Submitted:
26 July 2024
Posted:
29 July 2024
You are already at the latest version
Abstract
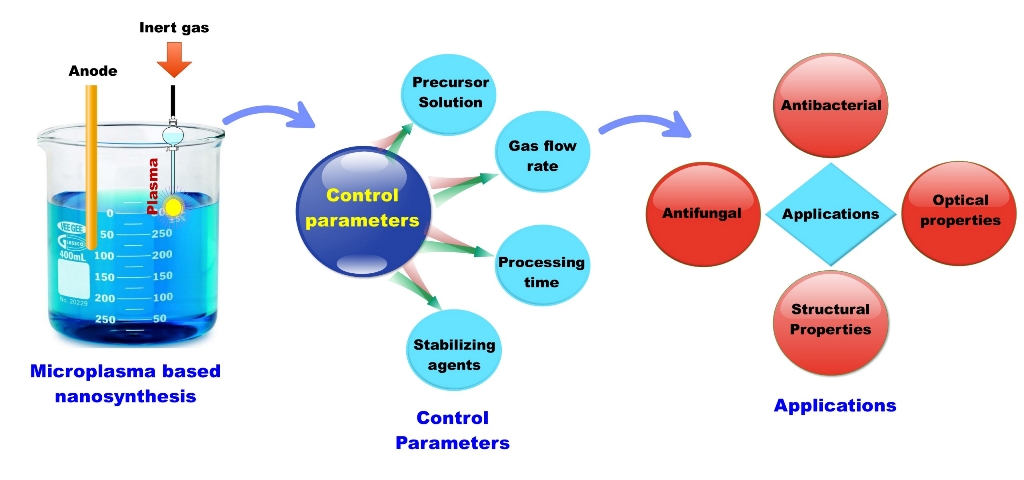
Keywords:
1. Introduction
2. Atmospheric Pressure Microplasma for Nanosynthesis
2.1. Background
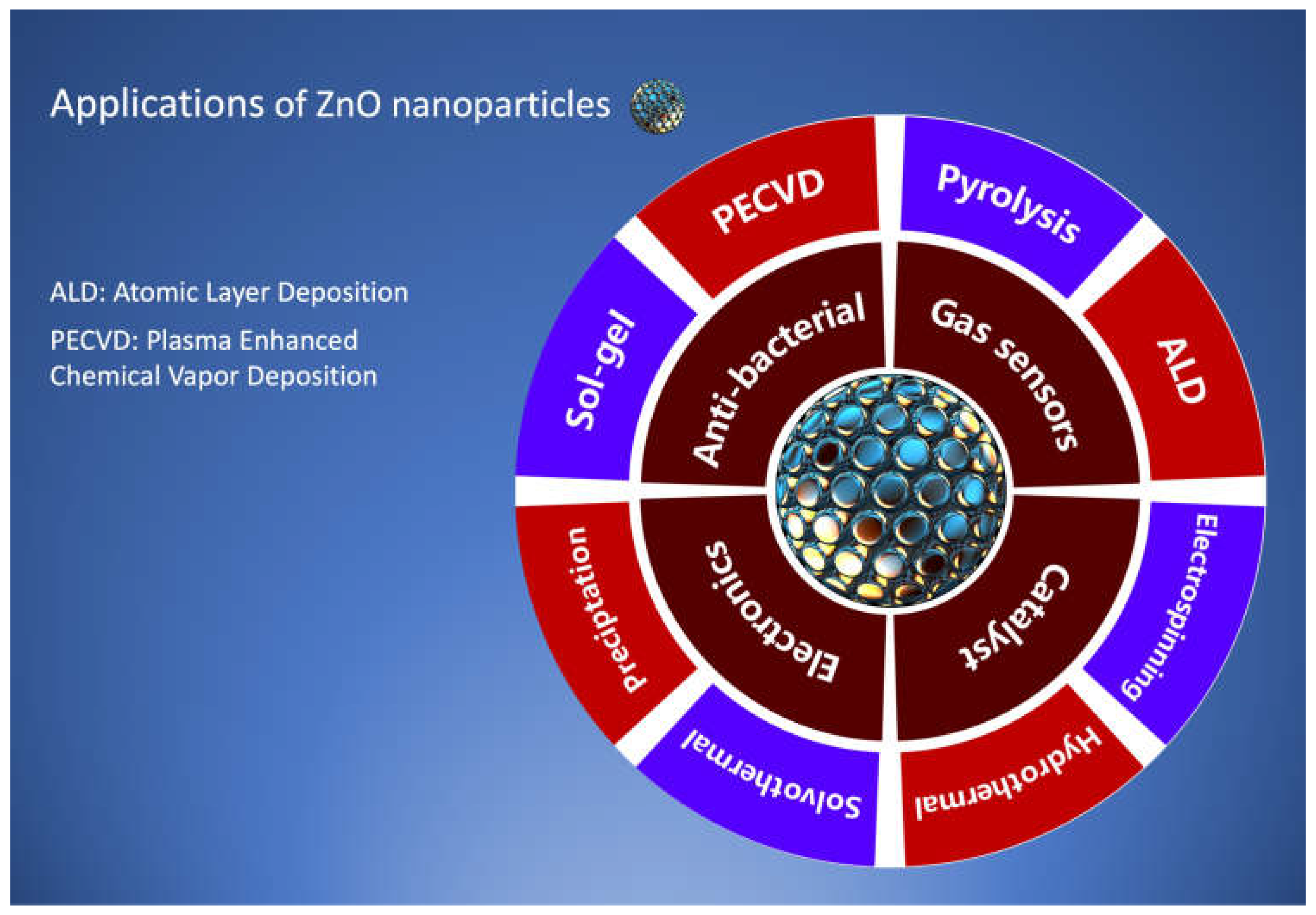
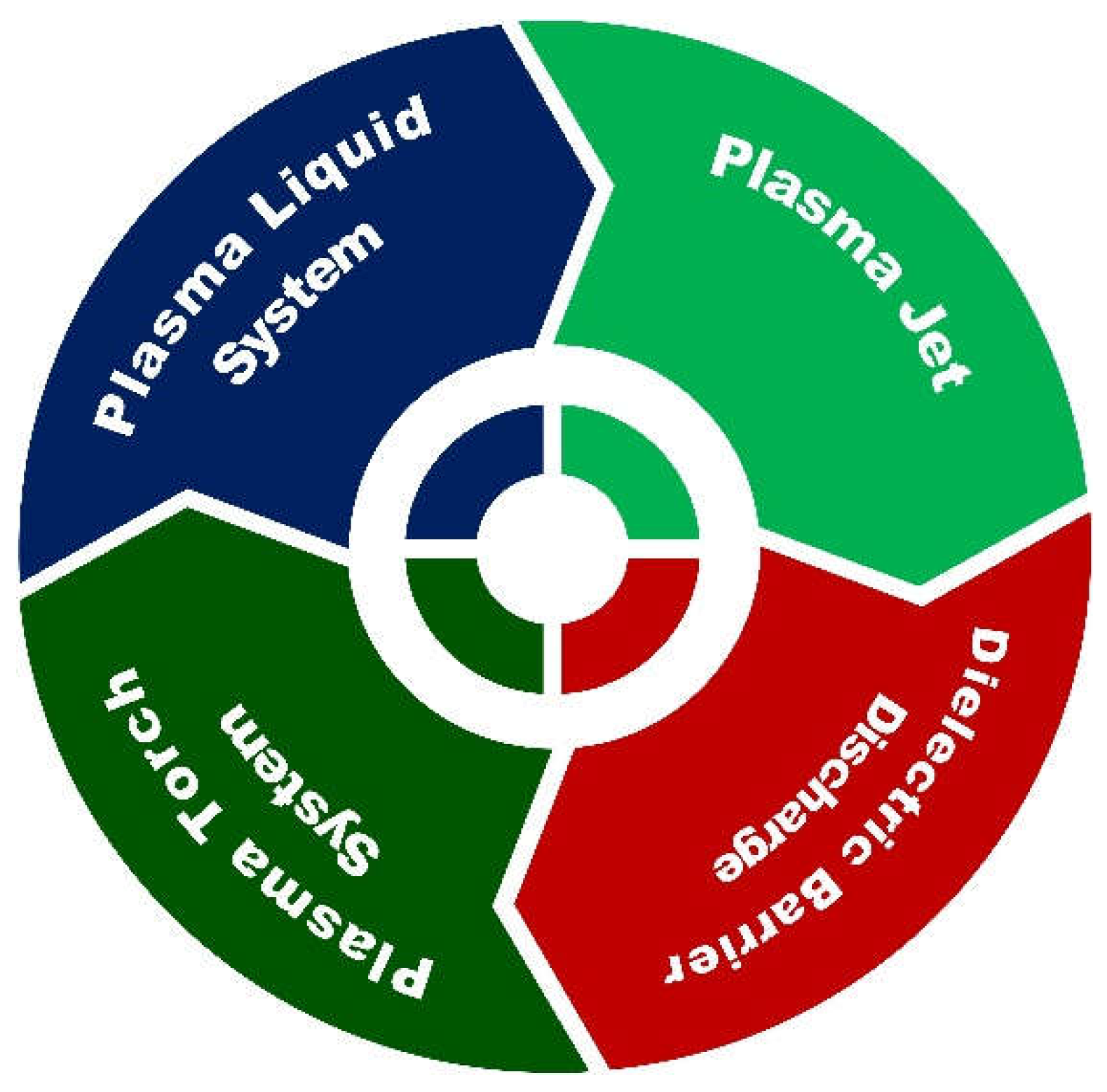
2.2. Synthesis of Nanomaterials
2.2.1. Plasma Jet System
2.2.2. Dielectric Barrier Discharge
2.2.3. Plasma Torch Method
2.2.4. Plasma-Liquid System
3. Literature Review
4. Summary of Review
5. Conclusion and final remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Jayeoye, T.J.; Mohite, P.; Singh, S.; Rajput, T.; Munde, S.; Eze, F.N.; Chidrawar, V.R.; Puri, A.; Prajapati, B.G.; et al. Sustainable and consumer-centric nanotechnology-based materials: An update on the multifaceted applications, risks and tremendous opportunities. Nano-Structures Nano-Objects 2024, 38, 101148. [Google Scholar] [CrossRef]
- Zhou, A.F.; Feng, P.X. One-Dimensional and Two-Dimensional Nanomaterials for Sensor Applications. 2024, MDPI. p. 622.
- Yamaguchi, T.; Kim, H.-J.; Park, H.J.; Kim, T.; Khalid, Z.; Park, J.K.; Oh, J.-M. Controlling the Surface Morphology of Two-Dimensional Nano-Materials upon Molecule-Mediated Crystal Growth. Nanomaterials 2023, 13, 2363. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-Y.; Sriram, B.; Wang, S.-F.; Kogularasu, S.; Chang-Chien, G.-P. Advanced Nanomaterial-Based Biosensors for N-Terminal Pro-Brain Natriuretic Peptide Biomarker Detection: Progress and Future Challenges in Cardiovascular Disease Diagnostics. Nanomaterials 2024, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Gatou, M.-A.; Vagena, I.-A.; Pippa, N.; Gazouli, M.; Pavlatou, E.A.; Lagopati, N. The Use of Crystalline Carbon-Based Nanomaterials (CBNs) in Various Biomedical Applications. Crystals 2023, 13, 1236. [Google Scholar] [CrossRef]
- Cao, M. Recent Development of Nanomaterials for Chemical Engineering. Nanomaterials 2024, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, L.; Huang, W.; Gao, Z.; Jin, M. Current Advances of Nanomaterial-Based Oral Drug Delivery for Colorectal Cancer Treatment. Nanomaterials 2024, 14, 557. [Google Scholar] [CrossRef] [PubMed]
- Gaci, Y.; Guittoum, A.; Hemmous, M.; Nez-Blanco, D.M.; Gorria, P.; Blanco, J.A.; Aouaroun, T. EFFECT OF Fe CONTENT ON THE STRUCTURAL AND MAGNETIC PROPERTIES OF TERNARY (Ni60Co40) 100−xFex NANOMATERIALS SYNTHESIZED BY HYDROTHERMAL ROUTE. Surface Review and Letters (SRL) 2024, 31, 1–9. [Google Scholar]
- Gatou, M.-A.; Skylla, E.; Dourou, P.; Pippa, N.; Gazouli, M.; Lagopati, N.; Pavlatou, E.A. Magnesium Oxide (MgO) Nanoparticles: Synthetic Strategies and Biomedical Applications. Crystals 2024, 14, 215. [Google Scholar] [CrossRef]
- Hadi, A.J.; Nayef, U.M.; Jabir, M.S.; Mutlak, F.A.-H. Titanium dioxide nanoparticles prepared via laser ablation: evaluation of their antibacterial and anticancer activityI. Surf. Rev. Lett. 2023, 30, 1–10. [Google Scholar] [CrossRef]
- Kayed, K.H.; The structural effects on silver nanoparticles plasma edges in optical reflectance spectra of Ag/Ag2O composites synthesized by oxygen plasma treatment of silver thin films. 2021.
- Casiano-Muñiz, I.M.; Ortiz-Román, M.I.; Lorenzana-Vázquez, G.; Román-Velázquez, F.R. Synthesis, Characterization, and Ecotoxicology Assessment of Zinc Oxide Nanoparticles by In Vivo Models. Nanomaterials 2024, 14, 255. [Google Scholar] [CrossRef]
- Alhoqail, W.A.; Alothaim, A.S.; Suhail, M.; Iqbal, D.; Kamal, M.; Asmari, M.M.; Jamal, A. Husk-like Zinc Oxide Nanoparticles Induce Apoptosis through ROS Generation in Epidermoid Carcinoma Cells: Effect of Incubation Period on Sol-Gel Synthesis and Anti-Cancerous Properties. Biomedicines 2023, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Zenkin, K.; Durmus, S.; Demir, A. Investigating Magnetic, Dielectric, Optic and Morphologic Properties of Nano-Nickel Oxide-Doped NdFeO3. Surface Review and Letters (SRL) 2024, 31, 1–16. [Google Scholar] [CrossRef]
- Kumar, P.; Ramesh, M.R.; Doddamani, M.; Suresh, J. Green synthesis of Fe/Ni/Cr oxide nanoparticles using Costus pictus plant extract: Microstructure and biological properties. Surf. Rev. Lett. 2024, 2450065. [Google Scholar] [CrossRef]
- Ounis, T.-D.; Rahmouni, K.; Aouar, L.Zaabat, M.; Optical properties and antibacterial activity of Ni, Mg and Fe-doped ZnO. Surface Review and Letters, 2024.
- Cho, N.-H.; Cheong, T.-C.; Min, J.H.; Wu, J.H.; Lee, S.J.; Kim, D.; Yang, J.-S.; Kim, S.; Kim, Y.K.; Seong, S.-Y. A multifunctional core–shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat. Nanotechnol. 2011, 6, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.M.K.; Prakash, V.; Gopinath, P.G. Influence of Precursor Molarity and Leaf Extract Concentration on Physical Properties of Zinc Oxide (ZnO) Nanoparticles Synthesized Using Moringa Oleifera Leaf Extract. Surf. Rev. Lett. 2024. [Google Scholar] [CrossRef]
- Abdulgafour, H.I.; Zainulabdeen, F.S.; Karam, G.S.; Magid, H.C.; Najim, A.A.; Hassan, F.M. Synthesis and characterization of Al-doped ZnO thin films as anti-reflection coatings for solar cell applications. Surf. Rev. Lett. 2024, 31, 1–7. [Google Scholar] [CrossRef]
- Wiesmann, N.; Mendler, S.; Buhr, C.R.; Ritz, U.; Kämmerer, P.W.; Brieger, J. Zinc Oxide Nanoparticles Exhibit Favorable Properties to Promote Tissue Integration of Biomaterials. Biomedicines 2021, 9, 1462. [Google Scholar] [CrossRef]
- Sugihartono, I.; Tan, S.T.; Arkundato, A.; Fahdiran, R.; Isnaeni, I.; Handoko, E.; Budi, S.; Budi, A.S. The Effect of Al-Cu Co-Dopants on Morphology, Structure, and Optical Properties of ZnO Nanostructures. Materials Research 2023, 26, e20220499. [Google Scholar] [CrossRef]
- Khan, S.; Zahoor, M.; Khan, R.S.; Ikram, M.; Islam, N.U. The impact of silver nanoparticles on the growth of plants: The agriculture applications. Heliyon 2023, 9, e16928. [Google Scholar] [CrossRef]
- Song, Y.; Yang, F.; Mu, B.; Kang, Y.; Hui, A.; Wang, A. Phyto-mediated synthesis of Ag nanoparticles/attapulgite nanocomposites using olive leaf extract: Characterization, antibacterial activities and cytotoxicity. Inorganic Chemistry Communications 2023, 151, 110543. [Google Scholar] [CrossRef]
- Hu, J.; Chen, F.; Mao, J.; Ni, L.; Lu, J. Direction regulation of interface carrier transfer and enhanced photocatalytic oxygen activation over Z-scheme Bi4V2O11/Ag/AgCl for water purification. Journal of Colloid and Interface Science 2023, 641, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-j.; Yin, Y.-g.; Liu, J.-f.J.E.S.P.Impacts; Silver nanoparticles in the environment. 2013. 15, 78-92.
- Jaswal, T.; Gupta, J. A review on the toxicity of silver nanoparticles on human health. Materials Today Proceedings 2023, 81, 859–863. [Google Scholar] [CrossRef]
- Neciosup-Puican, A.A.; Pérez-Tulich, L.; Trujillo, W.; Parada-Quinayá, C. Green Synthesis of Silver Nanoparticles from Anthocyanin Extracts of Peruvian Purple Potato INIA 328—Kulli papa. Nanomaterials 2024, 14, 1147. [Google Scholar] [CrossRef]
- Francisco, P.; Amaral, M.N.; Neves, A.; Ferreira-Gonçalves, T.; Viana, A.S.; Catarino, J.; Faísca, P.; Simões, S.; Perdigão, J.; Charmier, A.J.; et al. Pluronic® F127 Hydrogel Containing Silver Nanoparticles in Skin Burn Regeneration: An Experimental Approach from Fundamental to Translational Research. Gels 2023, 9, 200. [Google Scholar] [CrossRef]
- Holubnycha, V.; Husak, Y.; Korniienko, V.; Bolshanina, S.; Tveresovska, O.; Myronov, P.; Holubnycha, M.; Butsyk, A.; Borén, T.; Banasiuk, R.; et al. Antimicrobial Activity of Two Different Types of Silver Nanoparticles against Wide Range of Pathogenic Bacteria. Nanomaterials 2024, 14, 137. [Google Scholar] [CrossRef]
- Gong, P.; Li, H.; He, X.; Wang, K.; Hu, J.; Tan, W.; Zhang, S.Yang, X.J.N.; Preparation and antibacterial activity of Fe3O4@ Ag nanoparticles. 2007, 18, 285604.
- Naysmith, A.; Mian, N.S.; Rana, S. Development of conductive textile fabric using Plackett–Burman optimized green synthesized silver nanoparticles and in situ polymerized polypyrrole. Green Chemistry Letters and Reviews 2023, 16, 2158690. [Google Scholar] [CrossRef]
- Ghazwani, M.; Hani, U.; Alqarni, M.H.; Alam, A. Development and Characterization of Methyl-Anthranilate-Loaded Silver Nanoparticles: A Phytocosmetic Sunscreen Gel for UV Protection. Pharmaceutics 2023, 15, 1434. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Singh, S.K.; Singh, M. Green synthesis of silver nanoparticles: methods, biological applications, delivery and toxicity. Materials Advances 2023, 4, 1831–1849. [Google Scholar]
- Al-Serwi, R.H.; Eladl, M.A.; El-Sherbiny, M.; Saleh, M.A.; Othman, G.; Alshahrani, S.M.; Alnefaie, R.; Jan, A.M.; Alnasser, S.M.; Albalawi, A.E.; et al. Targeted Drug Administration onto Cancer Cells Using Hyaluronic Acid–Quercetin-Conjugated Silver Nanoparticles. Molecules 2023, 28, 4146. [Google Scholar] [CrossRef]
- Adam, A.; Mertz, D. Iron oxide@ mesoporous silica core-shell nanoparticles as multimodal platforms for magnetic resonance imaging, magnetic hyperthermia, near-infrared light photothermia, and drug delivery. Nanomaterials 2023, 13, 1342. [Google Scholar] [CrossRef]
- Kar, N.; McCoy, M.; Wolfe, J.; Bueno, S.L.A.; Shafei, I.H.; Skrabalak, S.E. Retrosynthetic design of core–shell nanoparticles for thermal conversion to monodisperse high-entropy alloy nanoparticles. Nat. Synth. 2023, 3, 175–184. [Google Scholar] [CrossRef]
- Rani, P.; Varma, R.S.; Singh, K.; Acevedo, R.; Singh, J. Catalytic and antimicrobial potential of green synthesized Au and Au@Ag core-shell nanoparticles. Chemosphere 2023, 317, 137841. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, X.; Zhang, J.; Gu, X.; Zhang, Y. Plasmon-activated NO2 sensor based on Au@MoS2 core-shell nanoparticles with heightened sensitivity and full recoverability. Sensors Actuators B Chem. 2023, 382. [Google Scholar] [CrossRef]
- Subbotina, J.; Rouse, I.; Lobaskin, V. In silico prediction of protein binding affinities onto core–shell PEGylated noble metal nanoparticles for rational design of drug nanocarriers. Nanoscale 2023, 15, 13371–13383. [Google Scholar] [CrossRef]
- Awiaz, G.; Lin, J.Wu, A. Recent advances of Au@ Ag core–shell SERS-based biosensors. Wiley Online Library.
- Liu, X.; Liang, X.; Yu, J.; Xu, K.; Shen, J.-W.; Duan, W.; Zeng, J. Recent development of noble metal-based bimetallic nanoparticles for colorimetric sensing. TrAC Trends Anal. Chem. 2023, 169, 117386. [Google Scholar] [CrossRef]
- Al-Rawi, B.K.Mazhir, S.N.J.I.J.o.N.; Evaluation of Antimicrobial Agents of Ag-ZnO Core-Shell Prepared by Micro-Jet Plasma Technique. 2023, 22, 2350044-16.
- Abuzeid, H.M.; Julien, C.M.; Zhu, L.; Hashem, A.M. Green synthesis of nanoparticles and their energy storage, environmental, and biomedical applications. Crystals 2023, 13, 1576. [Google Scholar] [CrossRef]
- Miao, W.; Hao, R.; Wang, J.; Wang, Z.; Lin, W.; Liu, H.; Feng, Z.; Lyu, Y.; Li, Q.; Jia, D. Architecture Design and Catalytic Activity: Non-Noble Bimetallic CoFe/fe3O4 Core–Shell Structures for CO2 Hydrogenation. Advanced Science 2023, 10, 2205087. [Google Scholar] [CrossRef]
- Chatterjee, K.; Sarkar, S.; Rao, K.J.; Paria, S. Core/shell nanoparticles in biomedical applications. Adv. Colloid Interface Sci. 2014, 209, 8–39. [Google Scholar] [CrossRef]
- Xinghao, L.I.U.; Cheng, C.; Zimu, X.U.; Shuheng, H.U.; Jie, S.; Yan, L.A.N.; Paul, K.C. Degradation of tetracycline in water by gas–liquid plasma in conjunction with rGO-TiO2 nanocomposite. Plasma Science and Technology 2021, 23, 115503. [Google Scholar]
- Iqbal, T.; Zahra, S.K.; Khan, M.A.R.; Shafique, M.; Raza, S.R.A.; Andleeb, S. Microplasma-assisted electrochemical synthesis of ZnO nanostructures for photocatalytic and antibacterial applications. Phys. Scr. 2021, 96, 125801. [Google Scholar] [CrossRef]
- Thai, V.-P.; Furuno, H.; Saito, N.; Takahashi, K.; Sasaki, T.; Kikuchi, T. The essential role of redox potential/equilibrium constant in the ability of non-equilibrium plasma for nano-synthesis in liquids. J. Appl. Phys. 2020, 128, 043305. [Google Scholar] [CrossRef]
- Ingsel, T.; Gupta, R.K., Plasma at the nanoscale: An introduction, in Plasma at the Nanoscale. 2022, Elsevier. p. 1-20.
- Abou El-Nour, K.M.; Eftaiha, A.a.; Al-Warthan, A.Ammar, R.A.J.A.j.o.c.; Synthesis and applications of silver nanoparticles. 2010. 3, 135-140.
- Skіba, M.; Pivovarov, A.; Vorobyova, V.; Derkach, T.Kurmakova, I.; Plasma-chemical formation of silver nanoparticles: The silver ions concentration effect on the particle size and their antimicrobial properties. 2019.
- Verma, S.; Rao, B.; Srivastava, A.; Srivastava, D.; Kaul, R.; Singh, B. A facile synthesis of broad plasmon wavelength tunable silver nanoparticles in citrate aqueous solutions by laser ablation and light irradiation. Colloids Surfaces A: Physicochem. Eng. Asp. 2017, 527, 23–33. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.Zolfaghari, B.J.R.i.p.s.; Synthesis of silver nanoparticles: chemical, physical and biological methods. 2014. 9, 385.
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B.J.A.c., nanomedicine,biotechnology; Microwave assisted green synthesis of silver nanoparticles using leaf extract of elephantopus scaber and its environmental and biological applications. 2018. 46, 795-804.
- Habib, T.; Caiut, J.M.A.Caillier, B.J.N.; Synthesis of silver nanoparticles by atmospheric pressure plasma jet. 2022. 33, 325603.
- Lin, L.; Li, S.; Hessel, V.; Starostin, S.A.; Lavrijsen, R.; Zhang, W. Synthesis of Ni nanoparticles with controllable magnetic properties by atmospheric pressure microplasma assisted process. AIChE J. 2018, 64, 1540–1549. [Google Scholar] [CrossRef]
- Mariotti, D.; Bose, A.C.Ostrikov, K.J.I.T.o.P.S.; Atmospheric-microplasma-assisted nanofabrication: Metal and metal–oxide nanostructures and nanoarchitectures. 2009, 37, 1027-1033.
- Shimizu, Y.; Kawaguchi, K.; Sasaki, T.Koshizaki, N.J.A.P.L.; Generation of room-temperature atmospheric H2/Ar microplasma jet driven with pulse-modulated ultrahigh frequency and its application to gold nanoparticle preparation. 2009, 94(19).
- Weerasinghe, J.; Li, W.; Zhou, R.; Zhou, R.; Gissibl, A.; Sonar, P.; Speight, R.; Vasilev, K.; Ostrikov, K. Bactericidal Silver Nanoparticles by Atmospheric Pressure Solution Plasma Processing. Nanomaterials 2020, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Bjelajac, A.; Phillipe, A.-M.; Guillot, J.; Fleming, Y.; Chemin, J.-B.; Choquet, P.; Bulou, S. Gold nanoparticles synthesis and immobilization by atmospheric pressure DBD plasma torch method. Nanoscale Adv. 2023, 5, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Du, C.Xiao, M.J.S.r.; Cu2O nanoparticles synthesis by microplasma. 2014. 4, 7339.
- Thong, Y.L.; Chin, O.H.; Ong, B.H.; Huang, N.M. Synthesis of silver nanoparticles prepared in aqueous solutions using helium dc microplasma jet. Jpn. J. Appl. Phys. 2015, 55, 01AE19. [Google Scholar] [CrossRef]
- Bisht, A.; Roshan Deen, G.Ilyas, U.; Synthesis of nanoparticles using atmospheric microplasma discharge. 2013.
- Kondeti, V.S.S.K.; Gangal, U.; Yatom, S.; Bruggeman, P.J. Ag+ reduction and silver nanoparticle synthesis at the plasma–liquid interface by an RF driven atmospheric pressure plasma jet: Mechanisms and the effect of surfactant. J. Vac. Sci. Technol. A 2017, 35, 061302. [Google Scholar] [CrossRef]
- Shepida, M.; Kuntyi, O.; Sukhatskiy, Y.; Mazur, A.; Sozanskyi, M.J.B.C.Applications; Microplasma synthesis of antibacterial active silver nanoparticles in sodium polyacrylate solutions. 2021. 2021.
- Huang, X.Z.; Zhong, X.X.; Lu, Y.; Li, Y.S.; E Rider, A.; A Furman, S.; Ostrikov, K. Plasmonic Ag nanoparticles via environment-benign atmospheric microplasma electrochemistry. Nanotechnology 2013, 24, 095604. [Google Scholar] [CrossRef] [PubMed]
- Shuaib, U.; Hussain, T.; Ahmad, R.; Zakaullah, M.; Mubarik, F.E.; Muntaha, S.T.; Ashraf, S. Plasma-liquid synthesis of silver nanoparticles and their antibacterial and antifungal applications. Mater. Res. Express 2020, 7, 035015. [Google Scholar] [CrossRef]
- Lin, L.; Li, X.; Zhou, J.; Zou, J.; Lai, J.; Chen, Z.; Shen, J.; Xu, H. Plasma-aided green and controllable synthesis of silver nanoparticles and their compounding with gemini surfactant. J. Taiwan Inst. Chem. Eng. 2021, 122, 311–319. [Google Scholar] [CrossRef]
- Vashistha, V.K.; Bala, R.; Das, D.K.; Mittal, A.; Pullabhotla, R.V. Transition metal Nanoparticles as Promising Antimicrobial Agents. Surf. Rev. Lett. 2023. [Google Scholar] [CrossRef]
- Saleem, M.T.; Bashir, S.Bashir, M.J.N.E.; Microplasma assisted synthesis of silver nanoparticles capped with PVA, PVP and Sucrose. 2021. 2, 020026.
- Skiba, M.; Vorobyova, V.; Pivovarov, A.; Trus, I. Preparation of silver nanoparticles using atmospheric discharge plasma for catalytic reduction of p-nitrophenol: the influence of pressure in the reactor. Pigment. Resin Technol. 2020, 49, 449–456. [Google Scholar] [CrossRef]
- Iqbal, T.; Mukhtar, M.; A Khan, M.; Khan, R.; Zaman, R.; Mahmood, H.; Zaka-Ul-Islam, M. Atmospheric pressure microplasma assisted growth of silver nanosheets and their inhibitory action against bacteria of clinical interest. Mater. Res. Express 2016, 3, 125019. [Google Scholar] [CrossRef]
- Iqbal, T.; Aziz, A.; Khan, M.; Andleeb, S.; Mahmood, H.; Khan, A.A.; Khan, R.; Shafique, M. Surfactant assisted synthesis of ZnO nanostructures using atmospheric pressure microplasma electrochemical process with antibacterial applications. Mater. Sci. Eng. B 2018, 228, 153–159. [Google Scholar] [CrossRef]
- Schwan, A.M.; Chwatal, S.; Hendler, C.; Kopp, D.; Lackner, J.M.; Kaindl, R.; Tscherner, M.; Zirkl, M.; Angerer, P.; Friessnegger, B.; et al. Morphology-controlled atmospheric pressure plasma synthesis of zinc oxide nanoparticles for piezoelectric sensors. Appl. Nanosci. 2023, 13, 6421–6432. [Google Scholar] [CrossRef]
- Jain, G.; Macias-Montero, M.; Velusamy, T.; Maguire, P.; Mariotti, D.J.P.P.Polymers; Porous zinc oxide nanocrystalline film deposition by atmospheric pressure plasma: Fabrication and energy band estimation. 2017. 14, 1700052.
- Abdullah, E.A.; Anber, A.A.; Edan, F.F.; Fraih, A.J. Synthesis of ZnO Nanoparticles by Using an Atmospheric-Pressure Plasma Jet. OALib 2018, 05, 1–7. [Google Scholar] [CrossRef]
- Khalid, A.; Murbat, H.H.; Shanan, Z.J.J.B.Archives, C.; SYNTHESIS AND CHARACTERIZATION OF GOLD–SILVER AU/AG-CORE-SHELL NANOPARTICLES BY COLD ATMOSPHERIC PRESSURE PLASMA. 2019. 19(2).
- Sun, D.; Turner, J.; Jiang, N.; Zhu, S.; Zhang, L.; Falzon, B.G.; McCoy, C.P.; Maguire, P.; Mariotti, D.; Sun, D.J.C.S.Technology; Atmospheric pressure microplasma for antibacterial silver nanoparticle/chitosan nanocomposites with tailored properties. 2020. 186: 107911.
- Aadim, K.A.Abbas, I.K.J.I.J.o.S.; Synthesis and Investigation of the Structural Characteristics of Zinc Oxide Nanoparticles Produced by an Atmospheric Plasma Jet. 2023: 1743-1752.
- Bose, A.C.; Shimizu, Y.; Mariotti, D.; Sasaki, T.; Terashima, K.; Koshizaki, N. Flow rate effect on the structure and morphology of molybdenum oxide nanoparticles deposited by atmospheric-pressure microplasma processing. Nanotechnology 2006, 17, 5976–5982. [Google Scholar] [CrossRef]
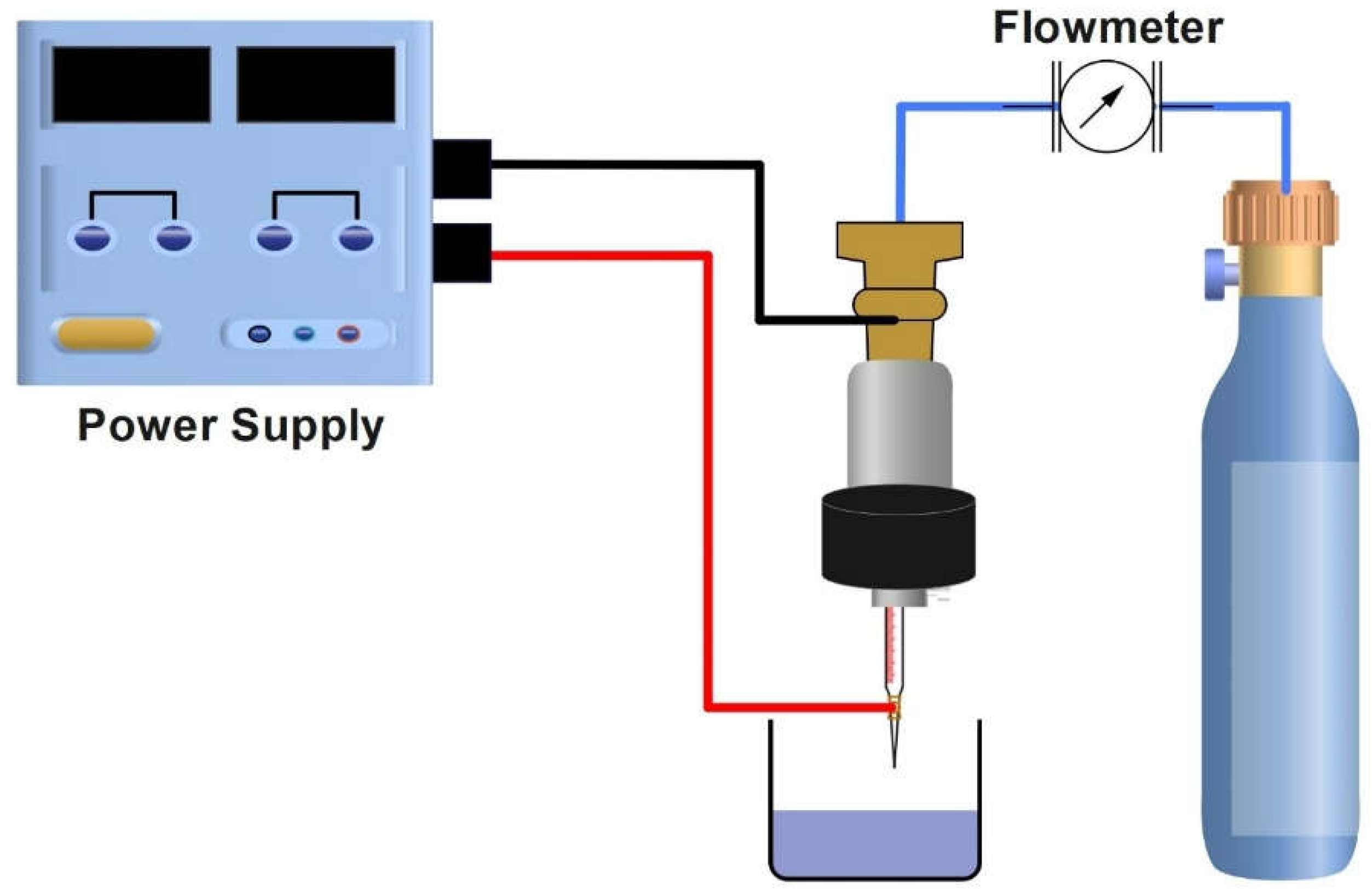
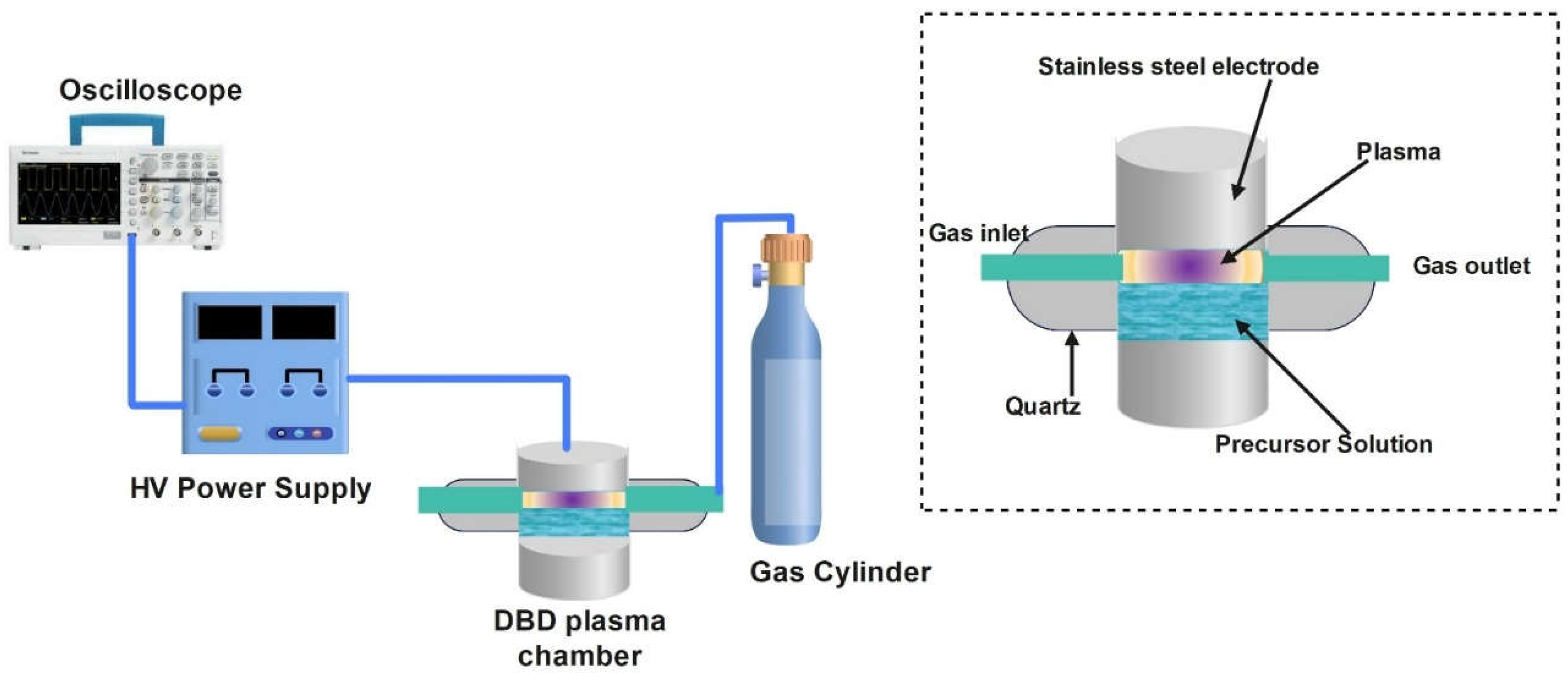
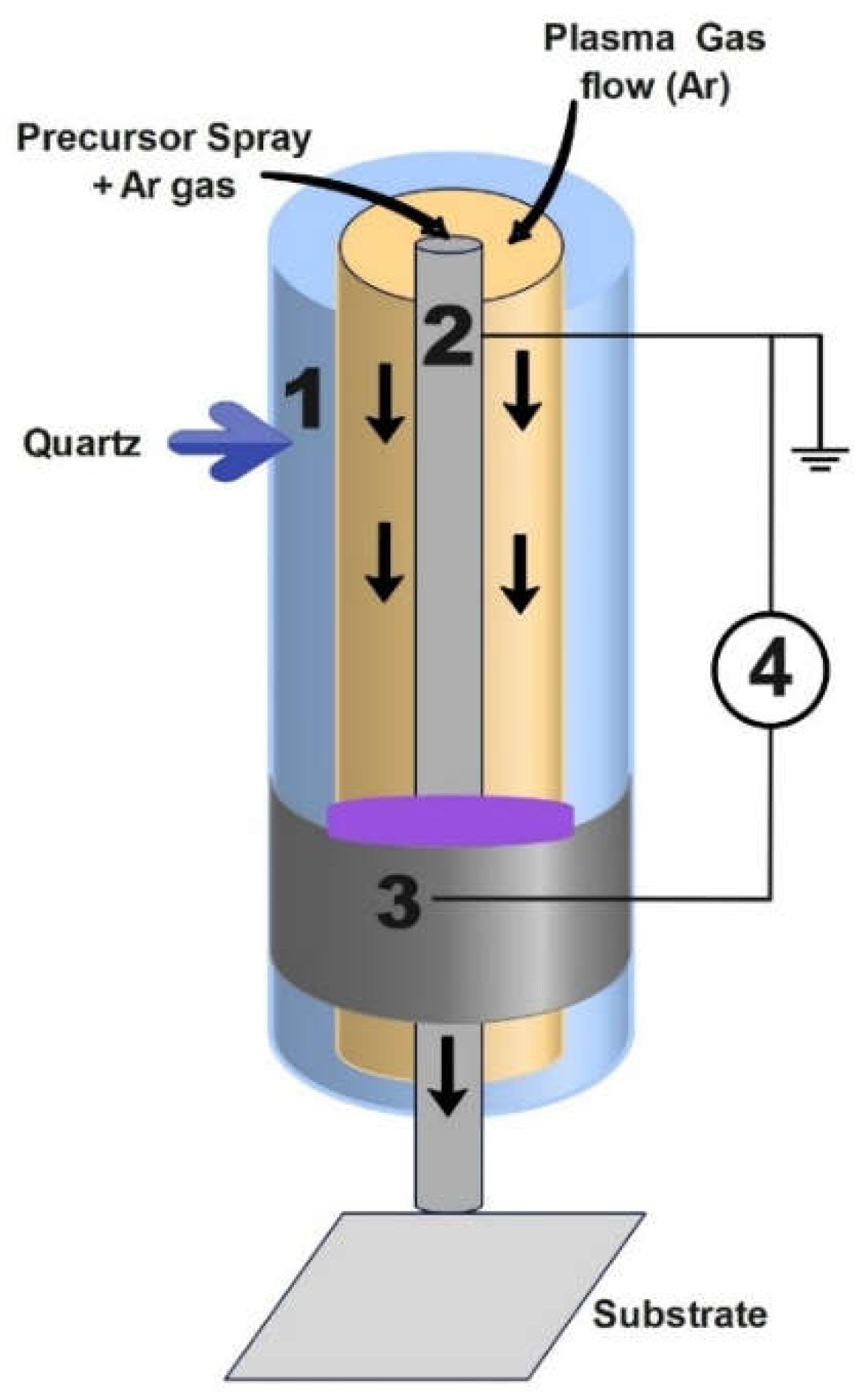
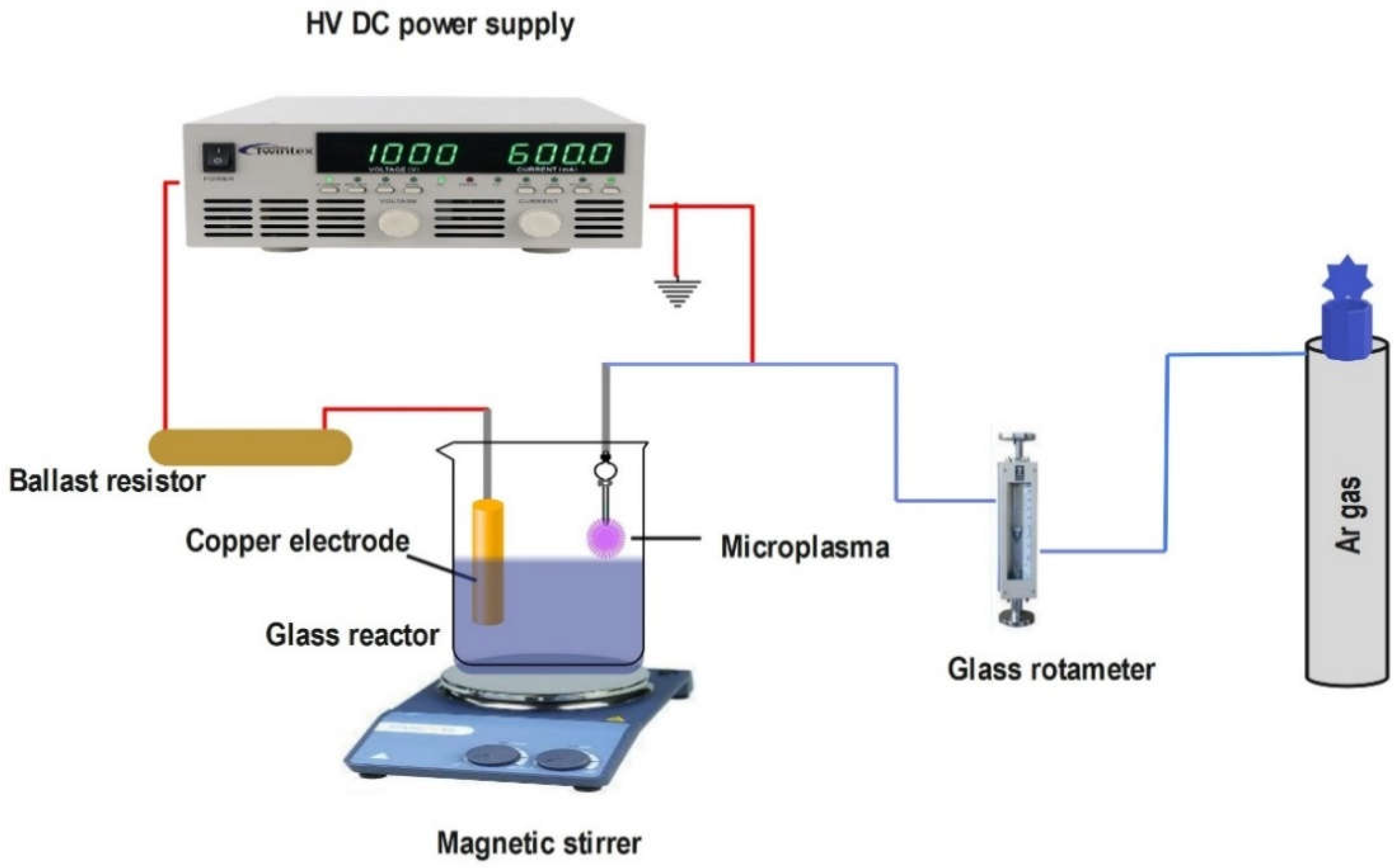
| No. | Plasma Configuration |
Nanomaterials | Applications | Capillary Diameter |
Precursor | Gas Flow Rate | Voltage & Current |
Ref. |
|---|---|---|---|---|---|---|---|---|
| 01 | Atmospheric Pressure Microplasma jet | Silver | Optoelectronics, sensing, biomedical applications | Internal diameter 0.26mm |
AgNO3 + sucrose | 26sccm | 2mA | [62] |
| 02 | Atmospheric Pressure Microplasma | Silver | Nanosensors | Internal diameter 0.7mm |
AgNO3 + sucrose | 25sccm | 0-15kV | [63] |
| 03 | R.F. atmospheric pressure Microplasma jet | Silver | Photovoltaic | Internal diameter 5.25mm |
AgNO3 | 1.5slm | [64] | |
| 04 | Microplasma Synthesis | Silver | Antibacterial activity | Internal diameter 0.1mm | AgNO3+ NaPA | 250V | [65] | |
| 05 | Atmospheric Microplasma electrochemistry | Silver | Plasmonic applications as sensing | Internal diameter 0.175mm | AgNO3 + fructose | 25sccm | 3mA and 2kV | [66] |
| 06 | Plasma liquid synthesis | Silver | Anti-bacterial and antifungal activities | Internal diameter 0.34mm | AgNO3 + fructose | 100 sccm | 15mA and 600V | [67] |
| 07 | Plasma-aided green and controllable synthesis | Silver | Antibacterial activity | Internal diameter 0.5 mm | AgNO3 + Acetone | 30 sccm | [68] | |
| 08 | Atmospheric pressure Plasma jet | Silver | Bioactivity, catalysis | Internal diameter 3.7mm |
AgNO3+ trisodium citrate | 3 L/min | 8A | [55] |
| 09 | Microplasma assisted synthesis | Silver | Cancer therapy | Internal diameter < 1mm | AgNO3+ PVA, PVP & sucrclose | 600 sccm | 3-5 kV | [70] |
| 10 | Atmospheric discharge plasma | Silver | Catalytic properties | Internal diameter 2.4mm | AgNO3+ AlgNa | 500-1000V | [71] | |
| 11 | Atmospheric pressure Microplasma | Silver | Anti-bacterial activity | Internal diameter 0.2 mm | AgNO3 + fructose | 150 sccm | 1000V | [72] |
| 12 | Atmospheric pressure Microplasma electrochemical process | Zinc oxide | Antibacterial applications | Internal diameter 0.2 mm | Zn (NO3)2 + surfactant | 150 sccm | 1000V | [73] |
| 13 | Atmospheric pressure plasma jet technique | Zinc oxide | Piezoelectric sensors | Zinc powder | 10L/min | 200-400A | [74] | |
| 14 | Atmospheric pressure plasma (R.F. Power) | Zinc oxide | Light-emitting diodes | Internal diameter 0.7mm |
Zinc wire | 150 sccm | [75] | |
| 15 | Atmospheric pressure plasma jet | Zinc oxide | Solar cells, Gas sensors | Internal diameter 0.6mm |
Zinc anode + NaOH+ HNO3+ sucrose | 60ml/min | 3kV 5-10 mA |
[76] |
| 16 | Atmospheric pressure Microplasma Jet | Ag-ZnO core shells | Antimicrobial activity | AgNO3 + Zn (NO3)2 | 13kV | [42] | ||
| 17 | Atmospheric pressure Microplasma | Au-Ag core shells | Optical and biological properties | Internal diameter 1mm |
AgNO3 + HAuCl4. 3H2O | 2 l/min | 10kV | [77] |
| Material | Control parameters | Effect of Parameters | Ref. |
|---|---|---|---|
| Silver | Solution concentration |
|
[72] |
|
[78] |
||
|
[66] |
||
| ZnO |
Processing time |
|
[76] |
|
[79] |
||
| Silver |
|
[63] |
|
| Silver | Stabilizing agent concentration |
|
[67] |
| Silver | Stabilizing agent concentration |
|
[62] |
| Molybdenum oxide | Gas flow rate |
|
[80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





