Submitted:
29 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. MTT Assay (Cell Viability) and IC50
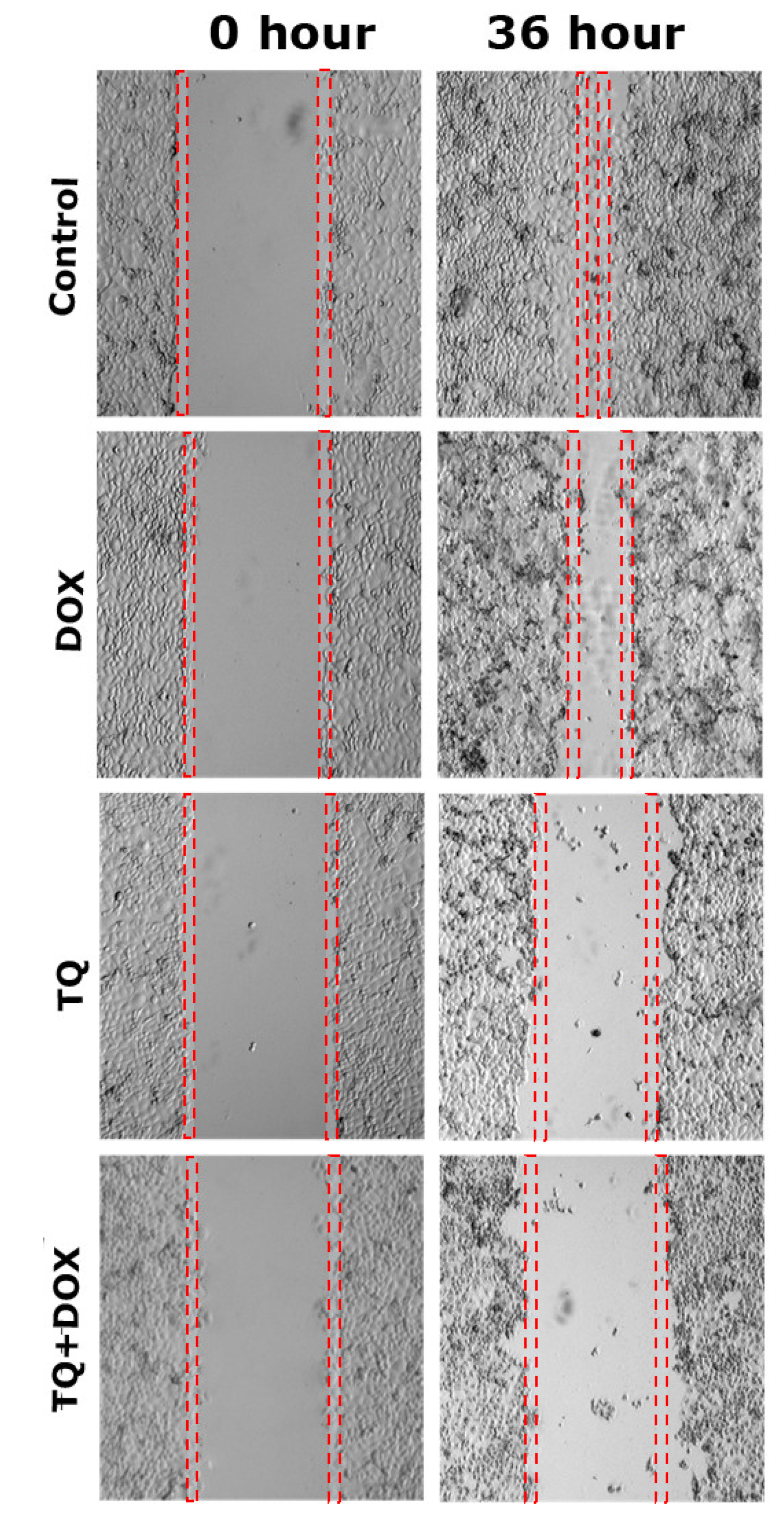
2.3. Wound Healing
2.4. Apoptotic Body Formation
2.5. RNA Isolation
2.8. Western Blot (EGFR, FOXP3)
2.9. Protein-Protein Interaction (PPI) Analysis
2.10. Enrichment Analysis
2.11. GO Functional Enrichment Analysis
2.11. Statistical Analysis
3. Results
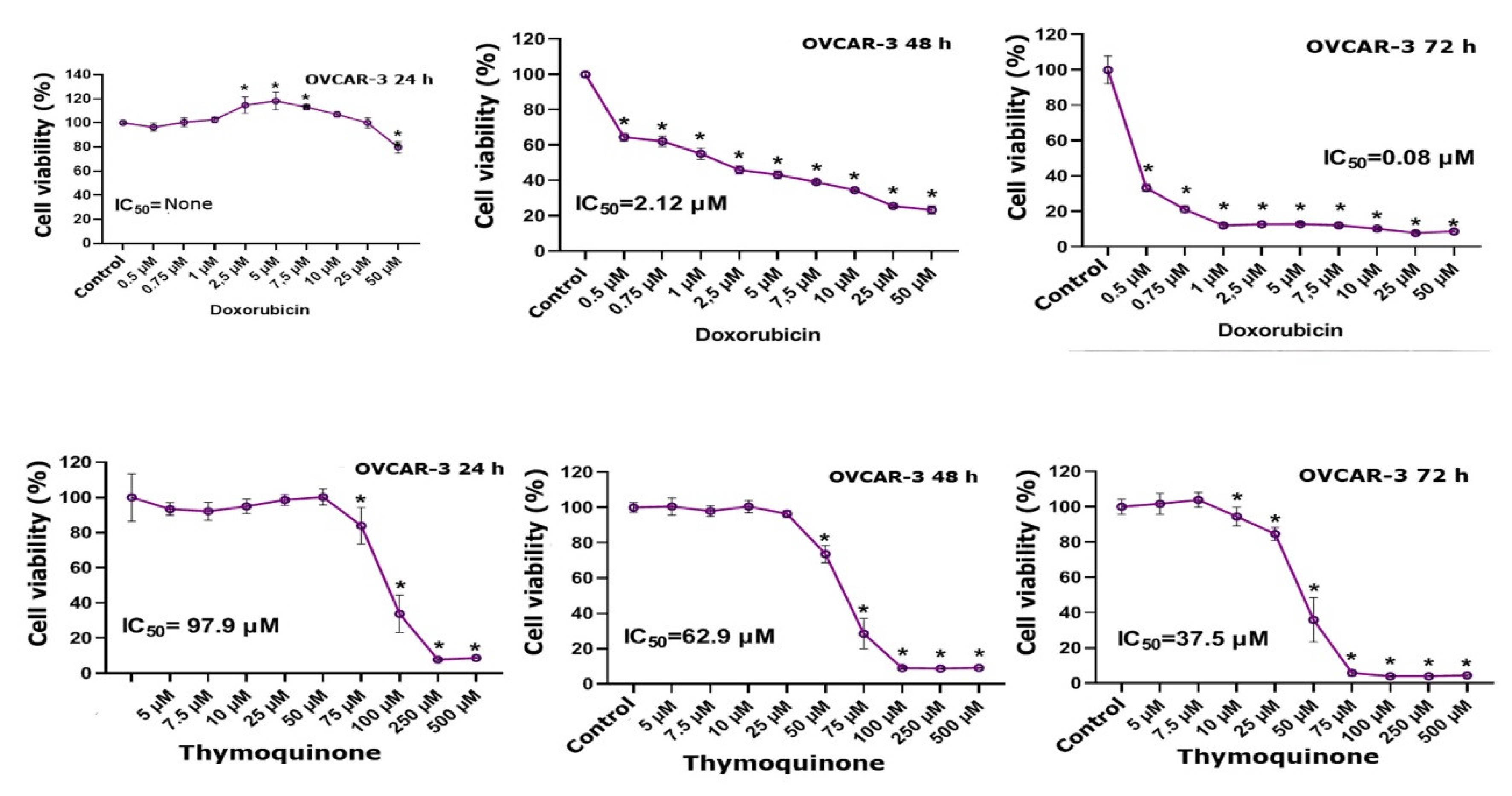
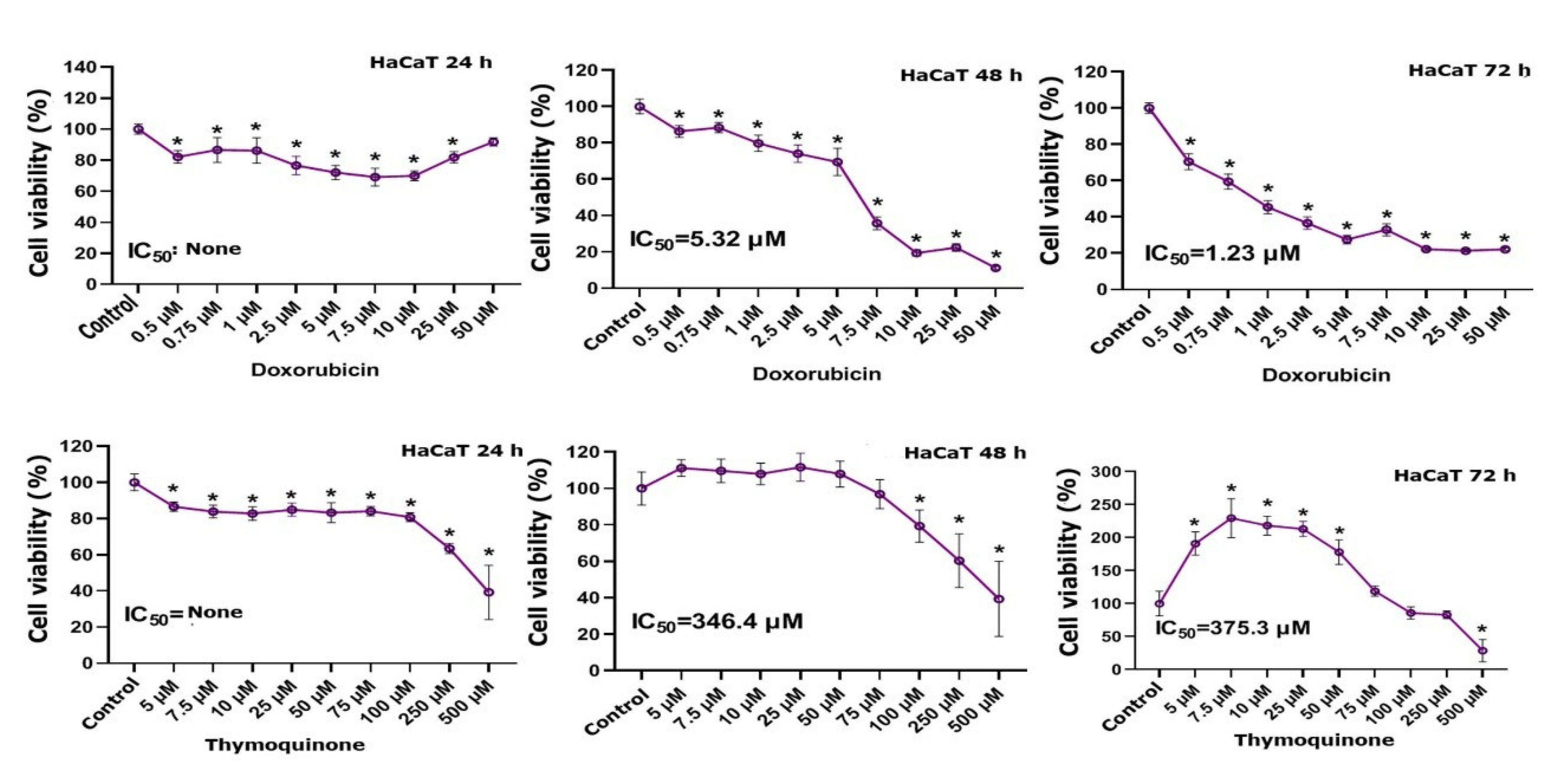
3.1. MTT Findings
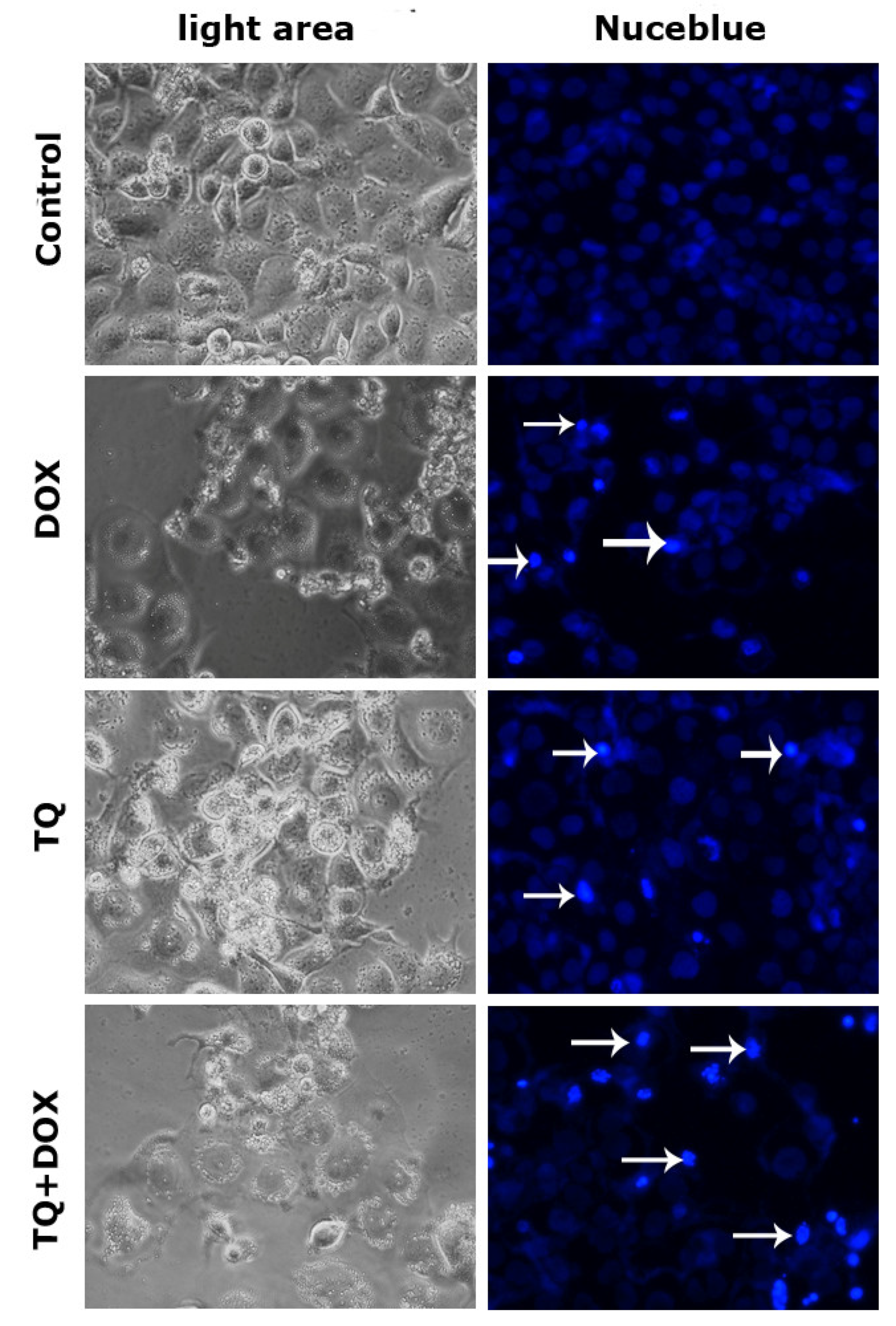
3.2. Nucblue Staining Findings
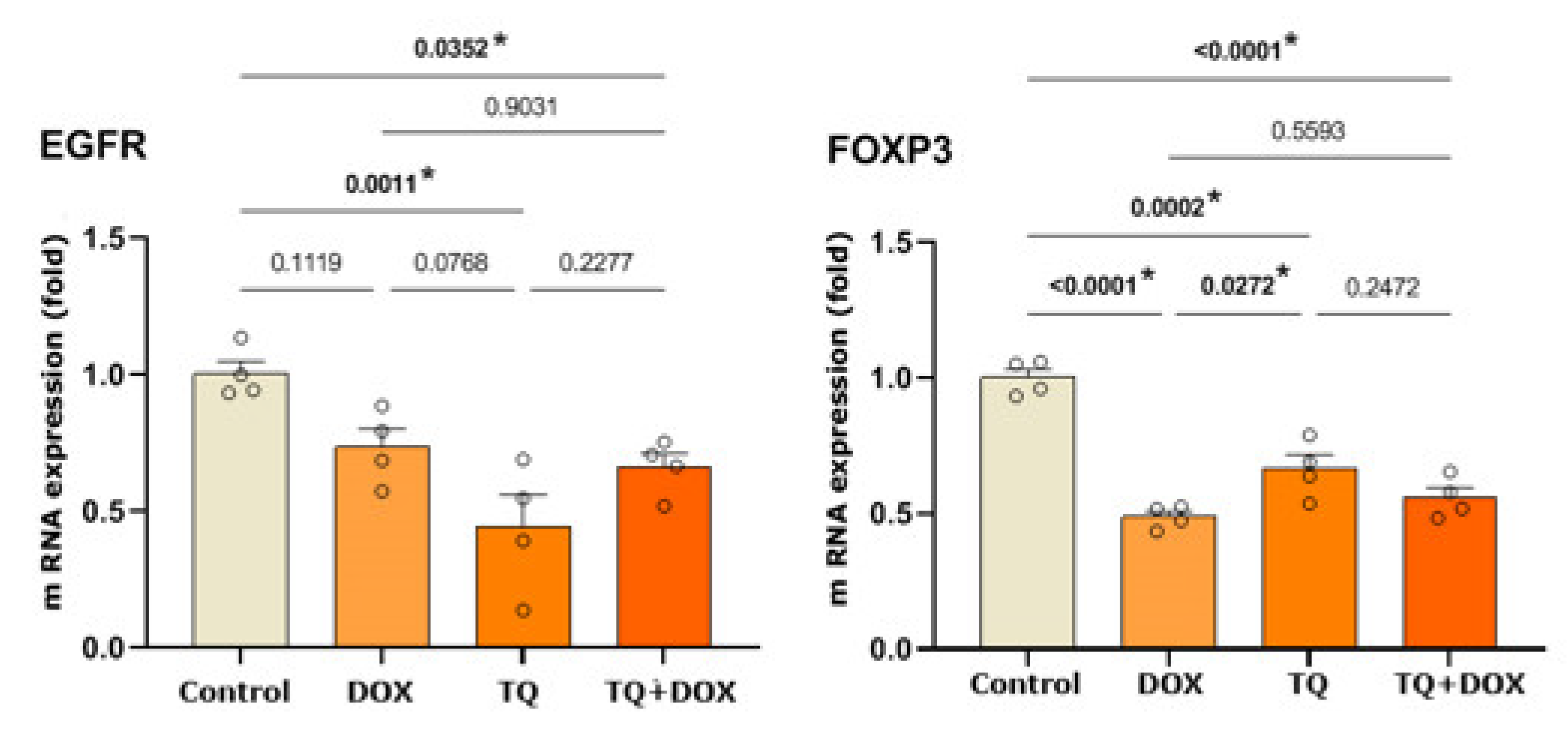
3.3. Western Blot Analysis
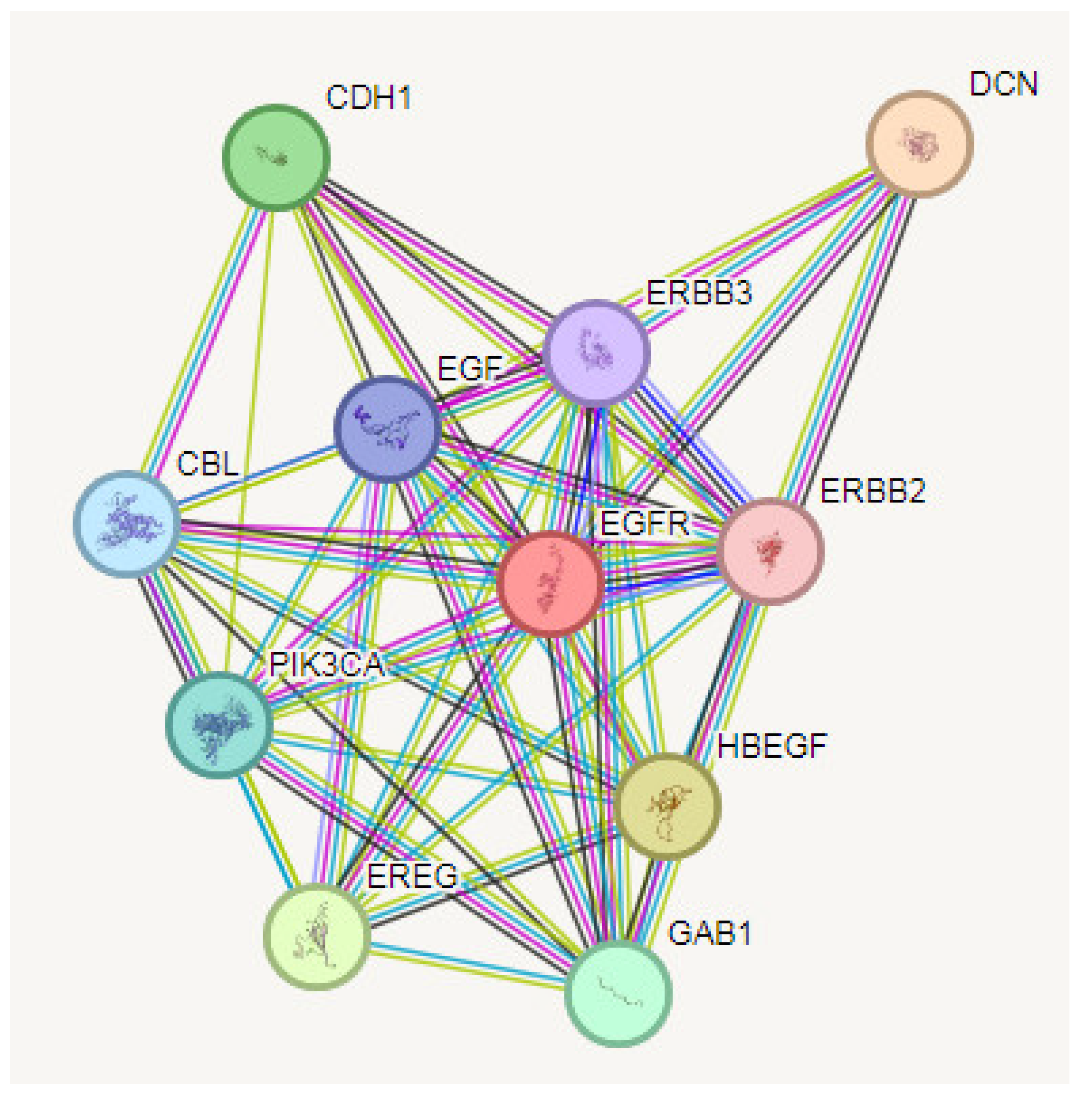
3.4. PPI Analysis
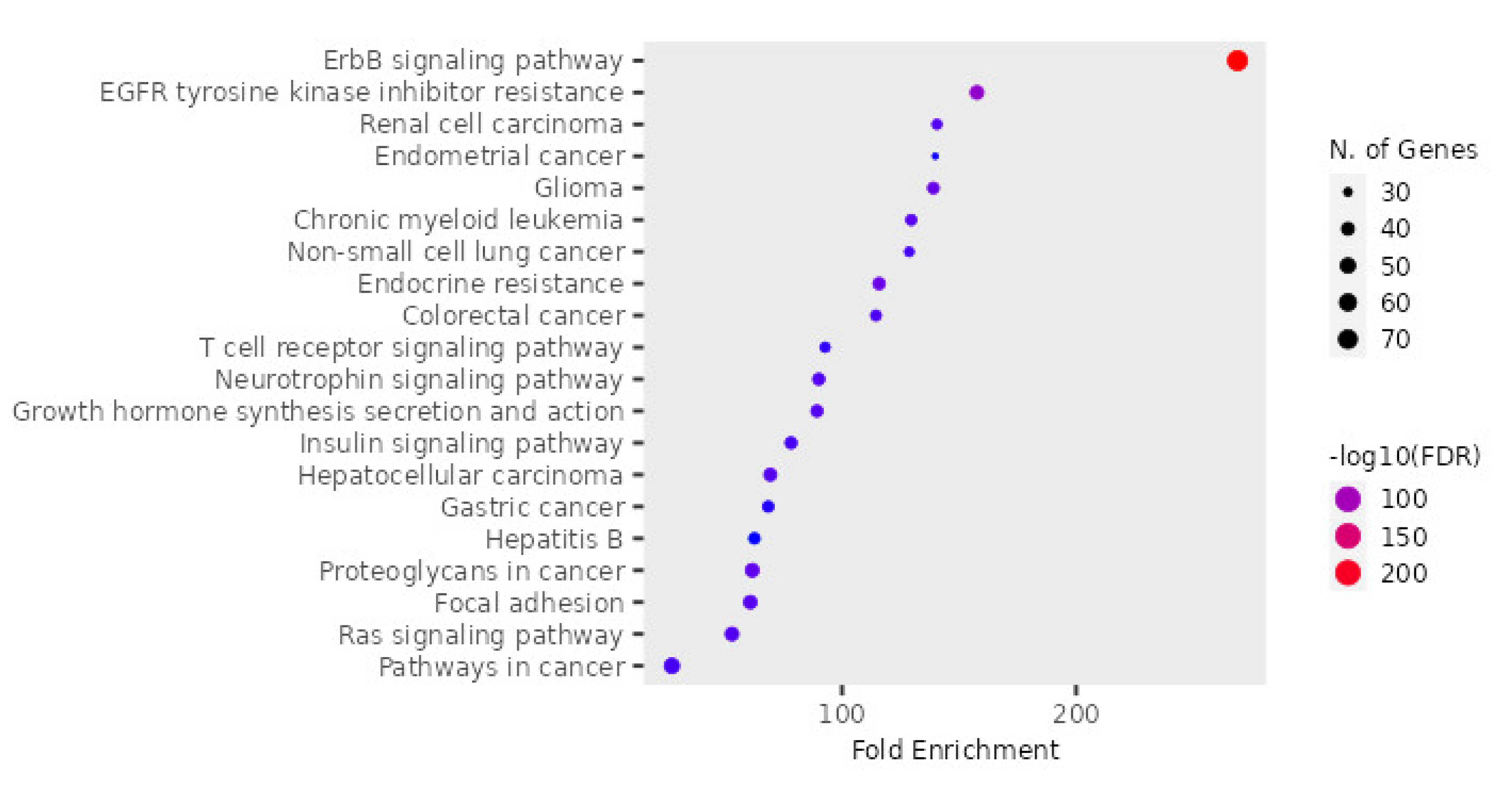
3.5. KEGG Pathway Enrichment Analysis
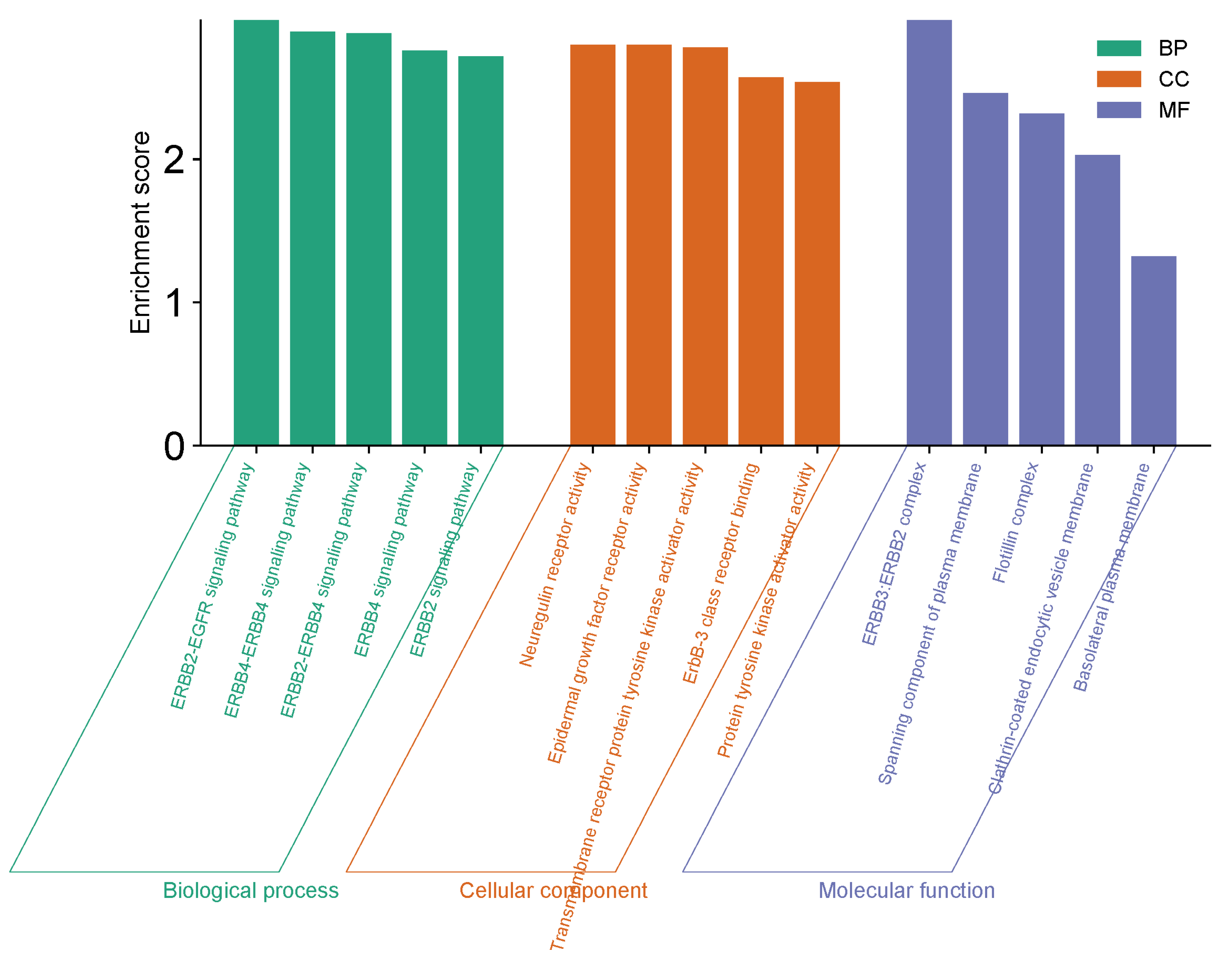
3.6. GO Functional Enrichment Analysis
4. Discussion
5. Conclusion
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019 Jan;69(1):7-34. [CrossRef]
- Moufarrij S, Dandapani M, Arthofer E, Gomez S, Srivastava A, Lopez-Acevedo M, Villagra A, Chiappinelli KB. Epigenetic therapy for ovarian cancer: promise and progress. Clin Epigenetics. 2019 Jan 15;11(1):7. [CrossRef]
- Chauhan SC, Kumar D, Jaggi M. Mucins in ovarian cancer diagnosis and therapy. J Ovarian Res. 2009;2:21.
- Bakhtiari H, Gheysarzadeh A, Ghanadian M, Aghaei M. 15-Hydroxy-8(17),13(E)-labdadiene-19-carboxylic acid (HLCA) inhibits proliferation and induces cell cycle arrest and apoptosis in ovarian cancer cells. Life Sci. 2021 Feb 15;267:118981. [CrossRef]
- Llueca A, Ibañez MV, Cascales P, Gil-Moreno A, Bebia V, Ponce J, Fernandez S, Arjona-Sanchez A, Muruzabal JC, Veiga N, Diaz-Feijoo B, Celada C, Gilabert-Estelles J, Aghababyan C, Lacueva J, Calero A, Segura JJ, Maiocchi K, Llorca S, Villarin A, Climent MT, Delgado K, Serra A, Gomez-Quiles L, Llueca M, On Behalf Of Spain Gog And Gecop Working Group. Neoadjuvant Chemotherapy plus Interval Cytoreductive Surgery with or without Hyperthermic Intraperitoneal Chemotherapy (NIHIPEC) in the Treatment of Advanced Ovarian Cancer: A Multicentric Propensity Score Study. Cancers (Basel). 2023 Aug 26;15(17):4271. [CrossRef]
- Hong L, Wang X, Zheng L, Wang S, Zhu G. Tumor-associated macrophages promote cisplatin resistance in ovarian cancer cells by enhancing WTAP-mediated N6-methyladenosine RNA methylation via the CXCL16/CXCR6 axis. Cancer Chemother Pharmacol. 2023 Jul;92(1):71-81. [CrossRef]
- Jafari SM, Nazri A, Shabani M, Balajam NZ, Aghaei M. Galectin-9 induces apoptosis in OVCAR-3 ovarian cancer cell through mitochondrial pathway. Res Pharm Sci. 2018;13(6):557-65.
- Raoufi-Nejad K, Javadi M, Torkamandi H, Rajabi M, Moeini A, Khanavi M, et al. Adverse drug reactions of herbal medicines during pregnancy amongst Iranian women. Res Pharm Sci. 2012;7(5):980.
- Almatroodi SA, Almatroudi A, Alsahli MA, Khan AA, Rahmani AH. Thymoquinone, an Active Compound of Nigella sativa: Role in Prevention and Treatment of Cancer. Curr Pharm Biotechnol. 2020;21(11):1028-1041. [CrossRef]
- Mehner C, Oberg AL, Goergen KM, Kalli KR, Maurer MJ, Nassar A, Goode EL, Keeney GL, Jatoi A, Radisky DC, Radisky ES. EGFR as a prognostic biomarker and therapeutic target in ovarian cancer: evaluation of patient cohort and literature review. Genes Cancer. 2017 May;8(5-6):589-599. [CrossRef]
- Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer. 2019 Nov;137:113-122. [CrossRef]
- Teplinsky E, Muggia F. EGFR and HER2: is there a role in ovarian cancer? Translational Cancer Research. 2015;4:10.
- Rendell A, Thomas-Bland I, McCuish L, Taylor C, Binju M, Yu Y. Targeting Tyrosine Kinases in Ovarian Cancer: Small Molecule Inhibitor and Monoclonal Antibody, Where Are We Now? Biomedicines. 2022 Aug 29;10(9):2113. [CrossRef]
- Parreno V, Loubiere V, Schuettengruber B, Fritsch L, Rawal CC, Erokhin M, Győrffy B, Normanno D, Di Stefano M, Moreaux J, Butova NL, Chiolo I, Chetverina D, Martinez AM, Cavalli G. Transient loss of Polycomb components induces an epigenetic cancer fate. Nature. 2024 May;629(8012):688-696. [CrossRef]
- Tajan M, Vousden KH. Dietary Approaches to Cancer Therapy. Cancer Cell. 2020 Jun 8;37(6):767-785. [CrossRef]
- Mohammadabadi M, Mozafari M. Enhanced efficacy and bioavailability of thymoquinone using nanoliposomal dosage form. J Drug Deliv Sci Technol. 2018;47:445-53.
- Khan MA, Tania M, Fu J. Epigenetic role of thymoquinone: impact on cellular mechanism and cancer therapeutics. Drug Discov Today. 2019;24(12):2315-22.
- Attoub S, Sperandio O, Raza H, Arafat K, Al-Salam S, Al Sultan MA, et al. Thymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam Clin Pharmacol. 2013;27(5):557-69.
- Alam M, Hasan GM, Ansari MM, Sharma R, Yadav DK, Hassan MI. Therapeutic implications and clinical manifestations of thymoquinone. Phytochemistry. 2022 Aug;200:113213. [CrossRef]
- Gholamnezhad Z, Rafatpanah H, Sadeghnia HR, Boskabady MH. Immunomodulatory and cytotoxic effects of Nigella sativa and thymoquinone on rat splenocytes. Food Chem Toxicol. 2015 Dec;86:72-80. [CrossRef]
- Arafa el SA, Zhu Q, Shah ZI, Wani G, Barakat BM, Racoma I, et al. Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat Res. 2011;706(1-2):28-35.
- Li ZL, Chen C, Yang Y, Wang C, Yang T, Yang X, et al. Gamma secretase inhibitor enhances sensitivity to doxorubicin in MDA-MB-231 cells. Int J Clin Exp Pathol. 2015;8(5):4378-87.
- Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H(2)O(2)- and p53-dependent pathways. J Biol Chem. 2004;279(24):25535-43.
- Ijaz H, Tulain UR, Qureshi J, Danish Z, Musayab S, Akhtar MF, Saleem A, Khan KK, Zaman M, Waheed I, Khan I, Abdel-Daim M. Review: Nigella sativa (Prophetic Medicine): A Review. Pak J Pharm Sci. 2017 Jan;30(1):229-234.
- Ashour AE, Ahmed AF, Kumar A, Zoheir KM, Aboul-Soud MA, Ahmad SF, et al. Thymoquinone inhibits growth of human medulloblastoma cells by inducing oxidative stress and caspase-dependent apoptosis while suppressing NF-κB signaling and IL-8 expression. Mol Cell Biochem. 2016;416(1-2):141-55.
- Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, et al. Drug resistance in cancer: an overview. Cancers (Basel). 2014;6(3):1769-92.
- Velho-Pereira R, Kumar A, Pandey BN, Jagtap AG, Mishra KP. Radiosensitization in human breast carcinoma cells by thymoquinone: role of cell cycle and apoptosis. Cell Biol Int. 2011 Oct;35(10):1025-9. [CrossRef]
- Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J Exp Clin Cancer Res. 2010 Jul 1;29(1):87. [CrossRef]
- Taha MM, Sheikh BY, Salim LZ, Mohan S, Khan A, Kamalidehghan B, et al. Thymoquinone induces apoptosis and increase ROS in ovarian cancer cell line. Cell Mol Biol (Noisy-le-grand). 2016;62(6):97-101.
- Liu X, Dong J, Cai W, Pan Y, Li R, Li B. The Effect of Thymoquinone on Apoptosis of SK-OV-3 Ovarian Cancer Cell by Regulation of Bcl-2 and Bax. Int J Gynecol Cancer. 2017;27(8):1596-601.
- Johnson-Ajinwo OR, Ullah I, Mbye H, Richardson A, Horrocks P, Li WW. The synthesis and evaluation of thymoquinone analogues as anti-ovarian cancer and antimalarial agents. Bioorg Med Chem Lett. 2018;28(7):1219-22.
- Bonello M, Sims AH, Langdon SP. Human epidermal growth factor receptor targeted inhibitors for the treatment of ovarian cancer. Cancer Biol Med. 2018;15(4):375-88.
- Dou L, Zou D, Song F, Jin Y, Li Y, Zhang Y. Bufalin suppresses ovarian cancer cell proliferation via EGFR pathway. Chin Med J (Engl). 2021;135(4):456-61.
- Shen LS, Jin XY, Wang XM, Tou LZ, Huang J. Advances in endocrine and targeted therapy for hormone-receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. Chin Med J (Engl). 2020;133(9):1099-108.
- Kang XH, Xu ZY, Gong YB, Wang LF, Wang ZQ, Xu L, et al. Bufalin Reverses HGF-Induced Resistance to EGFR-TKIs in EGFR Mutant Lung Cancer Cells via Blockage of Met/PI3k/Akt Pathway and Induction of Apoptosis. Evid Based Complement Alternat Med. 2013;2013:243859.
- Jiang Y, Zhang Y, Luan J, Duan H, Zhang F, Yagasaki K, et al. Effects of bufalin on the proliferation of human lung cancer cells and its molecular mechanisms of action. Cytotechnology. 2010;62(6):573-83.


| EGFR: F: GCCAAGGCACGAGTAACAAGC, R: GGGCAATGAGGACATAACC |
| FOXP3: F: GTGGCCGGATGTGAGAAG, R: GGAGCCCTTGTCGGATGATG |
| Β-Actin: F: CCTCTGAACCCTAAGGCCAAC, R: TGCCACAGGATTCCATACCC |
| GAPDH: F:CGGAGTCAACGGATTTGGTCGTAT, R:GCCTTCTCCATGGTGGTGAAGAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





