Submitted:
27 July 2024
Posted:
29 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Literature Search and Selection
2.3. Assembling and Assessing the 6PPD Quinone AEP Network
3. Results and Discussion
3.1. Essentiality of the Key Exposure States
3.2. Theoretical Plausibility of Key Transitional Relationships
3.3. Empirical Support for the Key Transitional Relationships
3.4. Quantititave Understanding of the Key Transitional Relationships
4. Applications of the Aggregate Exposure Pathway to Advance Risk Assessment
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and Health: A Progress Update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef]
- Wang, Z.; Walker, G.W.; Muir, D.C.G.; Nagatani-Yoshida, K. Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories. Environ. Sci. Technol. 2020, 54, 2575–2584. [Google Scholar] [CrossRef]
- Muir, D.C.G.; Getzinger, G.J.; McBride, M.; Ferguson, P.L. How Many Chemicals in Commerce Have Been Analyzed in Environmental Media? A 50 Year Bibliometric Analysis. Environ. Sci. Technol. 2023, 57, 9119–9129. [Google Scholar] [CrossRef]
- Persson, L.; Carney Almroth, B.M.; Collins, C.D.; Cornell, S.; de Wit, C.A.; Diamond, M.L.; Fantke, P.; Hassellöv, M.; MacLeod, M.; Ryberg, M.W.; et al. Outside the Safe Operating Space of the Planetary Boundary for Novel Entities. Environ. Sci. Technol. 2022, 56, 1510–1521. [Google Scholar] [CrossRef]
- Caplin, A.; Ghandehari, M.; Lim, C.; Glimcher, P.; Thurston, G. Advancing Environmental Exposure Assessment Science to Benefit Society. Nat. Commun. 2019, 10, 1236. [Google Scholar] [CrossRef]
- Lioy, P.J.; Smith, K.R. A Discussion of Exposure Science in the 21st Century: A Vision and a Strategy. Environ. Health Perspect. 2013, 121, 405–409. [Google Scholar] [CrossRef]
- Price, E.J.; Vitale, C.M.; Miller, G.W.; David, A.; Barouki, R.; Audouze, K.; Walker, D.I.; Antignac, J.-P.; Coumoul, X.; Bessonneau, V.; et al. Merging the Exposome into an Integrated Framework for “Omics” Sciences. iScience 2022, 25. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, H.; Peter, K.T.; Gonzalez, M.; Wetzel, J.; Wu, C.; Hu, X.; Prat, J.; Mudrock, E.; Hettinger, R.; et al. A Ubiquitous Tire Rubber–Derived Chemical Induces Acute Mortality in Coho Salmon. Science 2021, 371, 185–189. [Google Scholar] [CrossRef]
- Zoroufchi Benis, K.; Behnami, A.; Minaei, S.; Brinkmann, M.; McPhedran, K.N.; Soltan, J. Environmental Occurrence and Toxicity of 6PPD Quinone, an Emerging Tire Rubber-Derived Chemical: A Review. Environ. Sci. Technol. Lett. 2023, 10, 815–823. [Google Scholar] [CrossRef]
- Chen, X.; He, T.; Yang, X.; Gan, Y.; Qing, X.; Wang, J.; Huang, Y. Analysis, Environmental Occurrence, Fate and Potential Toxicity of Tire Wear Compounds 6PPD and 6PPD-Quinone. J. Hazard. Mater. 2023, 452, 131245. [Google Scholar] [CrossRef]
- Ihenetu, S.C.; Xu, Q.; Khan, Z.H.; Kazmi, S.S.U.H.; Ding, J.; Sun, Q.; Li, G. Environmental Fate of Tire-Rubber Related Pollutants 6PPD and 6PPD-Q: A Review. Environ. Res. 2024, 258, 119492. [Google Scholar] [CrossRef]
- Bohara, K.; Timilsina, A.; Adhikari, K.; Kafle, A.; Basyal, S.; Joshi, P.; Yadav, A.K. A Mini Review on 6PPD Quinone: A New Threat to Aquaculture and Fisheries. Environ. Pollut. 2024, 340, 122828. [Google Scholar] [CrossRef]
- Cao, G.; Zhang, J.; Wang, W.; Wu, P.; Ru, Y.; Cai, Z. Mass Spectrometry Analysis of a Ubiquitous Tire Rubber-Derived Quinone in the Environment. TrAC Trends Anal. Chem. 2022, 157, 116756. [Google Scholar] [CrossRef]
- Khan, F.R.; Rødland, E.S.; Kole, P.J.; Van Belleghem, F.G.A.J.; Jaén-Gil, A.; Hansen, S.F.; Gomiero, A. An Overview of the Key Topics Related to the Study of Tire Particles and Their Chemical Leachates: From Problems to Solutions. TrAC Trends Anal. Chem. 2024, 172, 117563. [Google Scholar] [CrossRef]
- Kazmi, S.S.U.H.; Xu, Q.; Tayyab, M.; Pastorino, P.; Barcelò, D.; Yaseen, Z.M.; Khan, Z.H.; Li, G. Navigating the Environmental Dynamics, Toxicity to Aquatic Organisms and Human Associated Risks of an Emerging Tire Wear Contaminant 6PPD-Quinone. Environ. Pollut. 2024, 356, 124313. [Google Scholar] [CrossRef]
- Jin, R.; Venier, M.; Chen, Q.; Yang, J.; Liu, M.; Wu, Y. Amino Antioxidants: A Review of Their Environmental Behavior, Human Exposure, and Aquatic Toxicity. Chemosphere 2023, 317, 137913. [Google Scholar] [CrossRef]
- Tan, Y.-M.; Leonard, J.A.; Edwards, S.; Teeguarden, J.; Egeghy, P. Refining the Aggregate Exposure Pathway. Environ. Sci. Process. Impacts 2018, 20, 428–436. [Google Scholar] [CrossRef]
- Teeguarden, J.G.; Tan, Y.-M.; Edwards, S.W.; Leonard, J.A.; Anderson, K.A.; Corley, R.A.; Kile, M.L.; Simonich, S.M.; Stone, D.; Tanguay, R.L.; et al. Completing the Link between Exposure Science and Toxicology for Improved Environmental Health Decision Making: The Aggregate Exposure Pathway Framework. Environ. Sci. Technol. 2016, 50, 4579–4586. [Google Scholar] [CrossRef]
- Tan, Y.-M.; Leonard, J.A.; Edwards, S.; Teeguarden, J.; Paini, A.; Egeghy, P. Aggregate Exposure Pathways in Support of Risk Assessment. Curr. Opin. Toxicol. 2018, 9, 8–13. [Google Scholar] [CrossRef]
- Hines, D.E.; Conolly, R.B.; Jarabek, A.M. A Quantitative Source-to-Outcome Case Study to Demonstrate the Integration of Human Health and Ecological End Points Using the Aggregate Exposure Pathway and Adverse Outcome Pathway Frameworks. Environ. Sci. Technol. 2019, 53, 11002–11012. [Google Scholar] [CrossRef]
- Hines, D.E.; Edwards, S.W.; Conolly, R.B.; Jarabek, A.M. A Case Study Application of the Aggregate Exposure Pathway (AEP) and Adverse Outcome Pathway (AOP) Frameworks to Facilitate the Integration of Human Health and Ecological End Points for Cumulative Risk Assessment (CRA). Environ. Sci. Technol. 2018, 52, 839–849. [Google Scholar] [CrossRef]
- Peng, G.; Lin, Y.; van Bavel, B.; Li, D.; Ni, J.; Song, Y. Aggregate Exposure Pathways for Microplastics (mpAEP): An Evidence-Based Framework to Identify Research and Regulatory Needs. Water Res. 2022, 209, 117873. [Google Scholar] [CrossRef]
- Clewell, R.A.; Leonard, J.A.; Nicolas, C.I.; Campbell, J.L.; Yoon, M.; Efremenko, A.Y.; McMullen, P.D.; Andersen, M.E.; Clewell, H.J.; Phillips, K.A.; et al. Application of a Combined Aggregate Exposure Pathway and Adverse Outcome Pathway (AEP-AOP) Approach to Inform a Cumulative Risk Assessment: A Case Study with Phthalates. Toxicol. In Vitro 2020, 66, 104855–104855. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, Z.; Liu, X.; Yang, L. Variation in Vegetation and Its Driving Force in the Pearl River Delta Region of China. Int. J. Environ. Res. Public. Health 2022, 19, 10343. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, J.; Zhang, Z.; Zhu, Y.; Hou, H.; Zhao, L.; Sun, Z.; Xue, W.; Shi, H. Linkage between Human Population and Trace Elements in Soils of the Pearl River Delta: Implications for Source Identification and Risk Assessment. Sci. Total Environ. 2018, 610–611, 944–950. [Google Scholar] [CrossRef]
- HKTDC Research PRD Economic Profile. Available online: https://research.hktdc.com/en/data-and-profiles/mcpc/provinces/guangdong/pearl-river-delta (accessed on 26 July 2024).
- Cao, G.; Wang, W.; Zhang, J.; Wu, P.; Qiao, H.; Li, H.; Huang, G.; Yang, Z.; Cai, Z. Occurrence and Fate of Substituted P-Phenylenediamine-Derived Quinones in Hong Kong Wastewater Treatment Plants. Environ. Sci. Technol. 2023, 57, 15635–15643. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, J.; Liu, X.; Deng, M.; Chen, X.; Shi, C.; Yao, L.; Wang, X.; Fang, M. Occurrence and Oxidation Kinetics of Antioxidant P-Phenylenediamines and Their Quinones in Recycled Rubber Particles from Artificial Turf. Environ. Sci. Technol. Lett. 2024, 11, 335–341. [Google Scholar] [CrossRef]
- Wang, W.; Cao, G.; Zhang, J.; Chen, Z.; Dong, C.; Chen, J.; Cai, Z. P-Phenylenediamine-Derived Quinones as New Contributors to the Oxidative Potential of Fine Particulate Matter. Environ. Sci. Technol. Lett. 2022, 9, 712–717. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Zhang, W.; Qi, Z.; Song, Y.; Zhu, L.; Dong, C.; Chen, J.; Cai, Z. P-Phenylenediamine Antioxidants in PM2.5: The Underestimated Urban Air Pollutants. Environ. Sci. Technol. 2022, 56, 6914–6921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, L.; Wang, L.; Zhang, H.; Chen, J.; Geng, N. A Nation-Wide Study for the Occurrence of PPD Antioxidants and 6PPD-Quinone in Road Dusts of China. Sci. Total Environ. 2024, 922, 171393. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Huang, J.; Qi, Y.; Chen, D.; Huang, W. Distribution Patterns of Rubber Tire-Related Chemicals with Particle Size in Road and Indoor Parking Lot Dust. Sci. Total Environ. 2022, 844, 157144. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Shi, Y.; Huang, J.; Deng, C.; Tang, S.; Liu, X.; Chen, D. Occurrence of Substituted P-Phenylenediamine Antioxidants in Dusts. Environ. Sci. Technol. Lett. 2021, 8, 381–385. [Google Scholar] [CrossRef]
- Cao, G.; Wang, W.; Zhang, J.; Wu, P.; Zhao, X.; Yang, Z.; Hu, D.; Cai, Z. New Evidence of Rubber-Derived Quinones in Water, Air, and Soil. Environ. Sci. Technol. 2022, 56, 4142–4150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Mei, Y.-X.; Liang, X.-N.; Ge, Z.-Y.; Huang, Z.; Zhang, H.-Y.; Zhao, J.-L.; Liu, A.; Shi, C.; Ying, G.-G. Small-Intensity Rainfall Triggers Greater Contamination of Rubber-Derived Chemicals in Road Stormwater Runoff from Various Functional Areas in Megalopolis Cities. Environ. Sci. Technol. 2024, 58, 13056–13064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Huang, Z.; Liu, Y.-H.; Hu, L.-X.; He, L.-Y.; Liu, Y.-S.; Zhao, J.-L.; Ying, G.-G. Occurrence and Risks of 23 Tire Additives and Their Transformation Products in an Urban Water System. Environ. Int. 2023, 171, 107715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, S.; Liu, X.; Tian, L.; Mo, Y.; Yi, X.; Liu, S.; Liu, J.; Li, J.; Zhang, G. Aquatic Environmental Fates and Risks of Benzotriazoles, Benzothiazoles, and p-Phenylenediamines in a Catchment Providing Water to a Megacity of China. Environ. Res. 2023, 216, 114721. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.-N.; Wu, N.-N.; Xu, R.; Liu, S.; Li, H.-X.; Lin, L.; Hou, R.; Xu, X.-R.; Zhao, J.-L.; Ying, G.-G. First Evidence of the Bioaccumulation and Trophic Transfer of Tire Additives and Their Transformation Products in an Estuarine Food Web. Environ. Sci. Technol. 2024, 58, 6370–6380. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Gao, Y.; Feng, S.; Cheng, Z.; Huang, H.; Xue, J.; Zhang, T.; Sun, H. Widespread Occurrence of Two Typical N, N’-Substituted p-Phenylenediamines and Their Quinones in Humans: Association with Oxidative Stress and Liver Damage. J. Hazard. Mater. 2024, 468, 133835. [Google Scholar] [CrossRef]
- Du, B.; Liang, B.; Li, Y.; Shen, M.; Liu, L.-Y.; Zeng, L. First Report on the Occurrence of N-(1,3-Dimethylbutyl)-N′-Phenyl-p-Phenylenediamine (6PPD) and 6PPD-Quinone as Pervasive Pollutants in Human Urine from South China. Environ. Sci. Technol. Lett. 2022, 9, 1056–1062. [Google Scholar] [CrossRef]
- Chen, C.; Gao, L.; Ding, P.; Zhang, S.; Wang, X.; Yang, K.; Zhou, Y.; Chi, B.; Tuo, X. The Potential Impact of 6PPD and Its Oxidation Product 6PPD-Quinone on Human Health: A Case Study on Their Interaction with Human Serum Albumin. Chemosphere 2024, 362, 142675. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.; Cao, G.; Wang, F.; Ru, Y.; Wang, B.; Zhang, Y.; Zhang, D.; Yan, J.; Xu, J.; et al. 6PPD-Quinone Exposure Induces Neuronal Mitochondrial Dysfunction to Exacerbate Lewy Neurites Formation Induced by α-Synuclein Preformed Fibrils Seeding. J. Hazard. Mater. 2024, 465, 133312. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-A.; Koh, B. Utilization of Road Dust Chemical Profiles for Source Identification and Human Health Impact Assessment. Sci. Rep. 2020, 10, 14259. [Google Scholar] [CrossRef] [PubMed]
- Hiki, K.; Yamamoto, H. Concentration and Leachability of N-(1,3-Dimethylbutyl)-N′-Phenyl-p-Phenylenediamine (6PPD) and Its Quinone Transformation Product (6PPD-Q) in Road Dust Collected in Tokyo, Japan. Environ. Pollut. 2022, 302, 119082. [Google Scholar] [CrossRef] [PubMed]
- Anh, N.T.; Can, L.D.; Nhan, N.T.; Schmalz, B.; Luu, T.L. Influences of Key Factors on River Water Quality in Urban and Rural Areas: A Review. Case Stud. Chem. Environ. Eng. 2023, 8, 100424. [Google Scholar] [CrossRef]
- Wang, H.; Xia, X.; Liu, R.; Wang, Z.; Zhai, Y.; Lin, H.; Wen, W.; Li, Y.; Wang, D.; Yang, Z.; et al. Dietary Uptake Patterns Affect Bioaccumulation and Biomagnification of Hydrophobic Organic Compounds in Fish. Environ. Sci. Technol. 2019, 53, 4274–4284. [Google Scholar] [CrossRef] [PubMed]
- Masset, T.; Ferrari, B.J.D.; Dudefoi, W.; Schirmer, K.; Bergmann, A.; Vermeirssen, E.; Grandjean, D.; Harris, L.C.; Breider, F. Bioaccessibility of Organic Compounds Associated with Tire Particles Using a Fish In Vitro Digestive Model: Solubilization Kinetics and Effects of Food Coingestion. Environ. Sci. Technol. 2022, 56, 15607–15616. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyk, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The Impact of Lipophilicity on Environmental Processes, Drug Delivery and Bioavailability of Food Components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, H. (Nina); Tian, Z.; Peter, K.T.; Dodd, M.C.; Kolodziej, E.P. Chemical Characteristics, Leaching, and Stability of the Ubiquitous Tire Rubber-Derived Toxicant 6PPD-Quinone. Environ. Sci. Process. Impacts 2023, 25, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Silva-Adaya, D.; Garza-Lombó, C.; Gonsebatt, M.E. Xenobiotic Transport and Metabolism in the Human Brain. NeuroToxicology 2021, 86, 125–138. [Google Scholar] [CrossRef]
- Hou, M.; Zhang, B.; Fu, S.; Cai, Y.; Shi, Y. Penetration of Organophosphate Triesters and Diesters across the Blood–Cerebrospinal Fluid Barrier: Efficiencies, Impact Factors, and Mechanisms. Environ. Sci. Technol. 2022, 56, 8221–8230. [Google Scholar] [CrossRef]
- Wager, T.T.; Chandrasekaran, R.Y.; Hou, X.; Troutman, M.D.; Verhoest, P.R.; Villalobos, A.; Will, Y. Defining Desirable Central Nervous System Drug Space through the Alignment of Molecular Properties, in Vitro ADME, and Safety Attributes. ACS Chem. Neurosci. 2010, 1, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Leung, M.C.K.; Rooney, J.P.; Sendoel, A.; Hengartner, M.O.; Kisby, G.E.; Bess, A.S. Mitochondria as a Target of Environmental Toxicants. Toxicol. Sci. 2013, 134, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Scatena, R.; Bottoni, P.; Botta, G.; Martorana, G.E.; Giardina, B. The Role of Mitochondria in Pharmacotoxicology: A Reevaluation of an Old, Newly Emerging Topic. Am. J. Physiol.-Cell Physiol. 2007, 293, C12–C21. [Google Scholar] [CrossRef] [PubMed]
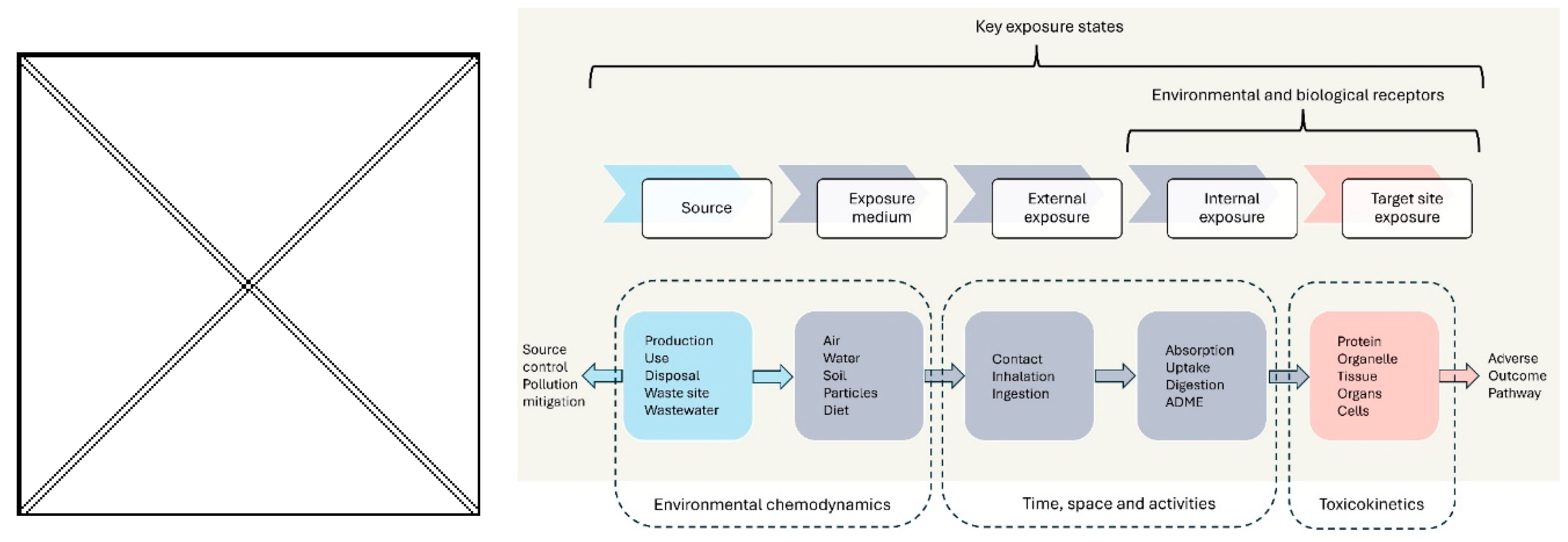
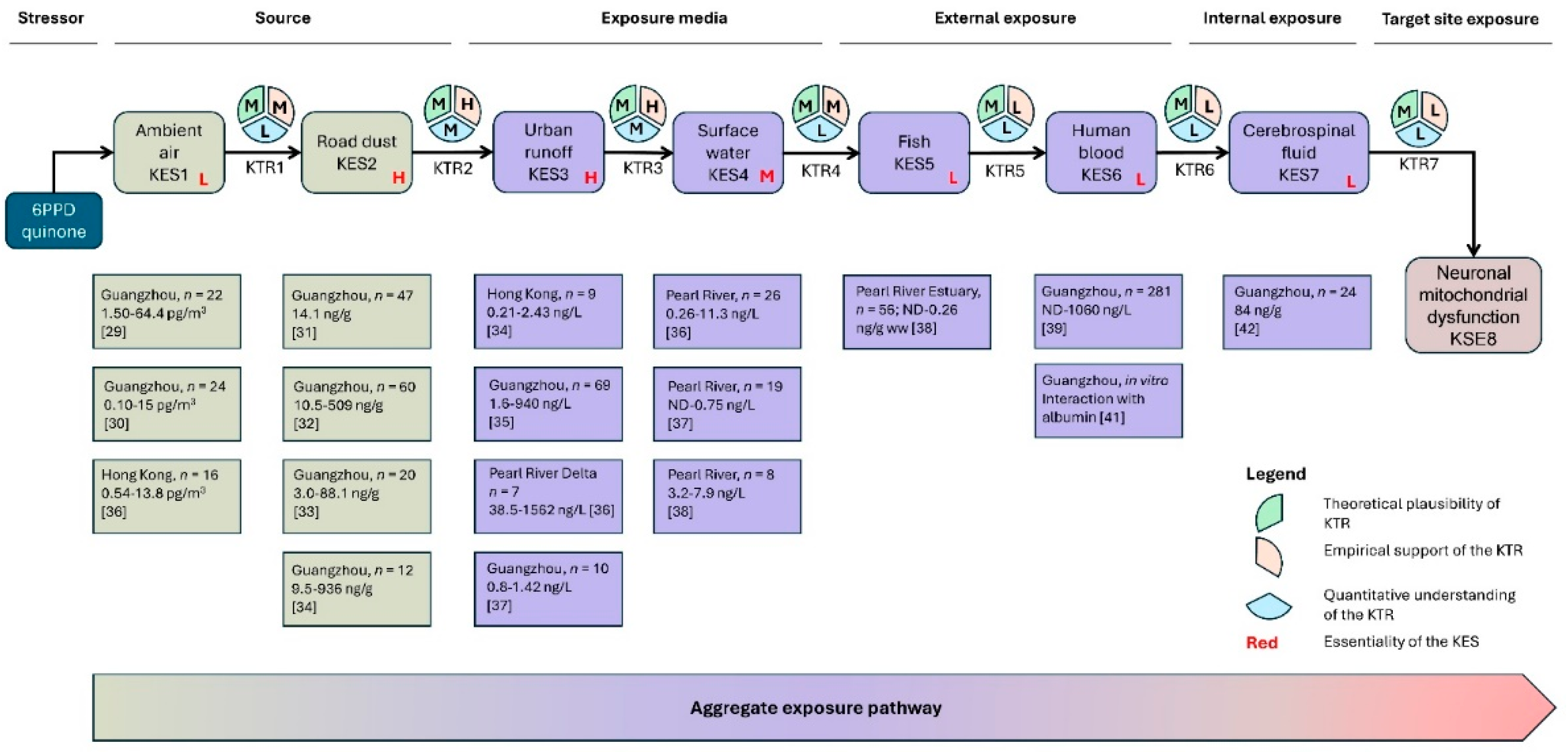
| Category | Criterion | Confidence level | ||
|---|---|---|---|---|
| High | Moderate | Low | ||
| Essentiality of the KES | KESdownstream will be reduced/will not take place if KESupstream is reduced/stopped. | Multiple lines of direct evidence, with no inconsistencies or contradictions. | Some direct evidence or multiple lines of indirect evidence, or limited number of inconsistencies or contradictions. | No direct evidence or considerable inconsistencies or contradictions. |
| Theoretical plausibility of the KTR | Theoretical knowledge supporting dependent and sequential change of two adjacent KESs. | Widely accepted and in-depth mechanistic understanding supporting the causal relationship between KESupstream and KESdownstream. | Partial mechanistic understanding with known knowledge gaps. | No or limited theoretical understanding. |
| Empirical support for the KTR | Empirical data supporting dependent and sequential change of two adjacent KESs, with temporal, spatial and incidence concordance. | Multiple lines of evidence with high temporal, spatial and incidence concordance, no or few data gaps or conflicting data. | Some direct evidence or multiple lines of indirect evidence, with some temporal, spatial and incidence concordance, and a limited number of inconsistencies or contradictions. | No or very limited evidence. |
| Quantitative understanding of the KTR | Quantitative model describing dependent and sequential change of two adjacent KESs. | Precise prediction of KESdownstream from KESupstream with a low uncertainty. Key modulating factors and feedback or feedforward are fully captured in the model. The model is generalized across the applicability domains of the AEP. | Precise prediction of KESdownstream from KESupstream with a high uncertainty. Key modulating factors and feedback or feedforward are not fully captured in the model. The model is only valid for a limited number of cases in the applicability domains of the AEP. | Only qualitative or semi-quantitative understanding. Known modulating factors and/or known feedback/feedforward mechanisms are not captured. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





