Submitted:
29 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Background on Long COVID and Symptoms
3. Materials and Methods
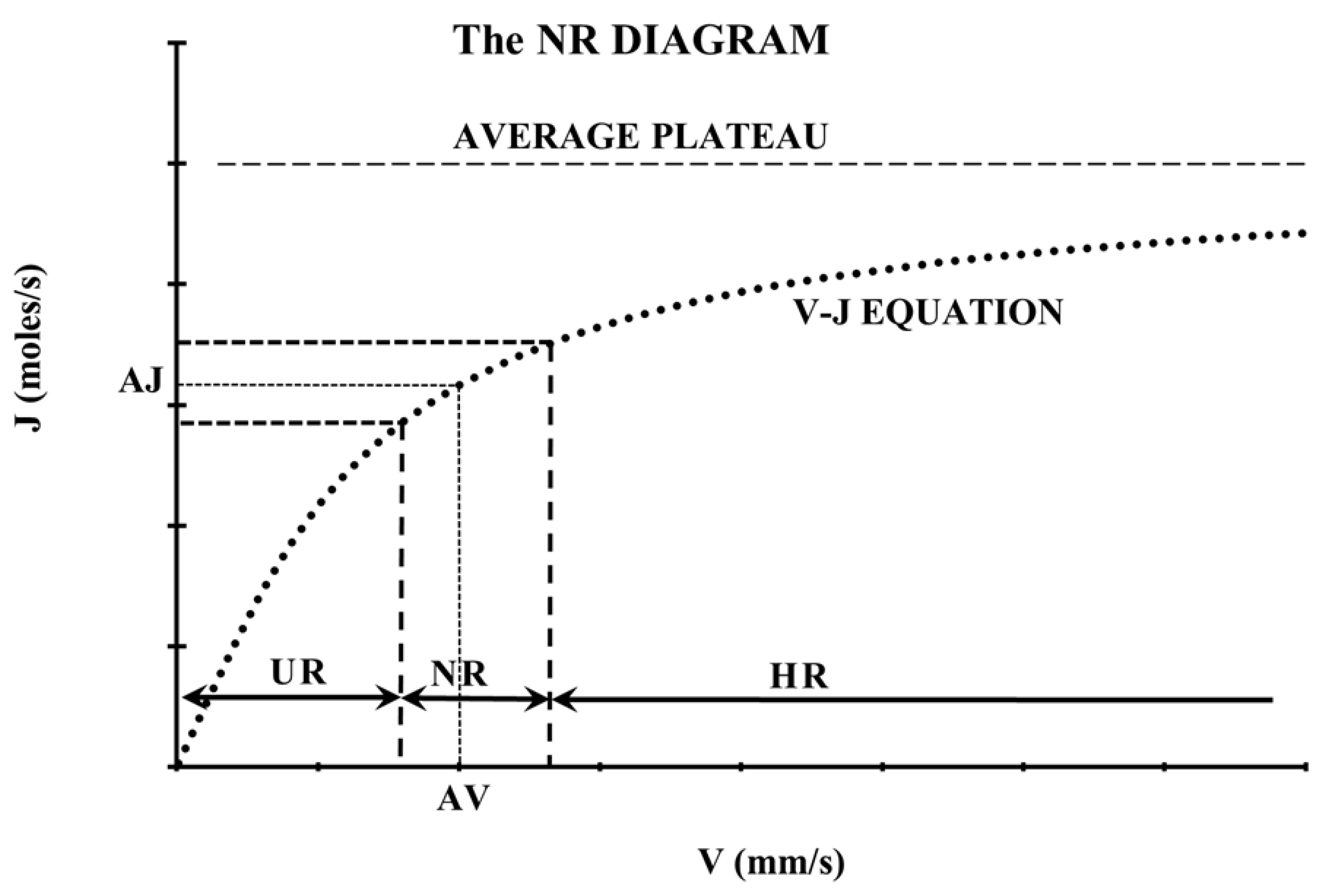
3.1. The Normative Range (NR) Diagram
3.2. Hemodynamic Decrease (HD)
3.3. Microvascular Loss (ML)
3.3.1. Vessel Density Reduction (VDR)
3.3.2. Foveal Avascular zone Enlargement (FAZE)
3.3.3. Capillary Density Reduction (CDR)
3.3.4. Percentage of Perfused Vessels Reduction (PPVR)
3.4. Blood Supply Reduction (SR)
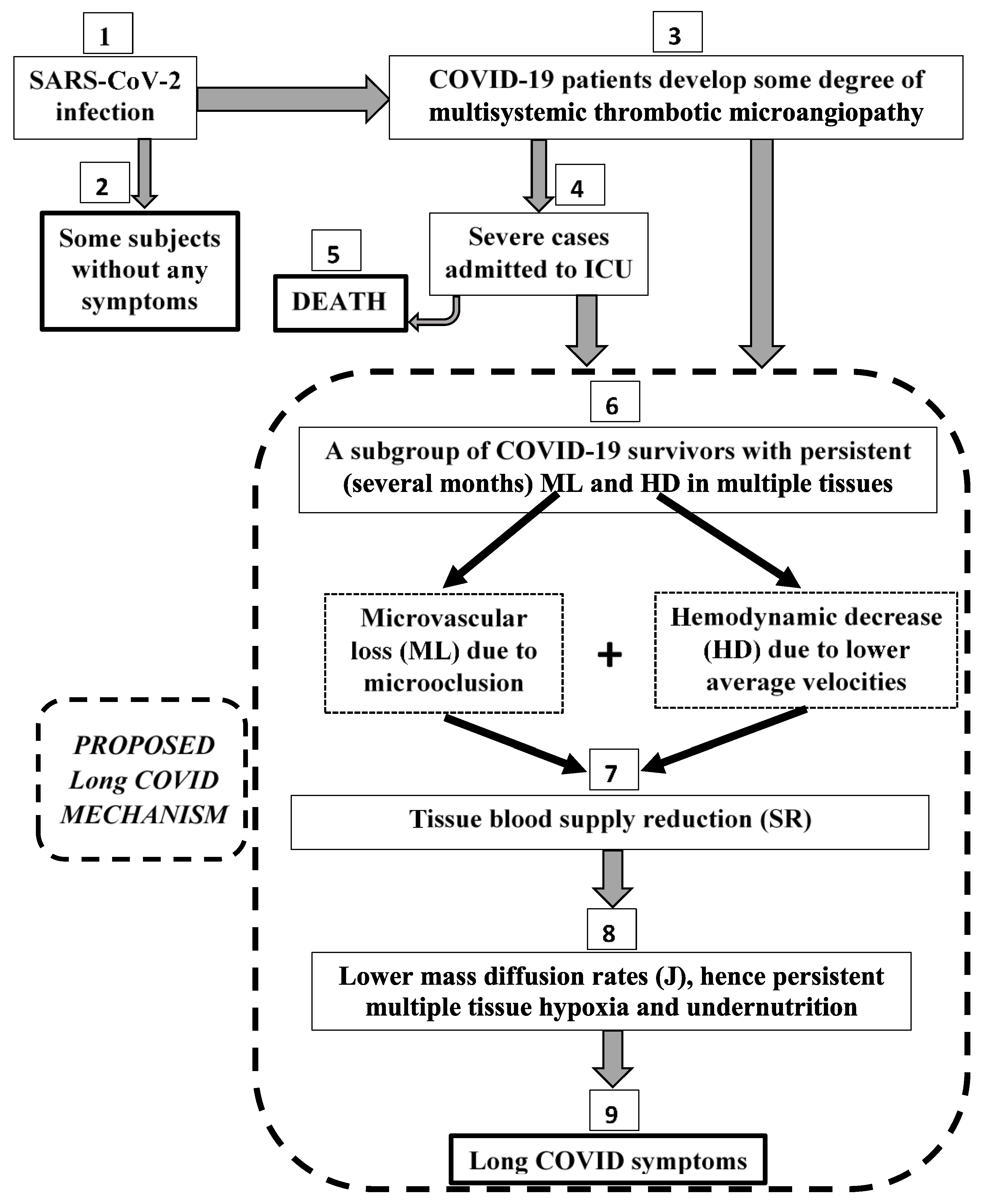
3.5. The Proposed Pathophysiological Microcirculatory Mechanism for Long COVID
3.6. Statistical Analysis
4. Results
4.1. Hemodynamic Decrease (HD) case-control studies [Table 1]
4.2. Microvascular Loss (ML) Case-Control Studies [Table 2, Table 3, Table 4 and Table 5]
4.2.1. Vessel Density Reduction (VDR) Case-Control Studies [Table 2]
4.2.2. FAZ Enlargement (FAZE) Case-Control Studies [Table 3]
4.2.3. Capillary Density Reduction (CDR) Case-Control Studies [Table 4]
4.2.4. Percentage of Perfused Vessels Reduction (PPVR) case-control studies [Table 5]
4.3. Blood SUPPLY REDUCTion (SR) [Table 6]
| TISSUE | STUDY | VASCULAR BED | VDR (%) | N | |
|---|---|---|---|---|---|
| Retina | Savastano et al. [30] | RPCP | 3 | 80 | |
| Gonzalez-Zamora et al. [31] | foveal SCP | 48 | 25 | ||
| foveal DCP | 33 | ||||
| Bilbao-Malavé et al. [32] | foveal SCP | 51 | 17 | ||
| Abrishami et al. [33] | foveal DCP | 13 | 31 | ||
| Guemes-Villahoz et al. [34] | SCP & DCP | 7 | 66 | ||
| Hazar et al. [35] | superior sector DCP | 2 | 50 | ||
| Cennamo et al. [36] | whole image RPCP | 8 | 40 | ||
| Erogul et al. [37] | whole image SCP | 3 | 32 | ||
| Kalaw et al. [40] | 3 inner retinal layers | 8 | 7 | ||
| Urfalioğlu et al. [41] | DCP | 3 | 72 | ||
| El-Hadad et al. [42] | Deep macular plexus | 11 | 50 | ||
| RPCP | 6 | ||||
| Choroid | Üçer & Cevher [43] | choroidal microvessels | 6 | 65 | |
| Sublingual | Osiaevi et al. [44] | sublingual microvessels | 41 | 27 | |
| MEDIAN | 8 | - | |||
| MEAN ± SEM | 16 ± 5 | - | |||
| RANGE | 49 | - | |||
| TOTAL | 562 | ||||
4.4. Results Supporting the Proposed Mechanism
| STUDY | VASCULAR BED | FAZE (%) | N |
|---|---|---|---|
| Gonzalez-Zamora et al. [31] | SCP | 55 | 25 |
| Bilbao-Malave et al. [32] | SCP | 65 | 17 |
| Abrishami et al. [33] | whole image (SCP and DCP) | 12 | 31 |
| Erogul et al. [37] | whole image (SCP and DCP) | 11 | 32 |
| Dipu et al. [38] | SCP | 19 | 35 |
| DCP | 15 | ||
| Kal et al. [39] | SCP | 30 | 63 |
| DCP | 51 | ||
| Urfalioğlu et al. [41] | DCP FAZ | 20 | 72 |
| MEDIAN | 20 | - | |
| MEAN ± SEM | 31 ± 7 | - | |
| RANGE | 54 | - | |
| TOTAL | 275 | ||
| STUDY | VASCULAR BED | CDR (%) | N |
|---|---|---|---|
| Çakmak et al. [45] | finger nailfold capillaries | 17 | 25 |
| Sulli et al. [46] | finger nailfold capillaries | 11* | 61 |
| MEDIAN | 14 | - | |
| MEAN ± SEM | 14 ± 3 | - | |
| RANGE | 6 | - | |
| TOTAL | 86 | ||
| STUDY | VASCULAR BED | PPVR (%) | N |
|---|---|---|---|
| Koutsiaris et al. [6] | eye conjunctiva | 21 | 17 |
| TISSUE (DATA SOURCE) |
α (%) | HD (%) | SR (%) | N | |
|---|---|---|---|---|---|
| PPVR (%) | VDR (%) | ||||
| Conjunctiva (Table 1 & Table 5) |
21 | - | 45 | 57 | 17 |
| Conjunctiva/Skin/Brain (Table 1) |
- | - | 37 | - | 72 |
| Retina/Choroid/Sublingual (Table 2) |
- | 16 | - | - | 562 |
| Multiple Tissues (Table 1 & Table 2) |
- | 16 | 37 | 47 | 634 |
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
References
- WHO (World Health Organization). COVID-19 Weekly Epidemiological Update. Edition 144, published 25 May 2023.
- WHO (World Health Organization). COVID-19 Weekly Epidemiological Update. Edition 168, published 17 June 2024.
- Blomberg, B.; Mohn, K.G.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.A.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef]
- Venkatesan, P. NICE guideline on long COVID. Lancet Respir. Med. 2021, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Sideratou, C.M.; Papaneophytou, C. Persisting Shadows: Unraveling the Impact of Long COVID-19 on Respiratory, Cardiovascular, and Nervous Systems. Infectious Disease Reports 2023, 15, 806–830. [Google Scholar] [CrossRef]
- Koutsiaris, A.G.; Riri, K.; Boutlas, S.; Panagiotou, T.N.; Kotoula, M.; Daniil, Z.; Tsironi, E.E. COVID-19 hemodynamic and thrombotic effect on the eye microcirculation after hospitalization: A quantitative case-control study. Clin. Hemorheol. Microcirc. 2022, 82, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Koutsiaris, A.G.; Riri, K.; Boutlas, S.; Daniil, Z.; Tsironi, E.E. A normative blood velocity model in the exchange microvessels for discriminating health from disease: Healthy controls versus COVID-19 cases. Clin. Hemorheol. Microcirc. 2023, 84, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Callard, F.; Perego, E. How and why patients made Long Covid. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network — United States, March–June 2020. Morb. Mortal. Wkly Rep. 2020, 69, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; Vehreschild, M.; Nagel, E. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. Erratum in: JAMA Cardiol. 2020, 5, 1308. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Rezel-Potts, E.; Douiri, A.; Sun, X.; Chowienczyk, P.J.; Shah, A.M.; Gulliford, M.C. Cardiometabolic outcomes up to 12 months after COVID-19 infection. A matched cohort study in the UK. PLoS Med. 2022, 19, e1004052. [Google Scholar] [CrossRef] [PubMed]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Jerndal, H.; Lundevaller, E.H.; Sund, M.; Lindmark, K.; Fors Connolly, A.M. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study. BMJ 2022, 377, e069590. [Google Scholar] [CrossRef]
- Percze, A.R.; Nagy, A.; Polivka, L.; Barczi, E.; Czaller, I.; Kovats, Z.; Varga, J.T.; Ballai, J.H.; Muller, V.; Horvath, G. Fatigue, sleepiness and sleep quality are SARS-CoV-2 variant independent in patients with long COVID symptoms. Inflammopharmacology 2023, 31, 2819–2825. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ Erratum in: BMJ 2021, 374, n1944. 2021, 374, n1648. [Google Scholar] [CrossRef]
- Baum, P.; Do, L.; Deterding, L.; Lier, J.; Kunis, I.; Saur, D.; Classen, J.; Wirtz, H.; Laufs, U. Cardiac function in relation to functional status and fatigue in patients with post-COVID syndrome. Sci. Rep. 2022, 12, 19575. [Google Scholar] [CrossRef]
- Merikanto, I.; Dauvilliers, Y.; Chung, F.; Wing, Y.K.; De Gennaro, L.; et al. Sleep symptoms are essential features of long-COVID - Comparing healthy controls with COVID-19 cases of different severity in the international COVID sleep study (ICOSS-II). J. Sleep. Res. 2023, 32, e13754. [Google Scholar] [CrossRef] [PubMed]
- Kalamara, E.; Pataka, A.; Boutou, A.; Panagiotidou, E.; Georgopoulou, A.; Ballas, E.; Chloros, D.; Metallidis, S.; Kioumis, I.; Pitsiou, G. Persistent Sleep Quality Deterioration among Post-COVID-19 Patients: Results from a 6-Month Follow-Up Study. J. Pers. Med. 2022, 12, 1909. [Google Scholar] [CrossRef] [PubMed]
- El-Tallawy, S.N.; Perglozzi, J.V.; Ahmed, R.S.; Kaki, A.M.; Nagiub, M.S.; LeQuang, J.K.; Hadarah, M.M. Pain Management in the Post-COVID Era-An Update: A Narrative Review. Pain Ther. 2023, 12, 423–448. [Google Scholar] [CrossRef]
- Peron, J.P.S. Direct and indirect impact of SARS-CoV-2 on the brain. Hum. Genet. 2023, 142, 1317–1326. [Google Scholar] [CrossRef]
- Koutsiaris, A.G. The velocity-diffusion equation in the exchange microvessels. Clin. Hemorheol. Microcirc. 2023, 84, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Renkin, E.M. B.W. Zweifach award lecture: regulation of the microcirculation. Microvasc. Res. 1985, 30, 251–63. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Körber, N.; Kiesewetter, H.; Prünte, C.; Wolf, S.; Reim, M. Measuring the microcirculation in the human conjunctiva bulbi under normal and hyperperfusion conditions. Graefes Arch. Clin. Exp. Ophthalmol. 1983, 220, 294–7. [Google Scholar] [CrossRef] [PubMed]
- Koutsiaris, A.G. Meta-analysis of conjunctival microvascular hemorheology metrics. Microvasc. Res. 2022, 142, 104369. [Google Scholar] [CrossRef] [PubMed]
- Zharkikh, E.V.; Loktionova, Y.I.; Fedorovich, A.A.; Gorshkov, A.Y.; Dunaev, A.V. Assessment of Blood Microcirculation Changes after COVID-19 Using Wearable Laser Doppler Flowmetry. Diagnostics(Basel) 2023, 13, 920. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wu, J.; Chen, T.; Li, J.; Zhang, G.; Wu, D.; Zhou, Y.; Zheng, N.; Cai, A.; Ning, Q.; Manyande, A.; Xu, F.; Wang, J.; Zhu, W. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J. Clin. Invest. 2021, 131, e147329. [Google Scholar] [CrossRef] [PubMed]
- Koutsiaris, A.G.; Batis, V.; Liakopoulou, G.; Tachmitzi, S.V.; Detorakis, E.T.; Tsironi, E.E. Optical Coherence Tomography Angiography (OCTA) of the eye: A review on basic principles, advantages, disadvantages and device specifications. Clin. Hemorheol. Microcirc. 2023, 83, 247–271. [Google Scholar] [CrossRef] [PubMed]
- Savastano, A.; Crincoli, E.; Savastano, M.C.; Younis, S.; Gambini, G.; De Vico, U.; Cozzupoli, G.M.; Culiersi, C.; Rizzo, S. Gemelli Against Covid-Post-Acute Care Study Group. Peripapillary Retinal Vascular Involvement in Early Post-COVID-19 Patients. J. Clin. Med. 2020, 9, 2895. [Google Scholar] [CrossRef]
- González-Zamora, J.; Bilbao-Malavé, V.; Gándara, E.; Casablanca-Piñera, A.; Boquera-Ventosa, C.; Landecho, M.F.; Zarranz-Ventura, J.; García-Layana, A. Retinal Microvascular Impairment in COVID-19 Bilateral Pneumonia Assessed by Optical Coherence Tomography Angiography. Biomedicines 2021, 9, 247. [Google Scholar] [CrossRef]
- Bilbao-Malavé, V.; González-Zamora, J.; Saenz de Viteri, M.; de la Puente, M.; Gándara, E.; Casablanca-Piñera, A.; Boquera-Ventosa, C.; Zarranz-Ventura, J.; Landecho, M.F.; García-Layana, A. Persistent Retinal Microvascular Impairment in COVID-19 Bilateral Pneumonia at 6-Months Follow-Up Assessed by Optical Coherence Tomography Angiography. Biomedicines 2021, 9, 502. [Google Scholar] [CrossRef]
- Abrishami, M.; Emamverdian, Z.; Shoeibi, N.; Omidtabrizi, A.; Daneshvar, R.; Saeidi Rezvani, T.; Saeedian, N.; Eslami, S.; Mazloumi, M.; Sadda, S.; Sarraf, D. Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: a case-control study. Can. J. Ophthalmol. 2021, 56, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Guemes-Villahoz, N.; Burgos-Blasco, B.; Vidal-Villegas, B.; Donate-López, J.; de la Muela, M.H.; López-Guajardo, L.; Martín-Sánchez, F.J.; García-Feijoó, J. Reduced macular vessel density in COVID-19 patients with and without associated thrombotic events using optical coherence tomography angiography. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Hazar, L.; Karahan, M.; Vural, E.; Ava, S.; Erdem, S.; Dursun, M.E.; Keklikçi, U. Macular vessel density in patients recovered from COVID 19. Photodiagnosis Photodyn. Ther. 2021, 34, 102267. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, G.; Reibaldi, M.; Montorio, D.; D'Andrea, L.; Fallico, M.; Triassi, M. Optical Coherence Tomography Angiography Features in Post-COVID-19 Pneumonia Patients: A Pilot Study. Am. J. Ophthalmol. 2021, 227, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Erogul, O.; Gobeka, H.H.; Dogan, M.; Akdogan, M.; Balci, A.; Kasikci, M. Retinal microvascular morphology versus COVID-19: What to anticipate? Photodiagnosis Photodyn. Ther. 2022, 39, 102920. [Google Scholar] [CrossRef]
- Dipu, T.; Goel, R.; Arora, R.; Thakar, M.; Gautam, A.; Shah, S.; Gupta, Y.; Chhabra, M.; Kumar, S.; Singh, K.; Kumar, S.; Garg, S.; Singh, H.; Pant, R. Ocular sequelae in severe COVID-19 recovered patients of second wave. Indian J. Ophthalmol. 2022, 70, 1780–1786. [Google Scholar] [CrossRef]
- Kal, M.; Winiarczyk, M.; Cieśla, E.; Płatkowska-Adamska, B.; Walczyk, A.; Biskup, M.; Pabjan, P.; Głuszek, S.; Odrobina, D.; Mackiewicz, J.; Zarębska-Michaluk, D. Retinal Microvascular Changes in COVID-19 Bilateral Pneumonia Based on Optical Coherence Tomography Angiography. J. Clin. Med. 2022, 11, 3621. [Google Scholar] [CrossRef]
- Kalaw, F.G.P.; Warter, A.; Cavichini, M.; et al. Retinal tissue and microvasculature loss in COVID-19 infection. Sci. Rep. 2023, 13, 5100. [Google Scholar] [CrossRef]
- Urfalioğlu, S.; Akkök, B.; Özdemir, G.; Daghan, B.; Guler, M. OCTA evaluation of posterior ocular blood flow in patients after COVID-19 infection without pneumonia. J Fr Ophtalmol. 2023, 46, 468–474. [Google Scholar] [CrossRef]
- El-Haddad, N.S.E.M.; Abd El-Wahed, E.; Abd El-Wahab, A.; Shalaby, S.; Farag, M.M.A.; Mohammed, N.S.; Shawky, S. The Effect of Post-Coronavirus Disease 2019 Infection on the Retinal Microvasculature. J. Curr. Ophthalmol. 2023, 35, 50–55. [Google Scholar] [CrossRef]
- Üçer, M.B.; Cevher, S. How does Covid-19 affect the choroidal structures at the early post-infectious period? J. Fr. Ophtalmol. 2023, 46, 106–113. [Google Scholar] [CrossRef]
- Osiaevi, I.; Schulze, A.; Evers, G.; Harmening, K.; Vink, H.; Kümpers, P.; Mohr, M.; Rovas, A. Persistent capillary rarefication in long COVID syndrome. Angiogenesis 2023, 26, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, F.; Demirbuga, A.; Demirkol, D.; et al. Nailfold capillaroscopy: A sensitive method for evaluating microvascular involvement in children with SARS-CoV-2 infection. Microvasc. Res. 2021, 138, 104196. [Google Scholar] [CrossRef] [PubMed]
- Sulli, A.; Gotelli, E.; Bica, P.F.; Schiavetti, I.; Pizzorni, C.; Aloè, T.; Grosso, M.; Barisione, E.; Paolino, S.; Smith, V.; Cutolo, M. Detailed videocapillaroscopic microvascular changes detectable in adult COVID-19 survivors. Microvasc. Res. 2022, 142, 104361. [Google Scholar] [CrossRef] [PubMed]
- Polak, S.B.; Van Gool, I.C.; Cohen, D.; von der Thüsen, J.H.; van Paassen, J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol. 2020, 33, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B. Prevalence of readily detected amyloid blood clots in 'unclotted' Type 2 Diabetes Mellitus and COVID-19 plasma: a preliminary report. Cardiovasc. Diabetol. 2020, 19, 193. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Stroszczynski, C.; Jung, F. Contrast enhanced ultrasonography (CEUS) to detect abdominal microcirculatory disorders in severe cases of COVID-19 infection: First experience. Clin. Hemorheol. Microcirc. 2020, 74, 353–361. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Bonaca, M.P. Studying the coagulopathy of COVID-19. Lancet 2022, 399, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, S.; Weiss, L.; Cullivan, S.; O’Rourke, E.; Murphy, C.A.; Toolan, S.; et al. Non-severe COVID-19 is associated with endothelial damage and hypercoagulability despite pharmacological thromboprophylaxis. J. Thromb. Haemost. 2022, 20, 1008–1014. [Google Scholar] [CrossRef]
- Watson, O.; Pillai, S.; Howard, M.; Zaldua, J.C.; Whitley, J.; Burgess, B.; Lawrence, M.; Hawkins, K.; Morris, K.; Evans, P.A. Impaired fibrinolysis in severe Covid-19 infection is detectable in early stages of the disease. Clin. Hemorheol. Microcirc. 2022, 82, 183–191. [Google Scholar] [CrossRef]
- Xu, S.W.; Ilyas, I.; Weng, J.P. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Badaras, I.; Laučytė-Cibulskienė, A. Vascular Aging and COVID-19. Angiology 2023, 74, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.W.; Brown, R.L.; Benjamin, L.; Nortley, R.; Wiethoff, S.; Bharucha, T.; et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 2020, 143, 3104–3120. [Google Scholar] [CrossRef]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef]
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Kotze, M.J.; Oladejo, S.O.; Watson, L.R.; Rajaratnam, K.; Watson, B.W.; Kell, D.B. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovasc. Diabetol. 2022, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Scheim, D.E.; Vottero, P.; Santin, A.D.; Hirsh, A.G. Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19. Int. J. Mol. Sci. 2023, 24, 17039. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, Long COVID, and ME/CFS: evidence, mechanisms, and therapeutic implications. Biochem. J. 2022, 479, 1653–1708. [Google Scholar] [CrossRef]
- Astin, R.; Banerjee, A.; Baker, M.R.; Dani, M.; Ford, E.; Hull, J.H.; Lim, P.B.; McNarry, M.; Morten, K.; O'Sullivan, O.; Pretorius, E.; Raman, B.; Soteropoulos, D.S.; Taquet, M.; Hall, C.N. Long COVID: mechanisms, risk factors and recovery. Exp. Physiol. 2023, 108, 12–27. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol. Psychiatry. 2023, 28, 564–578. [Google Scholar] [CrossRef]
- Lafetá, M.L.; Souza, V.C.; Menezes, T.C.F.; Verrastro, C.G.Y.; Mancuso, F.J.; Albuquerque, A.L.P.; Tanni, S.E.; Izbicki, M.; Carlstron, J.P.; Nery, L.E.; Oliveira, R.K.F.; Sperandio, P.A.; Ferreira, E.V.M. Exercise intolerance in post-coronavirus disease 2019 survivors after hospitalization. ERJ Open Res. 2023, 9, 00538–2022. [Google Scholar] [CrossRef]
- Gareau, M.G.; Barrett, K.E. Role of the microbiota-gut-brain axis in postacute COVID syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G322–G328. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Greene, C.; Dayaramani, C.; Rauchman, S.H.; Stecker, M.M.; De Leon, J.; Pinkhasov, A. Long COVID, the brain, nerves, and cognitive function. Neurol. Int. 2023, 15, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu-Bercu, A.; Lobiuc, A.; Căliman-Sturdza, O.A.; Oiţă, R.C.; Iavorschi, M.; Pavăl, N.E.; Șoldănescu, I.; Dimian, M.; Covasa, M. Long COVID: Molecular Mechanisms and Detection Techniques. Int. J. Mol. Sci. 2023, 25, 408. [Google Scholar] [CrossRef]
- Tziolos, N.R.; Ioannou, P.; Baliou, S.; Kofteridis, D.P. Long COVID-19 Pathophysiology: What Do We Know So Far? Microorganisms 2023, 11, 2458. [Google Scholar] [CrossRef] [PubMed]
- Noor, M.; McGrath, O.; Drira, I.; Aslam, T. Retinal Microvasculature Image Analysis Using Optical Coherence Tomography Angiography in Patients with Post-COVID-19 Syndrome. Journal of Imaging 2023, 9, 234. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. Erratum in: Nat. Rev. Microbiol. 2023, 21, 408. 2023, 21, 133–146. [Google Scholar] [CrossRef]
| TISSUE | STUDY | VASCULAR BED / METHOD | HD (%) | N |
|---|---|---|---|---|
| Conjunctiva | Koutsiaris et al. [6] | exchange microvessels / CVC | 45 | 17 |
| Skin | Zharkikh et al. [27] | wrist and shin microvessels / LDF | 29 | 23 |
| Brain | Qin et al. [28] | gray matter cortex, subcortical nuclei / MRI | - | 32 |
| MEDIAN | 37 | - | ||
| MEAN ± SEM | 37 ± 8 | - | ||
| RANGE | 16 | - | ||
| TOTAL | 72 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





