Submitted:
30 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
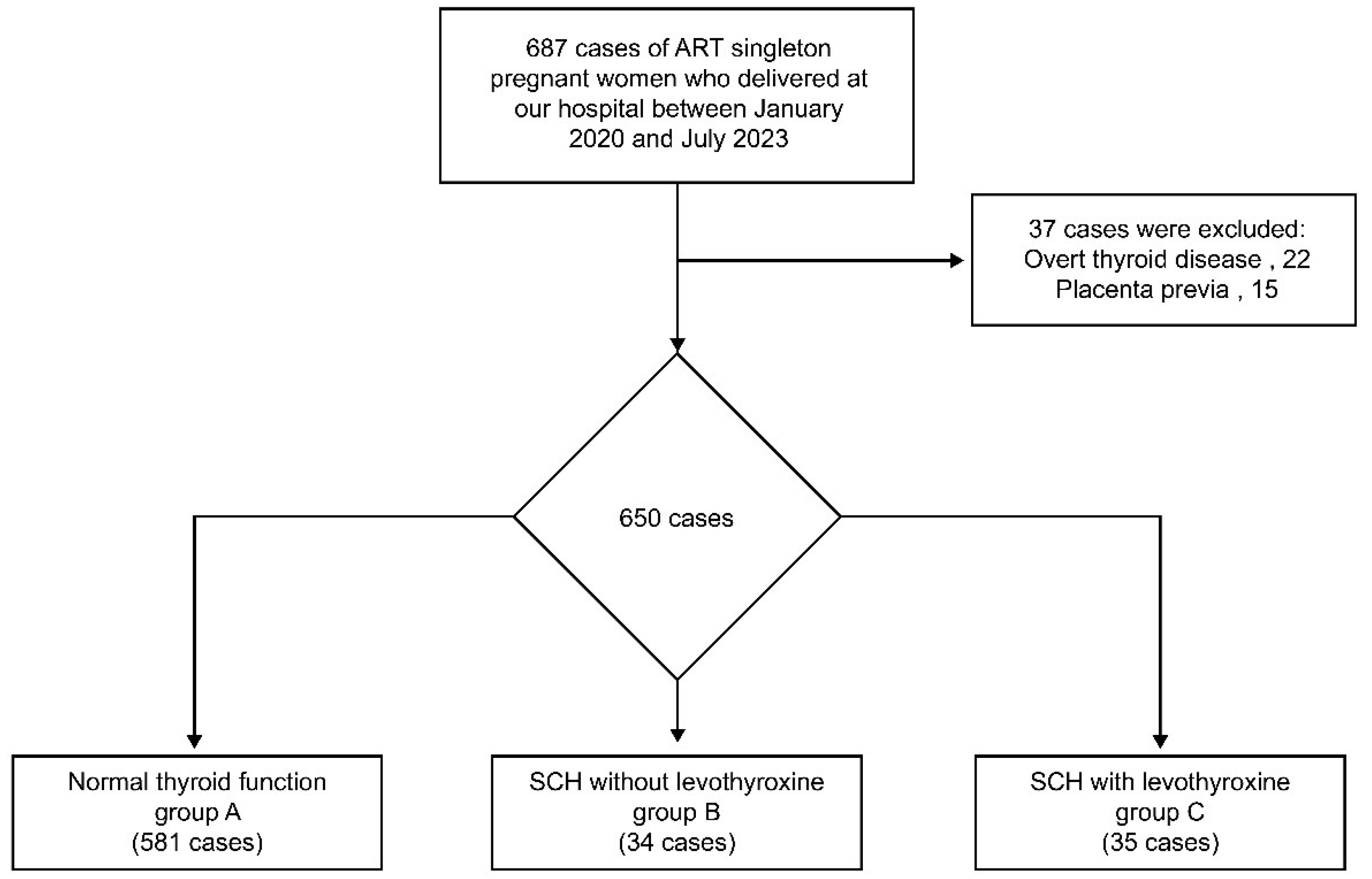
2.1. Participants
2.2. Data Collection and Definition
2.3. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasagi, K.; Takahashi, N.; Inoue, G.; Honda, T.; Kawachi, Y.; Izumi, Y. Thyroid function in Japanese adults as assessed by a general health checkup system in relation with thyroid-related antibodies and other clinical parameters. Thyroid 2009, 19, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Allan, W.C.; Haddow, J.E.; Palomaki, G.E.; Williams, J.R.; Mitchell, M.L.; Hermos, R.J.; Faix, J.D.; Klein, R.Z. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J. Med. Screen. 2000, 7, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.Z.; Haddow, J.E.; Faix, J.D.; Brown, R.S.; Hermos, R.J.; Pulkkinen, A.; Mitchell, M.L. Prevalence of thyroid deficiency in pregnant women. Clin. Endocrinol. 1991, 35, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shan, Z.; Li, C.; Mao, J.; Xie, X.; Wang, W.; Fan, C.; Wang, H.; Zhang, H.; Han, C.; et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid 2014, 24, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Cabral, H.J.; Aschengrau, A.; Pearce, E.N. Associations between maternal thyroid function in pregnancy and obstetric and perinatal outcomes. J. Clin. Endocrinol. Metab. 2020, 105, e2015–e2023. [Google Scholar] [CrossRef] [PubMed]
- Toloza, F.J.K.; Derakhshan, A.; Männistö, T.; Bliddal, S.; Popova, P.V.; Carty, D.M.; Chen, L.; Taylor, P.; Mosso, L.; Oken, E.; et al. Association between maternal thyroid function and risk of gestational hypertension and pre-eclampsia: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 2022, 10, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, F.M.; Donnelly, J.; Cooley, S.M.; Geary, M.; Malone, F.D. Subclinical hypothyroidism as a risk factor for placental abruption: evidence from a low-risk primigravid population. Aust. N.Z.J. Obstet. Gynaecol. 2013, 53, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Cleary-Goldman, J.; Malone, F.D.; Lambert-Messerlian, G.; Sullivan, L.; Canick, J.; Porter, T.F.; Luthy, D.; Gross, S.; Bianchi, D.W.; D’Alton, M.E. Maternal thyroid hypofunction and pregnancy outcome. Obstet. Gynecol. 2008, 112, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.F.; Zhang, M.; Chen, J.; Wei, Q.; Zeng, L.; Liu, D.; Zhang, C.; Li, H.; Zou, K.; Zhang, L.; et al. The impact of levothyroxine therapy on the pregnancy, neonatal and childhood outcomes of subclinical hypothyroidism during pregnancy: an updated systematic review, meta-analysis and trial sequential analysis. Front. Endocrinol. 2022, 13, 964084. [Google Scholar] [CrossRef]
- Consortium on Thyroid and Pregnancy—Study Group on Preterm Birth; Korevaar, T.I.M.; Derakhshan, A.; Taylor, P.N.; Meima, M.; Chen, L.; Bliddal, S.; Carty, D.M.; Meems, M.; Vaidya, B.; et al. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: A systematic review and meta-analysis. JAMA 2019, 322, 632–641. [Google Scholar] [CrossRef]
- Knøsgaard, L.; Andersen, S.; Hansen, A.B.; Vestergaard, P.; Andersen, S.L. Maternal hypothyroidism and adverse outcomes of pregnancy. Clin. Endocrinol. 2023, 98, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Baker, V.L.; Rone, H.M.; Pasta, D.J.; Nelson, H.P.; Gvakharia, M.; Adamson, G.D. Correlation of thyroid stimulating hormone (TSH) level with pregnancy outcome in women undergoing in vitro fertilization. Am. J. Obstet. Gynecol. 2006, 194, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Blatt, A.J.; Nakamoto, J.M.; Kaufman, H.W. National status of testing for hypothyroidism during pregnancy and postpartum. J. Clin. Endocrinol. Metab. 2012, 97, 777–784. [Google Scholar] [CrossRef]
- Vaidya, B.; Hubalewska-Dydejczyk, A.; Laurberg, P.; Negro, R.; Vermiglio, F.; Poppe, K. Treatment and screening of hypothyroidism in pregnancy: results of a European survey. Eur. J. Endocrinol. 2012, 166, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Matsubara, K.; Nakamoto, O.; Ushijima, J.; Ohkuchi, A.; Koide, K.; Makino, S.; Mimura, K.; Morikawa, M.; Naruse, K.; et al. Outline of the new definition and classification of “hypertensive disorders of pregnancy (HDP)”; a revised JSSHP statement of 2005. Hypertens. Res. Pregnancy 2018, 6, 33–37. [Google Scholar] [CrossRef]
- Yoshida, S.; Unno, N.; Kagawa, H.; Shinozuka, N.; Kozuma, S.; Taketani, Y. Prenatal detection of a high-risk group for intrauterine growth restriction based on sonographic fetal biometry. Int. J. Gynecol. Obstet. 2000, 68, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Jaques, A.M.; Amor, D.J.; Baker, H.W.G.; Healy, D.L.; Ukoumunne, O.C.; Breheny, S.; Garrett, C.; Halliday, J.L. Adverse obstetric and perinatal outcomes in subfertile women conceiving without assisted reproductive technologies. Fertil. Steril. 2010, 94, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Declercq, E.; Luke, B.; Belanoff, C.; Cabral, H.; Diop, H.; Gopal, D.; Hoang, L.; Kotelchuck, M.; Stern, J.E.; Hornstein, M.D. Perinatal outcomes associated with assisted reproductive technology: the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART). Fertil. Steril. 2015, 103, 888–895. [Google Scholar] [CrossRef]
- Murugappan, G.; Li, S.; Lathi, R.B.; Baker, V.L.; Luke, B.; Eisenberg, M.L. Increased risk of severe maternal morbidity among infertile women: analysis of US claims data. Am. J. Obstet. Gynecol. 2020, 223, 404.e1–404.e20. [Google Scholar] [CrossRef]
- Lazarus, J.H.; Bestwick, J.P.; Channon, S.; Paradice, R.; Maina, A.; Rees, R.; Chiusano, E.; John, R.; Guaraldo, V.; George, L.M.; et al. Antenatal thyroid screening and childhood cognitive function. N. Engl. J. Med. 2012, 366, 493–501. [Google Scholar] [CrossRef]
- Negro, R.; Schwartz, A.; Gismondi, R.; Tinelli, A.; Mangieri, T.; Stagnaro-Green, A. Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J. Clin. Endocrinol. Metab. 2010, 95, 1699–1707. [Google Scholar] [CrossRef]
- Thung, S.F.; Funai, E.F.; Grobman, W.A. The cost-effectiveness of universal screening in pregnancy for subclinical hypothyroidism. Am. J. Obstet. Gynecol. 2009, 200, 267.e1–267.e7. [Google Scholar] [CrossRef] [PubMed]
- Dosiou, C.; Barnes, J.; Schwartz, A.; Negro, R.; Crapo, L.; Stagnaro-Green, A. Cost-effectiveness of universal and risk-based screening for autoimmune thyroid disease in pregnant women. J. Clin. Endocrinol. Metab. 2012, 97, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.M.; Thom, E.A.; Peaceman, A.M.; Varner, M.W.; Sorokin, Y.; Hirtz, D.G.; Reddy, U.M.; Wapner, R.J.; Thorp, J.M., Jr.; Saade, G.; et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N. Engl. J. Med. 2017, 376, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed]
- Nazarpour, S.; Ramezani Tehrani, F.; Simbar, M.; Tohidi, M.; Alavi Majd, H.; Azizi, F. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur. J. Endocrinol. 2017, 176, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, M.; Li, T.; Yang, M.; Wang, W.; Chen, Y.; Ding, Y.; Liu, J.; Xu, X.; Zhang, J.; et al. High level of thyroid peroxidase antibodies as a detrimental risk of pregnancy outcomes in euthyroid women undergoing ART: A meta-analysis. Mol. Reprod. Dev. 2023, 90, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qiu, S.; Jiang, W.; Huang, Z.; Shi, H.; Du, S.; Sun, Y.; Zheng, B. Impact of thyroid autoimmunity on pregnancy outcomes in euthyroid women following fresh/frozen-thawed embryo transfer. Clin. Endocrinol. 2023, 99, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Abalovich, M.; Mitelberg, L.; Allami, C.; Gutierrez, S.; Alcaraz, G.; Otero, P.; Levalle, O. Subclinical hypothyroidism and thyroid autoimmunity in women with infertility. Gynecol. Endocrinol. 2007, 23, 279–283. [Google Scholar] [CrossRef]
- Sun, H.; Su, X.; Liu, Y.; Li, G.; Liu, X.; Du, Q. Association between abortion history and perinatal and neonatal outcomes of singleton pregnancies after assisted reproductive technology. J. Clin. Med. 2022, 12, 1. [Google Scholar] [CrossRef]
| Group A (n=581) | Group B (n=34) | Group C (n=35) | P-value | |
|---|---|---|---|---|
| Maternal age (years)* | 36.5 (±4.3) | 36.6 (±4.7) | 36.5 (±3.8) | 0.988 |
| Pre-pregnant BMI (kg/m2)* | 21.5 (±3.4) | 22.0 (±2.6) | 21.8 (±2.8) | 0.634 |
| History of abortion | 218/581 (37.5%) | 11/34 (32.4%) | 8/35 (22.9%) | 0.194 |
| Preterm birth at <37 weeks | 106/581 (18.2%) | 9/34 (26.5%) | 2/35 (5.7%) | 0.059 |
| Preterm birth at <34 weeks | 36/581 (6.2%) | 5/34 (14.7%) | 0/35 (0%) | 0.046 |
| Preterm birth at <32 weeks | 32/581 (5.5%) | 1/34 (2.9%) | 0/35 (0%) | 0.411 |
| Preterm birth at <28 weeks | 16/581 (2.8%) | 0/34 (0%) | 0/35 (0%) | 1 |
| Preeclampsia | 56/581 (9.6%) | 3/34 (8.8%) | 4/35 (11.4%) | 0.945 |
| Fetal growth restriction | 23/581 (4.0%) | 1/34 (2.9%) | 0/35 (0%) | 0.768 |
| Cesarean delivery | 272/581 (46.8%) | 15/34 (44.1%) | 19/34 (54.3%) | 0.641 |
| Blood loss at delivery (ml)* | 812 (±676) | 970 (±716) | 788 (±406) | 0.393 |
| Manual placental removal | 53/581 (9.1%) | 3/34 (8.8%) | 5/35 (14.3%) | 0.591 |
| Transfusion | 33/581 (5.7%) | 1/34 (2.9%) | 0/35 (0%) | 0.374 |
| Neonatal birth weight (g)* | 2901 (±1339) | 2819 (±640) | 3064 (±475) | 0.705 |
| TSH value (uIU/ml)* | 1.36 (±0.57) | 4.02 (±2.47) | 3.55 (±1.07) | <0.001 |
| Adjusted OR | 95% CI | P-value | |
|---|---|---|---|
| Maternal age | 1.00 | 0.956–1.05 | 0.880 |
| Pre-pregnant BMI | 1.00 | 0.939–1.07 | 0.991 |
| Nulliparous | 0.95 | 0.602–1.50 | 0.822 |
| History of abortion | 0.97 | 0.621–1.52 | 0.899 |
| Preeclampsia | 4.98 | 2.81–8.81 | <0.001 |
| Fetal growth restriction | 3.31 | 1.31–8.34 | 0.011 |
| Levothyroxine therapy | 1.36 | 0.378–1.36 | 0.230 |
| TSH value | 1.05 | 0.485–2.29 | 0.895 |
| Adjusted OR | 95% CI | P-value | |
|---|---|---|---|
| Maternal age | 1.00 | 0.926–1.07 | 0.915 |
| Pre-pregnant BMI | 1.03 | 0.942–1.13 | 0.506 |
| Nulliparous | 1.20 | 0.567–2.53 | 0.638 |
| History of abortion | 1.20 | 0.606–2.40 | 0.595 |
| Preeclampsia | 3.65 | 1.65–8.09 | 0.001 |
| Fetal growth restriction | 2.92 | 0.877–9.74 | 0.081 |
| Levothyroxine therapy | 0.117 | 0.015–0.948 | 0.044 |
| TSH value | 2.18 | 0.750–6.36 | 0.152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




