
- Hu, Z.W.; Li, W.G.; Zhao, Y.T. The Effect of Laser Power on the Properties of M3B2-Type Boride-Based Cermet Coatings Prepared by Laser Cladding Synthesis. MATERIALS. 2020, 13, 1867. [Google Scholar] [CrossRef]
- Wang, G.C.; Chong, X.Y.; Li, Z.L.; Feng, J.; Jiang, Y.H. Design of Fe2B-Based Ductile High Temperature Ceramics: First-Principles Calculations and Experimental Validation. CERAM INT. 2022, 48, 27163–27173. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Zhang, L.; Liu, H.; Zeng, C. Stability Investigation of Electrodeposited Zirconium Diboride Ceramic Coatings in Molten Zinc. MATER CORROS. 2019, 70, 492–502. [Google Scholar] [CrossRef]
- Lv, Y.H.; Wu, X.S.; Liu, Y.F.; Wu, Z.J. Preparation and Liquid Zinc Corrosion Resistance of Al2O3-TiB2 Composite Ceramic Coating. CHINA SURF ENG. 2011, 24, 30–33. [Google Scholar]
- Zhang, J.F.; Deng, C.M.; Song, J.B.; Deng, C.G.; Liu, M.; Zhou, K.S. MoB–CoCr as Alternatives to WC–12Co for Stainless Steel Protective Coating and Its Corrosion Behavior in Molten Zinc. SURF COAT TECH. 2013, 235, 811–818. [Google Scholar] [CrossRef]
- Tsipas, D.N.; Triantafyllidis, G.K.; Kipkemoi, J.; Flitris, Y. Thermochemical Treatments for Protection of Steels in Chemically Aggressive Atmospheres at High Temperatures. MATER MANUF PROCESS. 1999, 14, 697–712. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Zhang, L.; Liu, H.; Zeng, C. Stability Investigation of Electrodeposited Zirconium Diboride Ceramic Coatings in Molten Zinc. MATER CORROS. 2018, 70. [Google Scholar] [CrossRef]
- Lv, Y.H.; Wang, J.; Wu, Z.J. High Temperature Oxidation Resistance of High-Energy and High-Speed Plasma Sprayed MCrAlY Coatings. CHINA SURF ENG. 2013, 26, 24–28. [Google Scholar]
- He, Y.Y.; Xiao, X.J.; Zhai, J.Y. A Study on Electrochemical Method for Removing WC Coating by Supersonic Flame Spraying. Welding Technique. 2022, 51, 70–72. [Google Scholar]
- Sui, F.L. Study on the Method of Determining the Thickness of the Boron Permeable Layer. MET SCI HEAT TREAT. 1986, 11, 11–15. [Google Scholar]
- Jin, W.L.; Xiao, S.J.; Kou, Q.; Ding, D.S.; Zhang, J.; Fang, X.H.; Ge, C.T.; Zhong, C.; Zhu, H.M.; Haarberg, G.M. Preparation of Diboride Coatings by Electrophoretic Deposition in Nanoparticle-Containing Molten Inorganic Salts. MATER LETT. 2021, 306, 130908. [Google Scholar] [CrossRef]
- Pang, J.; Kou, Q.; Ge, C.T.; Xie, L.L.; Jin, W.L.; Qi, W.Q.; Zhang, J.; Zhu, H.M.; Xiao, S.J. Electrophoretic deposition of ZrB2 coatings in NaCl-KCl-AlF3 melt containing synthesized ZrB2 nanoparticles. J AM CERAM SOC. 2023, 106, 5147–5156. [Google Scholar] [CrossRef]
- Zhang, J.; Pang, J.; Jin, W.L.; Chu, S.J.; Haarberg, G.M.; Xiao, S.J. Production of TiB2 coatings on graphite substrates by electrophoretic deposition in NaF-AlF3 melt. PROCESS APPL CERAM. 2023, 17, 9–13. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, S.J.; Jin, W.L.; Cai, F.; Zhu, H.M.; Xiao, S.J. Fabrication of TiB2 coatings by electrophoretic deposition of synthesized TiB2 nanoparticles in molten salts. J MATER RES TECHNOL. 2022, 18, 2451–2457. [Google Scholar] [CrossRef]
- Sørlie, M.; Øye, H.A. Cathode in Aluminium Electrolysis. Aluminium-Verlag Marketing & Kommunikation GmbH Press. 2010.
- Zdaniewski, W.A. Solid Solubility Effect on Properties of Titanium Diboride. J AM CERAM SOC. 1987, 70, 793–797. [Google Scholar] [CrossRef]
- Petukhov, V.A.; Chekhovskoi, V.Y.; Zaichenko, V.M. Thermal Expansion of Molybdenum. HIGH TEMP, 1977.
- Pierson, H.O.; Randich, E. Titanium Diboride Coatings and their Interaction with the Substrates. THIN SOLID FILMS. 1978, 70, 119–128. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



