1. Introduction
Immunotherapy, emerging as a prominent strategy in cancer treatment, has garnered significant attention and research efforts [
1,
2,
3]. Innovative cancer immunotherapies, exemplified by monoclonal antibodies targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) [
4,
5,
6], have revolutionized the landscape of cancer therapy. Immune checkpoint inhibitors (ICI) circumvent inhibitory checkpoint molecules on tumor cells, thereby enhancing T-cell responsiveness and preventing immune exhaustion, thereby restoring immune surveillance [
7,
8,
9]. Despite achieving clinical success, these therapies demonstrate considerable variability in response rates across patients [
9,
10]. For instance, the response rate to ipilimumab is only about 15%, with rarely more than 25% in patients receiving anti-PD-1/PD-L1 ICI therapy [
12,
13,
14]. The efficacy of ICI appears correlated with pre-existing pro-inflammatory tumor microenvironments (TME), characterized by heightened immune cell infiltration or PD-L1 expression, while "immune-desert" or "cold" tumors exhibit limited responsiveness to ICI [
15,
16,
17]. To maximize the efficacy of ICI therapy, "cold" tumors must be reprogrammed into an immunogenic and pro-inflammatory or "hot" phenotype, which can reinvigorate anti-tumor immunity. One promising approach involves upregulating the stimulator of interferon genes (cGAS-STING) pathway, a major component of the innate immune system involved in antiviral and anti-tumor immunity [
18,
19]. cGAS-STING senses cytoplasmic double-stranded DNA (dsDNA) and triggers a cascade of downstream signaling events in response to infection [
20]. This pathway bridges innate and adaptive immunity, facilitating the activation and migration of dendritic cells (DCs) and subsequent initiation of cytotoxic T lymphocytes (CTLs) at tumor sites [
21]. Thus, this pathway has the potential to overcome immune-suppressive environments in certain cancers, rendering patients more responsive to immune checkpoint inhibitor (ICI) therapy [
22]. Activation of the innate immune response through exogenous stimulation with STING agonists, such as cyclic GMP-AMPP, has proven effective in enhancing the efficacy of immunotherapy [
23]. However, STING agonists based on cyclic dinucleotides (CDNs) are small molecules with hydrophilic negative charges, making them prone to enzymatic degradation and limiting their activation of the STING pathway in target tissues [
24,
25] Non-nucleotide STING agonists like SR-717 have shown potential in overcoming the instability associated with cyclic dinucleotides (CDN)-based agonists [
26]. Nevertheless, these drugs are hydrophobic, which contributes to their poor targeting and side effects, restricting their clinical utility [
27]. Therefore, there is a critical need to develop new treatment strategies capable of improving drug solubility, targeting specificity, and reducing side effects.
Targeted delivery system has been widely utilized in biomedical research to address these challenges [
28,
29,
30]. Many researchers have proposed various drug delivery strategies aimed at improving STING agonists utilization efficiency to enhance anti-tumor immune effects. For example, Zhou et al. used PMOF nanoparticles for light-triggered STING agonist SR-717 release, enhancing photodynamic-immune therapy [
31]. Similarly, Lu et al. developed hollow manganese dioxide nanoparticles with STING agonist MSA-2 and CRISPR-Cas9/sg-PD-L1 plasmid for sustained release in tumors, activating the cGAS-STING pathway and reducing PD-L1 [
32]. Additionally, a biomimetic cancer cell membrane-coated nano-vaccine delivery system (PLGA/STING@EPBM) effectively delivers STING agonists and tumor antigens to Clec9a
+ DCs, showing significant anti-tumor synergy with radiotherapy [
33]. These advancements in nanocarrier development effectively address the limitations of STING agonists in clinical applications caused by delivery barriers and adverse reactions. However, the complex preparation process and challenges in translation to clinical practice remain significant drawbacks.
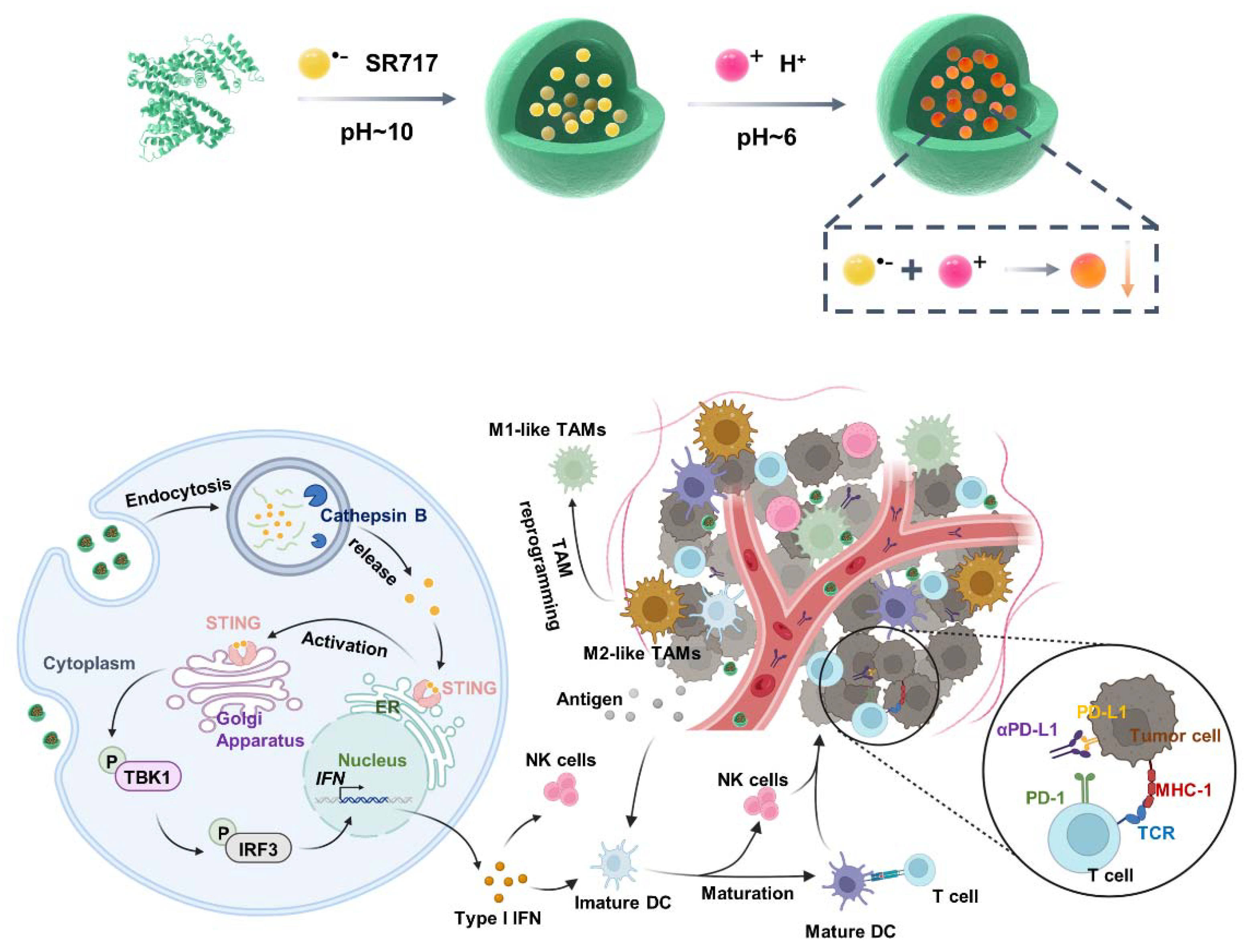
In recent years, our research has focused on developing innovative protein-based nanomedicine delivery systems with excellent targeting capabilities, high drug loading efficiency, and improved safety, significantly enhancing the efficacy of tumor therapy [
34,
35]. Building on this foundation, we have also explored the use of human serum albumin as nanoreactors at the single-molecule level. By leveraging biomineralization principles, we have achieved controlled nucleation and crystallization of therapeutic agents within the protein nanocage, resulting in multifunctional protein nanoparticles with tunable dimensions. These nanoparticles hold significant promise for targeted cancer therapies, including chemotherapy, photothermal therapy, and photodynamic therapy [
36,
37,
38]. Herein, we employ a biomineralization approach using a single-molecule albumin template to encapsulate the STING agonist SR717 (SH-NPs). These nanoparticles exhibited excellent tumor targeting ability with superior serum stability and efficient cellular uptake, thus considerably activating the STING signaling pathway, followed by relieving the immunosuppression and improving the immunogenetic feature of TME. Finally, SH-NPs boosts antitumor T-cell immunity and promotes the therapeutic efficacy of checkpoint blockade, which is further validated in freshly isolated human renal tumor tissues, highlighting their clinical translation potential. These findings pave the way towards targeted STING agonist delivery for reinvigorated immunotherapy of intractable cancers (
Scheme 1).
2. Materials and Methods
2.1. Materials
SR717 was purchased from Selleck Co., Ltd. Human serum albumin (HSA) was obtained from Aladdin Reagent. RPMI 1640 medium, fetal bovine serum (FBS), trypsin EDTA solution and Penicillin-streptomycin solution were purchased from Gibco Life Technologies (California, USA). IL-4 protein and IFN-γ protein were purchased from Peprotech. Cy5.5 N-hydroxysuccinimide (NHS) ester and LPS proteins were purchased from Sigma. ELISA kits for IFN-β, CXCL-10, IL-6, and TNF-α were purchased from Lianke. Anti-Phospho-TBK1 antibody (CST, catalog number 5483S, dilution: 1:1000), anti-Phospho-IRF3 antibody (CST, catalog number 29047S, dilution: 1:1000), anti-TBK1 antibody (CST, catalog number 38066S, dilution: 1:1000), anti-IRF3 antibody (CST, catalog number 4550S, dilution: 1:1000), and anti-GAPDH antibody (Abclonal, catalog number AC033, dilution: 1:5000) were purchased from CST. Goat anti-rabbit IgG H&L secondary antibody (Abcam, catalog number ab6702, dilution: 1:5000) was purchased from Abcam. PE anti-mouse CD80 (BioLegend, catalog number 104708, dilution: 1:200), APC anti-mouse CD86 (BioLegend, catalog number 105012, dilution: 1:200), PE anti-mouse CD86 (BioLegend, catalog number 105007, dilution: 1:200), PE anti-mouse CD206 (BioLegend, catalog number 141706, dilution: 1:200) FITC anti-mouse CD45 (BioLegend, catalog number 103108, dilution: 1:200), APC anti-mouse CD3 (BioLegend, catalog number 100236, dilution: 1:200), PE anti-mouse CD8a (BioLegend, catalog number 100708, dilution: 1:200), PE anti-mouse CD335 (BioLegend, catalog number 137604, dilution: 1:200), FITC anti-mouse CD11c (BioLegend, catalog number 117306, dilution: 1:200), PE anti-mouse CD80 (BioLegend, catalog number 104708, dilution: 1:200), FITC anti-mouse/human CD11b (BioLegend, catalog number 101206, dilution: 1:200), APC anti-mouse F4/80 (BioLegend, catalog number 123116, dilution: 1:200), FITC anti- human CD14 (BioLegend, catalog number 982502, dilution: 1:200), APC anti-human CD68 (BioLegend, catalog number 333809, dilution: 1:200), PE anti- human CD86 (BioLegend, catalog number 374205, dilution: 1:200), and PE anti-human CD206 (BioLegend, catalog number 321105, dilution: 1:200) were purchased from Biolegend.
2.2. Cell Lines
RENCA (catalog number CL-0568), DC2.4 (catalog number SCC142) and RAW264.7 (catalog number K1673) were purchased from Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). These cell lines were cultured in RPMI 1640 Medium supplemented with 1% penicillin-streptomycin and 10% FBS under 5% CO2 at 37 °C in a humidified incubator.
2.3. Animals and Ethics Statement
BALB/c mice (female, 18 ± 2 g, 6-8 weeks), were purchased from Shanghai SLAC Animal Technology Co., Ltd. (Shanghai China). Mice were housed in an animal facility under constant environmental conditions (room temperature, 21 ± 1 °C; relative humidity, 40-70% and a 12 h light-dark cycle). All mice had access to food and water. All animal experiments were carried out following protocols approved by Laboratory Animal Center of Soochow University (No. ECSU-2019000179). In our experiment, the maximum tumor burden was 1500 mm3 in mice, which is lower than the maximal tumor burden permitted by Laboratory Animal Center of Soochow University.
2.4. Synthesis
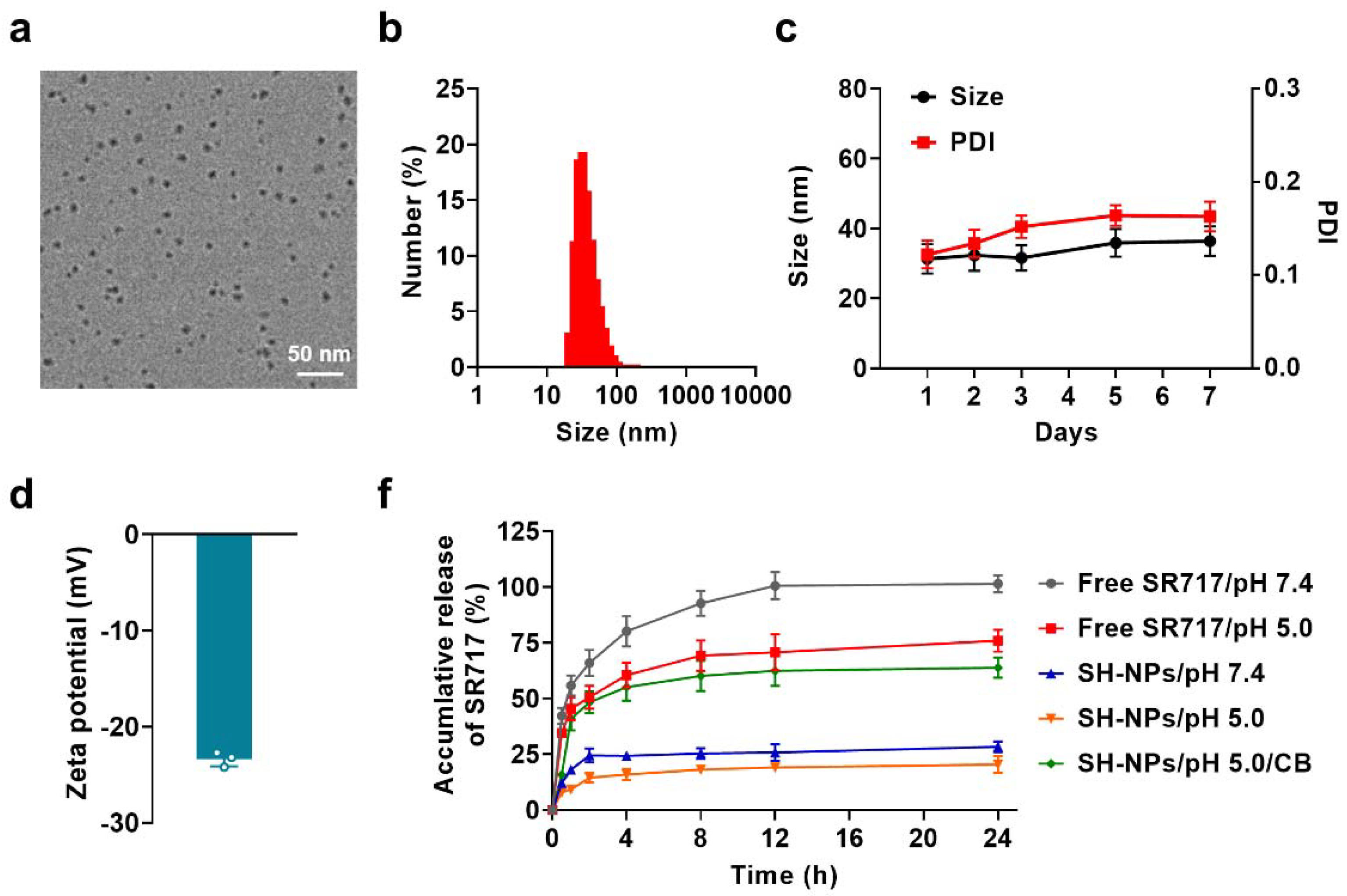
For the preparation of SH-NPs, 1.0 mL SR-717 solution (1.0 mg mL-1) was added into 30.0 mg human serum albumin (HSA) dispersing in 3.0 mL deionized water under vigorous stirring together with adjusting the pH to 10. After complete dissolution, adjusted the pH to 6 and followed by reaction at 55 °C for 4 h. Finally, SH-NPs were purified through centrifugation and ultrafiltration (100 kDa MWCO, 1000 g, 20 min/each) for 5 times and stored in PBS solution (pH 7.4, 10.0 mM).
2.5. Characterization
The morphology of SH-NPs was observed by transmission electron microscopy (TEM) (HT7700, Hitachi). The hydrodynamic diameter and zeta potential were quantified via dynamic light scattering (DLS) (Zetasizer ZS90, Malvern). The concentration of SR717 was determined through the application of reversed-phase high-performance liquid chromatography (HPLC) (Agilent 1100, Agilent).
2.6. Drug Release
To investigate the drug release kinetics, SR717 was evaluated in free SR717 and SH-NPs using the dialysis method. A volume of 1.0 mL of the sample (0.2 mg mL−1 of SR717) was placed in dialysis bags with a molecular weight cut-off of 3.5 kDa. These drug-loaded dialysis bags were then submerged in pH 7.4 phosphate buffer, pH 5.0 acetate buffer, and pH 5.0 acetate buffer containing 10 μg mL−1 of CB, and subsequently agitated using an oscillator shaker at 37 °C. The concentration of SR717 in the buffer was measured at 0, 0.5, 1, 2, 4, 8, 12, and 24 h.
2.7. Cellular Uptakes and Endocytic Pathway
For cellular uptake of SH-NPs, DC2.4 cells were seeded in plates (1.0 × 106 cells/well) followed by the addition of free SR717 and SH-NPs (10.0 µM SR717) and further incubation for 12 and 24 h. Following incubation, the cells were collected for cell counting and disruption under ultrasonication, and HPLC was used to determine the SR717 amount. For the endocytic pathway, the inhibitors including 10.0 µg·mL−1 chlorpromazine, 100.0 µg·mL−1 amiloride, 5.0 µg·mL−1 nystatin were added into DC2.4 cells (1.0 × 106 cells/well) followed by 1 h incubation at 37 °C or 4 °C in serum-free RPMI 1640 medium. Then, SH-NPs (10.0 µM SR717) were added into the medium for 12 h incubation. Afterward, the cells were collected through trypsin treatment, centrifugation, and lysis under ultrasonication. Finally, the amount of SR717 was determined by the HPLC.
2.8. In Vitro Cytotoxicity
To evaluate the cytotoxicity, DC2.4 cells (1.0 × 104 cells/well) were incubated with free SR717 and SH-NPs at the concentration of 0, 1.25, 2.5, 5.0, 10.0 and 20.0 µM SR717 for 24 h. The MTT assay was applied to evaluate the cell viability.
2.9. Western Blot
DC2.4 cells were incubated with free SR717 and SH-NPs (10.0 µM SR717) for 24 h. Cells were collected to extract total proteins using Protein Extraction Kit from Beyotime. Protein concentration was determined using a BCA assay. Then, 20.0 µg of each sample's proteins were run on the 10% SDS-polyacrylamide gel and transferred to a PVDF membrane. The membrane was blocked with 5% bovine serum albumin for 2 h, followed by incubation with anti-Phospho-TBK1 antibody (CST, 5483S, dilution: 1:1000), anti-Phospho-IRF3 antibody (CST, 29047S, dilution: 1:1000), anti-TBK1 antibody (CST, 38066S, dilution: 1:1000), anti-IRF3 antibody (CST, 4550S, dilution: 1:1000), and anti-GAPDH antibody (Abclonal, AC033, dilution: 1:5000) overnight at 4 °C. The membrane was then incubated with goat anti-rabbit IgG H&L secondary antibody (Abcam, ab6702, dilution: 1:5000) followed by visualization with ECL using a detection system (GE healthcare). For the tumor tissue protein extraction, SH-NPs (30.0 mg kg−1) were intravenously injected into the BALB/c mice (female, 6-8 weeks) bearing RENCA tumors (n=3 mice per group). After 72 h, the mice were euthanized and the tumors were harvested. Tissues were lysed with 0.5% (v/v) CHAPS containing protease and phosphatase inhibitors.
2.10. DC Maturation
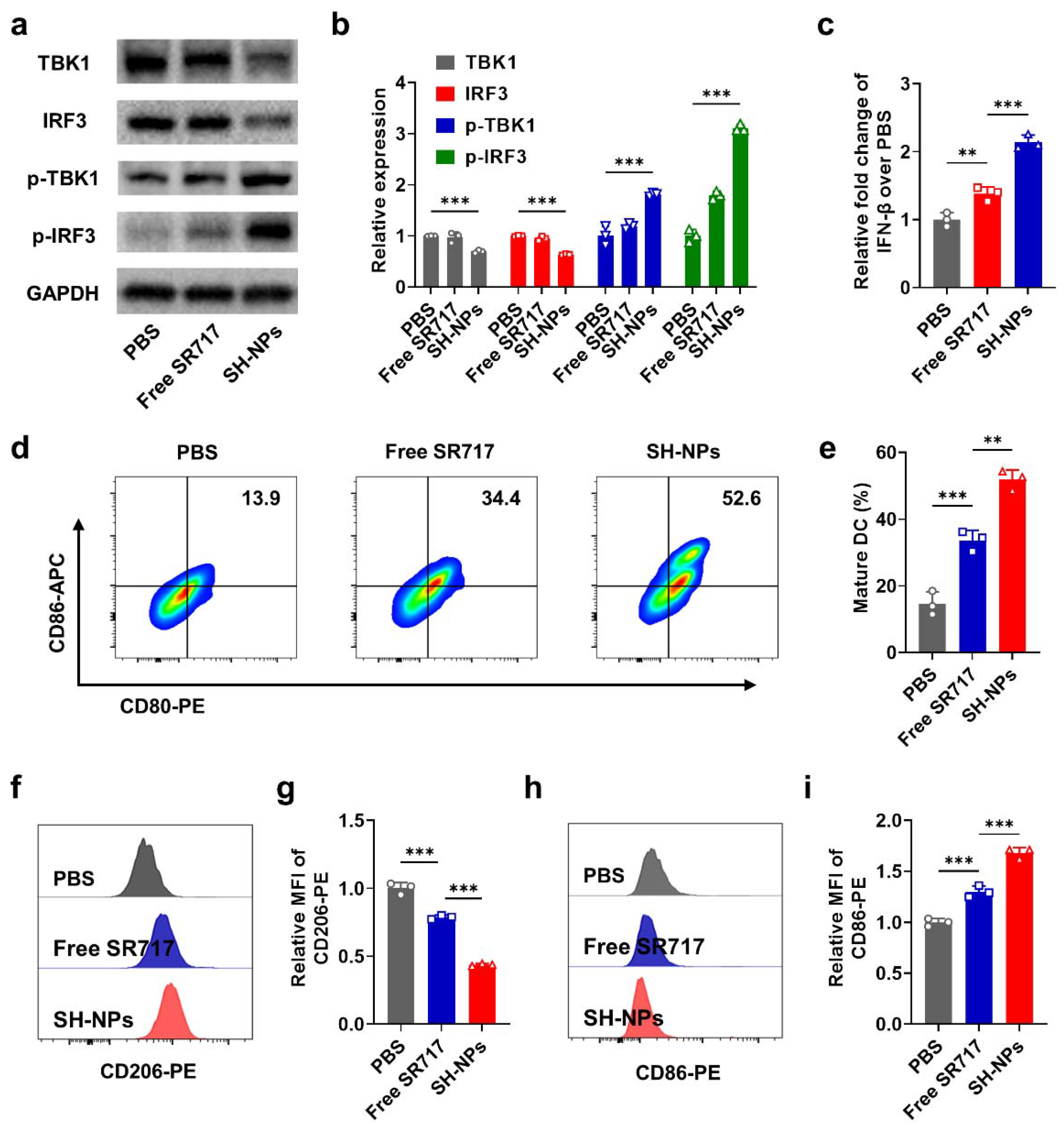
For DC stimulation experiments, DC2.4 cells were incubated with free SR717 and SH-NPs (10.0 µM SR717) for 24 h. Then DCs were stained with anti-CD86 PE and anti-CD80 APC, detected by flow cytometer to investigate their maturation via the surface expression of the costimulatory molecules CD86 and CD80. Additionally, the levels of IFN-β in the culture medium were measured by ELISA kits (Lianke).
2.11. Macrophage Polarization
For Macrophage polarization experiments, M1 macrophages were induced with 100.0 ng mL−1 of LPS and 20.0 ng mL−1 of IFN-γ, and M2 macrophages were induced with 20.0 ng mL−1 of IL-4. Polarized macrophage phenotypes were identified using flow cytometry. Subsequently, M2 macrophages were incubated with free SR717 and SH-NPs (10.0 µM SR717) for 24 h. Following incubation, RAW264.7 cells were stained with anti-CD206 PE and anti-CD86 PE, detected by flow cytometer to investigate their polarization via the surface expression of the costimulatory molecules CD206 and CD86.
2.12. Construction of RENCA Tumor Models
RENCA tumor cells (5.0 × 106 cells per mouse) were subcutaneously injected into the right back of the BALB/c mice (female, 6-8 weeks) to construct the above subcutaneous tumor models.
2.13. In Vivo Tumor Targeting
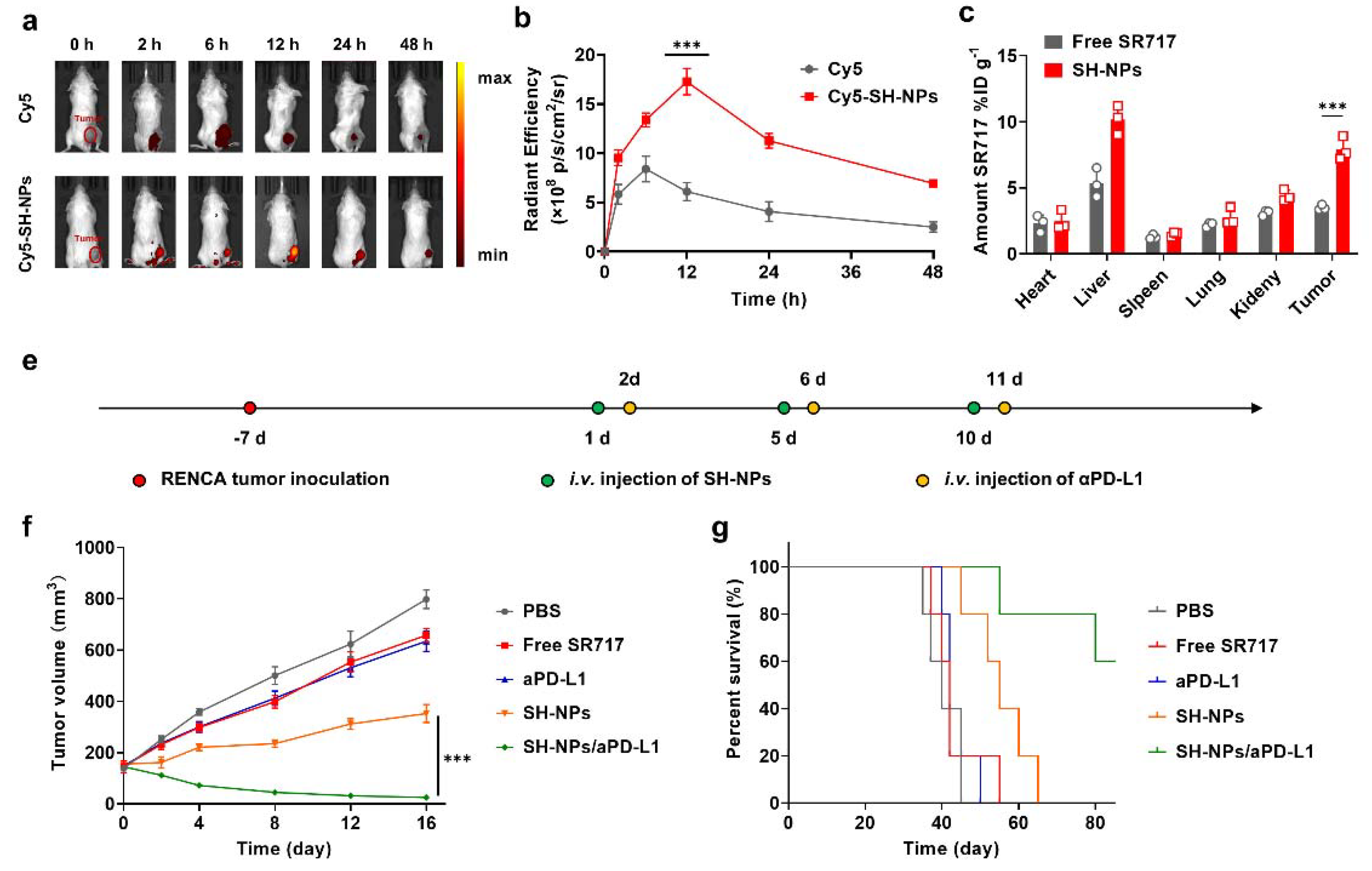
To evaluate the tumor targeting efficacy in RENCA tumors, Cy5-SH-NPs (30.0 mg kg−1 SR717) were intravenously injected into the mice bearing RENCA tumors (n=3 mice per group). Then fluorescence images were taken at intervals of 0, 2, 6, 12, 24, and 48 h, and analyzed using Living Imaging software.
2.14. In Vivo Biodistribution
To evaluate the biodistribution in RENCA tumors, free SR717 and SH-NPs (30.0 mg kg−1 SR717) were intravenously injected into the BALB/c mice (female, 6-8 weeks) bearing RENCA tumors (n=3 mice per group). Then, methanol was employed to extract SR717 from the heart, liver, spleen, lung, kidney, and tumor tissues at 12 h following injection. Finally, the amounts of SR717 were determined using HPLC.
2.15. In Vivo Antitumor Efficacy
To evaluate the antitumor efficacy, free SR717 and SH-NPs (30.0 mg kg−1 SR717) were intravenously injected into the mice bearing RENCA tumors (n=5 mice per group), followed by measurement of tumor volumes (V = 0.5 × L × W2, V, L, and W represent the tumor volume, long diameter, and short diameter, respectively.).
To evaluate the synergistic antitumor efficacy of SH-NPs and aPD-L1 against subcutaneous RENCA tumor models, SH-NPs (30.0 mg kg−1 SR717) and aPD-L1 (3.0 mg kg−1) were administered intravenously to mice with tumors measuring 75–100 mm³ (n=5 per group). Twenty-four hours after the initial injection, an additional dose of 3.0 mg/kg aPD-L1 was given intravenously. Tumor volumes were measured (V = 0.5 × L × W2, V, L, and W represent the tumor volume, long diameter, and short diameter, respectively.).
2.16. Cytokine Analysis
To evaluate the cytokine analysis, free SR717, SH-NPs (30.0 mg kg−1 SR717) and aPD-L1 (3.0 mg kg−1) were intravenously injected into the mice bearing RENCA tumors (n=3 mice per group). Afterward, 3.0 mg kg−1 aPD-L1 were intravenously injected after 24 h. The mice were euthanized and blood was collected at 3 days post-treatments, after which plasma was obtained by centrifugation. Then, the levels of IFN-β, CXCL-10, IL-6 and TNF-α were measured by ELISA kits (Lianke).
2.17. Antitumor Immune Response
Briefly, SH-NPs (30 mg kg−1 SR717) was administrated intravenously into mice bearing the RENCA tumor model (n = 3 mice per group). Afterward, 3.0 mg kg−1 aPD-L1 were intravenously injected 24 h later. To evaluate in vivo antitumor immune response, lymph nodes and tumors were harvested at 3 days post-treatments from BALB/c mice (female, 6-8 weeks) bearing subcutaneous and RENCA tumors. The samples were then processed through homogenization or digestion with enzymes in staining buffers to prepare single cell suspensions. For T-cell and NK cells analysis, tumor cells were stained with FITC anti-mouse CD45 (BioLegend, 103108, dilution: 1:200), APC anti-mouse CD3 (BioLegend, 100236, dilution: 1:200), PE anti-mouse CD8a (BioLegend, 100708, dilution: 1:200) and PE anti-mouse CD335 (BioLegend, 137604, dilution: 1:200) antibodies for 60 min followed by analysis of CD45+CD3+CD8+ CTLs and CD45+CD3−CD335+ NK cells. For DCs analysis, lymph nodes were stained with FITC anti-mouse CD11c (BioLegend, 117306, dilution: 1:200), PE anti-mouse CD80 (BioLegend, 104708, dilution: 1:200) and APC anti-mouse CD86 (BioLegend, 105012, dilution: 1:200) antibodies for 60 min followed by analysis of CD11c+CD80+CD86+ DCs.For TAMs analysis, tumor cells were stained with FITC anti-mouse/human CD11b (BioLegend, 101206, dilution: 1:200), APC anti-mouse F4/80 (BioLegend, 123116, dilution: 1:200), and PE anti-mouse CD206 (BioLegend, 141706, dilution: 1:200), PE anti-mouse CD86 (BioLegend, 105007, dilution: 1:200) antibodies for 60 min followed by analysis of CD11b+F4/80+CD86+ TAMs and CD11b+F4/80+CD206+ TAMs. These immune cells data were detected with flow cytometry (FACS Aria III, BD) and analyzed with FlowJo software (Version 10).
2.18. Evaluation of Antitumor Immune Response in Resected Human Tissues
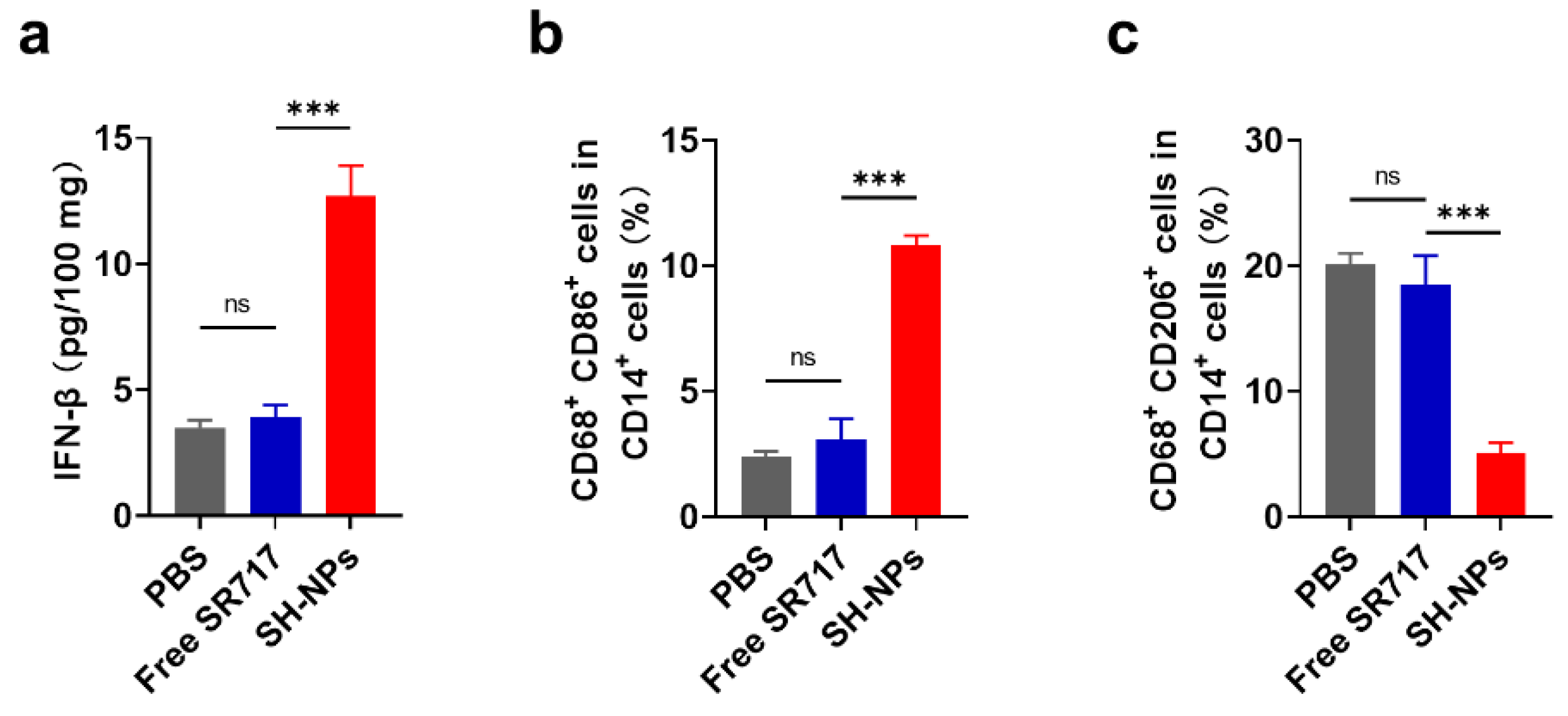
Patients provided consent for the use of biospecimens in research, as approved by the Fourth Affiliated Hospital of Soochow University. Freshly resected human tissues (primary RCC) were rinsed and divided into several sections (1–5 mm3) using a scalpel. Subsequently, these sections were injected with free SR717 and SH-NPs (500 µg SR717) in a 5% glucose solution within 30 minutes of resection. Each tissue section was cultured in 0.5 mL RPMI 1640 medium (supplemented with 10% heat-inactivated human serum, 1% insulin-transferrin-selenium, 1% GlutaMAX, and 1% penicillin–streptomycin) in a 24-well plate for 24 h. For TAMs analysis, tumour tissues were first digested by 1 mg mL−1 collagenase IV and 0.2 mg mL−1 DNase I (Sigma-Aldrich) for 45 min at 37 °C, then passed through a 70 μm nylon cell strainer to obtain single cells. Then, tumor cells were stained with FITC anti- human CD14 (BioLegend, 982502, dilution: 1:200), APC anti-human CD68 (BioLegend, 333809, dilution: 1:200), PE anti- human CD86 (BioLegend, 374205, dilution: 1:200), and PE anti-human CD206 (BioLegend, 321105, dilution: 1:200) antibodies for 60 min followed by analysis of CD14+CD68+CD86+ TAMs and CD14+CD68+CD206+ TAMs. The levels of IFN-β in the culture medium were measured by ELISA kits (Lianke).
2.19. Statistics and Reproducibility
Statistical analysis was performed using Graphpdad Prism (Version 8.0.2). All in vitro and in vivo experiments were repeated at least three times and data were presented as mean ± SD. Group size was determined on the basis of the preliminary experiments results, and no statistical method was used to predetermine sample size. The indicated sample size (n) represents biological replicates. For all studies, samples were randomly divided into different experimental groups. Group allocation and outcome assessment were not performed in a blinded manner. No data were excluded from the analyses. The statistical significance between two groups was calculated by unpaired two-tailed Student’s t test. The statistical significance between multiple groups was calculated by one-way ANOVA with Tukey’s post hoc test. Survival was measured using the Kaplan-Meier method and statistical significance was calculated by log-rank test. P < 0.05 was considered statistically significant.
4. Discussion
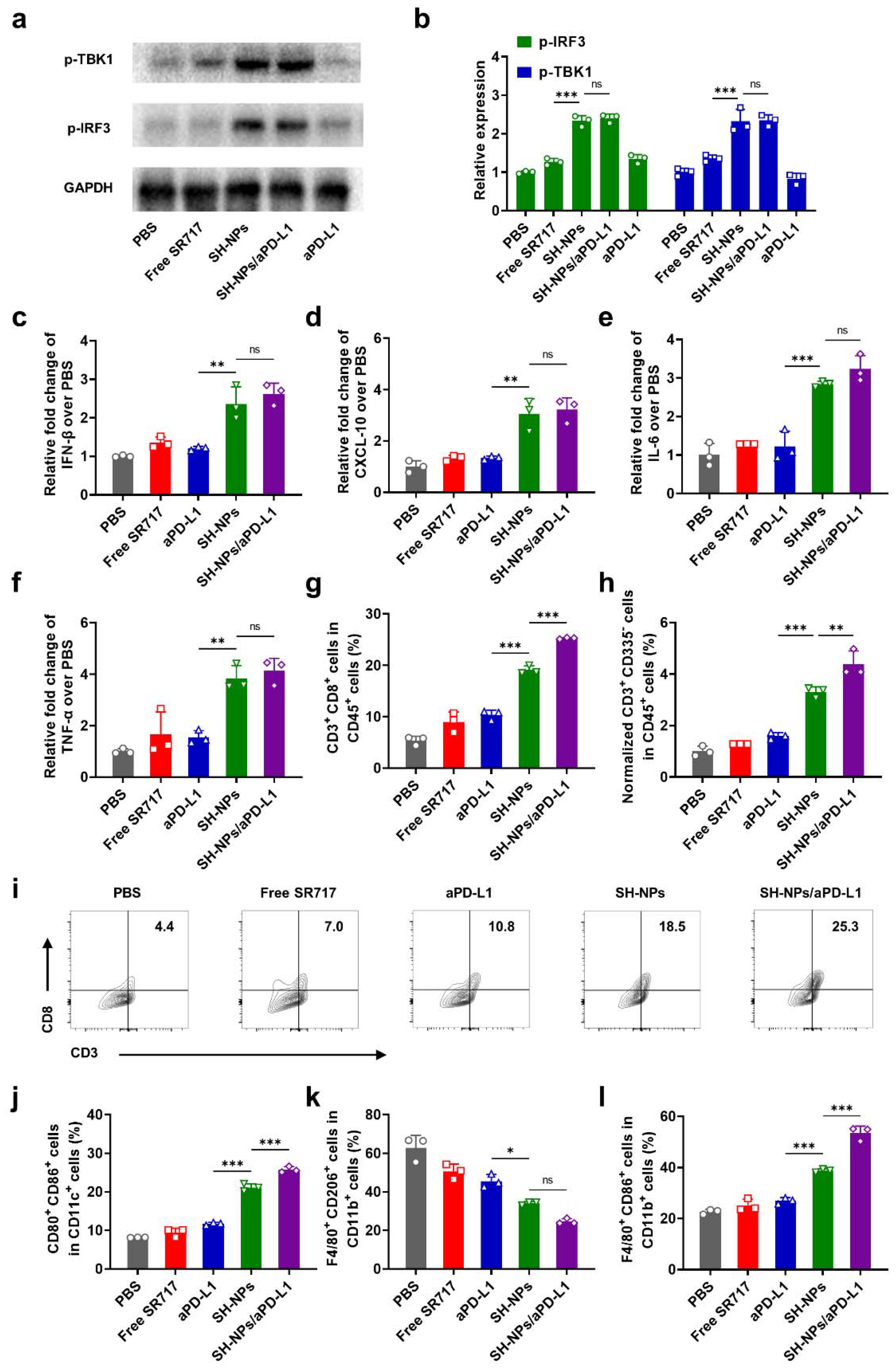
We have developed HSA-based nanoparticles as a highly effective approach to deliver the STING agonist SR717, which significantly improved the cellular uptake, stability, and STING activation of SR717, leading to enhanced anti-tumor immune responses both
in vitro and
in vivo. These findings support the potential of SH-NPs as a promising delivery system for cancer immunotherapy. Previous studies have highlighted the challenges associated with the delivery of STING agonists, particularly regarding their stability and targeting specificity. Traditional STING agonists, such as CDNs, suffer from rapid degradation and limited efficacy due to poor tissue penetration and off-target effects[
24,
25]. Non-nucleotide STING agonists like SR-717 have shown potential but still face issues related to hydrophobicity and targeting[
26,
41]. Our approach using HSA-based nanoparticles addresses these challenges by enhancing the solubility and targeting efficiency of SR717. Our research reveal that SH-NPs not only improve the stability and cellular uptake of SR-717 but also significantly activate the STING pathway, as evidenced by the elevated levels of phosphorylated TBK1 and IRF3 (
Figure 2a, b). This activation leads to the production of IFN-I proteins (
Figure 2c), crucial for initiating anti-tumor immune responses. The enhanced STING activation further promotes the maturation of DCs and the polarization of macrophages towards a pro-inflammatory phenotype (
Figure 2d, i). These immunological changes contribute to a more immunogenic tumor microenvironment, facilitating the recruitment and activation of CTLs and NK cells, which are essential for effective tumor eradication (
Figure 4g, h). The
in vivo studies in murine models demonstrated that SH-NPs significantly enhanced tumor targeting and retention of SR717, resulting in prolonged circulation time and higher tumor accumulation compared to free SR717 (
Figure 3a). This targeted delivery translated into superior anti-tumor efficacy, with SH-NPs effectively delaying tumor growth and improving survival rates in renal tumor-bearing mice. The combination of SH-NPs with anti-PD-L1 antibodies further potentiated the anti-tumor effects, leading to significant tumor regression in some cases (
Figure 3f). These results underscore the potential of SH-NPs in synergistic cancer immunotherapy.
In conclusion, this study demonstrates the potential of HSA-based nanoparticles for the targeted delivery of STING agonists, providing a promising strategy to enhance the efficacy of cancer immunotherapy. Our findings pave the way for the development of more effective and targeted therapeutic approaches, potentially transforming the landscape of cancer treatment.