Submitted:
29 July 2024
Posted:
29 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Manufacturing and Experimental Methods
3. Results and Discussion
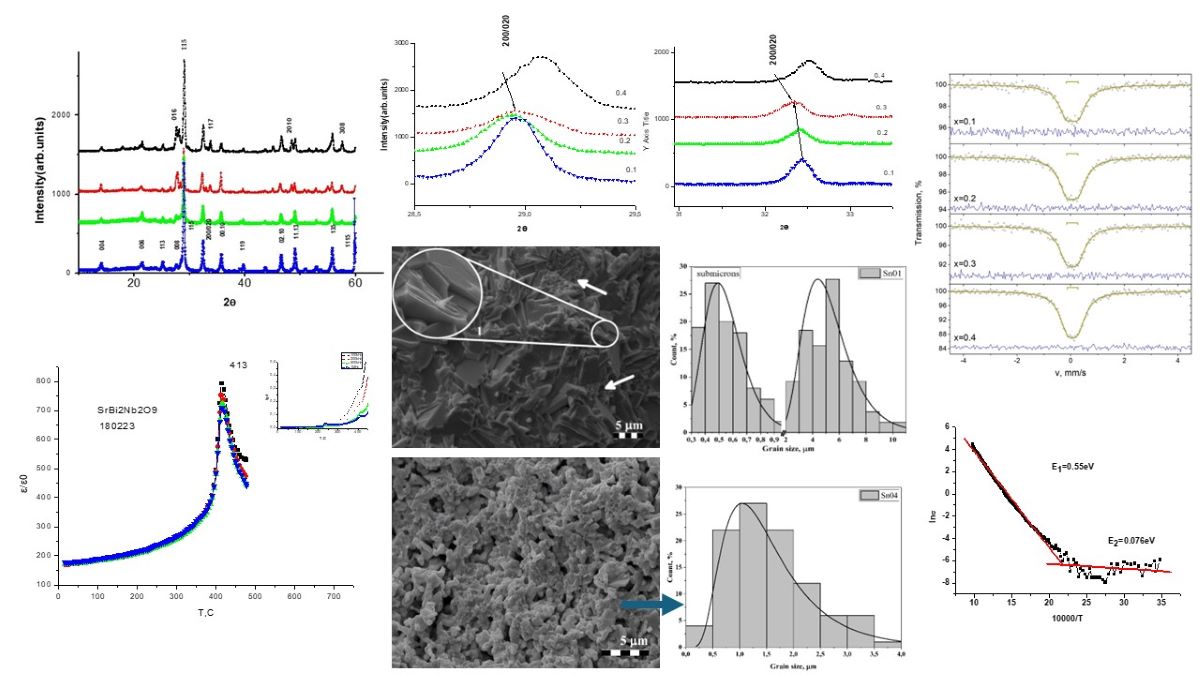
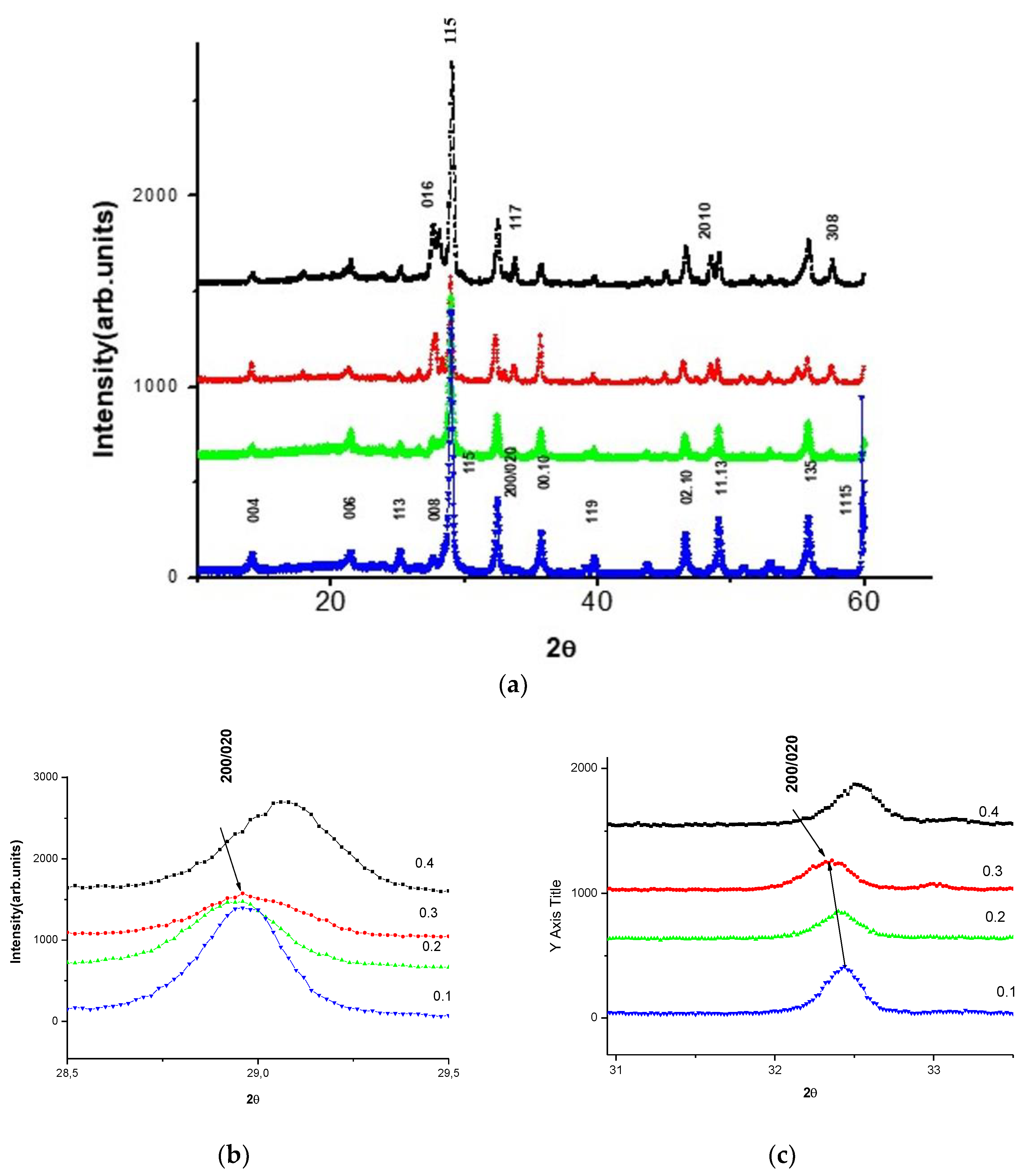
3.1. Diffraction
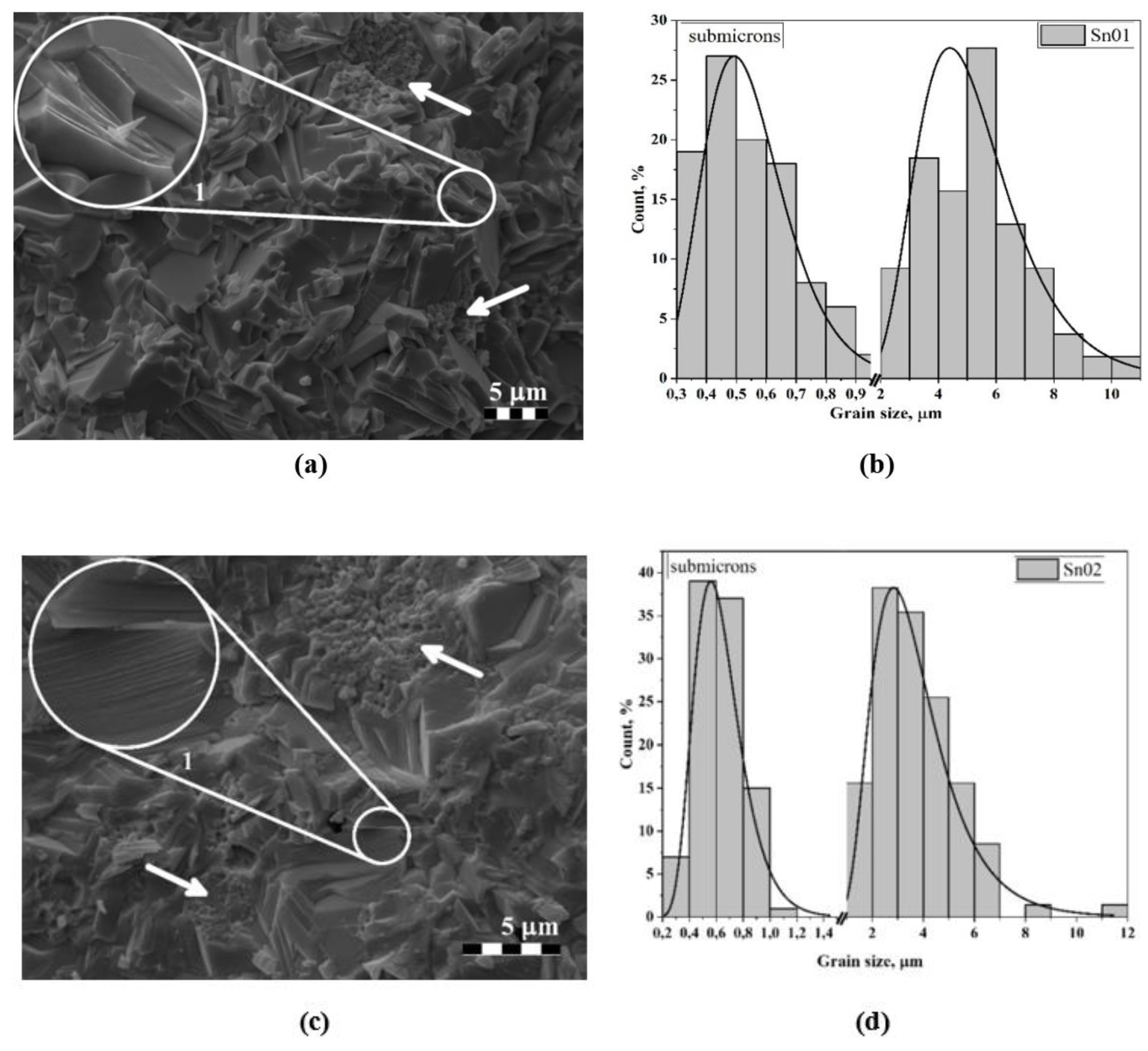
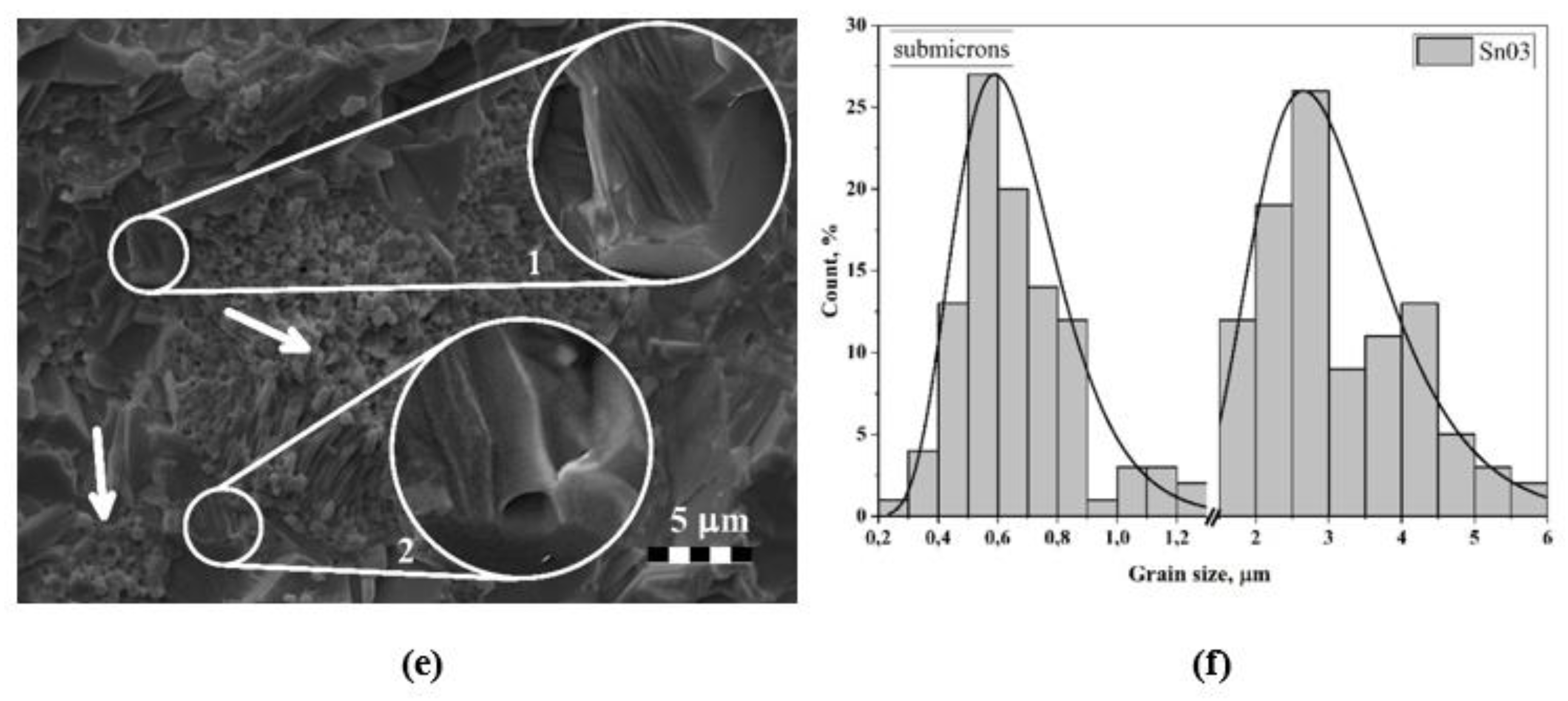
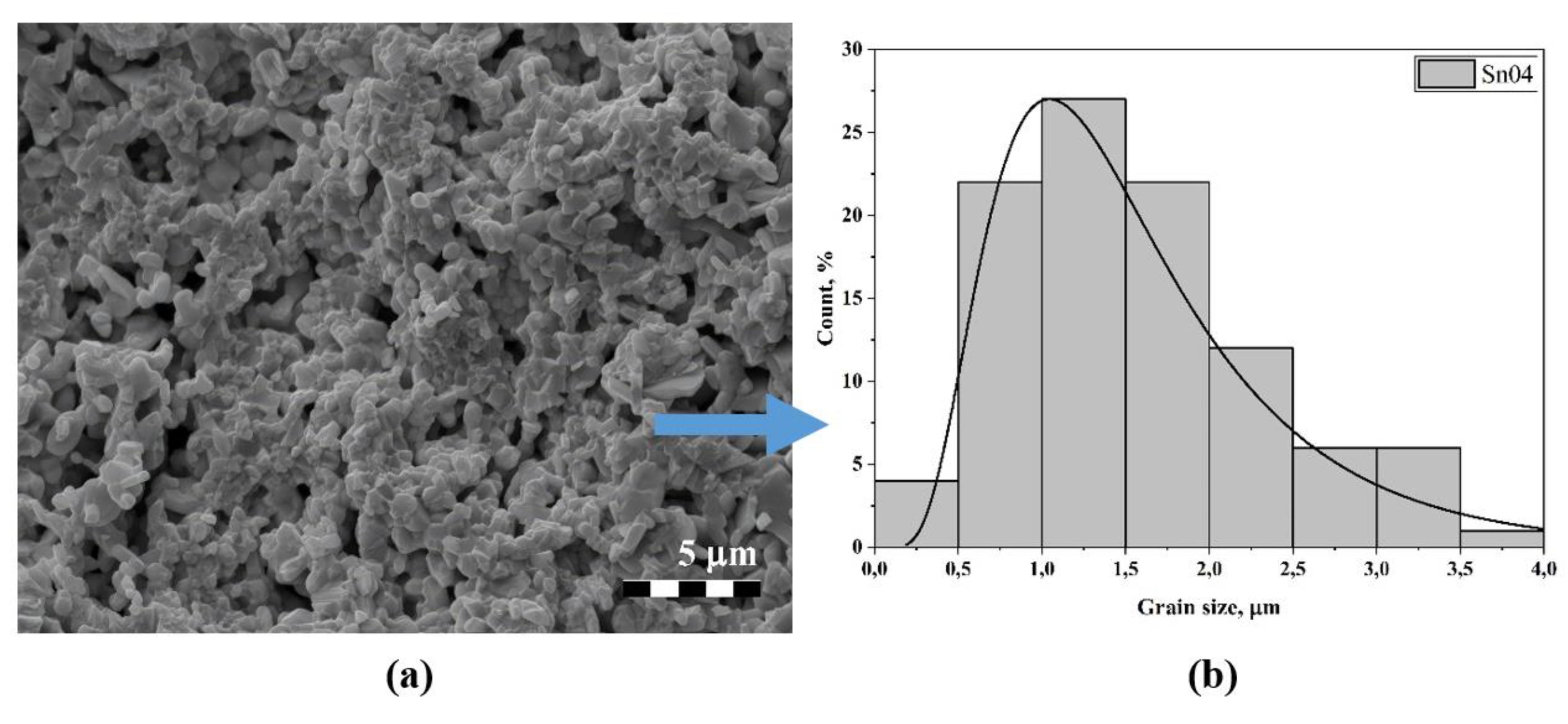
3.2. Microstructure
3.3. Crystalline Structure
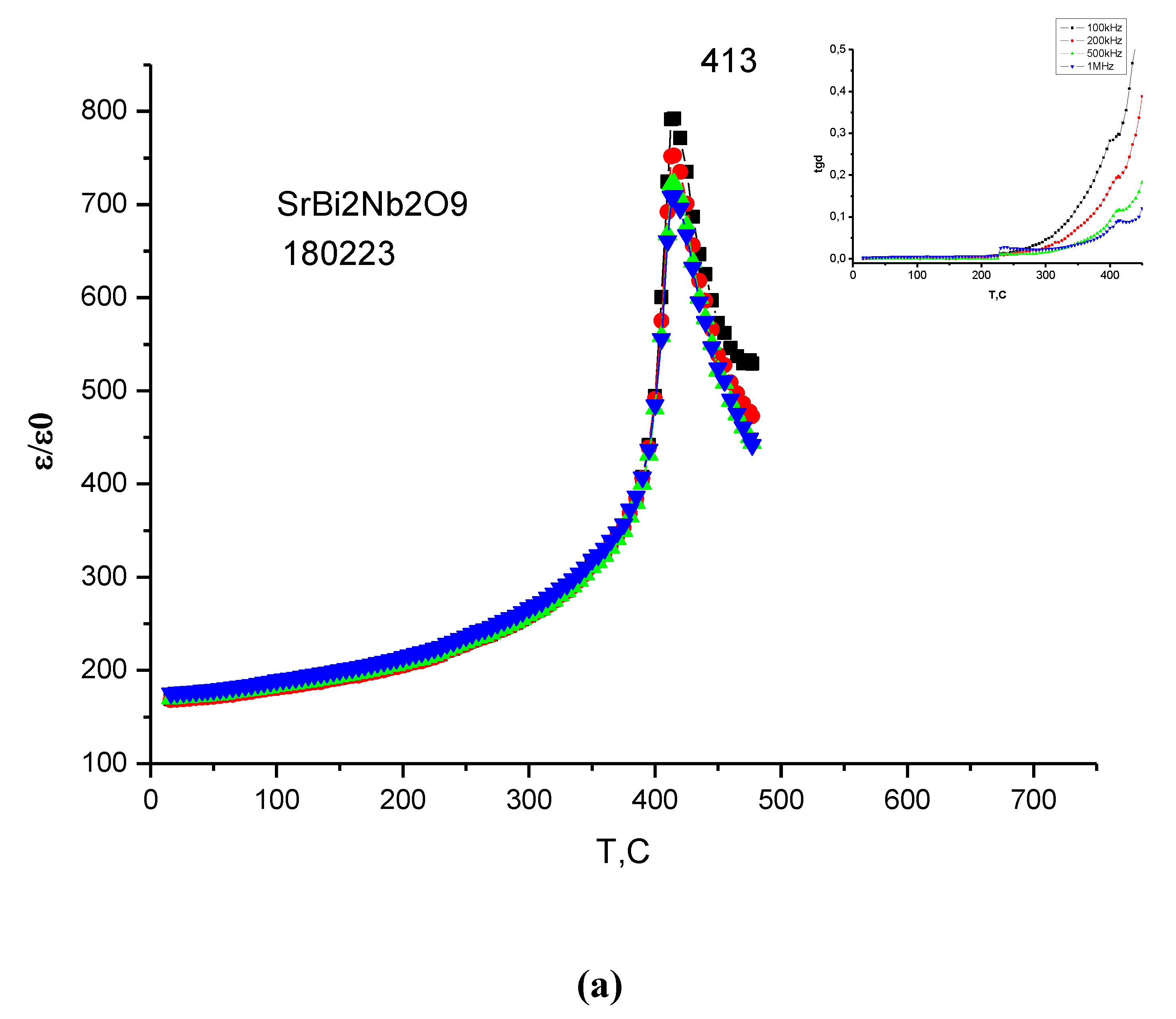
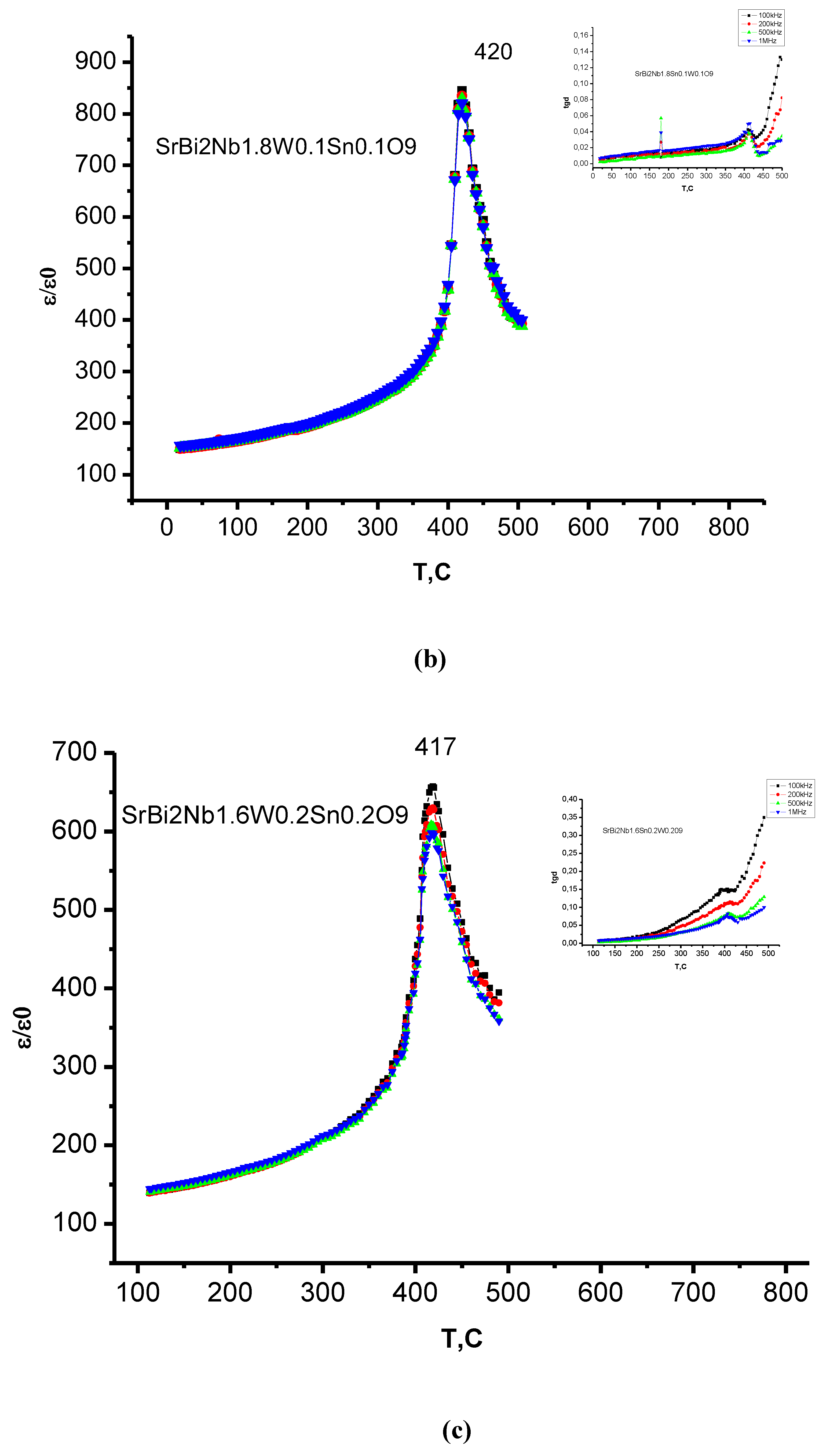
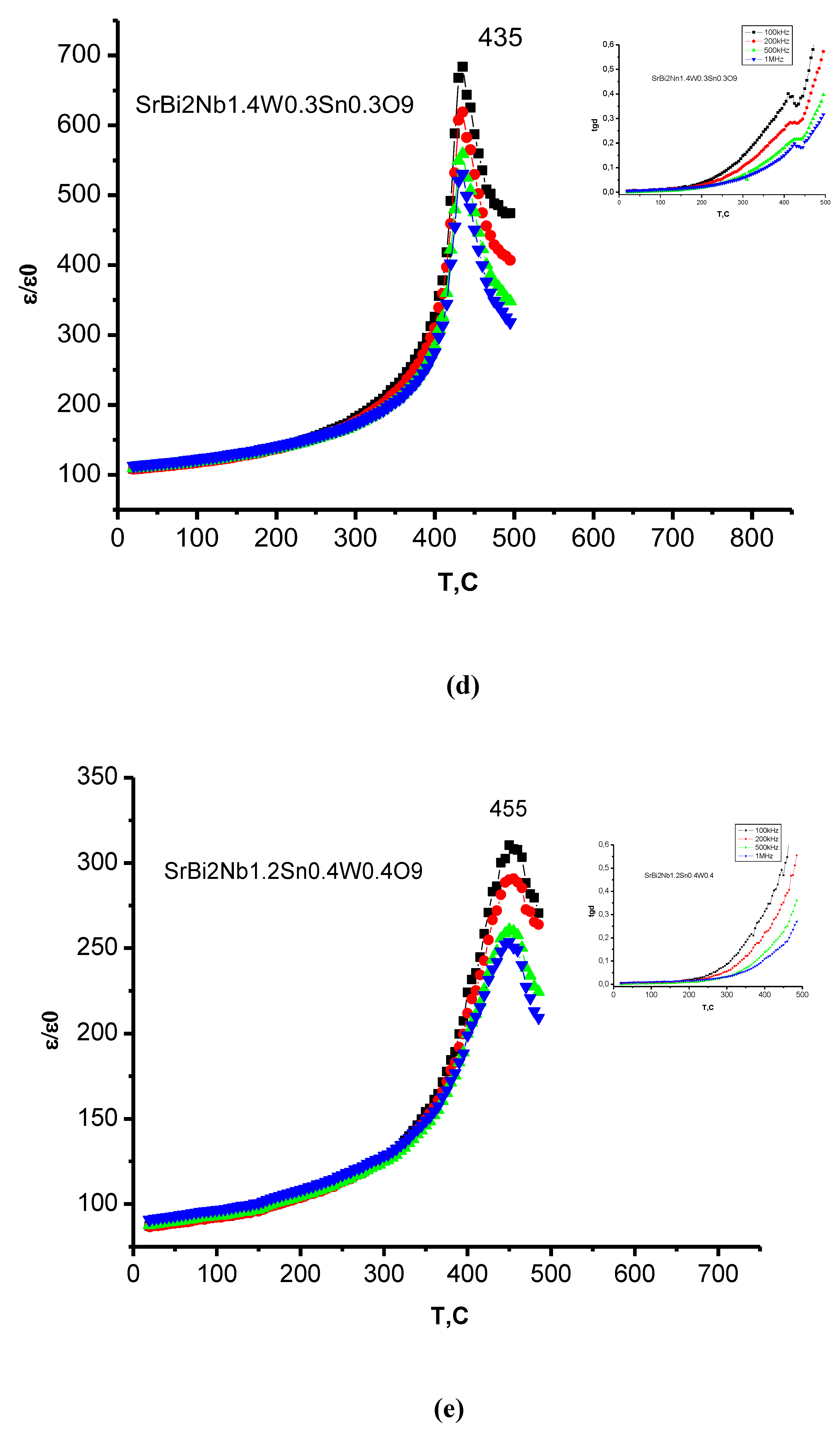
3.4. Dielectric Properties
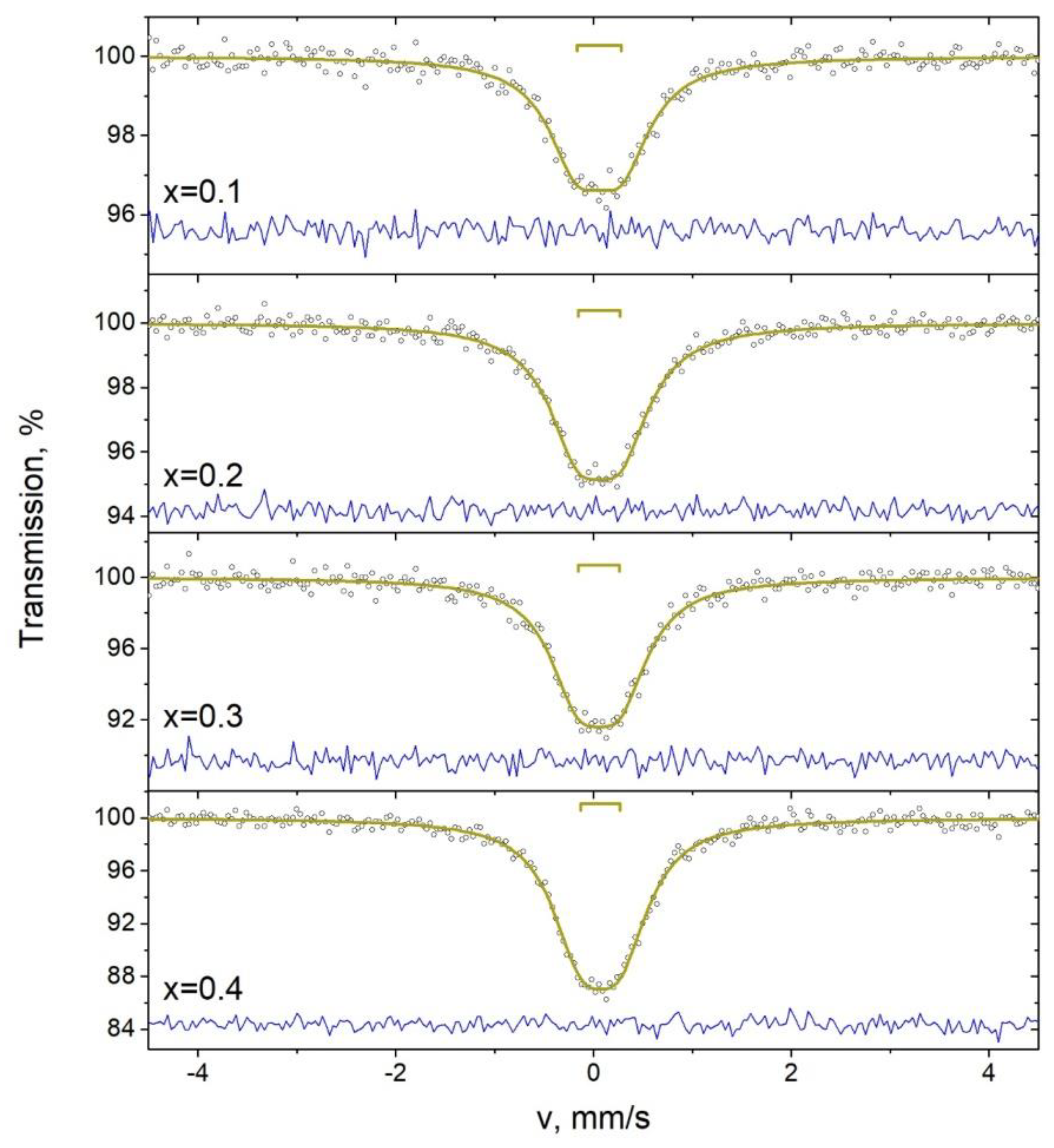
3.5. Mössbauer Studies
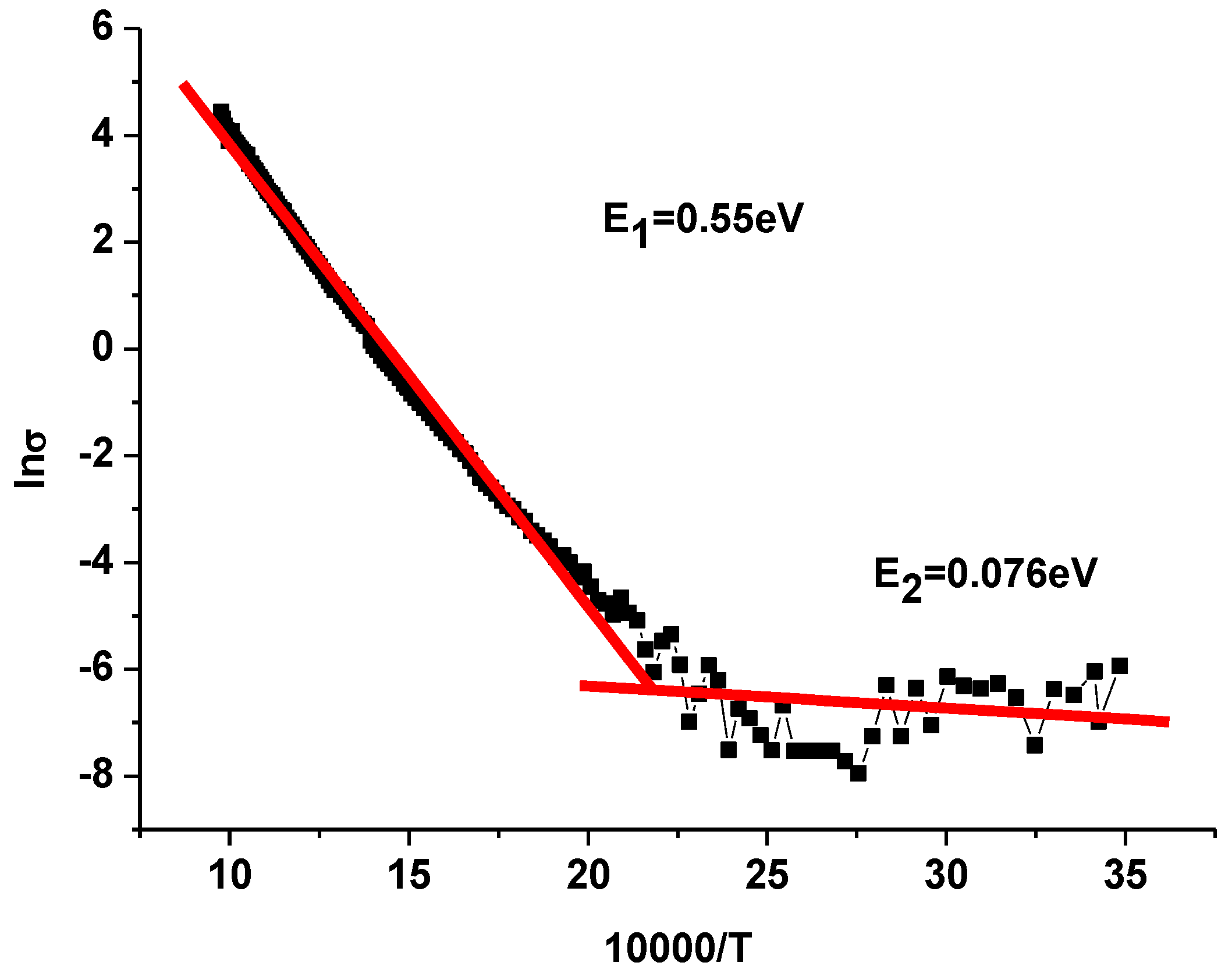
3.6. Activation Energy
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Aurivillius, B. Mixed Bismuth Oxides with Layer Lattices: I. Structure Type of CaBi2B2O9. Arkiv. Kemi. 1949, 54, 463–480. [Google Scholar]
- Aurivillius, B. Mixed Bismuth Oxides with Layer Lattices: II. Structure Type of Bi4Ti3O12. Arkiv. Kemi. 1949, 58, 499–512. [Google Scholar]
- Aurivillius, B. Mixed Bismuth Oxides with Layer Lattices: III. Structure Type of BaBi4Ti4O15. Arkiv. Kemi. 1950, 37, 512–527. [Google Scholar]
- Smolensky, G.A.; Isupov, V.A.; Agranovskaya, A.I. Ferroelectrics of the oxygen-octahedral type with layered structure, Soviet Physics Solid State. 3.
- Parinov, I.A.; Zubkov, S.V. Is it possible to speak about the two-dimensional nature of the dielectric properties of layered perovskite-like compounds of the family of Aurivillius–Smolensky phases? J. Adv. Dielectr. 2023, 13, 2340007. [Google Scholar] [CrossRef]
- Subbarao, E.C. Ferroelectricity in Mixed Bismuth Oxides With Layered-Type Structure. Chem. Physics, 1961, 34, 695–696. [Google Scholar] [CrossRef]
- Subbarao, E.C. Crystal chemistry of mixed bismuth oxides with layer-type structure. Am. Ceram. Soc, 1962, 45, 166–169. [Google Scholar] [CrossRef]
- Zubkov, S.V. Structure and dielectric properties of solid solutions Bi7−2xNd2xTi4NbO21 (x = 0.0, 0.2, 0.4, 0.6, 0.8, 1.0). J. Adv. Dielectr. 2021, 11, 2160018. [Google Scholar] [CrossRef]
- Zubkov, S.V. Crystal structure and dielectric properties of layered perovskite-like solid solutions Bi3−xGdxTiNbO9 (x = 0, 0. 1, 0.2, 0.3) with high Curie temperature, J. Adv. Dielectr. 2020, 10, 2060002. [Google Scholar] [CrossRef]
- Zubkov, S.V.; Vlasenko, V.G. Crystal structure and dielectric properties of layered perovskite-like solid solutions Bi3–xYxTiNbO9 x = 0, 0.1, 0.2, 0.3) with high Curie temperature. Phys. Solid State. 2017, 59, 2303–2307. [Google Scholar] [CrossRef]
- Zubkov, S.V.; Parinov, I.A.; Nazarenko, A.V.; Kuprina, Yu. A. Crystal structure, microstructure, piezoelectric and dielectric properties of high-temperature piezoceramics Bi3-xNdxTi1. 5W0.5O9 (x = 0, 0.1, 0.2), Phys. Solid State. 2022, 64, 1451–1458. [Google Scholar] [CrossRef]
- Zubkov, S.V.; Shevtsova, S.I. Crystal Structure and Dielectric Properties of Layered Perovskite-Like Solid Solutions Bi3−xLuxTiNbO9 (x = 0, 0.05, 0.1) with High Curie Temperature. Advanced Materials. Springer Proceedings in Materials 2020, 6, 173–182. [Google Scholar] [CrossRef]
- Zubkov, S.V. Structure and dielectric properties of solid solutions Bi7Ti4+xWxNb1−2x−0.1V0.1O21 (x = 0.1 − 0.4). J. Adv. Dielectr. 2020; 10, 2060008. [Google Scholar] [CrossRef]
- Zubkov, S.V.; Vlasenko, V.G.; Shuvaeva, V.A.; Shevtsova, S.I. Structure and dielectric properties of solid solutions Bi7Ti4+xWxTa1–2xO21 (x = 0–0.5). Phys. Solid State. 2016, 58, 44–50. [Google Scholar] [CrossRef]
- Vlasenko, V.G.; Zubkov, S.V.; Shuvaeva, V.A.; Abdulvakhidov, K.G.; Shevtsova, S.I. Crystal structure and dielectric properties of Aurivillius phases A0.5Bi4.5B0.5Ti3.5O15 (A = Na, Ca, Sr, Pb; B = Cr, Co, Ni, Fe, Mn, Ga). Phys. Solid State, 2014; 56, 1504–1510. Available online: https://journals.ioffe.ru/articles/40678.
- Goldschmidt, V.M. Die gesetze der krystallochemie. Naturwissenschaften, 1926; 14, 477–485, https://doi.org/10.1007/BF01507527. [Google Scholar] [CrossRef]
- Isupov, V.A. Curie temperatures of Am-1Bi2MmO3m+3 layered ferroelectrics. Inorg. Mater. 1997, 33, 936–940. (In Russsian) [Google Scholar]
- De Araujo, C.A.; Cuchlaro, J.D.; Mcmillan, L.D.; Scott, M.C.; Scott, J.F. Fatigue-free ferroelectric capacitors with platinum electrodes, Nature. 1995, 374, 6523, 627–629. [CrossRef]
- Desu, S.B.; Cho, H.S.; Joshi, P.C. Highly oriented ferroelectric CaBi2Nb2O9 thin films deposited on Si(100) by pulsed laser deposition, Appl. Phys. Lett. 1997, 70, 1393–1395. [Google Scholar] [CrossRef]
- Foschini, C.R.; Joshi, P.C.; Varela, J.A.; Desu, S.B. Properties of BaBi2Ta2O9 thin films prepared by chemical solution deposition technique for dynamic random-access memory applications, J. Mater. Res. 1999, 14, 1860–1864. [Google Scholar] [CrossRef]
- Ismunandar; Kennedy, B. J.; Gunawan; Marsongkohadi. Structure ABi2Nb2O9 (A = Sr, Ba): refinement of powder neutron diffraction data, J. Solid State Chem. 1996, 126, 135–141. [CrossRef]
- Shimakawa, Y.; Kubo, Y.; Nakagawa, Y.; Kamiyama, T.; Asano, H.; Izumi, F. Crystal structures and ferroelectric properties of SrBi2Ta2O9 and Sr0. 8Bi2.2Ta2O9, Appl. Phys. Lett. 1999, 74, 1904–1906. [Google Scholar] [CrossRef]
- Shulman, H.S.; Testorf, M.; Damjanovic, D.; Setter, N. Microstructure, electrical conductivity and piezoelectric properties of bismuth titanate, J. Am. Ceram. Soc. 1996, 79, 3124–3128. [Google Scholar] [CrossRef]
- Villegas, M.; Caballero, A.C.; Moure, C.; Duran, P.; Fernandez, J.F. Low temperature sintering and electrical properties of chemically W-doped Bi4Ti3O12 ceramics, J. Eur. Ceram. Soc. 1999, 19, 1183–1186. [Google Scholar] [CrossRef]
- Waser, R. Bulk conductivity and defect chemistry of acceptor-doped strontium titanate in the quenched state, J. Am. Ceram Soc. 1991, 74, 1934–1940. [Google Scholar] [CrossRef]
- Withers, R.L.; Thompson, J.G.; Rae, A.D. The crystal chemistry underlying ferroelectricity in Bi4Ti3O12, Bi3TiNbO9 and Bi2WO6, J. Solid State Chem. 1991, 94, 404–417. [Google Scholar] [CrossRef]
- Zhi, Y.; Chen, A.; Vilarinho, P.M.; Mantas, P.; Baptista, J.L. Dielectric properties of Bi doped SrTiO3 ceramics in the temperature range 500–800 K, J. Appl. Phys. 1998, 83, 4874–4877. [Google Scholar] [CrossRef]
- Gelfuso, M.V.; Thomazini, D.; Eiras, J.A. Synthesis and structural, ferroelectric, and piezoelectric properties of SrBi4Ti4O15 ceramics. J. Am. Ceram. Soc. 1999, 82, 2368–2372. [Google Scholar] [CrossRef]
- Forbess, M.J.; Seraji, S.; Wu, Y.; Nguyen, C.P.; Cao, G.Z. Dielectric properties of layered perovskite Sr1−xAxBi2Nb2O9 ferroelectrics (A = La, Ca and x = 0. 1), Appl. Phys. Lett. 2000, 76, 2934–2936. [Google Scholar] [CrossRef]
- Noguchi, Y.; Miyayama, M.; Oikawa, K.; Kamiyama, T. Cation-vacancy- induced low coercive field in La-Modified SrBi2Ta2O9, J. Appl. Phys. 2004, 95, 4261–4266. [Google Scholar] [CrossRef]
- Bidault, O.; Goux, P.; Kchikech, M.; Belkaoumi, M.; Maglione, M. Space-charge relaxation in perovskites, J. Physical Review B, 1994, 49, 7868–7873. [Google Scholar] [CrossRef]
- Hervoches, C.H.; Snedden, A.; Riggs, R.; Kilcoyne, S.H.; Manuel, P.; Lightfoot, P. Structural behavior of the four-layer Aurivillius-phase ferroelectrics SrBi4Ti4O15 and Bi5Ti3FeO15, Journal of Solid State Chemistry, 2002, 164, 280–291. Journal of Solid State Chemistry. [CrossRef]
- Wu, Y.; Forbess, M.J.; Seraji, S.; Limmer, S.J.; Chou, T.P.; Nguyen, C.; Cao, G. Doping effect in layer structured SrBi2Nb2O9 ferroelectrics, J. Appl. Phys. 2001, 90, 5296–5302. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, G.Z. Influence of vanadium doping on ferroelectric properties of strontium bismuth niobates, J. Mater. Sci. Lett. 2000, 19, 267–269. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, G.Z. Enhanced ferroelectric properties and lowered bismuth niobates with vanadium doping, Appl. Phys. Lett. 1999, 75, 2650–2652. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, G.Z. Ferroelectric and dielectric properties of strontium bismuth niobate vanadates, J. Mat. Research. 2000, 15, 1583–1590. [Google Scholar] [CrossRef]
- Wu, Y.; Forbess, M.; Seraji, S.; Limmer, S.J.; Chou, T.P.; Cao, G.Z. Impedance study of strontium bismuth tantalate vanadate ferroelectrics, Materials Science and Engineering: B. 2001, 86, 70–78. [CrossRef]
- Wu, Y.; Seraji, S.; Forbess, M.J.; Limmer, S.J.; Chou, T.; Cao, G.Z. Oxygen-vacancy-induced dielectric relaxation in SrBi2(Ta0. 9V0.1)2O9 ferroelectrics, J. Appl. Phys. 2001, 89, 5647–5652. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. PowderCell for Windows. Powder Diffr, 1998; 13, 256–259. [Google Scholar] [CrossRef]
- Zubkov, S.V.; Parinov, I.A.; Kubrin, S.P. Crystal structure and dielectric properties of a new series of perovskite-like solid solutions of the Aurivillius phase family Bi2SrNB2-xSnxWxO9 (x = 0.1, 0.2, 0.3, 0.4). In: 2023 International Conference on “Physics and Mechanics of New Materials and Their Applications” (PHENMA 2023): Abstracts and Schedule (Surabaya, Indonesia, October 3–8, 2023), Parinov, I.A.; Putri, E.P.; Chang, S.-H. (eds.), Rostov-on-Don, Taganrog, Southern Federal University Press, 2023, 331–332. https://www.elibrary.ru/item.asp?id=63103906&pff=1.
- Millan, P.; Ramirez, F.; Castro, A. Substitutions of smaller Sb3+ and Sn3+ cations for Bi3+ in Aurivillius-like phase, J. Materials Science Letters. 1995, 14, 1657–1660. [Google Scholar] [CrossRef]
- Isupov, V.A. Crystal chemical aspects of the bithmus –containing layered compounds of the Am-1Bi2BmO3m-3type. Ferroelectrics. 1996, 189, 211–217. [Google Scholar] [CrossRef]
- Zubkov, S.V. Crystal structure and dielectrical properties of complex perovskite-like solid solutions Bi3Ti1−xSnxNbO9 (x = 0.0, 0.1, 0.35). Advanced Materials. Springer Proceedings in Physics. 2019, 224, 231–238. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr., Sect. A. 1976, 32, 751–767. [Google Scholar] [CrossRef]
| Compounds | a0, Å | b0, Å | c0, Å | V, Å3 | at,% | c', Å | δc',% | δb0,% |
|---|---|---|---|---|---|---|---|---|
| SrBi2Nb209 | 5.55 | 5.48 | 25.261 | 768.23 | 3.899 | 3.79 | -2.8 | -1.2 |
| SrBi2Nb1.8W0.1Sn0.109 | 5.49 | 5.45 | 25.16 | 754.20 | 3.87 | 3.77 | -2.52 | -0.8 |
| SrBi2Nb1.6W0.2Sn0.209 | 5.52 | 5.51 | 25.16 | 766.18 | 3.90 | 3.77 | -2.52 | -0.2 |
| SrBi2Nb1.4W0.3Sn0.309 | 5.50 | 5.57 | 25.34 | 776.13 | 3.91 | 3.80 | -2.87 | 1.27 |
| SrBi2Nb1.2W0.4Sn0.409 | 5.53 | 5.51 | 25.16 | 766.78 | 3.90 | 3.77 | -2.87 | -0.2 |
| Compound | TС, °C | t | ε/ε0 (at 100 kHz) | E1/E2, eV |
|---|---|---|---|---|
| SrBi2Nb209 | 455 | 0.9778 | 960 | 0.67/0.06 |
| SrBi2Nb1.8W0.1Sn0.109 | 420 | 0.97 | 850 | 0.55/0.076 |
| SrBi2Nb1.6W0.2Sn0.209 | 417 | 0.97 | 660 | 0.49/0.26 |
| SrBi2Nb1.4W0.3Sn0.309 | 435 | 0.97 | 690 | 0.54/0.13 |
| SrBi2Nb1.2W0.4Sn0.409 | 450 | 0.97 | 290 | 0.52/0.093 |
| x | Component | δ ± 0.02, mm/s | Δ ± 0.02, mm/s | Γ ± 0.02, mm/s | A ± 1, % |
|---|---|---|---|---|---|
| 0.1 | D | 0.06 | 0.45 | 0.73 | 100 |
| 0.2 | D | 0.06 | 0.43 | 0.72 | 100 |
| 0.3 | D | 0.05 | 0.48 | 0.73 | 100 |
| 0.4 | D | 0.07 | 0.39 | 0.73 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





