Introduction
GTN was first discovered in 1847 by Sobrero [
1]. Tolerance seen in its treatment of patients was first described in 1888 [
2]. Over the ensuing years many hypotheses to explain and eliminate tolerance in the treatment of cardiovascular diseases have been advanced. Recently superoxide from mitochondria in response to GTN treatment has been discovered [
3]. The purpose of this paper is to review the involvement of mitochondrial complex I as the site of synthesis of the first superoxide radical in response to GTN and the mechanism whereby it leads to glutathione (GSH) dependent tolerance. The paper also will explain the rationale for GSH based GTN formulations and approaches, that not only address tolerance, and vasodilator heart failure treatment failures involving redox induced diuretic resistance, but also address the rational for their use to target underlying redox disturbances of other diseases treated by GTN.
Nitric Oxide Is the Cause of GSH Dependent GTN Tolerance
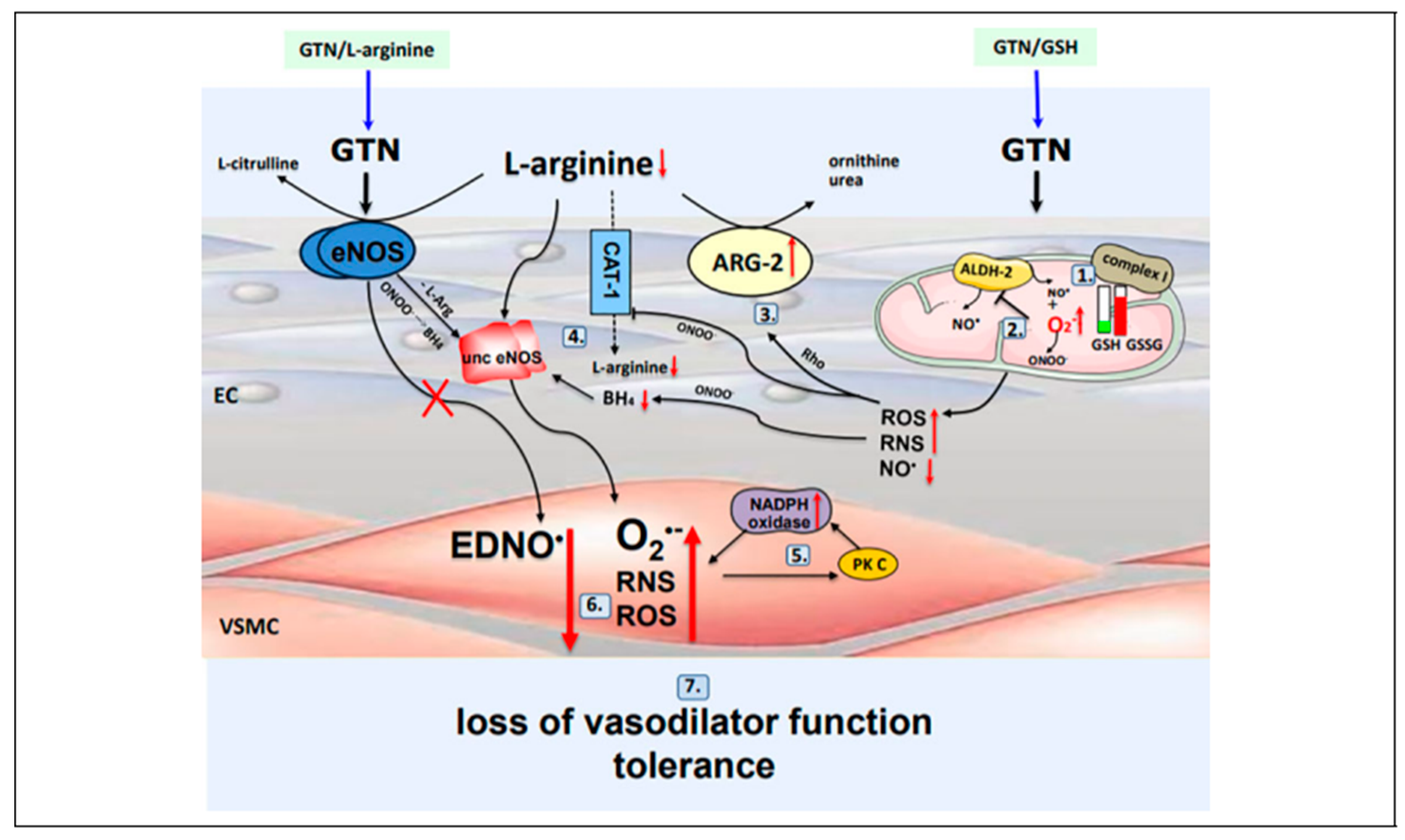
The cause of GTN tolerance is nitric oxide (NO). GTN tolerance is a process that begins at mitochondrial complex I with its uncoupling by S-nitrosylation [
4,
5] from NO donated and generated [
6,
7,
8] by GTN. The resulting superoxide formed depletes GSH by S-glutathiolation thereby leading to further superoxide which is GSH suppressible [
4,
9,
10]. This process ends at NADPH oxidase. In between mitochondrial complex I and NADPH oxidase there is uncoupling of aldehyde dehydrogenase II (ALDH II) and eNOS, that involves its S-glutathiolation [
13], which follows complex I uncoupling and substrate depletion by arginase II [
6,
12], all of which is mediated by ROS and RNS from uncoupled complex I. This is followed by activation of protein PKC [
6,
13] which completes the process by activating NADPH oxidase with superoxide production from a third and final site.
Therefore, the site of formation of the first superoxide radical is mitochondrial complex I and NADPH oxidase is the site of the last superoxide radical’s formation. The net effect of superoxide from all 4 sites is formation of peroxynitrite [
6,
14] in place of NO from ALDH-2 and EDNO from eNOS. Tolerance with loss of vasodilator function is the result. This entire GSH dependent-reversible process is illustrated and summarized in
Figure 1 and discussed in complete detail in reference [
6].
A Chronology of Studies Showing the Actions of GTN and Tolerance
Are GSH Dependent
Beginning as early as 1969, Needleman showed the vasodilator actions of nitroglycerin (GTN) are glutathione (GSH) dependent [
15]. This was followed by studies showing GTN tolerance involves depletion of sulphydryl groups and lead to the “sulphydryl-depletion hypothesis” of GTN tolerance [
16]. In 1983 it was shown that the hemodynamic actions of GTN could potentiated by n-acetylcysteine (NAC), a GSH prodrug, in patients undergoing cardiac catheterization for evaluation of chest pain [
17]. In 1987 it was shown that NAC prevents GTN tolerance in heart failure [
18]. In 1995, the parenteral administration of 4.2 grams of GSH over a 7 day period was found to normalize primary tolerance to the rheologic effects of GTN in 40 patients with diabetes [
19]. A 1998 study of GSH administered directly into the angiographically normal coronary arteries of 26 patients was found to enhance the epicardial coronary vasodilator response to GTN in addition to suppressing the coronary constrictor response and enhancing the increase in coronary flow response to acetylcholine [
20]. A 1999 study of patients with femoral artery atherosclerosis revealed prevention of GTN induced vasoconstrictor responses, similar to those seen in diabetics in 1995 [
19], with intra-arterial GSH pretreatment [
19]. Finally, in 2006 it was shown tolerance to GTN involves respiratory complex I and can be prevented by GSH [
5].
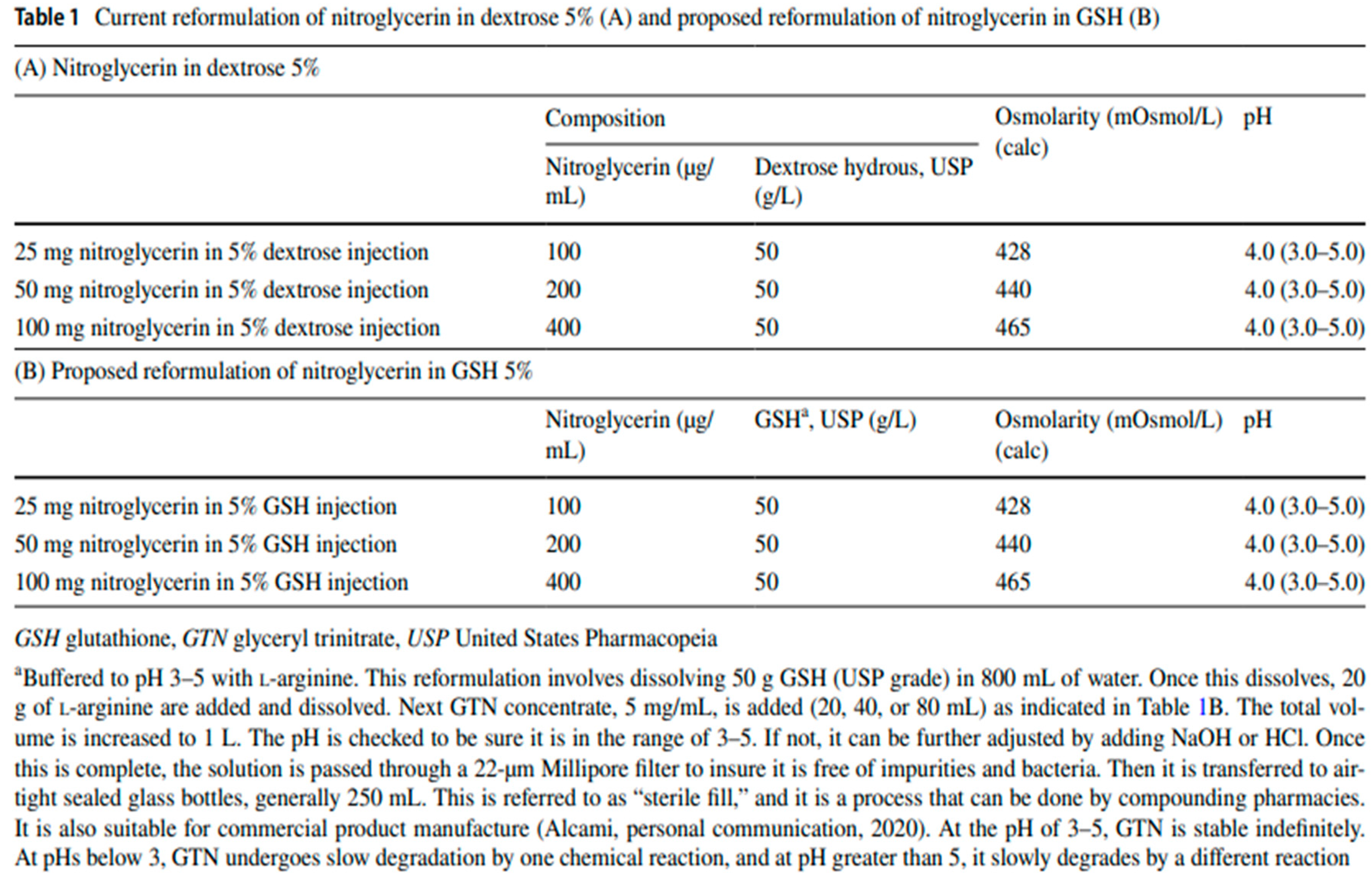
Backround for a Study of the Impact of IV GTN in 5% GSH on Diuretic
Resistance in Patients with Congestive Heart Failure
The background for this is a recent study of cimlanod, a new nitroxl anion donor vasodilator being evaluated as a treatment for acute heart failure [
27]. The purpose of the study was to assess the effect of cimlanod on urine output and sodium excretion in response to diuretic treatment in stable patients with HFrEF with EF </= 45%. This study found that, patients with HF, congestion and a reduced LVEF, the infusion of cimlanod reduced urine volume and sodium excretion, both before and after the administration of furosemide, accompanied by plasma volume expansion and an increase in total body water, findings seen with other vasodilators, to include nesiritide, serelaxin, minoxidil, nitroprusside and IV GTN in 5% dextrose, studied in heart failure patients. As a result it was concluded infusion of cimlanod attenuates a furosemide-induced diuresis in patients with heart failure and an LVEF <45%, which might have adverse consequences for those who are already congested, i.e. ADHF patients. Also, that development of interventions for acute heart failure should include research focused on assessing effects on water balance and potential interactions with diuretic agents.
Rationale for a Study of the Impact of IV GTN in 5% GSH on Diuretic Resistance in Patients with Acute Heart Failure
Regarding the assessment of effects of vasodilators on water balance and interactions with diuretic agents, heart failure itself, unrelated to treatment, leads to increased renal superoxide levels via hypoperfusion, eNOS uncoupling, NADPH oxidase upregulation and NO/superoxide peroxinitrite formation [
28]. Therefore, those vasodilators previously mentioned, to include cimlanod, that failed in previous clinical ADHF trials would either have had no benefit on diuretic responses or impaired responses leading to diuretic resistance (cimlanod).
As discussed previously [
6], cimlanod is a nitroxyl anion donor and and nitroxyl (HNO) reacts with molecular oxygen and forms peroxynitrite at physiological pH [
29]. Therefore, one can view cimlanod as a peroxynitrite prodrug. Furthermore, peroxynitrite is a highly reactive radical which can uncouple eNOS by oxidizing BH4 like superoxide from which it can be formed. All of this as discussed below explains cimlanod’s effect to blunt Na+ and water excretion as in the above discussed study [
27].
Mitochondrial superoxide has been found to reduce urine flow and sodium excretion in a rat kidney study [
30]. And this was prevented using a superoxide dismutase mimetic. In so far as GSH similarly suppresses mitochondrial superoxide from GTN induced mitochondrial complex I uncoupling, it is proposed that IV GTN in 5% GSH be used in place of cimlanod in a repeat of the previously discussed cimlanod diuretic study [
27]. This is to improve sodium excretion and diuretic responses thereby reducing or preventing diuretic resistance, in addition to preventing tolerance. Elimination of diuretic resistance and tolerance in the treatment of ADHF would be a significant improvement.
As for a reason to anticipate a positive response to IV GTN in 5% GSH, this is based on the positive effect seen with the SGLT inhibitors in acute heart failure. Similarly to GSH, dapagliflozin, a SGLT inhibitor, has been found to reduce oxidative stress in human proximal tubular cells [
31]. This may explain the results of empagliflozin, another SGLT inhibitor, on the response to diuretics and urine output seen in the EMPAG-HF trial [
32], all of which suggests a rationale for combining these two agents in the treatment of ADHF.
Treating Right Heart Failure Following Left Ventricular Assist Device Placement with IV GTN in 5% GSH
Similarly to CHF from left heart failure, RHF may be another disease with mitochondrial superoxide in its underlying pathogenesis. A recent RHF pulmonary artery banding study done in mice revealed the transition of maladaptive RV remodeling into RHF involved mitochondrial superoxide [
33]. This may be process that can be blocked or reduced by GSH based GTN formulations used in the treatment of pulmonary hypertension.
In patients requiring LVADs for treatment of advanced HF, there is the additional problem of RHF from pulmonary hypertension induced by the operation of the device. RHF was seen in upwards of 20% of patients in the MOMENTUM-3 study. The use of an RVAD was required in 4% [
34]. Treatment approaches for this problem include the use of diuretics, hemofiltration, inotropes, rhythm synchronization in addition to pulmonary vasodilators for reducing post capillary pulmonary hypertension.
Multiple approaches for treating pulmonary hypertension leading to RHF seen with LVADs have been tried with only short- term success. This includes inhaled nitric oxide, IV GTN in dextrose 5%, nitroprusside, inotropes, sildenafil and endothelin antagonists. The vasodilator most frequently used has been nitroprusside. As discussed previously [
6], IV GTN in 5% GSH would be the next logical vasodilator that could be considered on the basis that superoxide from the use of GTN in dextrose 5% may have limited previous attempts to control RHF, or promoted RV remodeling.
For those patients who have responded favorably with extended use of LVADs there is a need to improve the process for explantation to reduce the need for heart transplantation. Currently up to five oral drugs are required to maintain patients hemodynamically stable during the weening process which is successful in about 50 % of attempts after 18 months [
35]. Therefore this paper proposes studying the use of IV GTN in 5% GSH to reduce the need for multiple oral agents and to reduce weening time.
Treating Refractory Angina with Sublingual and Transdermal GTN Combined with Sublingual GSH
Refractory angina (RA) is conventionally defined as a chronic condition (≥3 months in duration) characterized by angina in the setting of coronary artery disease (CAD), which cannot be controlled by a combination of optimal medical therapy, angioplasty or bypass surgery, and where reversible myocardial ischaemia has been clinically established to be the cause of the symptoms [
36]. And this condition is described by the term refractory because, until recently, multiple treatment approaches have been unsuccessful. However it has very recently been shown in the ORBITA-COSMIC study that, via a mechanism unrelated to increased blood flow, a reduction in angina frequency beginning at 10 weeks post insertion may be achieved using a coronary sinus reducer (CSR) [
37].
As for the mechanism explaining the benefit seen ORBITA-COSMIC one explanation consistent with the delayed onset of benefit beginning 10 weeks, is reduction in inflammation. There are no studies of inflammation in refractory angina but inflammation is thought to play a major pathological role in the in the severity of unstable angina [
38]. If inflammation is involved, treatment with previously discussed sublingual and transdermal GSH based GTN approaches, following and in combination with CSR, may further improve results seen in the ORBITA-COSMIC study. This is because mitochondrial superoxide leads to activation of the NLRP3 inflammasome [
39] and supplementing GSH is a way of preventing activation of the NLRP3 inflammasome which leads to vascular inflammation [
40].
Summary and Conclusions
1]Uncoupled mitochondrial complex I from GTN mediated S-nitrosylation is the site of the first superoxide radical that leads to a cascade involving ALDH II and eNOS uncoupling, activation of PKC, activation of NADPH oxidase that ends in peroxynitrite formation with loss of vasodilator function and GTN tolerance.
2] baseline pre-treatment renal Na+ excretory function and urine output of heart failure patients is reduced secondary to the inhibitory effect of mitochondrial superoxide and related redox species on renal tubular function.
3] previous vasodilator drugs, which failed in the treatment of ADHF, failed because those agents did not suppress superoxide, and underlying renal tubule redox disturbances, or made it worse creating diuretic resistance as a result.
4] New GSH based GTN formulations and related treatment approaches, may, because of suppressing mitochondrial superoxide from GTN, and redox disturbances of diseases treated by GTN, improve the treatment of those diseases.
Abreviations
| ADHF |
acute decompensated heart failure |
| AHF |
acute heart failure |
| BH4 |
tetrahydrobiopterin |
| eNOS |
endothelial nitric oxide synthase |
| GSH |
glutathione |
| GTN |
nitroglycerin, glycerol trinitrate |
| HF |
heart failure |
| IV GTN/GSH |
intravenous nitroglycerin in glutathione 5% |
| LVAD |
left ventricular assist device |
| NAC |
n-acetylcysteine |
| NO |
nitric oxide |
| OONO |
peroxynitrite |
| PKC |
protein kinase C |
| RHF |
right heart failure |
| ROS |
reactive oxygen species |
| RNS |
reactive nitrogen species |
| RVAD |
right ventricular assist device |
| SGLT |
sodium glucose transporter |
Funding
There was no funding for this manuscript
Conflicts of Interest
The author has no conflicts to delare.
Availability of Data and Material (data transparency)
The manuscript does not include data
Code Availability (software application or custom code)
Microsoft word
Author Contributions
the author of this paper researched, organized, wrote and proofread the paper as its sole author.
Ethics Approval (include appropriate approvals or waivers)
The manuscript did not involved studies of animal or human subjects
Consent to Participate (include appropriate statements)
The manuscript did not require consent to participate as it did not involve studies of animal or human subjects
Consent for Publication (include appropriate statements)
the author of the paper consents in its publication.
Conflicts
none to declare
References
-
Sobrero A (1847). "Sur plusieur composés détonants produits avec l'acide nitrique et le sucre,la dextrine, la lactine, la mannite et la glycérine" [On several detonating compounds produced with nitric acid and sugar, dextrin, lactose, mannitol, and glycerine]. Comptes Rendus (in French). 24: 247–248. From p. 248.
- Stewart DD. Remarkable tolerance to nitroglycerin. Philadelphia Polyclinic. 1888;6:43. [CrossRef]
- Mollnau H, Wenzel P, Oelze M, et al. Mitochondrial oxidative stress and nitrate tolerance comparison of nitroglycerin and pentaerithrityl tetranitrate in Mn-SOD+/- mice. BMC Cardiovasc Disord. 2006 Nov 8;6:44. https://doi.org/10.1186/1471-2261-6-44. [CrossRef] [PubMed] [PubMed Central]
- Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci USA. 1998;95(13):7631-7636. [CrossRef]
- Esplugues JV, Rocha M, Nunez C, et al. Complex I dysfunction and tolerance to nitroglycerin: an approach based on mitochondrial-targeted antioxidants. Circ Res. 2006;99(10): 1067-1075. [CrossRef]
- Kaesemeyer W and Suvorava T. Nitric Oxide is the Cause of Nitroglycerin Tolerance : Providing an Old Dog New Tricks For Acute Heart Failure. J Cardiovasc Pharmacol Ther. 2022; 27, 1-9. [CrossRef]
- Kaesemeyer W, Suvorava T. Treating Acute Decompensated Heart Failure in Patients with COVID-19 Using Intravenous Nitroglycerin in 5% Glutathione. Am J Cardiovasc Drugs. 2021 Nov;21(6):589-593. [CrossRef] [PubMed] [PubMed Central]
- Bonini MG, Stadler K, Silva SO, et al. Constitutive nitric oxide synthase activation is a significant route for nitroglycerin-mediated vasodilation. Proc Natl Acad Sci U S A. 2008 Jun 24;105(25):8569-74. Epub 2008 Jun 18. [CrossRef] [PubMed] [PubMed Central]
- Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biolog Chem. 2003;278(22): 19603-19610. [CrossRef]
- Tsou P-S, Addanki V, Haas JA, et al. Role of Glutaredoxin-Mediated Protein S-Glutathionylation in Cellular Nitroglycerin Tolerance. Journal of Pharmacology and Experimental Therapeutics.2009; 329(2):649-56. [CrossRef]
- Chen CA, Wang TY, Varadharaj S, et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010 Dec 23;468(7327):1115-8. [CrossRef] [PubMed] [PubMed Central]
- Chandra S, Romero MJ, Shatanawi A, et al. Oxidative species increase arginase activity in endothelial cells through the RhoA/ Rho kinase pathway. Br J Pharmacol. 2012;165(2):506-519. [CrossRef]
- Abou-Mohamed G, Johnson JA, Jin L, et al. Roles of superoxide, peroxynitrite, and protein kinase C in the development of tolerance to nitroglycerin. J Pharmacol Exp Ther. 2004;308(1): 289-299. [CrossRef]
- Mao M, Varadarajan S, Fukai T, et al. Nitroglycerin tolerance in caveolin-1 deficient mice. PLoS One. 2014;9(8):e104101. [CrossRef]
- Needleman P, Blehm D J, Rotskoff K S. Relationship between glutathione dependent denitration and the vasodilator effectiveness of organic nitrates. J Pharmacol Exp Ther. 1969; 165: 2, 286-8.
- Needleman P, Johnson EM. Mechanism of tolerance development to organic nitrates. J Pharmacol Exp Ther. 1973;184:709-713.
- Horowitz J D, Antman E M, Lorell B H et al. Potentiation of the cardiovascular effects of nitroglycerin by N-acetylcysteine. Circulation. 1983; 68: 6, 1247-53. [CrossRef]
- Packer M, Lee WH, Kessler PD, et al.et al. Prevention and reversal of nitrate tolerance in patients with congestive heart failure. N Engl J Med. 1987;317(13):799-804. [CrossRef]
- Giugliano D, Marfella R, Verrazo G et.al. Abnormal rheologic effects of glyceryl trinitrate in patients with non-insulin-dependent diabetes mellitis and reversal by antioxidants. Ann Intern Med. 1995; 123:5,338-43. [CrossRef]
- Kugiyama K, Ohgushi M, Motoyama T, et al. Intracoronary infusion of reduced glutathione improves endothelial vasomotor response to acetylcholine in human coronary circulation. Circulation. 1998;97(23):2299-22301. 10.1161/01.cir.97.23.2299.
- Prasad A, Andrews N P, Padder F A et al. Glutathione reverses endothelial dysfunction and improves nitric oxide bioavailability. J Am Coll Cardiol. 1999; 34: 2,507-514. [CrossRef]
- Venardos K, Zhang WZ, Lang C, Kaye DM. Effect of peroxynitrite on endothelial L-arginine transport and metabolism. Int J Biochem Cell Biol. 2009;41(12):2522-2527. [CrossRef]
- Kaesemeyer WH, Ogonowski AA, Jin L, et al. Endothelial nitric oxide synthase is a site of superoxide synthesis in endothelial cells treated with glyceryl trinitrate. Br J Pharmacol. 2000;131(5):1019-1023. [CrossRef]
- Schmitt B, Vicenzi M, Garrel C, et al. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 2015 Dec;6:198-205. Epub 2015 Jul 29. [CrossRef] [PubMed] [PubMed Central]
- Jonathan Abrams.New nitrate delivery systems: Buccal nitroglycerin. American HeartJournal. 1983;105:5, 848-854. ISSN 0002-8703. (https://www.sciencedirect.com/science/article/pii/000287038390251X). [CrossRef]
- Parker JO, Parker JD, Caldwell RW, Farrell B, Kaesemeyer WH. The effect of supplemental L-arginine on tolerance development during continuous transdermal nitroglycerin therapy. J Am Coll Cardiol. 2002;39(7):1199-1203. [CrossRef]
- Pellicori P, Cleland JGF, Borentain M, et al. Impact of vasodilators on diuretic response in patients with congestive heart failure: A mechanistic trial of cimlanod (BMS-986231). Eur J Heart Fail. 2024 Jan;26(1):142-151. Epub 2023 Dec 28. [CrossRef] [PubMed]
- Ho HJ, Shirakawa H. Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells. 2022 Dec 25;12(1):88. [CrossRef] [PubMed] [PubMed Central]
- Smulik R, Debski D, Zielonka J, et al. Nitroxyl (HNO) reacts with molecular oxygen and forms peroxynitrite at physiological pH. Biological Implications. J Biol Chem. 2014;289(51):35570-35581. [CrossRef]
- Zou AP, Li N, Cowley AW Jr. Production and actions of superoxide in the renal medulla. Hypertension. 2001 Feb;37(2 Pt 2):547-53. [CrossRef] [PubMed]
- Zaibi N, Li P, Xu SZ. Protective effects of dapagliflozin against oxidative stress-induced cell injury in human proximal tubular cells. PLoS One. 2021 Feb 19;16(2):e0247234. [CrossRef] [PubMed] [PubMed Central]
- Schulze PC, Bogoviku J, Westphal J, et al. Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients With Acute Decompensated Heart Failure (EMPAG-HF). Circulation. 2022 Jul 26;146(4):289-298. Epub 2022 Jun 29. [CrossRef] [PubMed]
- Müller M, Bischof C, Kapries T, et al. Right Heart Failure in Mice Upon Pressure Overload Is Promoted by Mitochondrial Oxidative Stress. JACC Basic Transl Sci. 2022 Jul 6;7(7):658-677. [CrossRef] [PubMed] [PubMed Central]
- Mehra M R, Uriel N, Naka Y et al. A Fully Magnetically Levitated Left Ventricular Assist Device – Final Report. N Engl J Med. 2019;380: 1618-1627. [CrossRef]
- Birks EJ, Drakos SG, Patel SR, et al. Prospective multicenter study of myocardial recovery using left ventricular assist devices (RESTAGE-HF [Remission from Stage D Heart Failure]): medium-term and primary end point results. Circulation. 2020; 142(21):2016-2028. [CrossRef]
- Cheng K., Sainsbury P., Fisher M., and de Silva R. Management of Refractory Angina Pectoris. Eur Cardiol. 2016 Dec; 11(2): 69–76. [CrossRef]
- Foley MJ, Rajkumar CA, Ahmed-Jushuf F, et al. Coronary sinus reducer for the treatment of refractory angina (ORBITA-COSMIC): a randomised, placebo-controlled trial. Lancet. 2024 Apr 20;403(10436):1543-1553. Epub 2024 Apr 8. [CrossRef] [PubMed]
- Kojima S, Nonogi H, Morii I, Sumida H, Sutani Y, Yasuda S, Daikoku S, Goto Y, Miyazaki S. Is inflammation related to the clinical severity of unstable angina? Jpn Circ J. 2001 May;65(5):414-8. [CrossRef] [PubMed]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011 Jan 13;469(7329):221-5. Epub 2010 Dec 1. Erratum in: Nature. 2011 Jul 7;475(7354):122. [CrossRef] [PubMed]
- Zhang T, Tsutsuki H, Li X, Sawa T. New insights into the regulatory roles of glutathione in NLRP3-inflammasome-mediated immune and inflammatory responses. J Biochem. 2022 Mar 31;171(4):367-377. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).