1. Introduction
Huanglongbing (HLB or citrus greening), the destructive disease that has decimated the citrus industry in Florida, USA, is associated with the phloem-limited bacterium
Candidatus Liberibacter asiaticus (
CLas) and is now widespread and endemic in the state [
1]. Despite the endemic nature of HLB in Florida, citrus growers continue to plant new trees. Controlling HLB and the spread of the vector, the Asian Citrus Psyllid (ACP,
Diaphorina citri), is critical, especially in newly planted citrus trees, to ensure survival and productivity in an environment of high ACP pressure and neighboring mature infected trees, allowing rapid spread of the disease. Young citrus trees are more vulnerable to both ACP infestation and CLas infection due to more frequent flushing, and protection from the vector psyllid is crucial. Importantly, infection during the early growth stage prevents trees from ever becoming productive [
2]. Vector exclusion is an effective strategy for HLB management. Growing citrus under protective screens (CUPS) is effective in excluding psyllids and allows for rapid, healthy growth and production of high-quality fruit [
3,
4]. However, due to the high cost, this production system is only viable for fresh fruit production and not feasible for large-scale production of varieties used for juice processing, such as Valencia or Hamlin (
Citrus sinensis L. Osbeck), which represent more than 90% of citrus grown in Florida. An alternative method is the use of individual protective covers (IPCs) for young trees. IPCs are polyethylene mesh bags with a pore diameter smaller than the size of a psyllid (≈0.6 mm), preventing the insects from accessing the new flushes and hence impeding pathogen transmission. Both in solid blocks of newly planted trees or as resets of scattered new trees occupying vacant planting spots after removal of dead or unproductive trees in infected mature groves, this system is being increasingly adopted by citrus growers in Florida. We have shown previously that IPCs are effective in protecting newly planted trees from HLB by excluding the CLas carrying ACP vector [
5,
6]. In addition, IPCs altered microclimatic conditions in the tree canopy and positively influenced most of the horticultural traits measured, increasing tree growth, flush expansion, and leaf size. At the same time, less leaf drop was observed, and the canopy was denser in trees under IPCs compared to non-covered trees [
6]. Finally, IPCs enabled trees to sustain a balanced carbohydrate metabolism by preventing CLas infection and induced higher leaf chlorophyll levels [
7]. Since leaf chlorophyll levels are positively correlated with the photosynthesis rate and plant productivity [
8], it is expected that IPC-covered trees will be more productive compared to trees without IPCs as they reach maturity and enter fruit-bearing age.
Typically, young trees may be covered with IPCs for up to three years before they are removed, but this depends on the variety, the rootstock-scion combination, growth pattern, grove management, and size of the IPCs [
5]. After IPC removal, trees become exposed to the ACPs and ultimately become infected. The rate of infection in such trees has not been determined to date. At the same time, many varieties that do not need cross-pollination may have begun to bloom and set fruit already. ‘Valencia’ sweet orange (
Citrus sinensis), which is the most cultivated variety in Florida, is self-fertile and can therefore set fruit in an IPC-enclosed environment. This opens the possibility of setting an earlier-than-usual crop, accelerating, and increasing the return on investment for growers. One main consequence of HLB is the great reduction in fruit yield and quality. HLB-affected fruits have less soluble solids and a higher titratable acidity, among other unfavorable qualities resulting from changes in secondary metabolites [
9]. This results in juice that is described as distinctly bitter, sour, salty/umami, metallic, musty, and lacking in sweetness and fruity/orange flavor [
10]. Fruit yield has been dramatically reduced in recent years, mostly due to extensive preharvest drop, smaller fruit size, and general decline of HLB-affected trees [
11]. It has been determined that in ‘Valencia’ sweet orange, the drop rate of mature fruit is significantly greater for trees exhibiting severe HLB symptoms than for mildly symptomatic trees [
12].
Here we investigated how IPC protection of newly planted trees for 30 months influenced fruit quality and yield of ‘Valencia’ trees for three consecutive seasons after IPC removal compared to non-covered trees. We also determined the CLas infection rate after IPC removal. We found that delay in infection provided by the IPCs provided a kick-start advantage to newly planted trees that resulted in significantly more yield and better internal fruit quality after three years, even though the trees became infected within 12 months after IPC removal.
2. Materials and Methods
2.1. Plant Material
The experiment was conducted at the University of Florida, Southwest Florida Research and Education Center (SWFREC) research farm in Immokalee, Collier County, FL (26°27'51.4"N, 81°26'39.9"W). HLB-free 18-month-old trees composed of sweet orange ‘Valencia’ (Citrus sinensis L. Osbeck) scion grafted on ‘Cleopatra’ mandarin (C. reticulata) rootstock obtained from a commercial citrus nursery (Southern Citrus Nurseries, Dundee, FL, USA) were used for establishing a new plot for the experiment.
2.2. Experimental Design
Ninety trees were planted in January 2018 in six rows of 15 trees each at a spacing of 8 feet (2.4 m) within rows and 22 feet (6.7 m) between rows. The trees were arranged in a completely randomized 2 x 3 factorial design with five replications, each consisting of linear plots of 3 trees, as described in a previous work [
6]. The first factor (tree cover) had two levels: 1) IPC and 2) no IPC. The second factor (insecticide rate) had three levels: 1) full recommended rate (Full), 2) half the recommended rate (Half), and 3) no insecticides (Zero). We, therefore, had total of 6 treatment combinations/interactions.
2.3. Treatments and Experiment Timeline
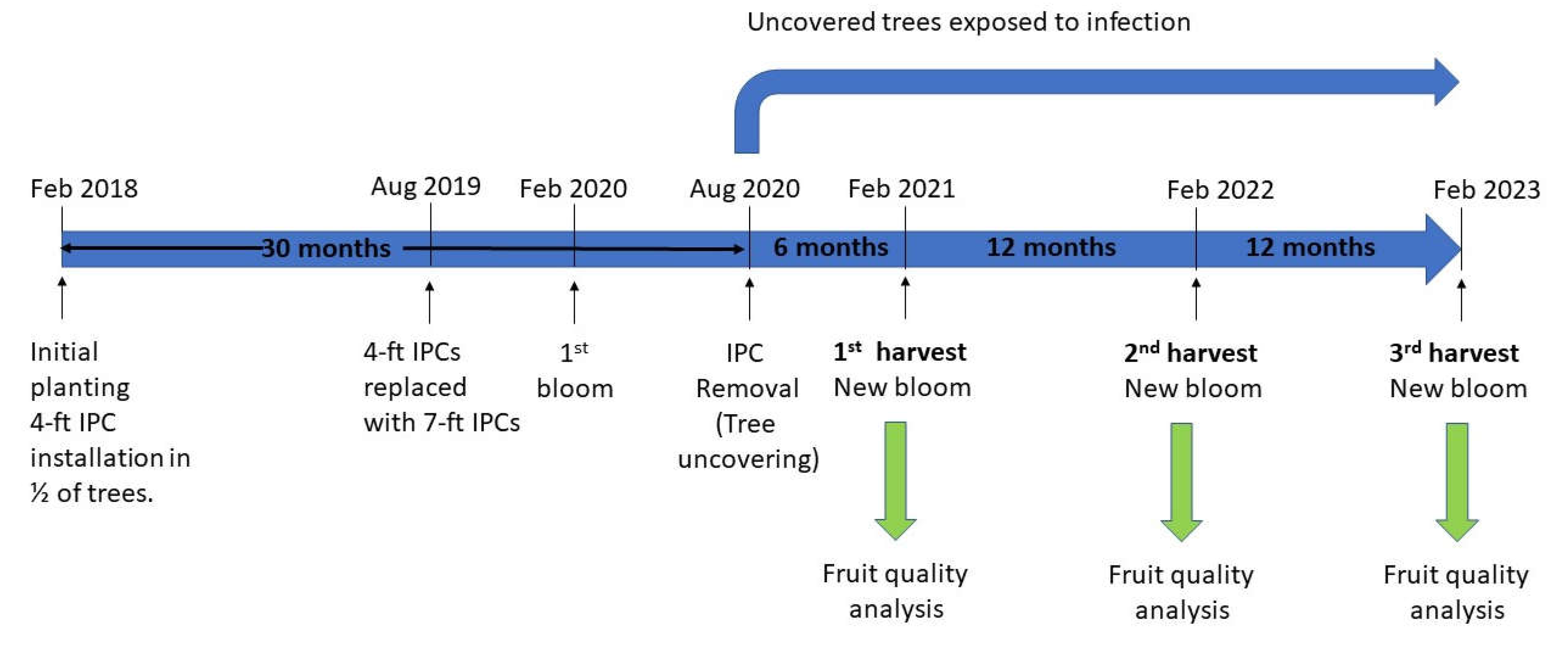
Individual protective covers (IPCs, Tree defender Inc., Dundee, FL, USA) were installed on 45 randomly selected trees at the time of planting (January 2018). IPCs were composed of monofilament high-density polyethylene (HDPE) with a mesh size of 50 (0.297 mm diameter holes). A PVC pole was installed to support each IPC and the covers were tied with zip ties at the base of the trunk to prevent psyllids and other insects from entering. During the first 18 months, trees were covered with 4-ft (1.2 m) tall IPCs until the cover restricted canopy expansion. The 4-ft IPCs were then replaced with 7-ft (2.1 m) tall IPCs to allow the canopy to further expand. For these large-size IPCs, the supporting PVC poles were equipped with metal spreaders to support the net. The 7-ft IPCs remained on the trees for an additional 12 months until removal in August 2020. The timeline of the experiment is depicted in
Figure 1.
Insecticide treatments were performed as described before [
6]. Insecticides were diluted in water and each tree received a soil drench of 300 ml material per application. Trees were irrigated three times a week by under-tree microjets. Diamond-R 8-8-8 young tree blend (Diamond R Fertilizer, Fort Pierce, FL, USA) was applied at the rate of 227 g per tree in year 1, and 454 g per tree in years 2 and 3. Diamond R CitriBlend 12-8-6 control release fertilizer (Diamond R Fertilizer, Fort Pierce, FL, USA) was applied at the rate of 227 g per tree in year 4. Weeds were uniformly managed in the experimental plot as needed using standard grower practices. Upon IPC removal 30 months after planting, insecticide treatments and irrigation continued without changes for the remainder of the experiment (30 additional months).
2.4. Candidatus Liberibacter asiaticus (CLas) Detection
Quantitative real-time polymerase chain reaction (qRT-PCR) was conducted for CLas detection using a 7500 Fast Real-Time PCR system (Applied Biosystem, Foster City, CA, USA). Trees were sampled for CLas detection during spring and summer of 2019, and 2020. Three to four mature leaves from recent flushes were randomly collected from the middle tree of each 3-tree plot. Petioles and midribs of leaves were excised, minced with a razor blade, lyophilized in a FreeZone 6 freeze dry system (Labconco, Kansas City, MO, USA), and pulverized using a mini bead beater (Biospec products, INC. OK, USA). DNA from 100 mg of pulverized leaves was extracted using the Wizard Magnetic 96 DNA Plant System (Promega Corporation, Madison, WI, USA) according to the manufacturer’s instructions. DNA was quantified using a microplate reader (Synergy HTX Multimode Reader, Biotek instruments, INC, VT, USA) and normalized to 10 ng/µl. The analysis was conducted using primers (HLBas and HLBr), and probe HLBp [
6]. In our experiment, Ct-value of less than 36 was considered as CLas-positive and those that were equal or greater than 36 were considered CLas-negative. Samples for which no qPCR product was detected after 40 cycles were assigned a value of 41. IPCs were briefly removed for leaf sample collection and replaced immediately thereafter at each time point.
2.5. Tree Size
Tree height, canopy volume, trunk diameter (scion and rootstock), and leaf area index were assessed on every middle tree of each 3-tree plot as previously described [
6]. Tree height was measured from the soil surface to the top of the tree using a tape measurer (Komelon, Waukesha, WI, USA) and avoiding any erratic shoots. Canopy diameter was measured parallel and perpendicular to the row, and the canopy volume was calculated using the formula by Wutscher and Hill (1995) [
13]:
canopy volume = [(diameter parallel to row x diameter perpendicular to row) x height]/4.
The leaf area index (LAI) was determined using a LIA-2200C Plant Canopy Analyzer (LI-COR Biosciences, Lincoln, NE, USA). Tree height, canopy volume and LAI were measured during summer 2020, 2021 and 2022.
Scion and rootstock diameters were measured at 5 cm above and below the graft union respectively, during summer (July) of 2020, 2021, and 2022 using a Vernier caliper (Fowler high precision, Auburndale, MA, USA). Trunk diameters were measured in two perpendicular directions and averaged.
2.6. Fruit Quality and Yield
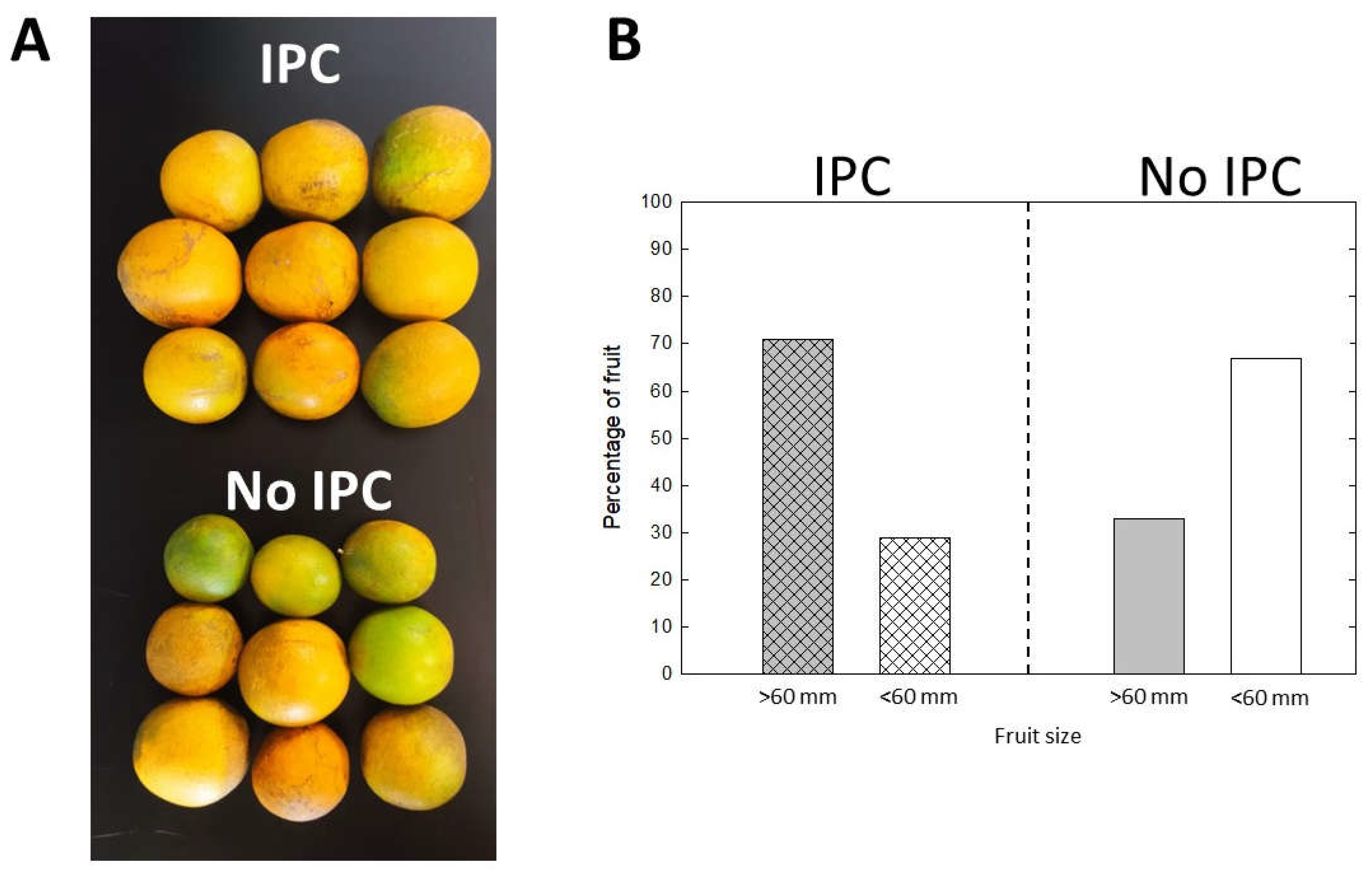
Fruits (four replicates of five fruit) were randomly collected from the 3 IPC-covered trees in each replicated plot of 5 trees in February of every year. Fruit was so scarce in the non-covered trees that all harvested fruit were used for quality determination. Juice was extracted with a hand reamer. °Brix was determined by using a table-model refractometer with temperature compensation (Hanna Instruments, Smithfield, Rhode Island, USA). Percentage of titratable acidity (TA) was determined by titrating sodium hydroxide to a phenolphthalein endpoint. Results are presented as the Brix to acid ratio. Fruit peel color was measured using a portable colorimeter (Konica Minolta, CR-400, Tokyo, Japan). For this, three different readings were obtained along the equatorial circumference of each fruit. Results are presented as the a*/b* color ratio. Yield was assessed in three consecutive years on the second week of February by counting the number of fruits in each 3-tree plot at harvest. Fruit diameter was measured with a digital caliper and fruit was classified as either large (commercially acceptable) when the diameter was >60 mm or small (not commercially acceptable) when the diameter was smaller than 60 mm.
2.7. Statistical Analysis
Analysis of variance (ANOVA) was conducted to determine the effect of tree cover, and insecticide rate. Mean separation was done by Tukey’s honestly significant difference (HSD) test. Pearson’s correlations coefficients were calculated for selected response variables. Differences were defined as statistically significant when P < 0.05.
4. Discussion
One major concern for the citrus industry in Florida has been that newly planted trees under endemic HLB conditions, either in solid blocks or as resets, will never produce harvestable fruit, or fruit quality will be poor due to CLas infection [
2]. Infection greatly reduces fruit quality and exacerbates fruit drop resulting in growers having to harvest earlier than pre-HLB. The premature harvest times greatly impacts juice quality, as maturation is critical for achieving commercially acceptable juice quality [
10]. We have previously shown that IPCs effectiveness in preventing newly planted trees from becoming infected with CLas by excluding the ACP [
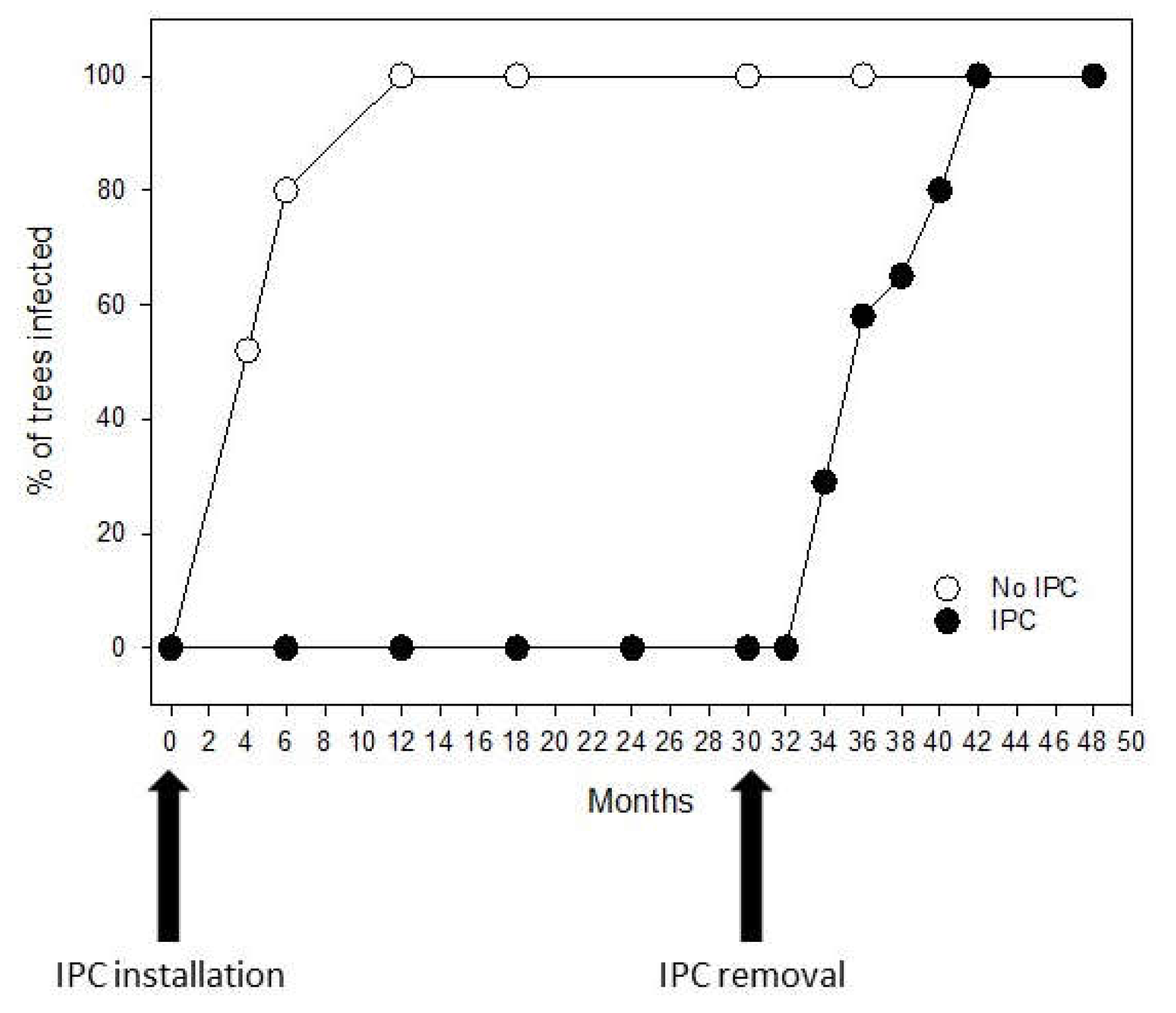
6]. Uncovering trees after 30 months of IPC protection resulted in a trend of infection similar to that of trees that were not covered at planting. As a result, 100% infection after IPC removal was observed in about 10 months of exposure to the ACPs. This reinforces the notion that covering trees with IPCs at planting is currently the best option for growers to keep the young trees free from HLB.
Tree growth was promoted by IPCs. Trunk diameter, tree height and canopy volume were larger than in non-covered trees after 30 months, irrespective of the insecticide dosage [
6]. It was not clear if this advantage conferred by IPCs is the result of maintaining trees free from HLB, shading effect, or both combined. Shading can promote tree growth in citrus and improve photosynthesis [
14] and may ameliorate HLB symptoms [
15]. This possibility merits further evaluation, which is currently being performed in our labs. Some favorable differences in tree growth were maintained for at least 6 months after IPC removal; tree height and LAI were still significantly improved in trees that were covered with IPCs. After IPC removal insecticide treatment had a significant effect on tree growth, but only for trunk diameter, not tree height. This reinforces that it is critical to continue to protect trees from the ACPs once IPCs are removed and trees are left exposed to natural ACP pressures. In any case, the differences observed in tree health parameters between protected and non-protected trees influenced the ability of trees to produce fruit and determined fruit production upon IPC removal. We monitored fruit set, yield, and quality for three consecutive seasons after IPC removal in both types of trees: trees non-covered at the time of planting and hence, exposed to ACPs (and CLas infection) from the beginning of the experiment, and trees protected with IPCs for 30 months and only then exposed to ACPs (and CLas infection) for another 30 months after IPC removal. Some varieties, including the most widely planted in Florida for juice production such as ‘Hamlin’ and ‘Valencia’ sweet oranges can set fruit under IPCs, without the need for external pollinators. This was the case in the first season in our study when trees bloomed and set fruit while being under IPC covers. After IPC removal in August 2020, during the rapid fruit growth stage due to cell expansion in phase II of fruit development [
16], trees were left exposed to psyllids and therefore, CLas infection. However, no fruit drop was observed in this first season even though infection likely occurred during this phase II of fruit development. It has been suggested that cell division and/or expansion are impeded in response to HLB-induced changes in hormone homeostasis [
17], and that the likeliness of fruit drop before harvest might be determined before fruit reach maturity, and as early as stage I or stage II of fruit development and growth [
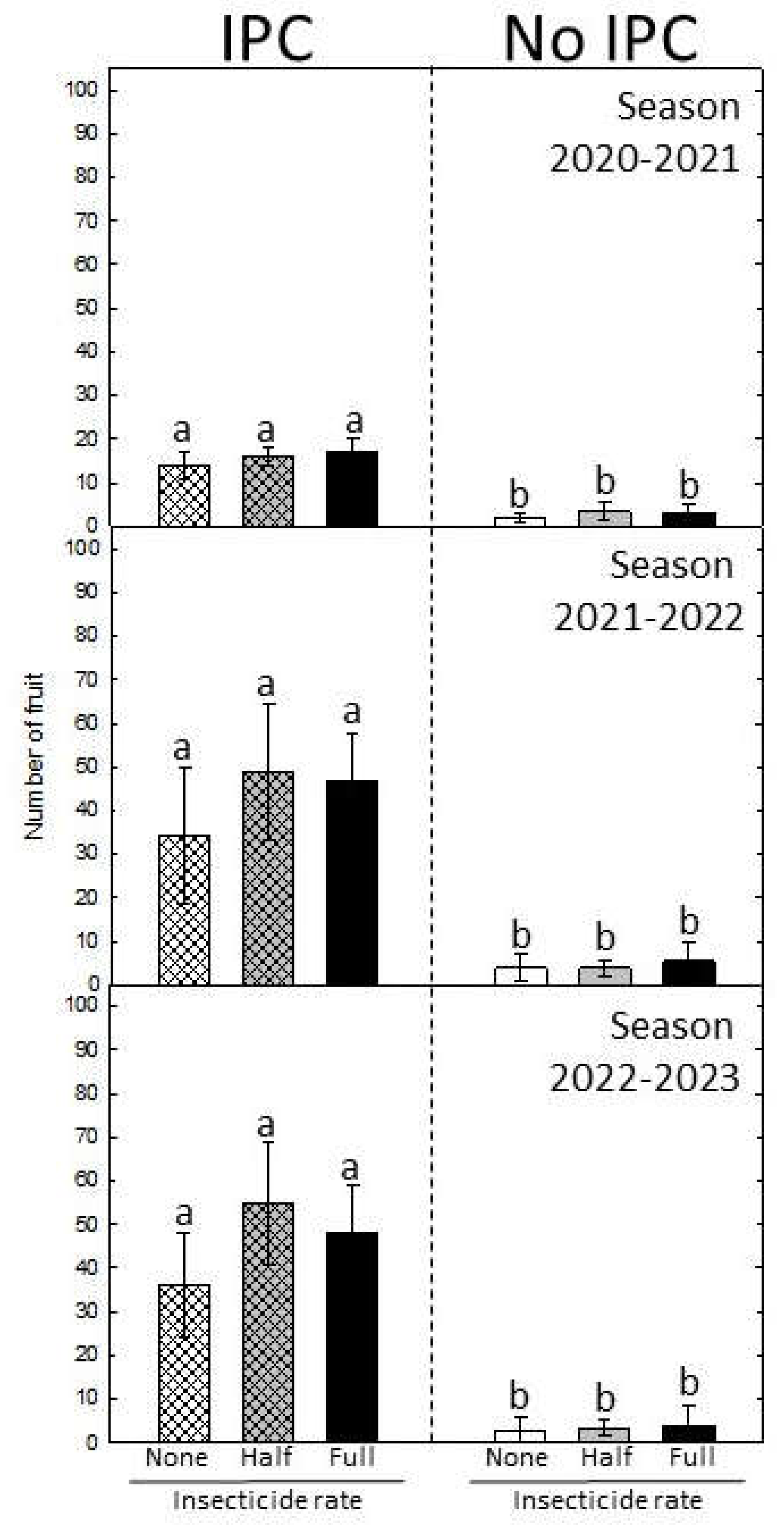
12]. Fruit yield was consistently higher in trees protected by IPCs in all three seasons. A combination of factors may explain this. First, fruit set was more abundant in all three seasons in trees that were initially covered with IPCs even though these trees became infected after IPC removal. Second, fruit drop was absent in the first season in IPC-protected trees, and significantly lower than in non-protected trees in the following two seasons, despite CLas infection . Fruit drop was around 60% in non-protected trees in all three seasons, which is consistent with observations in commercial groves [
18]. Finally, fruit from trees that were initially covered with IPCs were significantly larger than fruit from non-covered trees. It is worth noting that generally, HLB symptomatic fruit from infected trees are smaller in diameter as compared to asymptomatic fruit from infected or from healthy trees [
10]. In addition, larger fruits consistently have a higher fruit detachment force (i.e., the force necessary to detach a fruit from the stem through the abscission zone), resulting in less fruit drop [
12].
One of the most important effects of HLB is the reduced fruit quality. Symptomatic fruit from HLB-affected trees show higher titratable acidity, lower soluble solids, and sugar/acids ratio [
10]. This results in enormous economic losses for the industry, as juice quality is negatively affected. In Florida, growers are paid by pound solids, the weight of the total dissolved sugar solids in each 90-lb (40.82 Kg) box of fruit; therefore, juice quality is a very important parameter for the viability of a farm. Peel color is affected by HLB as well. Flavedo in HLB symptomatic fruit tends to be greener than flavedo from healthy fruit, as color development may only ‘break’ on the stem end, leaving most of the fruit surface green [
18]. We monitored the internal and external fruit quality for the three consecutive seasons. Consistently, fruit quality was improved in IPC-covered trees although eventually, trees became infected after IPC removal. Brix and sugar to acid ratio were significantly higher in fruit from IPC trees in all three seasons. In the first season harvest, at the time the trees previously covered with IPCs had been exposed to psyllid infestation for 6 months, and most of the trees (around 60%) were infected, Brix in fruit from these trees was significantly higher at 10.9 as compared to 7.6 in fruit from control trees, that were exposed to HLB infection for 36 months. Significant differences were the maintained in subsequent two seasons, although a clear decline in Brix and sugar to acid ratio was observed with each season. This was expected, as the health of the trees was declining with infection, despite the continued insecticide treatments after IPC removal. However, it is worth noting that despite the decline in internal quality, IPCs provided a kick-start to trees resulting in better attributes such as healthier tree development and retention of fruit quality under HLB conditions. We also consistently observed a better coloration in flavedo from fruit in IPC-covered trees in all three seasons. ‘Valencia’ is a late maturation variety, and fruit color was not fully developed at the time of harvest in early February. In Florida, under HLB-free conditions, ‘Valencia’ fruit reach their full color and internal quality by April or May, but under HLB is nearly impossible that trees bear fruit that late in the season, mainly due to the extensive pre-harvest fruit drop. This was the main reason of harvesting fruit in our experiments in February. It is not clear that the color we measured was the result of advanced degreening or less regreening, both due to healthier tree conditions. In any case, IPC protection during the first 30 months after planting imparted favorable conditions that improved the color of the flavedo.
In conclusion, our data support previous reports that IPCs improve tree health under HLB- endemic conditions and show that this tool kick-started the newly planted citrus trees, resulting in more yield and fruits with better internal and external quality. Based on the results from this study, benefits from IPC protection may last for at least three consecutive seasons once trees enter the productive age despite CLas-infection within 12 months after IPC removal. Sustained tree health is a goal after IPC removal so quality fruit production can be attained over time. Additional management practices should be considered after IPC removal to prolong its positive effects. Currently, we are investigating the use of foliar sprays of homobrassinolides which appear to be promising for retaining health via induction of SAR immunity and improving young tree vigor [
19,
20,
21]. Research has also shown that oxytetracycline (OTC) administered by trunk injection can effectively reduce fruit drop, increase fruit yield and fruit size, and improve juice quality in young and mature HLB-affected trees [
22,
23]. We envision a novel Integrated Pest Management (IPM) strategy that would employ IPCs for young tree protection followed by treatment with homobrassinolide or other plant growth regulators to improve growth further and delay CLas infection or alleviate HLB symptoms as the tree transition to full commercial fruit production (4–5-year-old trees). Once trees become infected, injection of OTC or other antibacterials/immunity inducers would prevent tree decline and ensure the continued production of high-quality fruit. Studies are already in progress to assess these strategies and determine the economic benefits.