Submitted:
29 July 2024
Posted:
29 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
| No. | Normal/hyperhydricity in subcultured plantlets (%) | Nodal segment-induced shoots | LNC (%) | LN (%) | |||
|---|---|---|---|---|---|---|---|
| Primary/secondary (cm) ** | Hyperhydricity (%) *** | Surv | Rege | Surv | Rege | ||
| 1. LO(W0) * | Normal (68.7%) | Primary (4.5) | 41.7 | 76.7 | 66.7 | 74.2 | 64.2 |
| Secondary (2.0) | 91.7 | 60.0 | 50.0 | 38.3 | 31.7 | ||
| Hyperhydric (31.3%) | Primary (4.5) | 100 | 80.0 | 50.0 | 60.0 | 50.0 | |
| Secondary (1.5) | 100 | 60.0 | 30.0 | 30.0 | 20.0 | ||
| 4. LO(W2) | Normal (95.8%) | Primary (6.5) | 0 | 100.0 | 83.3 | 93.8 | 80.0 |
| Secondary (2.5) | 25 | 70.0 | 60.0 | 55.0 | 50.0 | ||
| Hyperhydric (4.2%) | Primary (6.5) | 100 | 90.0 | 60.0 | 75.0 | 60.0 | |
| Secondary (1.5) | 100 | 70.0 | 40.0 | 45.0 | 35.0 | ||
| 7. LX | Normal (100%) | Primary (4.0) | 0 | 60.0 | 55.0 | 62.1 | 56.8 |
| Secondary (2.0) | 0 | 50.0 | 30.0 | 40.0 | 25.0 | ||
| Hyperhydric (0%) | - | - | - | - | - | - | |
2. Results
2.1. In Vitro Propagation of Scrophularia kakudensis
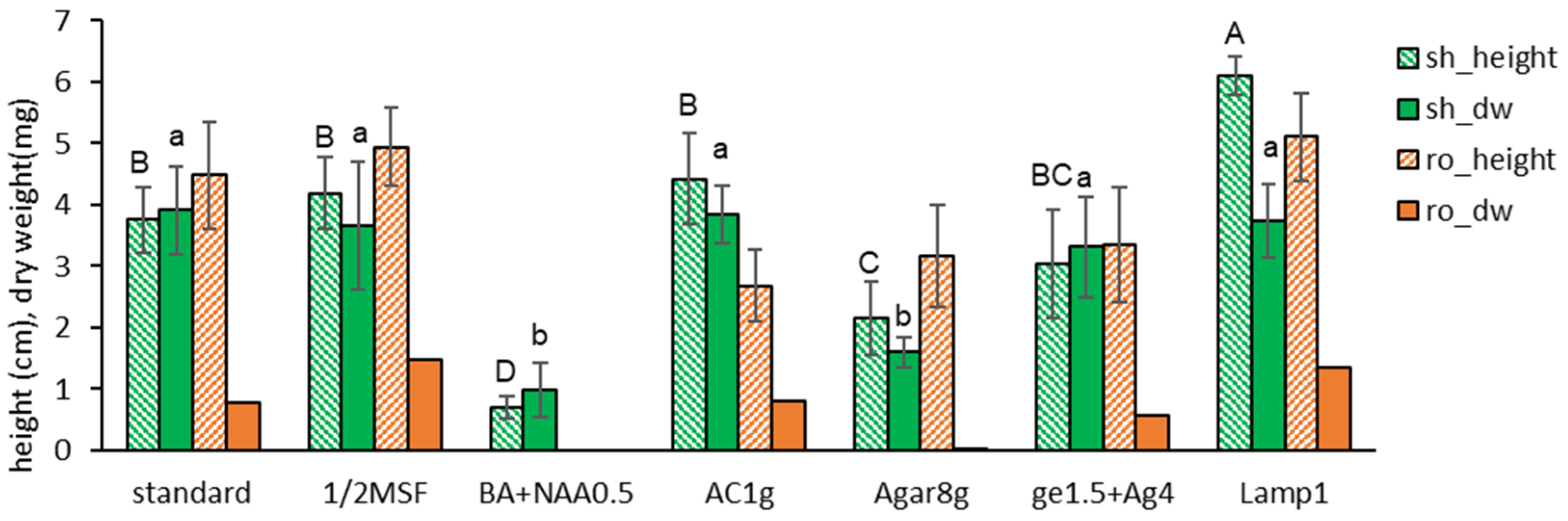
2.1.1. Effect of Subculture Medium and Conditions
| Set | No. | Medium strength | Growth regulators (mg L−1) | Gelling agents (g L−1) ** |
AC (g L−1) ** |
Liquid overlay*** | Light (Lamp) **** | Code |
|---|---|---|---|---|---|---|---|---|
| Subculture medium and conditions | 1 | MS | − | Gellan gum 3 | − | LX | 2 | MSF*, standard |
| 2 | 1/2MS | − | Gellan gum 3 | − | LX | 2 | 1/2MSF | |
| 3 | MS | BA0.5+NAA0.5 | Gellan gum 3 | − | LX | 2 | B0.5N0.5 | |
| 4 | MS | − | Gellan gum 3 | 1 | LX | 2 | AC1 | |
| 5 | MS | − | Agar 8 | − | LX | 2 | Agar8 | |
| 6 | MS | − | Gell 1.5+Agar 4 | − | LX | 2 | Gel1.5+Agar4 | |
| 7 | MS | − | Gellan gum 3 | − | LX | 1 | L1 | |
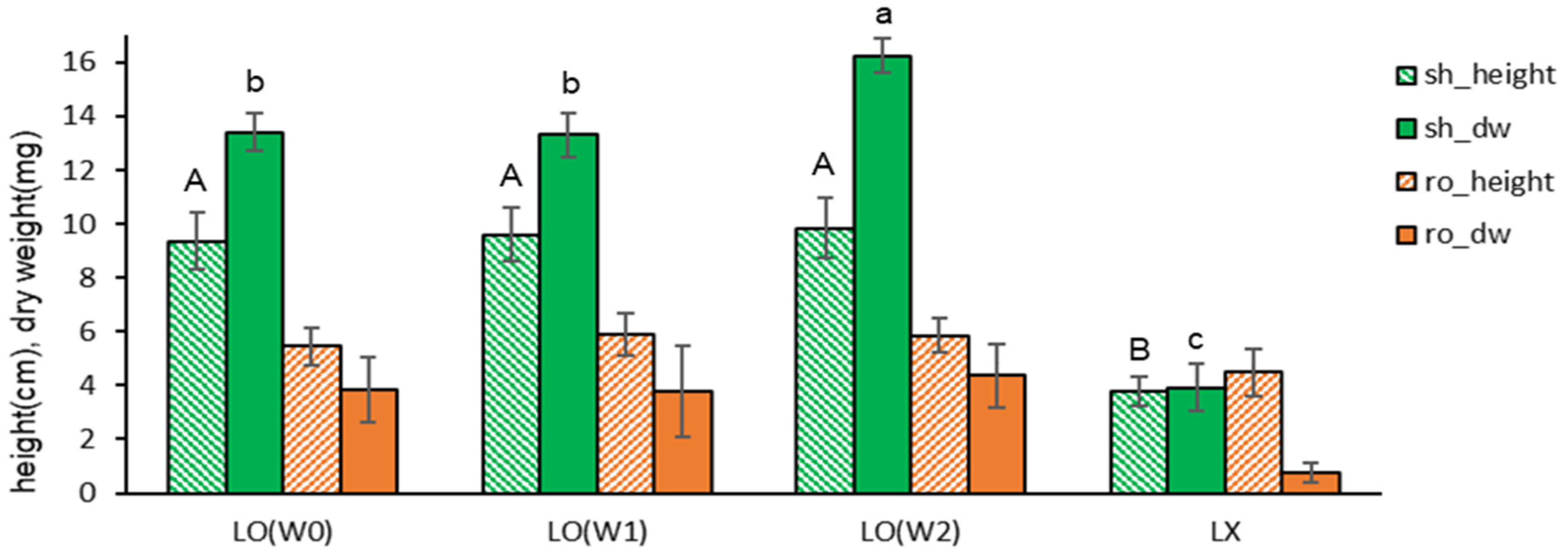
| Timing of liquid overlay | 1 | MS | − | Gellan gum 3 | − | LX | 2 | LX |
| 2 | MS | − | Gellan gum 3 | − | LO(W0) | 2 | LO(W0) | |
| 3 | MS | − | Gellan gum 3 | − | LO(W1) | 2 | LO(W1) | |
| 4 | MS | − | Gellan gum 3 | − | LO(W2) | 2 | LO(W2) |
2.1.2. Effect of Liquid Overlay on Top of the Gelled Medium
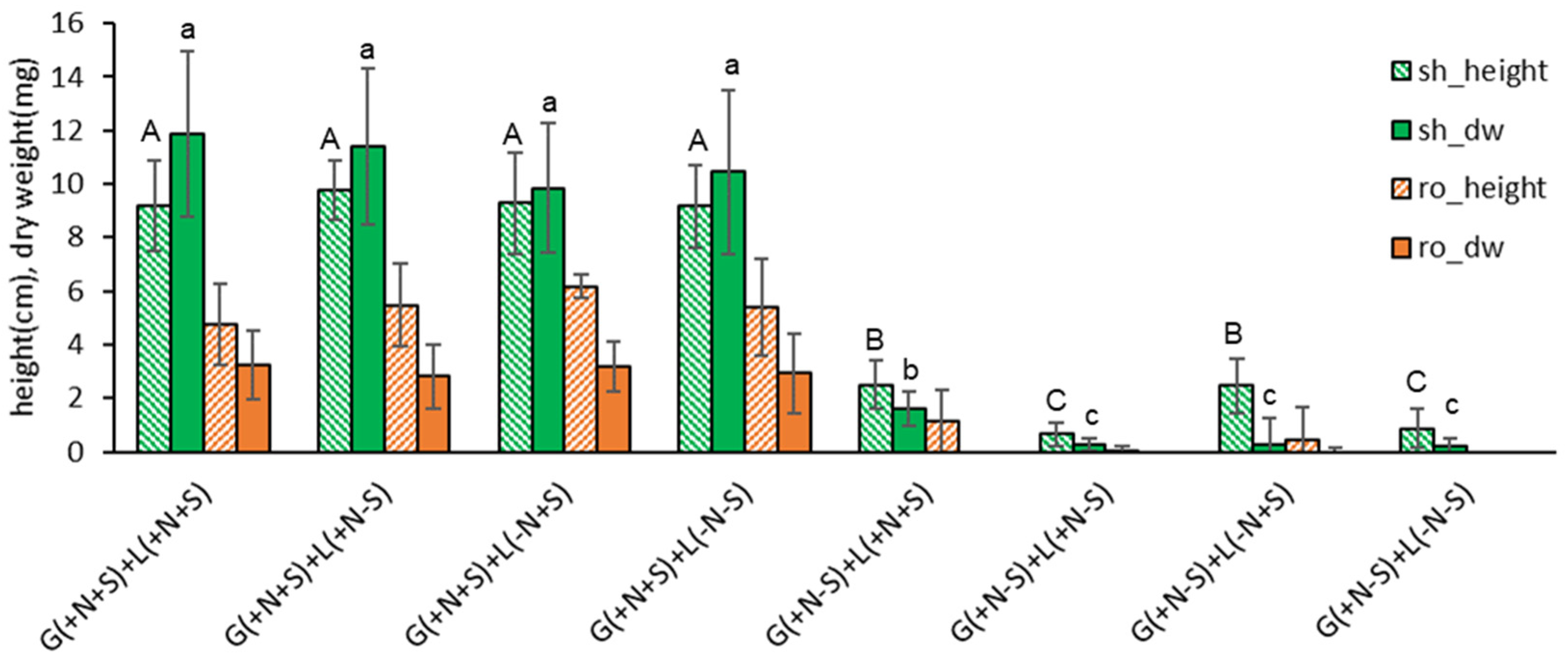
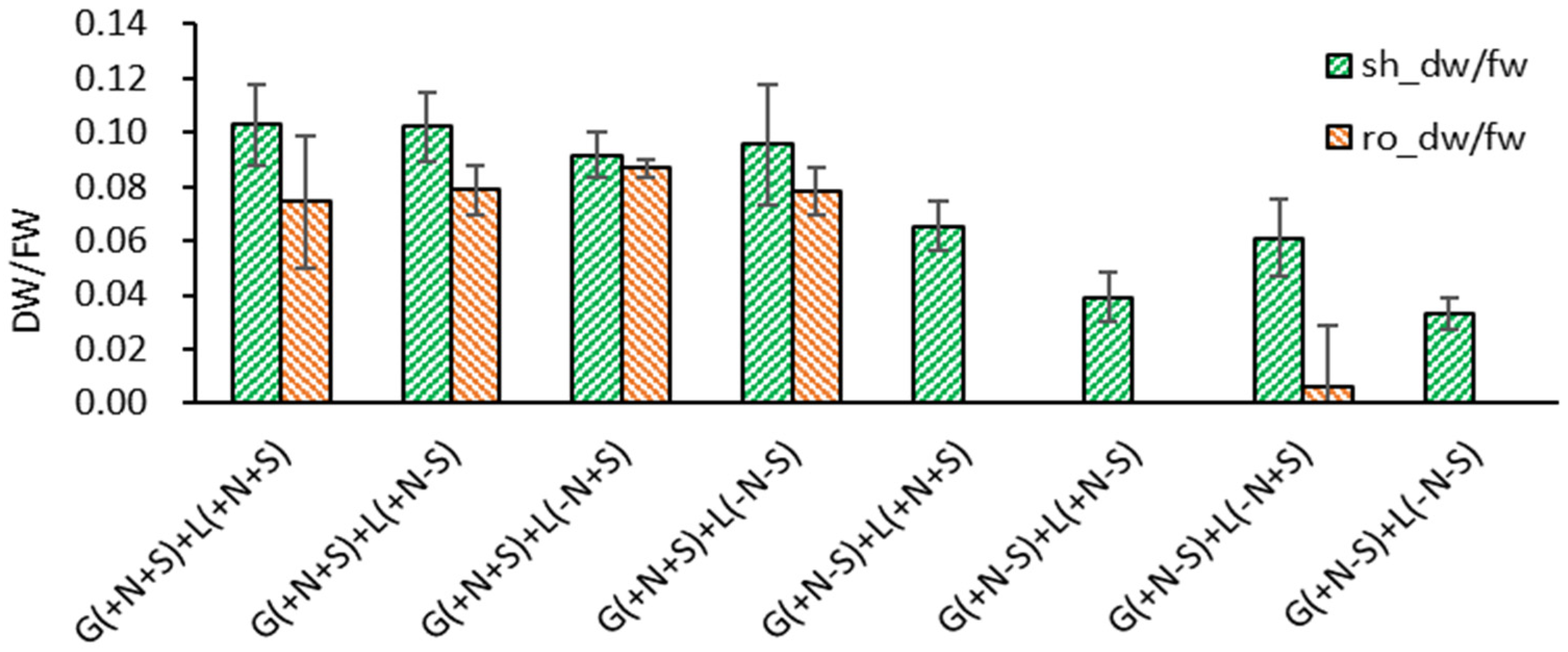
2.1.3. Effect of Liquid Overlay Composition (Nutrients and Sucrose)
| No. | Gellan gum-gelled medium | Liquid-overlay medium | Code |
|---|---|---|---|
| A1 | G(+N, +S) | +L(+N, +S) | G(+N+S)+L(+N+S) |
| A2 | G(+N, +S) | +L(+N, −S) | G(+N+S)+L(+N−S) |
| A3 | G(+N, +S) | +L(−N, +S) | G(+N+S)+L(−N+S) |
| A4 | G(+N, +S) | +L(−N, −S), ddw | G(+N+S)+L(−N−S) |
| B1 | G(+N, −S) | +L(+N, +S) | G(+N−S)+L(+N+S) |
| B2 | G(+N, −S) | +L(+N, −S) | G(+N−S)+L(+N−S) |
| B3 | G(+N, −S) | +L(−N, +S) | G(+N−S)+L(−N+S) |
| B4 | G(+N, −S) | +L(−N, −S) | G(+N−S)+L(−N−S) |
2.2. Cryopreservation of Scrophularia kakudensis Shoot Tips
2.2.1. Pre-LN Stages in Droplet-Vitrification Procedure
2.2.2. Three-Step Regrowth in Droplet-Vitrification Procedure
2.2.3. Effect of Liquid Overlay (Timing & Composition) in Droplet-Vitrification Procedure
3. Discussion
3.1. In Vitro Propagation System of Endangered Wild Species
3.2. Droplet-Vitrification Procedure—Protocol Development
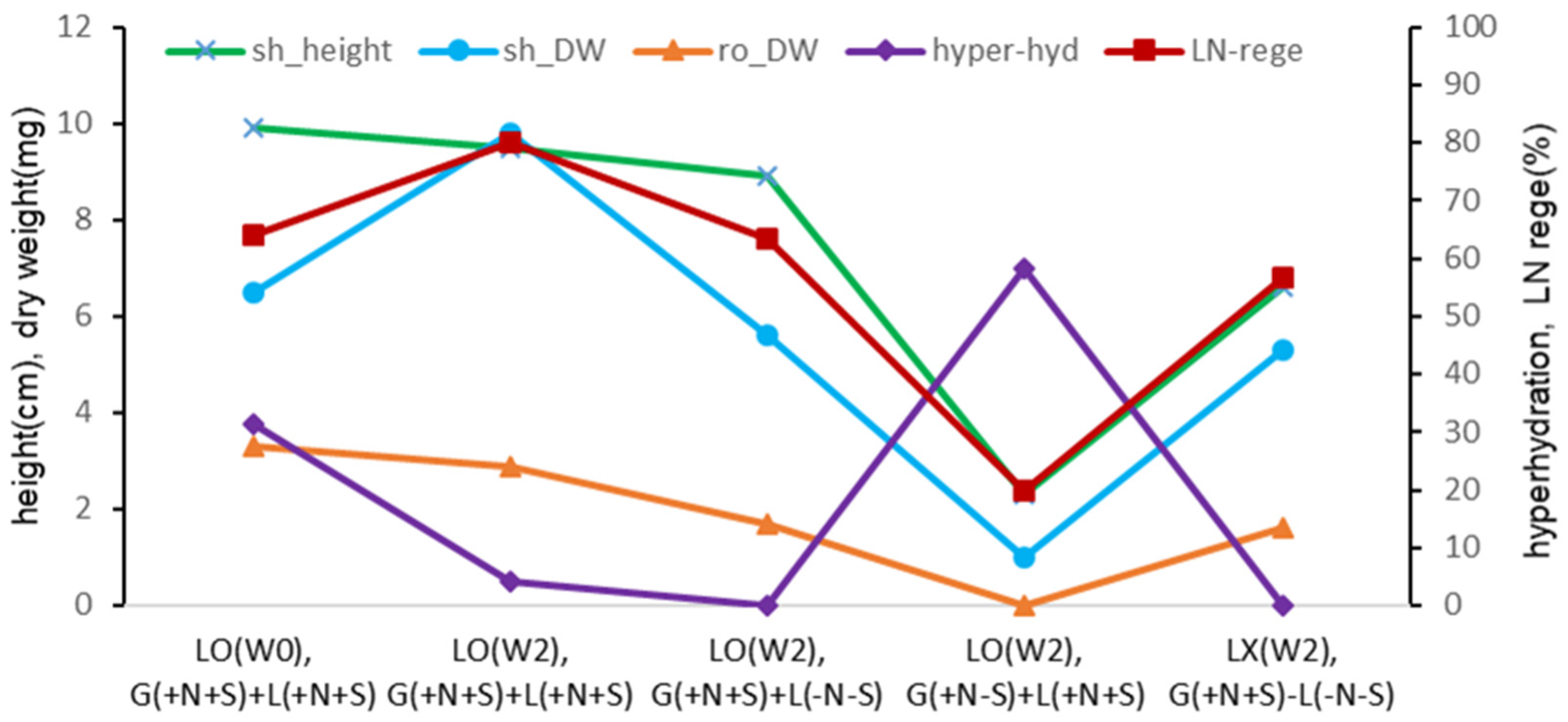
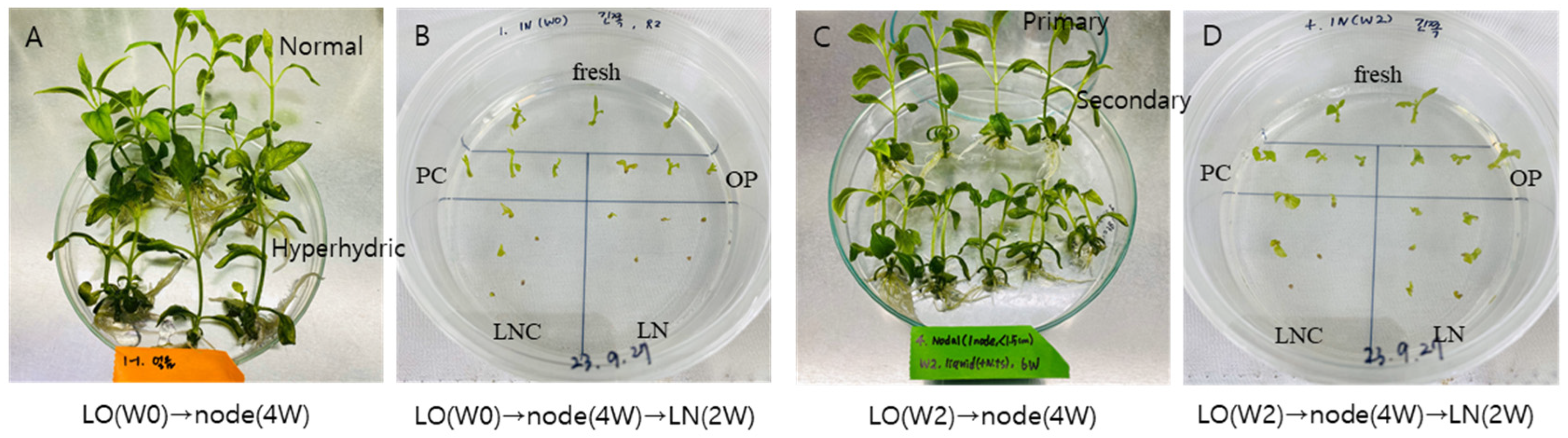
3.3. Droplet-Vitrification Procedure—Liquid Overlay-Induced Donor Plant Vigor
4. Materials and Methods
4.1. Plant Material, In Vitro Establish and Preparation of Donor Plants
| Procedure | Treatment Conditions | Code | ||
|---|---|---|---|---|
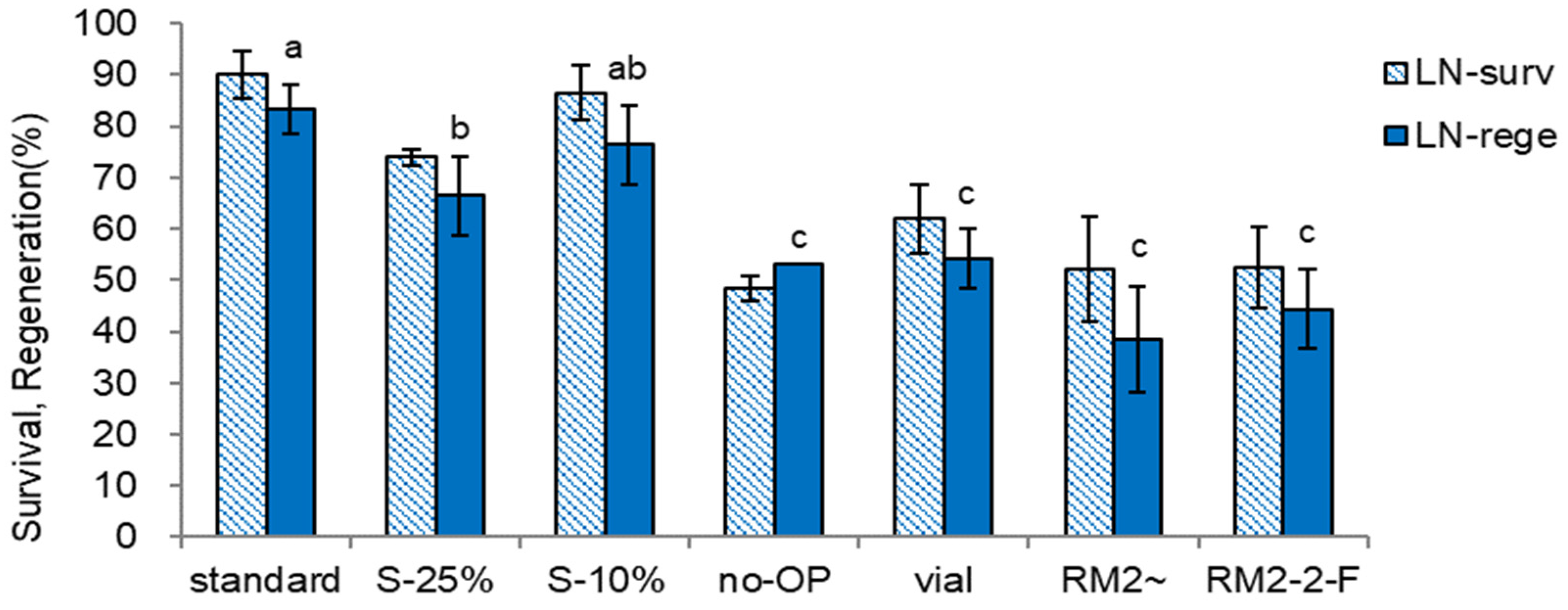
| Pre-LN, LN | Preculture, | 10% sucrose, 31 h → 17.5% sucrose, 16 h | S-17.5%, st *** | |
| 10% sucrose, 31 h → 25% sucrose, 16 h | S-25% | |||
| 10% sucrose, 48 h | S-10% | |||
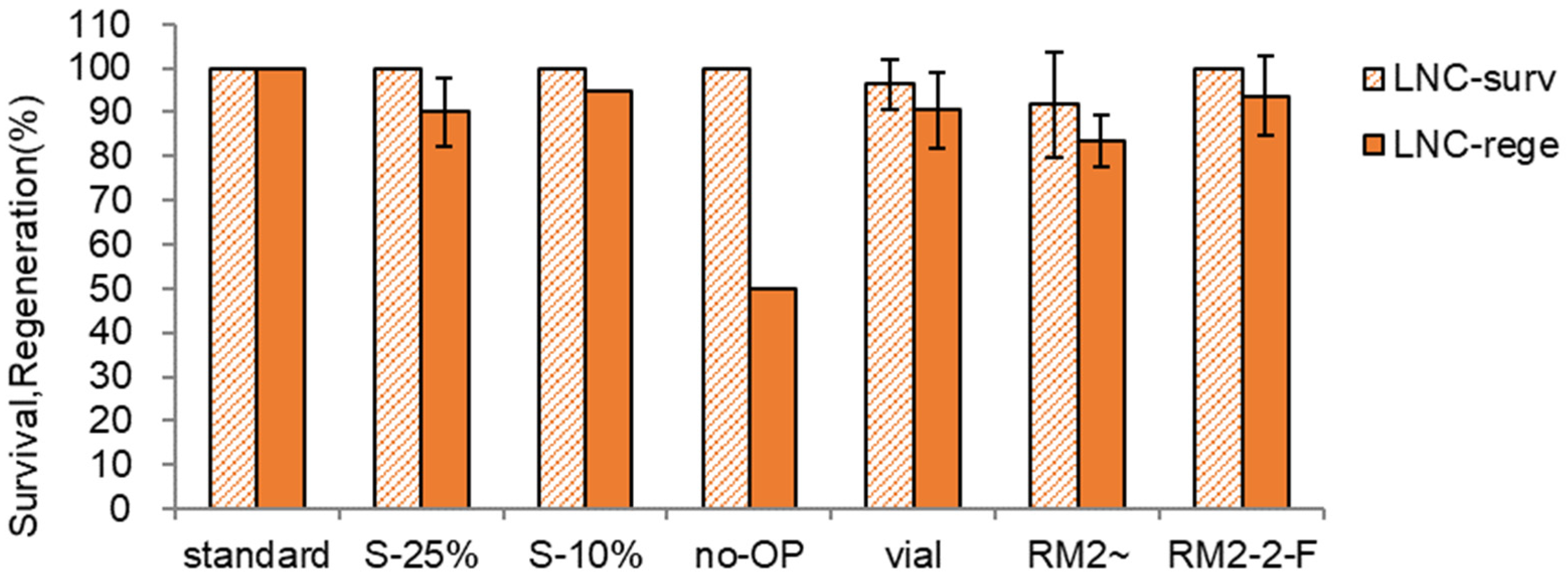
| Osmoprotection | C4-35%, 30 min | OP, st | ||
| No osmoprotectant | no-OP | |||
| Cooling/warming container | Aluminum foil-strips | Foil, st | ||
| Cryovial (2 mL) | Vial | |||
| Regrowth medium | RM1-RM2-MSF | RM1-2-F, st | ||
| RM2~ | RM2~ | |||
| RM2-RM2-MSF | RM2-2-F | |||
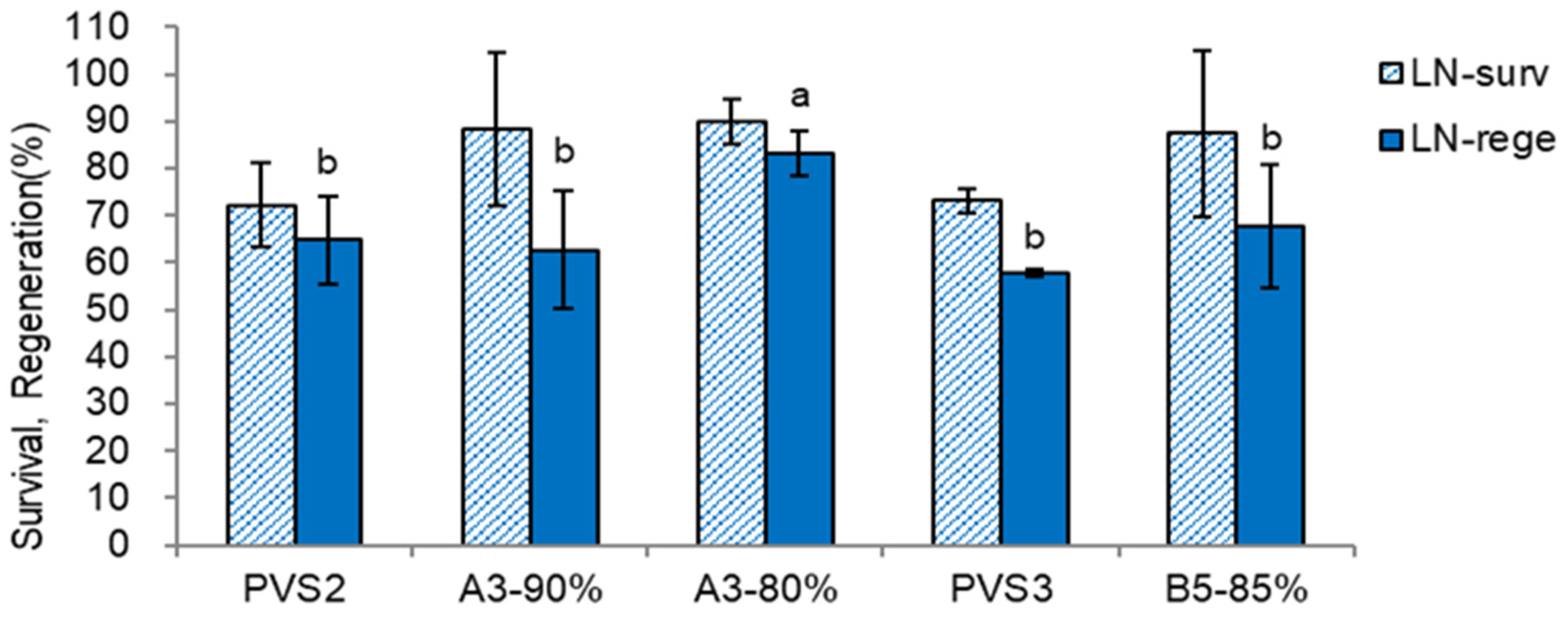
| Cryoprotection | A1-73.7% (PVS2) * ice, 60 min | PVS2 | ||
| A3-90% ice, 60 min | A3-90% | |||
| A3-80% ice, 60 min | A3-80%, st | |||
| B1-100 (PVS3) 25 °C, 60 min | PVS3 | |||
| B5-85% 25 °C, 60 min | B5-85% | |||
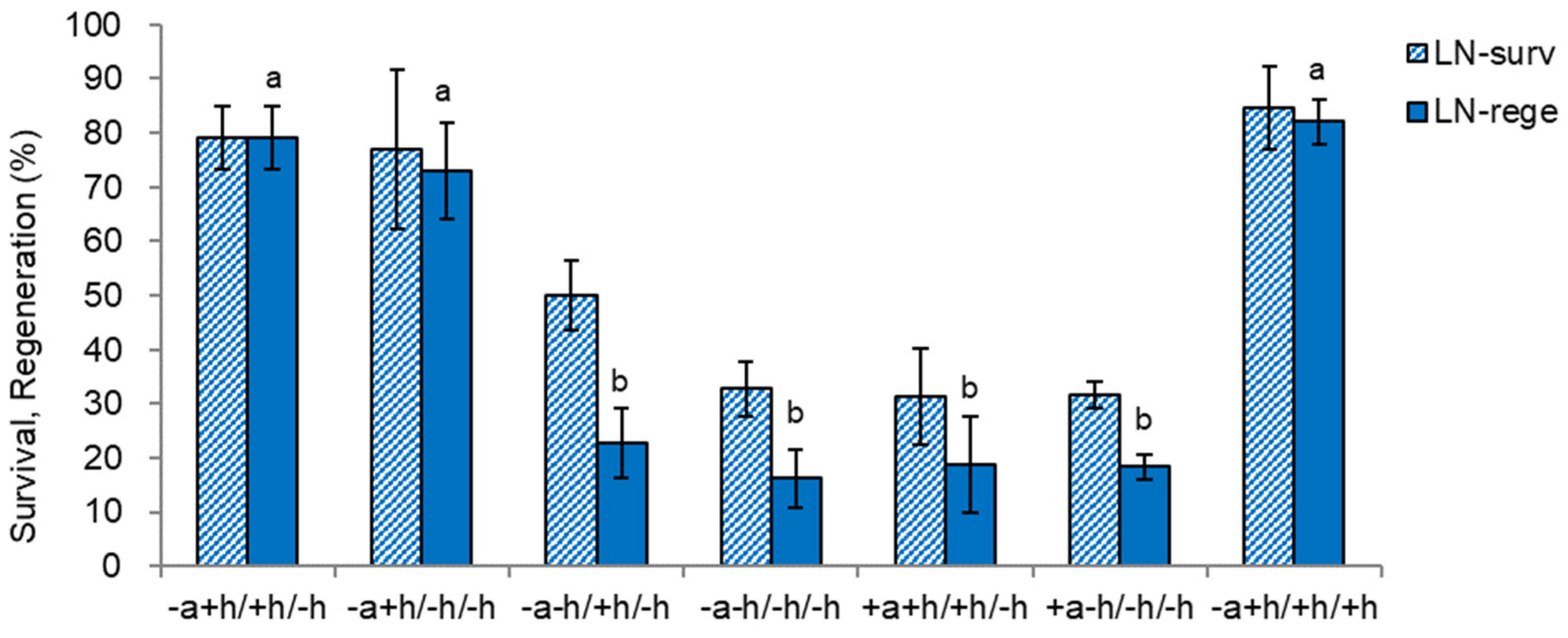
| Post-LN | Ammonium ion and growth hormones in regrowth medium | Regrowth Step 1 Step 2 Step 3 | ||
| 1. NH4NO3-free + GA1+BA1 → GA1+BA1 → MSF ** | −a+h/+h/−h, st | |||
| 2. NH4NO3-free + GA1+BA1 → MSF → MSF | −a+h/−h/−h | |||
| 3. NH4NO3-free + MSF → GA1+BA1 → MSF | −a−h/+h/−h | |||
| 4. NH4NO3-free + MSF → MSF → MSF | −a−h/−h/−h | |||
| 5. NH4NO3-containing + GA1+BA1 → GA1+BA1 → MSF | +a+h/+h/−h | |||
| 6. NH4NO3-containing + MSF → MSF → MSF | +a-h/−h/−h | |||
| 7. H4NO3-free + GA1+BA1 → GA1+BA1 → GA1+BA1 | −a+h/+h/+h | |||
| Donor plant vigor | Liquid overlay | 1. Liquid overlay at week 0, G(+N+S)+L(+N+S) | LO(W0), G(+N+S)+L(+N+S) | |
| 2. Liquid overlay at week 2, G(+N+S)+L(+N+S) | LO(W2), G(+N+S)+L(+N+S), st | |||
| 3. Liquid overlay at week 2, G(+N+S)+L(−N−S) (ddw) | LO(W2), G(+N+S)+L(−N−S) | |||
| 4. Liquid overlay at week 2, G(+N−S)+L(+N+S) | LO(W2), G(+N−S)+L(+N+S) | |||
| 5. no Liquid overlay, G(+N+S)−L(−N−S) | LX, G(+N+S)−L(−N−S) | |||
4.2. Effect of Subculture Conditions in In Vitro Propagation of Scrophularia kakudensis
4.3. Experimental Design of Treatments in the Droplet-Vitrification (DV) Procedure
4.3.1. Standard Droplet-Vitrification Procedure
4.3.2. Sets of Experimental Conditions—Pre-LN Stages, Three-Step Regrowth, and Liquid Overlay
4.4. Recovery Assessment and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, G.; So, S.; Lee, J.H.; Kim, M. Taxonomy of the genus Scrophularia (Scrophulariaceae) in Korea. Korean J. Plant Taxon. 2009, 39, 237–246. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, T.H.; Oh, B.U. A taxonomic review of Scrophularia kakudensis Franch. and its relatives. Korean J. Plant Taxon. 2011, 41, 345–352. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Jeong, B.R. In vitro propagation, phytochemical analysis, and evaluation of free radical scavenging property of Scrophularia kakudensis Franch tissue extracts. Biomed. Res. Int. 2015, 480564. [Google Scholar] [CrossRef]
- Bunn, E., Turner, SR. & Dixon, K.W. Biotechnology for saving rare and threatened flora in a biodiversity hotspot. In Vitro Cell. Dev.Biol.-Plant 2011, 47, 188–200. [CrossRef]
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Dixon, K.W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.C. Cryobiotechnologies: Tools for expanding long-term ex situ conservation to all plant species. Biol. Conserv. 2020, 250, 108736. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic plant species conservation: Biotechnological approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef]
- Breman, E.; Ballesteros, D.; Castillo-Lorenzo, E.; Cockel, C.; Dickie, J.; Faruk, A.; O’Donnell, K.; Offord, C.A.; Pironon, S.; Sharrock, S.; et al. Plant Diversity Conservation Challenges and Prospects—The Perspective of Botanic Gardens and the Millennium Seed Bank. Plants 2021, 10, 2371. [Google Scholar] [CrossRef]
- Reed, B.M. Implementing cryogenic storage of clonally propagated plants. CryoLetters 2001, 22, 97–104. [Google Scholar]
- Yoon, J.W.; Kim, H.H.; Ko, H.C.; Hwang, H.S.; Cho, E.G.; Sohn, J.K.; Engelmann, F. Cryopreservation of cultivated and wild potato varieties by droplet vitrification: Effect of subculture of mother-plants and of preculture of shoot tips. CryoLetters 2006, 27, 211–222. [Google Scholar]
- Lee, H.; Park, H.; Popova, E.; Lee, Y.Y.; Park, S.U.; Kim, H.H. Ammonium-free medium is critical for regeneration of shoot tips of the endangered species Pogostemon yatabeanus cryopreserved using droplet-vitrification. CryoLetters 2021, 45, 290–299. [Google Scholar]
- Association of Official Seed Analysts (AOSA). Seed Vigor Testing Handbook; Baalbaki, R.Z.B., Ed.; Ithaca, NY, USA; 2009; Volume 32. Available online: https://books.google.co.kr/books?id=UO1UswEACAAJ (accessed on 3 February 2023).
- Kim, H. Optimizing the Droplet-Vitrification Procedure by Balancing the Cryoprotection and Cytotoxicity of Alternative Plant Vitrification Solutions Based on the Nature of Donor Plant Vigor. Plants 2023, 12, 4040. [Google Scholar] [CrossRef]
- Lee, H.-E.; Popova, E.; Park, H.-N.; Park, S.-U.; Kim, H.-H. Optimization of a cryopreservation method for the endangered Korean species Pogostemon yatabeanus using a systematic approach: The key role of ammonium and growth regulators. Plants 2021, 10, 2018. [Google Scholar] [CrossRef]
- Hussien, F.A.; Osman, M.A.; Idris, T.I.M. The influence of liquid media support, gelling agents and liquid overlays on performance of in vitro cultures of ginger (Zingiber officinale). Intl. J. Sci. Res. Pub. 2014, 4, 2250–3153. [Google Scholar]
- Choi, C.H.; Popova, E.; Lee, H.; Park, S.U.; Ku, J.; Kang, J.H.; Kim, H.H. Cryopreservation of endangered wild species, Aster altaicus var. uchiyamae Kitam, using droplet-vitrification procedure. CryoLetters 2019, 40, 113–122. [Google Scholar]
- Lee, H.; Kim, H. Vigorous growing of donor plantlets by liquid overlay in subcultures is the key to cryopreservation of endangered species Pogostemon yatabeanus. Plants (Basel) 2022, 11, 3127. [Google Scholar] [CrossRef]
- Zilani, R.A.K.M.; Lee, H.; Popova, E.; Kim, H. In vitro multiplication and cryopreservation of Penthorum chinense shoot tips. Life 2022, 12, 1759. [Google Scholar] [CrossRef]
- Kang, K.S.; Veeder, G.T.; Mirrasoul, P.J.; Kaneko, T.; Cottrell, I.W. Agar-like polysaccharide produced by a Pseudomonas species: Production and basic properties. Appl. Environ. Microbiol. 1982, 43, 1086–1091. [Google Scholar] [CrossRef]
- Franck, T.; Kevers, C.; Gaspar, T.; Dommes, J.; Deby, C.; Greimers, R.; Serteyn, D.; Deby-Dupont, G. Hyperhydricity of Prunus avium shoots cultured on gelrite: A controlled stress response. Plant Physiol. Biochem. 2004, 42, 519–527. [Google Scholar] [CrossRef]
- Fira, A.; Clapa, D. The influence of the gelling agent upon multiplication rate in Sequoia sempervirens. Bull. UASVM 2008, 65, 463. [Google Scholar]
- Benjelloun, J.; Taoufyq, A.; El Abidine Triqui, Z.; Alami, Q.L.; Layachi, R.; Smouni, A.; Bouzroud, S.; Guedira, A. Improvement of in vitro germination of Cycas revoluta zygotic embryos using gelrite as gelling agent. Adv. Hort. Sci. 2020, 34, 349–354. [Google Scholar] [CrossRef]
- Klimaszewska, K., bernier-Cardou, M., Cyr, D.R. et al. Influence of gelling agents on culture medium gel strength, water availability, tissue water potential, and maturation response in embryogenic cultures of Pinus strobus L.. In Vitro Cell.Dev.Biol.-Plant 36, 279–286 (2000). [CrossRef]
- Sah, S.K.; Kaur, A.; Jagdeep, S.S. High frequency embryogenic callus induction and whole plant regeneration in Japonica rice Cv. kitaake. J. Rice Res. 2014, 2, 125. [Google Scholar] [CrossRef]
- Toaima, N.; Bosila, H.; El-Ateeq, A.E. In vitro growth regulators, gelling agents and sucrose levels affect micropropagation of Gypsophila paniculate L. Mid. E. J. Agri. 2016, 5, 313. [Google Scholar]
- Polivanova, O.B.; Bedarev, V.A. Hyperhydricity in Plant Tissue Culture. Plants 2022, 11, 3313. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Martinez, L.I.; Visser, R.G.F.; de Klerk, G.J.M. The hyperhydricity syndrome: waterlogging of plant tissues as a major cause. Propagation of ornamental plants 2010, 10, 169–175. [Google Scholar]
- Kemat, N.; Visser, R.G.F.; Krens, F.A. Hypolignification: A Decisive Factor in the Development of Hyperhydricity. Plants 2021, 10, 2625. [Google Scholar] [CrossRef]
- Pullman, G.S.; Skryabina, A. Liquid medium and liquid overlays improve embryogenic tissue initiation in conifers. Plant Cell Rep. 2007, 26, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.Y.; Lee, S.Y.; Lee, G.A.; Jeong, J.W.; Cho, J.H.; Kim, H.H. Improvement of the droplet-vitrification method for the cryopreservation of cultivated potato shoot tips. Kor. J. Breed. Sci. 2012, 44, 94–99. [Google Scholar]
- Vujović, T.; Ružić, Đ.; Cerović, R. Effect of the duration of liquid nitrogen storage on the regrowth of blackberry cryopreserved by droplet vitrification. Contemp. Agric. 2017, 66, 44–50. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.; Park, S.-U.; Kim, H. Alternative plant vitrification solution A3-80% and initial ammonium-free regrowth medium enable cryobanking of chrysanthemum germplasm. Plants 2023, 12, 1059. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Lee, Y.G.; Shin, D.J.; Ko, H.C.; Gwag, J.G.; Cho, E.G.; Engelmann, F. Development of alternative plant vitrification solutions in droplet-vitrification procedures. CryoLetters 2009, 30, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Montero, M.E.; Harding, K. Cryobionomics: Evaluating the Concept in Plant Cryopreservation. In PlantOmics: The Omics of Plant Science; Barh, D., Khan, M., Davies, E., Eds.; Springer: New Delhi, India, 2015. [Google Scholar] [CrossRef]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification. CryoLetters 2007, 28, 151–172. [Google Scholar] [PubMed]
- Kim, H.H.; Yoon, J.W.; Park, Y.E.; Cho, E.G.; Sohn, J.K.; Kim, T.S.; Engelmann, F. Cryopreservation of potato cultivated and wild species: Critical factors in droplet vitrification. CryoLetters 2006, 27, 223–234. [Google Scholar] [PubMed]
- Touchell, D.; Turner, S.R.; Senaratna, T.; Bunn, E.; Dixon, K.W. Cryopreservation of Australian species — the role of plant growth regulators. In Cryopreservation of Plant Germplasm II. Biotechnology in Agriculture and Forestry, vol. 50; Towill, L.E., Bajaj, Y.P.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 373–390. [Google Scholar] [CrossRef]
- Mukherjee, P.; Mandal, B.B.; Bhat, K.V.; Biswas, A.K. Cryopreservation of Asian Dioscorea bulbifera L. and D. alata L. by vitrification: importance of plant growth regulators. CryoLetters 2009, 30, 100–111. [Google Scholar]
- Turner, S.R.; Touchell, D.H.; Senaratna, T.; Bunn, E.; Tan, E.; Dixon, K.W. Effects of plant growth regulators on survival and recovery growth following cryopreservation. CryoLetters 2001, 22, 163–174. [Google Scholar] [PubMed]
- Popova, E.; Kulichenko, I.; Kim, H.-H. Critical role of regrowth conditions in post-cryopreservation of in vitro plant germplasm. Biology 2023, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Whelehan, L.M.; Dalziell, E.L.; Bunn, E.; Mancera, R.L.; Funnekotter, B. How does metabolic rate in plant shoot tips change after cryopreservation? Cryobiology 2022, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, B.; Oh, S.; Park, H.; Popova, E.; Paik, M.-J.; Kim, H. Dynamics of Organic Acids during the Droplet-Vitrification Cryopreservation Procedure Can Be a Signature of Oxidative Stress in Pogostemon yatabeanus. Plants 2023, 12, 3489. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.K.; Fleming, S.D.; Aiken, R.J.; De Iuliis, G.N.; Zieschang, J.A.; Clark, A.M. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum. Reprod. 2009, 24, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Funnekotter, B.; Colville, L.; Kaczmarczyk, A.; Turner, S.R.; Bunn, E.; Mancera, R.L. Monitoring of oxidative status in three native Australian species during cold acclimation and cryopreservation. Plant Cell Rep. 2017, 36, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Pennycooke, J.C.; Towill, L.E. Medium alterations improve regrowth of sweet potato (Ipomea batatas L. Lam.) shoot cryopreserved by vitrification and encapsulation-dehydration. CryoLetters 2001, 22, 381–389. [Google Scholar] [PubMed]
- Decruse, S.W.; Seeni, S. Ammonium nitrate in the culture medium influences regeneration potential of cryopreserved shoot tips of Holostemma annulare. CryoLetters 2002, 23, 55–60. [Google Scholar] [PubMed]
- Park, S.U.; Kong, H.J.; Shin, D.J.; Bae, C.H.; Lee, S.C.; Bae, C.H.; Rha, E.S.; Kim, H.H. Development of vitrification protocol in Rubia akane (Nakai) hairy roots using a systematic approach. CryoLetters 2014, 35, 377–384. [Google Scholar]
- Jitsopakul, N.; Thammasiri, K.; Ishikawa, K. Cryopreservation of Bletilla striata mature seeds, 3-day germinating seeds and protocorms by droplet-vitrification. CryoLetters 2008, 29, 517–526. [Google Scholar] [PubMed]
- Yi, J.Y.; Balaraju, K.; Baek, H.J.; Yoon, M.S.; Kim, H.H.; Lee, Y.Y. A successful regeneration from shoot tips of Chrysanthemum morifolium (Ramat.) following cryopreservation by droplet-vitrification. Kor. J. Plant Res. 2018, 31, 675–683. [Google Scholar] [CrossRef]
- Yi, J.Y.; Balaraju, K.; Baek, H.J.; Yoon, M.S.; Kim, H.H.; Lee, Y.Y. Cryopreservation of Citrus limon (L.) Burm. F shoot tips using a droplet-vitrification method. Kor. J. Plant Res. 2018, 31, 684–694. [Google Scholar]
- Engelmann, F. Cryopreservation of clonal crops: A review of key parameters. Acta Hortic. 2014, 1039, 31–39. [Google Scholar] [CrossRef]
- Wu, Y.; Engelmann, F.; Zhao, Y.; Zhou, M.; Chen, S. Cryopreservation of apple shoot tips: Importance of cryopreservation technique and of conditioning of donor plants. CryoLetters 1999, 20, 121–130. [Google Scholar]
- Pathirana, R.; Mathew, L.; McLachlan, A. A simplified method for high recovery of kiwifruit (Actinidia spp.) shoot tips after droplet vitrification cryopreservation suitable for long-term conservation. Plant Cell Tiss. Organ Cult. 2021, 144, 97–102. [Google Scholar] [CrossRef]
- Guerra, P.A.; Souza, E.H.; Max, D.A.S.; Rossi, M.L.; Villalobos-Olivera, A.; Ledo, C.A.S.; Martinez-Montero, M.E.; Souza, F.V.D. Morphoanatomical aspects of the starting material for the improvement of pineapple cryopreservation by the droplet-vitrification technique. An. Acad. Bras. Cienc. 2021, 93, e20190555. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco cell cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





