Article Summary
Strengths and Limitations:
The study is designed with a focus on low- and middle-income countries, where the burden of paediatric pneumonia is high and access to advanced diagnostic tools is limited.
The structured and iterative process of the modified Delphi method allows for thorough evaluation and refinement of guidelines, ensuring robustness and reliability.
The guidelines developed will be directly applicable and easily integrated into the Integrated Management of Childhood Illness (IMCI) framework.
Limitations inherent of the modified Delphi method may introduce bias. Structural limitations of point of care ultrasound experts from resource-limited settings to participate in the survey may bias the results.
Background
In 2019, a total of 2.6 million deaths globally were attributed to lower respiratory tract infections (LRTI). LRTI-related deaths disproportionately affect children in low-resource settings and pneumonia is the leading infectious cause of death in children [
1,
2]. LRTIs, including pneumonia, are the most common reason for children to seek acute outpatient care. Current pneumonia management guidelines, based on the World Health Organization’s (WHO) Integrated Management of Childhood Illness (IMCI) guidelines, recommend antibiotics for all suspected cases [
3]. These guidelines rely on clinical signs such as cough, respiratory rate, and lower chest indrawing, which may not be specific to pneumonia and do not effectively distinguish between bacterial and viral infections, leading to over-prescription of antibiotics [
3,
4,
5]. Furthermore, recent evidence estimates the incidence of bacteria as a causative agent of pneumonia among children in sub-Saharan Africa (SSA) is as low as 2-4% in primary [
6,
7], and 23.3-31.6% in [
8] hospital settings.
Antimicrobial resistance (AMR) is a growing concern, accounting for an estimated 1.27 million deaths globally in 2019, with the highest rates in SSA [
9]. Inappropriate and excessive prescription of antibiotics is a significant contributor to AMR [
10,
11,
12]. In low-resource settings, over 50% of sick children receive antibiotics when visiting health facilities; however, as many as 80-90% of these prescriptions are deemed inappropriate and respiratory infections are the highest contributing illness to inappropriate prescribing [
13,
14,
15,
16,
17].
Improving IMCI diagnostic criteria to better identify children with pneumonia who would benefit from antibiotic therapy, utilizing more disease-specific tools, is therefore a WHO research priority [
18]. Previous studies highlighted the limitations of pathogen-specific approaches due to complexity, high costs, and delayed results of microbiological assays [
8]. Broader strategies, such as point-of-care C-reactive protein testing, have had limited impact in reducing antibiotic prescriptions among children with respiratory infections [
19,
20].
Lung Point of Care Ultrasound (L-POCUS): L-POCUS has been proposed as an approach to improve the specificity of pneumonia diagnosis. Incorporation of L-POCUS into diagnostic pathways has the potential to improve antibiotic prescription practices in low- and middle-income countries (LMICs). Portable and affordable ultrasound-on-a-chip devices, compatible with mobile phones, allow L-POCUS to be integrated into standard clinical exams without significant additional costs or specialist consultation. Although L-POCUS is used in high-resource settings and is gaining popularity in SSA [
21,
22,
23,
24], robust evidence on its impact on health outcomes, such as pneumonia management, is lacking in LMICs. This evidence gap hampers the integration of ultrasound into current pneumonia management guidelines, which limits investment in POCUS devices and efforts to scale up its use.
Validity of L-POCUS Compared to Other Imaging Modalities: Several studies have compared L-POCUS to chest x-ray (CXR) and/or computerized tomography for diagnosing pneumonia. A multi-center European trial involving 362 adults with pneumonia reported a sensitivity of 93.4% and a specificity of 97.7% for L-POCUS compared to CXR [
25]. Studies in children, largely conducted in high-income countries, have shown sensitivity of 95% and specificity of 96% for L-POCUS compared to CXR for the diagnosis of pneumonia [
26,
27,
28]. However, L-POCUS may be overly sensitive as it can detect subcentimeter subpleural consolidations, of unclear clinical significance, that are not visible on CXR [
29,
30,
31,
32,
33]
Differentiating Bacterial from Viral Pneumonia: The ability of CXR to differentiate bacterial from viral pneumonia has yielded mixed results. Features like alveolar infiltrate are more suggestive of bacterial pneumonia, and some guidelines advise against the prescription of antibiotics if no consolidation is seen on CXR [
34,
35,
36]. However, translating these findings into treatment changes is challenging due to poor interoperability and clinicians’ failure to act on results [
37,
38]. Some studies have explored the use of L-POCUS to determine the etiological causes of consolidations, but the lack of microbiologically confirmed diagnoses limits definitive conclusions [
39,
40]. Findings from a multi-country study in SSA and South Asia (the Pneumonia Etiology Research in Children [PERCH] study) emphasized the challenges of reliable reference standards in paediatric pneumonia [
8,
41,
42], underscoring the need for targeted study designs to assess L-POCUS within management pathways.
Initial studies on L-POCUS in LMICs, conducted in Nepal, Peru, and Egypt, are promising but limited in scope [
43,
44,
45,
46,
47]. To date, there is no evidence on the clinical impact of L-POCUS-assisted diagnoses on antimicrobial use or health outcomes in outpatient settings.
Current Definitions of Pneumonia: Despite increasing evidence supporting L-POCUS, CXR remains the gold standard in most national guidelines, with ultrasound primarily used to identify complications like effusion or abscesses [
48,
49,
50,
51]. Studies on L-POCUS vary in their pneumonia definitions, highlighting the need for standardized criteria. For instance, Lissaman et al. (2019) noted the potential for over-diagnosing pneumonia using L-POCUS due to the similarity of atelectasis and pneumonia, suggesting a threshold of >1 cm for consolidation to improve specificity [
52].
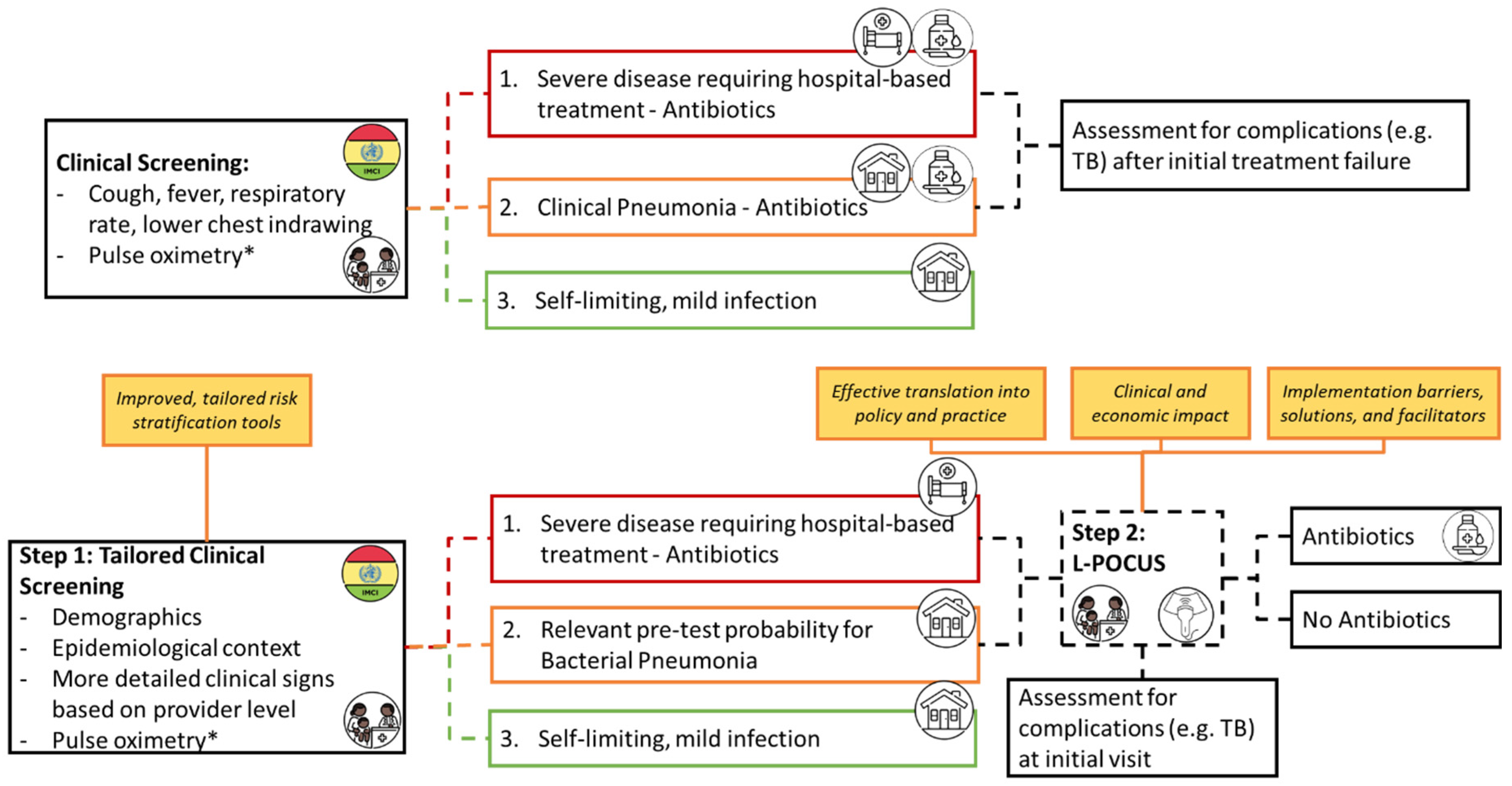
Hypothesized Value of L-POCUS for IMCI Guidelines: IMCI-defined pneumonia encompasses various clinical entities, often failing to differentiate bacterial from viral illnesses. The PERCH study revealed that only half of the children admitted with IMCI severe/very severe pneumonia had infiltrates on CXR, with likely bacterial etiology in only one-third to one-fourth of cases [
8]. Improved diagnostic criteria using L-POCUS could enhance the IMCI framework’s ability to identify children needing antibiotics, especially in setttings where CXR is not readily available.
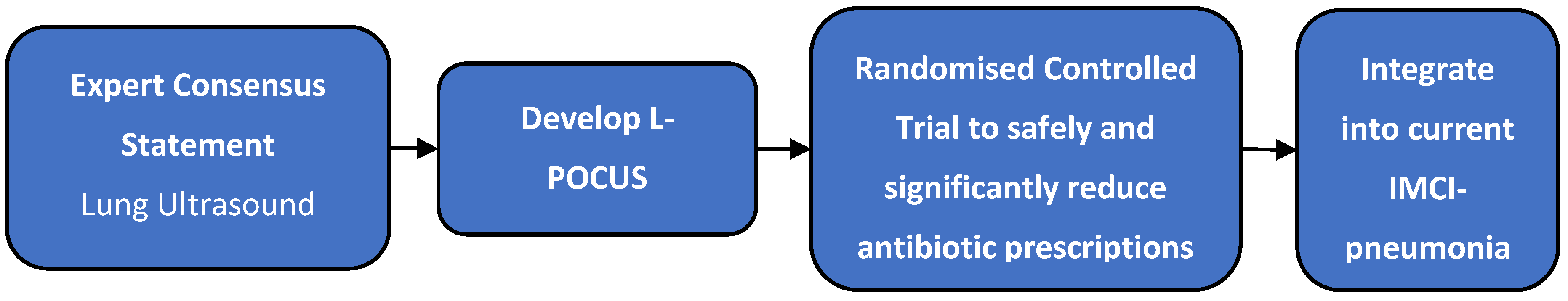
This study, therefore, aims to develop an expert consensus statement on the best practices for L-POCUS interpretation in paediatric acute respiratory tract infections that would constitute a framework for effective guidelines (on criteria/thresholds) to guide judicious antibiotic prescribing. The intended use of these findings is to have them integrated into the IMCI management pathway of paediatric pneumonia - a system in wide use in LMICs where access to CXR and laboratory tests is often severely limited. Once generated, this research group will conduct a randomised controlled trial to assess if these guidelines can safely and significantly reduce antibiotic prescriptions in children aged 2 months to 12 years that present for acute management of IMCI-defined pneumonia in outpatient settings (
Figure 1).
Current IMCI guidelines rely on clinical symptoms to categorise cases of cough/difficulty breathing into 3 categories:
Red – the most severe, high likelihood of pneumonia warranting immediate antibiotics and hospital referral/admission
Yellow – moderate severity, presence of tachypnoea/chest in-drawing warranting oral antibiotics and either hospital referral or home care
Green – no signs of pneumonia or severe disease warranting symptomatic home care.
We propose that findings from this could add further nuance to those classified as red/yellow and further define those that would not benefit from antibiotic therapy in IMCI guidelines (
Figure 2).
Methods and Analysis
Study Design: Considering the novelty of using L-POCUS in paediatric pneumonia diagnosis, it is essential to develop standardized guidelines for effective application. Due to discrepancies in available literature from diverse clinical settings, a group consensus method is required to determine sonographic findings that are consistent with pneumonia that requires antibiotic treatment. The modified Delphi method is suitable for creating clinical guidelines, using an iterative process to enable expert-based judgment in scenarios in which there is absence of strong evidence to support one approach over another [
53,
54,
55,
56,
57,
58]. This study uses the RAND/UCLA modified Delphi method, incorporating an initial literature summary to standardize the knowledge context [
59].
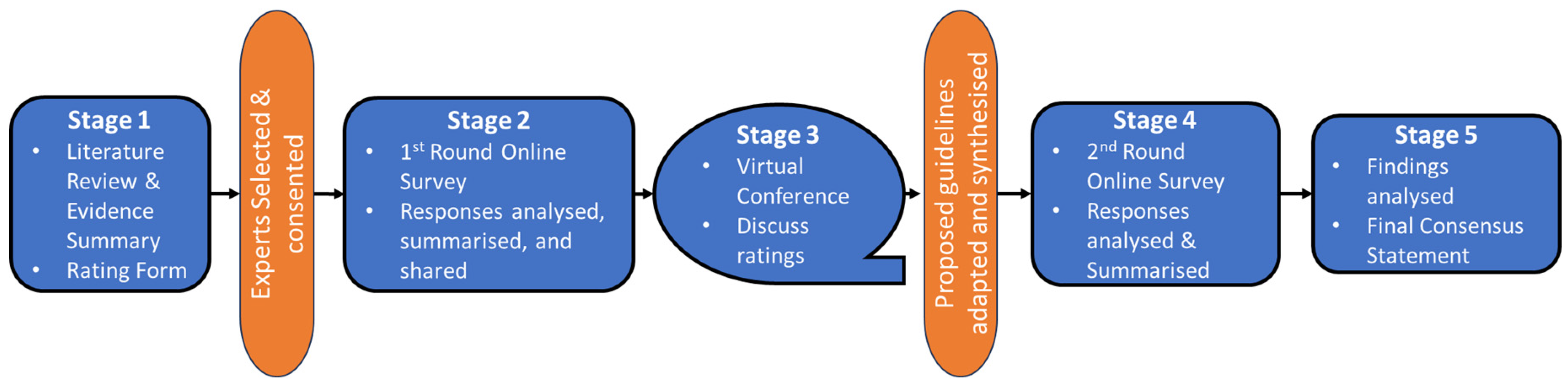
Figure 3 summarizes the study flow.
Stage 1: Literature Summary and Consensus Statement Creation: A targeted literature search will be conducted on paediatric lung ultrasound and relevant adult L-POCUS studies. This will result in a conceptual interpretation framework for L-POCUS use within the IMCI framework with corresponding statements for voting among L-POCUS experts.
Stage 2: First Round of Online Surveys: Experts will review the literature summary, consensus statements, and conceptual framework. They will provide feedback and vote on the statements using a Likert-type scale through an online survey. The input will be analyzed, summarized into a ratings report, and shared with all participants for review.
Stage 3: Virtual Expert Meeting: Given that the modified Delphi method has been shown to be as effective whether virtual or in person [
60,
61], experts will discuss the survey results in 1-2 virtual sessions, focusing on areas lacking consensus. The conference will be facilitated by a study investigator, with anonymous polling used to facilitate consensus if necessary. On issues with very divergent opinion and evidence of dominating players, panelists will not be required to vote/finalise decisions during the virtual conference session to avoid the discussion stalling or a false consensus being reached. Discussion points and suggestions will inform adjustments to the proposed guidance and rating form. The updated ratings report and proposed L-POCUS guidelines will be shared with all participants for their review.
Stage 4: Second Round of Online Surveys: Experts will review the revised consensus statements and proposed guidelines and complete a final rating form. The input will be reviewed to develop the final consensus statements and guidance statement.
Stage 5: Final Analysis and Consensus Statement: Survey responses will be analyzed using descriptive statistics. Consensus can be defined in many ways, however, the most frequently used is percentage agreement, with a systematic review of Delphi studies showing a median threshold of above 75% [
56,
62]. However, as this threshold is essentially arbitrary, items falling closely under this threshold at the time of analysis may be included pending their perceived clinical importance. Where there is consensus against the inclusion of an item in the first round, it will be dropped, and the opportunity will be made at the virtual conference for suggestions for its replacement.
Participant Selection: To ensure guidelines represent diverse contexts and patient populations, and to maximize interaction, 12-15 expert participants will be selected [
63,
64]. Criteria include significant paediatric L-POCUS experience, geographic diversity, and gender balance. Participants will be recruited through POCUS practitioner and research networks, such as Paediatric Emergency Research Networks (PERN). An invitation survey will be circulated, and respondents will be screened according to our inclusion criteria. Established experts will also be approached directly where appropriate. Special effort will be made to limit selection bias despite the use of some purposive sampling techniques.
The criteria for participant selection will be:
- 1)
-
Significant paediatric POCUS experience – defined as either:
- a)
A clinician (Sonographer, Professional Nurse, Clinical Associate, Doctor, or equivalents) with formal training in POCUS and experience conducting L-POCUS in a clinical setting
- b)
Experience conducting L-POCUS trainings as a supervisor
- c)
Experience conducting clinical research in the field of L-POCUS
- d)
Involvement in L-POCUS didactic curriculum development or previous contributions toward POCUS guideline development
- e)
Research or didactic activity in national and international POCUS-networks
Note: We acknowledge that professional activities in LMICs are different and hence an equivalent activity will be considered [
65].
- 2)
-
The group must meet the following overall composition criteria:
Invitation and Reminders: Selected participants will receive an email inviting them to partake in the Delphi study, including the literature summary, expected time commitment, study timelines, proposed virtual conference timeframe, and study process details. Weekly reminder emails will be sent for 2 weeks to non-respondents. Those willing to participate will receive emails at each subsequent stage. For Stage 2, a link to the survey/rating form will be provided, open for 2 weeks. For Stage 3, an email will share feedback on the initial survey and coordinate the virtual conference schedule. For Stage 4, an email will summarize the virtual conference outcomes, highlight changes in the proposed guidelines, and provide a link to the second-round survey. For Stage 5, the final consensus statement and guidelines will be shared, with details on anticipated publication. Weekly reminders will be sent to non-respondents, and by week 2, the principal investigator will contact the expert telephonically to determine if they wish to withdraw from the study.
Advisory group: We anticipate that response rates may be higher in certain geographical areas and may need to restrict the number of participants from certain regions. These international experts will be invited to take part in an advisory group. The advisory group will review the material provided to the panelists, participate in online meetings as observers, and contribute to the publication. The advisory group will not participate in the surveys or in the determination of consensus of the statements.
Data Collection and Analysis: Survey responses will be analyzed using descriptive statistics.
Ethical Considerations: Participation is voluntary and expressed consent will be obtained at the time of invitation. Participants can withdraw at any time without negative consequences. Participant responses will remain anonymous to prevent coercion. The study was approved by the Ethics Commission of Bern, Switzerland (BASEC-Nr: Req-2024-00102) on 22/01/2024.
Dissemination: The findings will be disseminated through open access publication. We intend to publish the findings from the modified Delphi study in an open access format reported according to the best available reporting guidelines, such as the Accord (ACcurate COnsensus Reporting Document) reporting guidelines [
66]. This will ensure all methods and techniques used to reach consensus are outlined in a transparent and thorough manner.
Discussion
This study seeks to develop expert consensus-based guidelines for the interpretation of L-POCUS in the diagnosis and management of paediatric pneumonia in low-resource settings. L-POCUS has emerged as a valuable tool in paediatric respiratory care. Its advantages over traditional CXR include portability, real-time imaging, lower costs, and the absence of radiation exposure. Despite these benefits, L-POCUS is not yet widely integrated into clinical guidelines, partly due to a lack of standardized interpretation criteria. This modified Delphi study seeks to fill this gap by establishing expert consensus on the interpretation of L-POCUS in children with suspected pneumonia in these settings for further testing in interventional studies.
Previous Delphi studies have focused on various aspects of L-POCUS. Topics addressed include clinical areas of application, training aspects, acquisition protocols, overall recommendation of use, as well standardizing terminology of key ultrasound features [
67,
68]. These studies, although extensive in their scope, primarily draw from adult literature and offer minimal guidance relating to the field of paediatrics and more specifically to paediatric pneumonia. A Delphi study conducted by Jaworska et al., focused on paediatric respiratory infections, outlined consensus statements on the validity of L-POCUS compared to CXR. The study confirmed the reliability of identifying ultrasound features associated with pneumonia and bronchiolitis but offered limited statements around these diagnostic features and did not address the differentiation of cases that would benefit from antibiotic treatment from those that would not [
69]. These studies also highlight the heterogeneity in L-POCUS interpretation and the need for standardized guidelines for image interpretation as it applies to clinical management.
Strengths and Limitations: Given the current lack of consensus in this area, we are conducting the first critical step to ensure consistency in the criteria used to define pneumonia using L-POCUS. With the use of the modified Delphi method we aim to ensure that the guidance to be tested in future L-POCUS studies in SSA are based on the best available literature and the collective expertise of a diverse group of international L-POCUS experts. The study is designed with a focus on LMICs, where access to advanced diagnostic tools is limited, and the burden of paediatric pneumonia is high. The structured and iterative process of the modified Delphi method allows for thorough evaluation and refinement of key evidence-based statements that can be used in the formation of guidelines, ensuring robustness and reliability. The guidelines developed will be directly applicable to the IMCI framework, facilitating their integration into existing clinical pathways.
While efforts are made to include a geographically and professionally diverse group of experts, the selection process may still introduce some bias. Given the scope of this process, the findings may be less applicable to high-income settings where the diagnostic infrastructure and clinical practices differ significantly from those in LMICs. The modified Delphi method relies on achieving consensus, which may not always reflect the most scientifically rigorous standards but rather the collective opinion of the panel.
Conclusion: The integration of L-POCUS into healthcare settings in LMICs has the potential to revolutionize paediatric respiratory care by providing a reliable, cost-effective diagnostic tool for pneumonia and reducing unnecessary antibiotic prescription. This modified Delphi study aims to establish standardized guidelines for L-POCUS interpretation, addressing a critical need in paediatric pneumonia management.
Author Contributions
JD Du Toit: Conceptualization, methodology, writing – original draft, and project administration. JD Du Toit led the design and execution of the protocol, and contributed significantly to drafting and revising the manuscript. F Romano: Methodology, writing – review & editing, and supervision. F Romano contributed to the study design, and provided critical revisions to the manuscript, ensuring scientific accuracy and clarity. CA Rees: Methodology, review, and editing. CA Rees also provided substantial feedback on manuscript drafts and ensured alignment with the overall research goals. K Keitel: Conceptualization, supervision, funding acquisition, writing – review & editing. K Keitel oversaw the project, secured funding, and contributed to the study’s conceptual framework. K Keitel also provided substantial feedback on manuscript drafts and ensured alignment with the overall research goals. All authors read and approved the final manuscript.
Funding
This study is funded by the Swiss National Science Foundation 19-090 - SNF ToolCap. Dr. Chris A. Rees was supported by the United States National Institutes of Health (K23HL173694).
Data Availability Statement
Data generated under this study will be available from corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no competing interests. The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Dr. Keitel served on the Guideline Development Group of the World Health Organization for the Diagnosis and Management of Pneumonia and Diarrhea in Children. Dr. Neuman served on the Guideline Development Group of the World Health Organization for the Diagnosis and Management of Pneumonia and Diarrhea in Children. He also serves as an expert panel member on Infectious Diseases Society of America Clinical Practice Guideline for the Management of Community-Acquired Pneumonia in Children. Dr. Neuman receives royalties from Wolters Kluwer for contributions to UpToDate and for serving as co-Editor-in-Chief for Paediatric Emergency Care.
References
- World Health Organisation (WHO). WHO Fact Sheet: The top 10 causes of death (2020) [Internet]. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 29 March 2023).
- WHO. Pneumonia Fact Sheet [Internet]. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/pneumonia (accessed on 7 March 2023).
- Gera T, Shah D, Garner P, Richardson M, Sachdev HS. Integrated management of childhood illness (IMCI) strategy for children under five. Vol. 2016, Cochrane Database of Systematic Reviews. John Wiley and Sons Ltd.; 2016.
- Risk R, Naismith H, Burnett A, Moore SE, Cham M, Unger S. Rational prescribing in paediatrics in a resource-limited setting. Arch Dis Child. 2013;98 (7):503–9.
- Shah SN, Bachur RG, Simel DL, Neuman MI. Does this child have pneumonia? The rational clinical examination systematic review. Vol. 318, JAMA - Journal of the American Medical Association. American Medical Association; 2017. p. 462–71.
- Keitel K, Kagoro F, Samaka J, Masimba J, Said Z, Temba H, et al. A novel electronic algorithm using host biomarker point-of-care tests for the management of febrile illnesses in Tanzanian children (e-POCT): A randomized, controlled non-inferiority trial. Tumwine JK, editor. PLoS Med. 2017;14 (10):e1002411.
- D’Acremont V, Kilowoko M, Kyungu E, Philipina S, Sangu W, Kahama-Maro J, et al. Beyond Malaria — Causes of Fever in Outpatient Tanzanian Children. New England Journal of Medicine. 2014;370 (9):809–17.
- O’Brien KL, Baggett HC, Brooks WA, Feikin DR, Hammitt LL, Higdon MM, et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. The Lancet. 2019;394 (10200):757–79.
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022;399 (10325):629–55.
- Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Vol. 13, The Lancet Infectious Diseases. Elsevier; 2013. p. 1057–98.
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. Vol. 340, BMJ (Online). British Medical Journal Publishing Group; 2010. p. 1120.
- Holmes AH, Moore LSP, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance [Internet]. Vol. 387, The Lancet. Lancet Publishing Group; 2016. p. 176–87.
- van de Maat J, De Santis O, Luwanda L, Tan R, Keitel K. Primary Care Case Management of Febrile Children: Insights From the ePOCT Routine Care Cohort in Dar es Salaam, Tanzania. Front Pediatr. 2021;9:626386.
- Levine GA, Bielicki J, Fink G. Cumulative Antibiotic Exposure in the First 5 Years of Life: Estimates for 45 Low- and Middle-Income Countries From Demographic and Health Survey Data. Clin Infect Dis. 2022;75 (9):1537–47.
- Sulis G, Adam P, Nafade V, Gore G, Daniels B, Daftary A, et al. Antibiotic prescription practices in primary care in low- And middle-income countries: A systematic review and meta-analysis. PLoS Med. 2020;17 (6):e1003139.
- Fink G, D’Acremont V, Leslie HH, Cohen J. Antibiotic exposure among children younger than 5 years in low-income and middle-income countries: a cross-sectional study of nationally representative facility-based and household-based surveys. Lancet Infect Dis. 2020;20 (2):179–87.
- Ardillon A, Ramblière L, Kermorvant-Duchemin E, Sok T, Zo AZ, Diouf JB, et al. Inappropriate antibiotic prescribing and its determinants among outpatient children in 3 low- and middle-income countries: A multicentric community-based cohort study. PLoS Med. 2023;20 (6):e1004211.
- World Health Organisation (WHO). Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022.
- Althaus T, Greer RC, Swe MMM, Cohen J, Tun NN, Heaton J, et al. Effect of point-of-care C-reactive protein testing on antibiotic prescription in febrile patients attending primary care in Thailand and Myanmar: an open-label, randomised, controlled trial. Lancet Glob Health. 2019;7 (1):e119–31.
- Keitel K, Samaka J, Masimba J, Temba H, Said Z, Kagoro F, et al. Safety and Efficacy of C-reactive Protein-guided Antibiotic Use to Treat Acute Respiratory Infections in Tanzanian Children: A Planned Subgroup Analysis of a Randomized Controlled Noninferiority Trial Evaluating a Novel Electronic Clinical Decision Algorithm (ePOCT). Clinical Infectious Diseases. 2019;69 (11):1926–34.
- Cortellaro F, Colombo S, Coen D, Duca PG. Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emergency Medicine Journal. 2012;29 (1):19–23.
- Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure the BLUE protocol. Chest. 2008;134 (1):117–25.
- Chavez MA, Shams N, Ellington LE, Naithani N, Gilman RH, Steinhoff MC, et al. Lung ultrasound for the diagnosis of pneumonia in adults: A systematic review and meta-analysis. Respir Res. 2014;15 (1).
- Stewart KA, Navarro SM, Kambala S, Tan G, Poondla R, Lederman S, et al. Trends in Ultrasound Use in Low and Middle Income Countries: A Systematic Review. International Journal of Maternal and Child Health and AIDS (IJMA). 2020;9 (1):103–20.
- Reissig A, Copetti R, Mathis G, Mempel C, Schuler A, Zechner P, et al. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia: A prospective, multicenter, diagnostic accuracy study. Chest. 2012;142 (4):965–72.
- Heuvelings CC, Bélard S, Familusi MA, Spijker R, Grobusch MP, Zar HJ. Chest ultrasound for the diagnosis of paediatric pulmonary diseases: a systematic review and meta-analysis of diagnostic test accuracy. Br Med Bull. 2019;129 (1):35–51.
- Balk DS, Lee C, Schafer J, Welwarth J, Hardin J, Novack V, et al. Lung ultrasound compared to chest X-ray for diagnosis of pediatric pneumonia: A meta-analysis. Pediatr Pulmonol. 2018 Aug 1;53 (8):1130–9.
- Orso D, Ban A, Guglielmo N. Lung ultrasound in diagnosing pneumonia in childhood: a systematic review and meta-analysis. J Ultrasound. 2018;21 (3):183–95.
- Shah VP, Tunik MG, Tsung JW. Prospective evaluation of point-of-care ultrasonography for the diagnosis of pneumonia in children and young adults. Vol. 167, JAMA Pediatrics. 2013. p. 119–25.
- González MM, García de Casasola Sánchez G, Muñoz FJT, Proud K, Lourdo D, Sander JV, et al. Comparison of lung ultrasound versus chest x-ray for detection of pulmonary infiltrates in covid-19. Diagnostics. 2021 Feb 1;11 (2).
- Lorio G, Capasso M, Prisco S, De Luca G, Mancusi C, Laganà B, et al. Lung Ultrasound Findings Undetectable by Chest Radiography in Children with Community-Acquired Pneumonia. Ultrasound Med Biol. 2018;44 (8):1687–93.
- Claes AS, Clapuyt P, Menten R, Michoux N, Dumitriu D. Performance of chest ultrasound in pediatric pneumonia. Eur J Radiol. 2017 Mar 1;88:82–7.
- Pereda MA, Chavez MA, Hooper-Miele CC, Gilman RH, Steinhoff MC, Ellington LE, et al. Lung ultrasound for the diagnosis of pneumonia in children: A meta-analysis. Vol. 135, Pediatrics. American Academy of Pediatrics; 2015. p. 714–22.
- Tew J, Calenoff L, Berlin BS. Bacterial or Nonbacterial Pneumonia: Accuracy of Radiographic Diagnosis1. 1977;124 (3):607–12. [CrossRef]
- Ellis SM, Flower Christopher, Ostensen Harald, Pettersson H, International Society of Radiology., World Health Organization. The WHO manual of diagnostic imaging: radiographic anatomy and interpretation of the chest and the pulmonary system. Published by the World Health Organization in collaboration with the International Society of Radiology; 2006. 147 p.
- Canadian Paediatric Society - Infectious Diseases and Immunization Committee. Uncomplicated pneumonia in healthy Canadian children and youth: Practice points for management. 2018.
- Neuman MI, Lee EY, Bixby S, Diperna S, Hellinger J, Markowitz R, et al. Variability in the interpretation of chest radiographs for the diagnosis of pneumonia in children. J Hosp Med. 2012 Apr;7 (4):294–8.
- van de Maat JS, Garcia Perez D, Driessen GJA, van Wermeskerken AM, Smit FJ, Noordzij JG, et al. The influence of chest X-ray results on antibiotic prescription for childhood pneumonia in the emergency department. Eur J Pediatr. 2021;180 (9):2765–72.
- Malla D, Rathi V, Gomber S, Upreti L. Can lung ultrasound differentiate between bacterial and viral pneumonia in children? Journal of Clinical Ultrasound. 2021 Feb 1;49 (2):91–100.
- Ginsburg AS, Vitorino P, Qasim Z, Lenahan JL, Hwang J, Lamorte A, et al. Lung ultrasound patterns in paediatric pneumonia in Mozambique and Pakistan. ERJ Open Res. 2021 Jan;7 (1):00518–2020.
- McIntosh, K. Community-Acquired Pneumonia in Children. New England Journal of Medicine. 2002;346 (6):429–37.
- Duke, T. What the PERCH study means for future pneumonia strategies. Vol. 394, The Lancet. Lancet Publishing Group; 2019. p. 714–6.
- Amatya Y, Russell FM, Rijal S, Adhikari S, Nti B, House DR. Bedside lung ultrasound for the diagnosis of pneumonia in children presenting to an emergency department in a resource-limited setting. Int J Emerg Med. 2023;16 (1).
- Ellington LE, Gilman RH, Chavez MA, Pervaiz F, Marin-Concha J, Compen-Chang P, et al. Lung ultrasound as a diagnostic tool for radiographically-confirmed pneumonia in low resource settings. Respir Med. 2017;128:57–64.
- Chavez MA, Naithani N, Gilman RH, Tielsch JM, Khatry S, Ellington LE, et al. Agreement Between the World Health Organization Algorithm and Lung Consolidation Identified Using Point-of-Care Ultrasound for the Diagnosis of Childhood Pneumonia by General Practitioners. Lung. 2015;193 (4):531–8.
- Pervaiz F, Chavez MA, Ellington LE, Grigsby M, Gilman RH, Miele CH, et al. Building a Prediction Model for Radiographically Confirmed Pneumonia in Peruvian Children: From Symptoms to Imaging. Chest. 2018;154 (6):1385–94.
- Seif El Dien HM, Abd Ellatif DAK. The value of bedside Lung Ultrasonography in diagnosis of neonatal pneumonia. Vol. 44, Egyptian Journal of Radiology and Nuclear Medicine. Elsevier B.V.; 2013. p. 339–47.
- Pereda MA, Chavez MA, Hooper-Miele CC, Gilman RH, Steinhoff MC, Ellington LE, et al. Lung Ultrasound for the Diagnosis of Pneumonia in Children: A Meta-analysis. Pediatrics. 2015;135 (4):714–22.
- Ramnath RR, Heller RM, Ben-Ami T, Miller MA, Campbell P, Neblett WW, et al. Implications of early sonographic evaluation of parapneumonic effusions in children with pneumonia. Pediatrics. 1998;101 (1 Pt 1):68–71.
- Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: Update 2011. Vol. 66, Thorax. 2011.
- Zar HJ, Moore DP, Andronikou S, Argent AC, Avenant T, Cohen C, et al. Diagnosis and management of community-acquired pneumonia in children: South African thoracic society guidelines. African Journal of Thoracic and Critical Care Medicine. 2020;26 (3):95–116.
- Lissaman C, Kanjanauptom P, Ong C, Tessaro M, Long E, O’Brien A. Prospective observational study of point-of-care ultrasound for diagnosing pneumonia. Arch Dis Child. 2019 Jan 1;104 (1):12–8.
- Niederberger M, Spranger J. Delphi Technique in Health Sciences: A Map. Front Public Health. 2020 Sep 22;8:561103.
- Constantine E, Levine M, Abo A, Arroyo A, Ng L, Kwan C, et al. Core Content for Pediatric Emergency Medicine Ultrasound Fellowship Training: A Modified Delphi Consensus Study. Gottlieb M, editor. AEM Educ Train. 2020 Apr 12;4 (2):130–8.
- Arishenkoff MD S, Blouw MD M, Card MD S, Conly MD J, Gebhardt MD C, Gibson MD N, et al. Expert Consensus on a Canadian Internal Medicine Ultrasound Curriculum. Canadian Journal of General Internal Medicine. 2014 Jan 1;9 (3):106–11.
- Rahaghi FF, Baughman RP, Saketkoo LA, Sweiss NJ, Barney JB, Birring SS, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. European Respiratory Review. 2020 Mar 1;29 (155).
- Clyne B, Tyner B, O’Neill M, Jordan K, Carty PG, Phillips MK, et al. ADAPTE with modified Delphi supported developing a National Clinical Guideline: stratification of clinical risk in pregnancy. J Clin Epidemiol. 2022 Jul 1;147:21–31.
- Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: Characteristics and guidelines for use. Am J Public Health [Internet]. 1984 Oct 7;74 (9):979–83.
- Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care. 1986;2 (1):53–63.
- Sparks JB, Klamerus ML, Caverly TJ, Skurla SE, Hofer TP, Kerr EA, et al. Planning and Reporting Effective Web-Based RAND/UCLA Appropriateness Method Panels: Literature Review and Preliminary Recommendations. Vol. 24, Journal of Medical Internet Research. JMIR Publications Inc.; 2022. p. e33898. Available online: https://www.jmir.org/2022/8/e33898.
- Beiderbeck D, Frevel N, von der Gracht HA, Schmidt SL, Schweitzer VM. Preparing, conducting, and analyzing Delphi surveys: Cross-disciplinary practices, new directions, and advancements. MethodsX. 2021 Jan 1;8:101401.
- Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, et al. Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014 Apr 1;67 (4):401–9.
- Murphy, Black, Lamping, McKee, Sanderson, Askham, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess (Rockv). 1998 Mar 6;2 (3).
- Black N, Murphy M, Lamping D, McKee M, Sanderson C, Askham J, et al. Consensus development methods: A review of best practice in creating clinical guidelines. J Health Serv Res Policy. 1999 Oct 1;4 (4):236–48.
- American College of Emergency Physicians. Ultrasound Guidelines: Emergency, Point-of-care, and Clinical Ultrasound Guidelines in Medicine. 2023.
- Gattrell WT, Hungin AP, Price A, Winchester CC, Tovey D, Hughes EL, et al. ACCORD guideline for reporting consensus-based methods in biomedical research and clinical practice: a study protocol. Res Integr Peer Rev. 2022 Dec 7;7 (1):3.
- Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. In: Intensive Care Medicine. 2012. p. 577–91.
- Demi L, Wolfram F, Klersy C, De Silvestri A, Ferretti VV, Muller M, et al. New International Guidelines and Consensus on the Use of Lung Ultrasound. Vol. 42, Journal of Ultrasound in Medicine. John Wiley and Sons Ltd.; 2023. p. 309–44.
- Jaworska J, Komorowska-Piotrowska A, Pomie’cko AP, Wi´sniewski JW, Wo´zniak MW, Zej Littwin B, et al. Consensus on the Application of Lung Ultrasound in Pneumonia and Bronchiolitis in Children. Diagnostics. 2020;10:935.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).