1. Introduction
Central line-associated bloodstream infections (CLABSI) and catheter-related bloodstream infections (CRBSI) are the most critical central line-correlated infectious complications among pediatric hematology oncology patients.[
1,
2] The incidence of CLABSI among pediatric oncological patients is 1-4.6 per 1000 catheter days for external CVC, engaging about 25% of this specific population with an estimated mortality rate between 12.5% and 25%.[
3,
4,
5] Patients are particularly susceptible to infectious complications during episodes of chemotherapy-induced neutropenia, but also non-neutropenic outpatients can suffer from CVC-related infections.[
6,
7]
The incidence of CLABSI is variable according to adjustable and unadjustable factors. If patient characteristics such as age, low body weight, disease status, and comorbidities cannot be changed, the “human factor,” which determines how a CVC is managed, is improvable. The correct management of the central line, the proper preparation and administration of infusion liquids, the correct dressing, and the appropriate drug delivery technique are all modifiable factors that have a certain weight in the incidence of catheter-related infections.[
8,
9,
10]
We aimed to analyze the impact of a nurse-designed CVC management protocol on the incidence of CLABSI in one pediatric oncologic facility.
2. Materials and Methods
2.1. Study Design and Population
This single-center retrospective cohort study reviewed CVC data routinely collected from January 2012 to July 2022. Three hundred twenty-six consecutive children and adolescents with cancer and hematological disease who initiated care at the Department of Pediatric Hematology Oncology and Bone Marrow Transplantation of urban, tertiary referral, academic medical center Burlo Garofolo Children’s Hospital were included in the study group.
This was a unit-based quality improvement project designed by nurses with long-standing experience. The study's primary aim was to assess whether the nurses' CVC management recommendations would result in a lower incidence of infectious complications than the rates reported in the literature. The secondary aim was to determine if, among the analyzed variables, it was possible to identify any additional risk factors for CVC-related infectious in a pediatric oncological setting.
Demographic and clinical characteristics data were collected on patients with an oncologic or hematologic diagnosis and placed in a CVC.
2.2. Ethical Approval and Informed Consent
The study protocol was approved by the Institutional Review Board of the IRCCS Burlo Garofolo (reference No. IRB RC 32/2023), and the study was conducted following the Declaration of Helsinki. The data were collected according to the Authorization to Process Personal Data for Scientific Research Purposes (Authorization No. 9/2014).[
11]
Parents and custodians signed informed consent at the first visit, agreeing that clinical data may be used for clinical research purposes, epidemiology, the study of pathologies, and training to improve knowledge, care, and prevention.
2.3. Nurse-Led Quality Improvement CVC-Management Protocol
Following a critical evaluation of the current best evidence, the oncological nurse team developed changes to the unit's nursing practice on CVC handling guidelines to reduce CLABSI incidence. The differences in modified guidelines compared to the in-force departmental CVC maintenance protocol were the following: the ordinary management of the CVC is exclusively the responsibility of the trained personnel; two-person dressing approach; use an aseptic technique during any CVC manipulation that requires the lumen opening or removal of the CVC exit-site dressing; preparation of injecting drugs or solutions to be infused into the CVC under a laminar flow hood using an aseptic technique; 2% chlorhexidine and 70% isopropyl alcohol disinfection of needle-free connectors (NFC) and cover with a double layer of sterile gauze for biological fluid contamination protection; maintenance of the closed circuit during the use of infusion lines; replacement of infusion lines every 24 hours if blood products, total parenteral nutrition (TPN), propofol administration, or closed-circuit interruption; otherwise, replacement is carried out every 72 hours; production of the patient CVC logbook, which includes placement information, dressing according to the eventual patient's preferences, any maintenance problems, and any infectious event reported; newly hired and satellite hospital personnel training to prepare them to work independently, maintaining uniform CVC management.
2.4. Central-Line Data
We documented all CVCs, along with device type, number of lumens, tube size, data of placement (laterality, cannulated vein), duration of CVC, dressing frequency, CVC use (TPN, transfusions, blood sampling, apheresis), and removal indication. CVCs that had been in place for less than one day were excluded. If a patient underwent multiple CVC placements, it was considered a different entry in the database. CVCs were categorized as tunneled (cuffed and non-cuffed), non-tunneled, and implanted subcutaneously.
2.5. CVC Dressing Protocol
CVC dressings were performed according to the GAVeCeLT protocol (Italian Expert group on long-term central venous accesses. They were changed every seven days (planned) or whenever they became damp, detached, or visibly soiled (unplanned). If the dressing and CVC exit-site appeared perfect in inpatients, the dressing was postponed to 10 days.
After the adhesive tissue was removed with Remove© wipes (Smith and Nephew, England), the dressing area's skin was cleaned using a BD ChloraPrepTM applicator (Becton, Dickinson, and Company, USA) that delivers a solution of 2% w/v chlorhexidine gluconate and 70% v/v isopropyl alcohol without orange dye. BD Chloraprep™ was applied by rubbing the skin around the exit-site back and forth to cover the whole dressing area and allowed to dry for 30 seconds. When the skin was dry, adhesive transparent dressing IV3000 (Smith&Nephew, Germany) or Tegaderm™ I.V. Advanced (3M, Germany) was attached.
2.6. CVC-Associated Infectious Data
CLABSI is defined as a culture of recognized pathogens from one or more blood cultures, with the organism not related to an infection at another site, in patients with a CVC in place within 48 hours before detection. If a common skin contaminant is cultured, two or more blood cultures drawn on separate occasions are required, along with specific symptoms.[
12] We considered CLABSI in the inpatient setting if the first positive blood culture occurred > 48 hours after admission or < 48 hours after hospital discharge. In the outpatients, CLABSIs were considered if the first positive blood culture occurred > 48 hours after hospital discharge or < 48 hours after admission.[
13] We considered the mucosal barrier injury to reduce the potential overestimation of true CRSBI incidence.[
14] The occurrence of skin irritation was defined as the presence of areas of skin loss, erythematous plaques, or vesicles in the site of CVC insertion. We have defined a CVC exit-site infection as hyperemia, induration, and/or tenderness ≤ 2 cm from the catheter exit-site with negative blood cultures. The exit site was evaluated using a visual exit-site score.[
15]
2.7. Statistical Analysis
All data was divided by the outcome of interest, and the two cohorts were compared. Data were statistically analyzed using R software version 4.3.1 (2023-06-16 ucrt) for Windows.[
16] Descriptive statistics were used to analyze demographic data, CVC characteristics, and details on CVC insertion, usage, and complications using the “gtsummary” package.[
17] Categorical data were presented as frequency and percentage, while continuous data were presented as the median and interquartile range (IQR). Tests defaulted were the Wilcoxon rank sum test for continuous variables, Pearson’s Chi-squared test without Yates’ correction for categorical variables with all expected cell counts > = 5, and Fisher’s exact test for categorical variables with any expected cell count < 5. Incomplete observations were removed from the analysis.
Alternative definitions of outcomes using the same method were proposed and explored to conduct sensitivity analyses. In this descriptive analysis, and elsewhere in the article, OR and 95% CI are reported running a univariate logistic regression on the relative outcome for each variable taken into consideration. In the supplements, a survival analysis was conducted to establish the Cumulative incidence of infection among different groups with the help of the “survival” package.[
18]
The statistical significance was arbitrarily set to < 0.05.
3. Results
3.1.1. Patient Population and Device Characteristics
In total, 236 pediatric oncological and hematological patients had a CVC inserted between January 2012 and December 2019, with the last follow-up occurring in December 2022. The most common primary diagnoses were acute lymphoblastic leukemia (n = 145, 45%) and solid tumors (n = 100, 31%). Twenty-six (8%) patients were affected by non-malignant hematological diseases. 122 (37.8%) patients underwent hematopoietic stem cell transplantation (HSCT), and 245 (75.9%) patients received TPN.
The total number of CVCs inserted during the study period was 323, with 66 (20%) patients requiring one or more CVC replacements. All CVCs were inserted according to local standard operating procedures. The median age at placement was 6.7 years (range, 0 – 18). The most common central device type (n = 210, 6%) was Broviac® —Hickman® (Bard Access Systems). All central devices were tunneled and cuffed. The median lumen density was 1.46, and the median duration of the central device was 228 days (IQR, 103 – 320). The following indications for removal were observed: 200 (62%) due to end of therapy, 31 (10%) due to CVC-related infections, 31 (10%) due to malposition and dislodgement, 27 (8.5%) due to death, 19 (6%) due to occlusion and malfunction, 11 (3.5%) due to breakage. Patient and central device characteristics are shown in
Table 1.
3.1.2. Central-Line-Associated Infectious Events
During the study period, we identified 24 proven CLABSI events in the hospital's electronic database. The rate of CLABSI was 0.32 infections per 1000 catheter days. We analyzed patient and CVC characteristics to find factors that might increase the risk of infection (
Table 2).
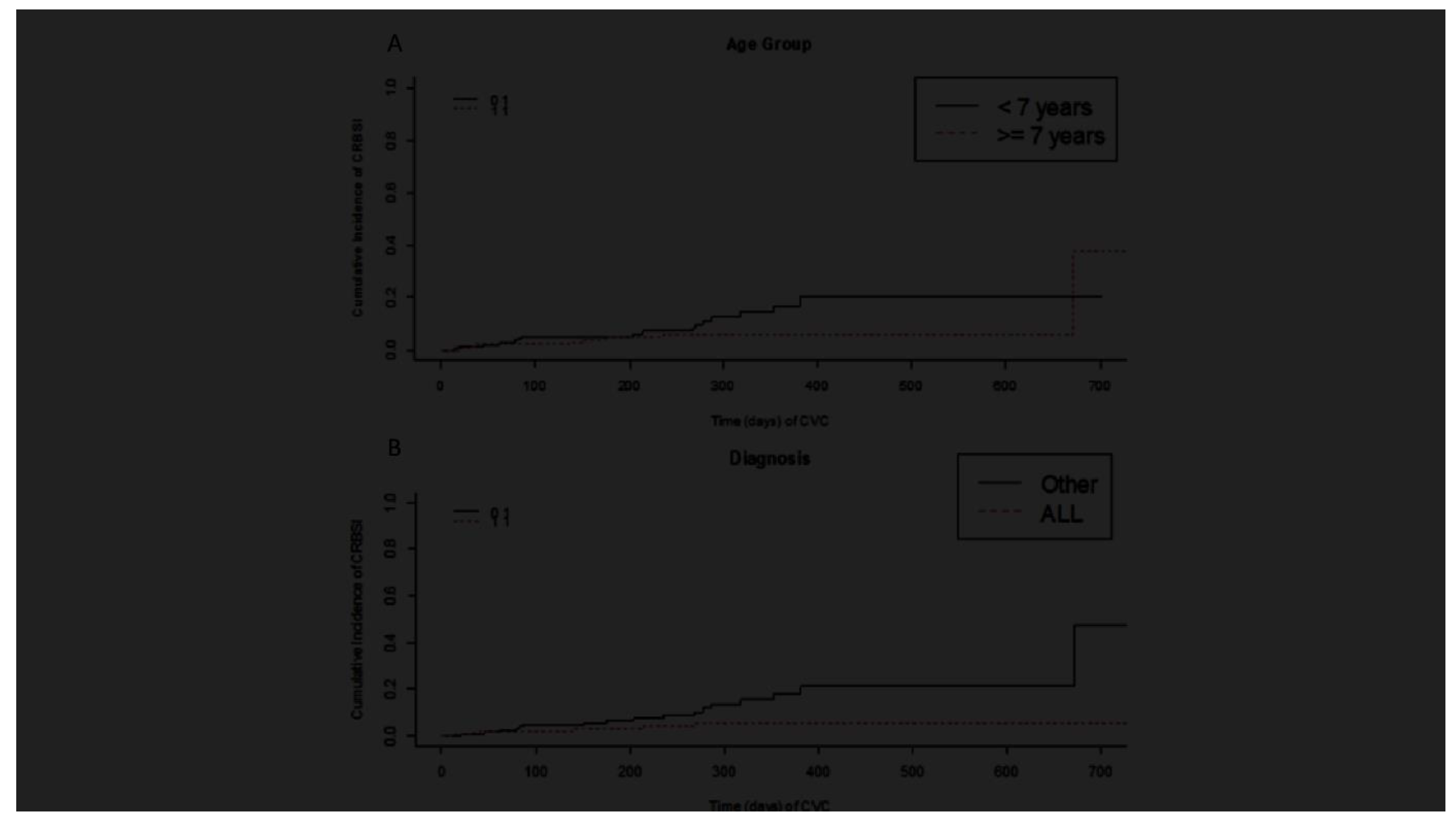
Only ALL diagnosis was associated with a lower incidence of CLABSI (
p = 0.042). Even if no other statistically significant association with infection was found, older age (> seven years) and insertion in the subclavian vein appear to be protective factors (
Figure 1). We found no correlation net even between CLABSI and total parenteral nutrition (TPN) or CVC occlusion events in our cohort. The reliability of the results was confirmed through sensitivity analysis. The sensitivity analysis was also extended to patients with possible CVC-related infection and CVCs removed due to infection (
Table 3). Fifty-one cases of possible infection were identified, and in this population, the patients' ages were also lower than the mean cohort age. The incidence of possible infection was 0.67 events per 1000 days of CVC. Thirty-one infected CVCs (10%) were removed because of the infection.
The majority of the germs involved in the CLABSI were Staphylococcus aureus (45%), Staphylococcus epidermidis (17%), Pseudomonas aeruginosa (13%), Candida albicans (13%), and Candida parapsilosis (13%). Ten percent (32 events) of all CVCs analyzed had a positive exit-site culture, with the prevalence of Staphylococcus aureus (50%). The skin site colonization and CLABSI were correlated in 9 cases (28%). A strong correlation was found between CVC tip colonization and bloodstream infection. The same pathogen was isolated from blood cultures in 11 out of 18 cases (61%).
3.1.3. Dressing and CVC Exit-Site Score
We assessed whether the frequency of dressings correlates with CVC-related infections (
Table 2). The median percentage of dressings with 1- 6 day intervals was significantly higher in the CLABSI group than in the non-infected group (50%, IQR 37%- 68% vs. 30%, IQR 22% - 43%;
p < 0.001). The median percentage of dressings performed once a week was significantly higher in the non-infected group compared to the CLABSI group (50%, IQR 38% - 61% vs. 31%, IQR 11% - 45%;
p < 0.001). No significant differences were found in both groups for dressing performed every ten days (mean 16% vs. 12%,
p = 0.3). We obtained comparable, statistically significant results by analyzing the correlation between the frequency of dressings and the number of CVCs removed due to infection: 54% vs. 29% for 1 – 6 day intervals and 31% vs. 50% for once weekly dressing in the CLABSI group and the non-infected group, respectively;
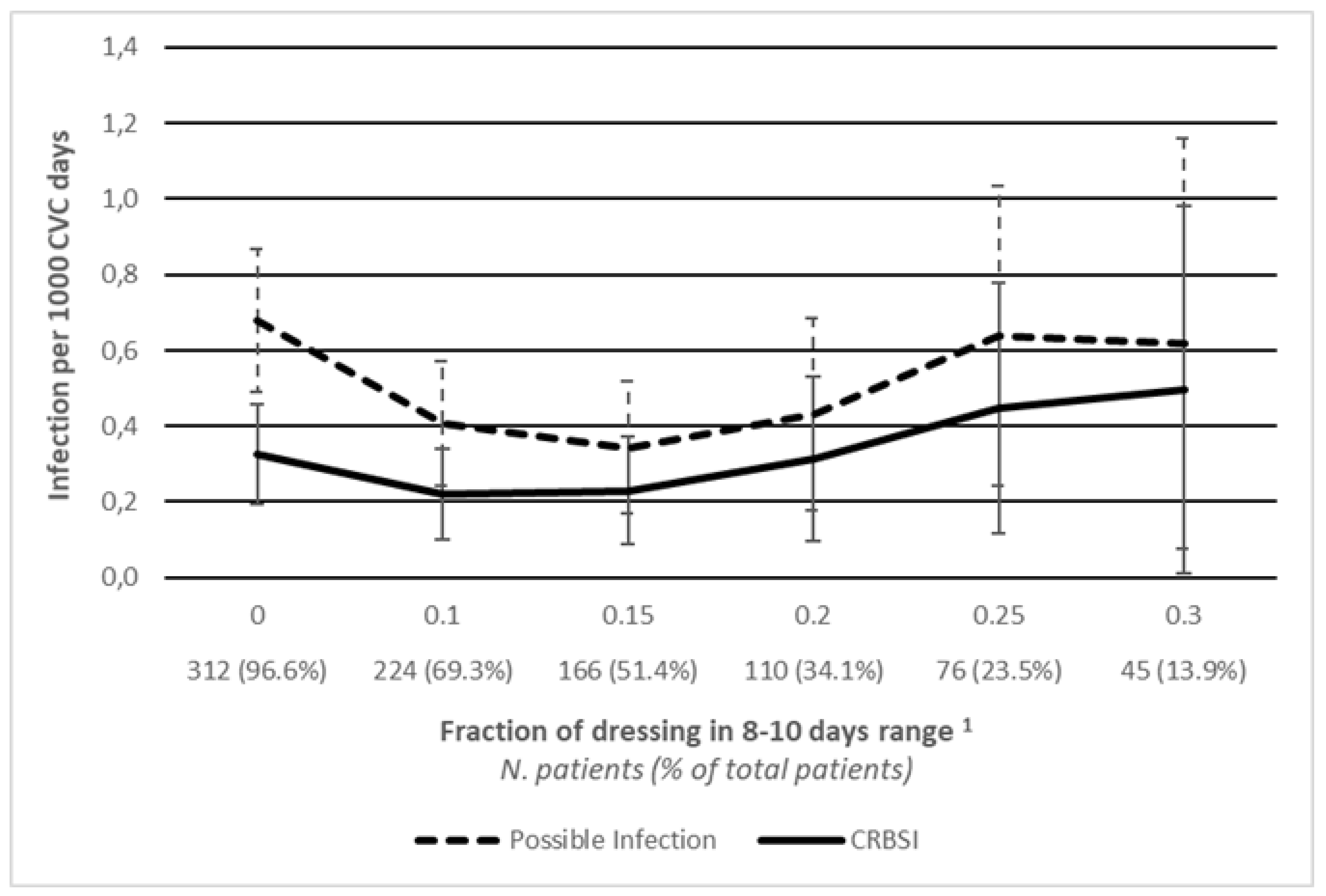
p < 0.001. To further explore the relationship between dressing frequency and infection, we calculated different infection rates in subsets of patients whose percentage of dressings falling within the 8-10 days range exceeded a variable threshold. The infection rate initially reduced even if patients were subject to less frequent dressing, and then seems to stabilize around the value of 0.495 cases per 1000 CVC days considering patients who were dressed with an 8-10 days range of more than 30% of the time (
Figure 2).
In the event of suspected or confirmed exit-site infection, it is customary to perform CVC dressings in the pediatric hematology oncology department. Patients with a CVC exit-site score of 0 and without malfunction problems received the medication at home from the appropriately trained staff of the satellite hospitals. For this reason, the relationship between the dressing location and CLABSI was predictable (
Table 2). The median percentage of dressings performed at our department on patients with CLABSI compared to other locations was significantly higher (100%, IQR 69%-100% vs. 0%, IQR 0%-31%;
p < 0.001).
We investigated a possible relationship between the visual exit-site score grading and CLABSI. A statistically significant correlation exists between CLABSI and exit-site score ≥ 1. In the patients without CLABSI, the median number of dressings with a score of 0 was 0.93, compared to 0.81 in the CLABSI group (p = 0.001).
3.1.4. CVC Removal Infection-Related Indication
Thirty-one (9.6%) CVCs were removed due to CLABSI. The only variables correlating with CVC removal for infection are ALL and long-time catheterization. ALL diagnosis is a protective factor, with only 26% of CVCs removed due to infection compared to 47% removed due to other reasons (
p = 0.025). CVCs that remain in placement longer are frequently removed for reasons other than infection (
p = 0.019). Other variables, such as CVC model and type, method of insertion, number of lumens, HSCT, TPN, and occlusion events, are not correlated with infection-related removal indication (
Table 3).
4. Discussion
Literature data report an incidence of central CLABSI between 1.7 and 11.3 cases for 1000 days catheter. In the oncology and hematology wards, the incidence is 1 – 4.6 per 1000 catheter days for external CVCs. CLABSI affects about 25% of pediatric patients with oncological and hematological disease, and its estimated mortality rate is between 12.5% and 25%.[
3] Infected CVC management differs in pediatric patients compared to adults, particularly in the oncology setting. The necessity of peripheral vein cultures for diagnosing CVC-related infection remains controversial in children because of the poorer venous assets. Conservative, pharmacologically focused management through CVC remains mandatory, with CVC removal only in selected cases.[
19] Therefore, the prevention of CLABSI in this setting of patients becomes a more effective approach.
The nursing initiative was carried out to reduce the incidence of CLABSI through education and bundled intervention implementation in our pediatric oncology hematology department. Their effort and determination achieved significant and sustained decreases in CLABSI over ten years, bringing the incidence consistent with national data to 0.32 proven events for 1000 catheter days only. The protocol's capillary distribution within the satellite hospitals that manage the patients treated in our department and frequent retraining of the healthcare teams were probably among the most important factors contributing to its effectiveness.
The creation of the CVC logbook is an innovation that allows healthcare workers in any hospital to safely manage every central device inserted in our department. This logbook is compiled for each CVC placed in our department. It reports all insertion details and specifics of each dressing performed in and outside the department beyond occlusion, dislocation, or exit-site infection episodes. Our study is the first report in the literature of a personal CVC logbook filled out at each notable catheter handling. Another critical point is carefully evaluating the exit-site during each CVC dressing. A section where the exit site is classified according to the Visual Exit Site Score has been inserted in the CVC logbook.[
20] This tool helps identify early signs of local infection, prevents microorganism migration and colonization of the CVC, avoiding CLABSI.
In our cohort, 32 (10%) of the 323 catheters with a positive exit-site swab resulted in a match with a positive blood culture. Of these, only 9 (28%) presented with CLABSI, which means that less than one-third of exit-site infections resulted in an extraluminal migration of the pathogen. The sterile technique of handling the infusion lines under a laminar flow hood guarantees a low risk of intraluminal bacterial dissemination.
TPN, multi-lumen devices, chemotherapy treatment, immunosuppression, and the number of days of catheterization were recognized as modifiable risk factors that increased the probability of developing CLABSI.[
21] Among the non-modifiable factors is known significant negative surviving correlation with diabetes mellitus, cardiovascular disease, lung disease, chronic kidney disease, the presence of ≥ 3 comorbidities, gut or skin graft versus host disease, patients within 15 days after stem cell transplant, high-risk neuroblastoma, AML, relapsed ALL, dressing/line concerns or issues within the past 72 h.[
22,
23] In our study, only a few variables were associated with infection. Presumably, such a low incidence of infections affected the identification of risk factors and discouraged the implementation of further analysis. The association between ALL and a lower infection rate is probably linked to the intensity of chemotherapy protocols. While other malignancies have a strictly programmed time interval for chemotherapy cycles, ALL protocols require a normalized white blood cell count before starting a new cycle, thus exposing patients to a milder infectious risk. In the years ahead, by expanding the data pool, it could be possible to investigate risk factors and patterns of infection in our setting to be included in new surveillance protocols. Furthermore, even if some patients underwent less frequent dressing, they were adequately selected since the incidence of infection didn’t change considerably. In fact, in patients who underwent more than 20% of dressing in a range of 8-10 days, proven infections were 0.314 per 1000 days of CVC, similar to the incidence in the whole cohort.
Although we comprehensively examined the factors that led to the significant reduction in CLABSI, our study has several limitations. First, it is a retrospective, one-facility study. Given our cohort's low incidence of CLABSI, a much larger sample size is needed to perform multivariate analyses, considering all the variables examined. Future projects could explore some interesting data on the life of the catheter that have yet to be considered, such as the influence of different therapeutic protocols.
In conclusion, targeted interventions with protocols produced by highly trained professionals, their sharing, and intensive training of satellite hospital staff lead to a significant drop in infectious complications among pediatric hematological oncology patients with CVC.
Author Contributions
Conceptualization, A.L. and N.M.; Methodology, M.S., D.V. and N.D.; Software, F.T.; Validation, N.D., S.T. and L.Z.; Formal Analysis, F.T.; Investigation, M.S. and D.V; Resources, A.L.; Data Curation, S.T., L.Z. and N.D.; Writing – Original Draft Preparation, F.T. and M.S.; Writing – Review & Editing, N.M.; Visualization, A.L.; Supervision, N.M.; Project Administration, A.L.; Funding Acquisition, N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the Declaration of Helsinki and approved by the Institutional Review Board of the Institute for Maternal and Child Health—IRCCS Burlo Garofolo (reference No. IRB RC 32/2023).
Informed Consent Statement
Written informed consent was obtained from all parents and custodians of patients involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Public Involvement Statement
No public involvement in any aspect of this research.
Guidelines and Standards Statement
This manuscript was drafted against the STROBE guidelines for a cross-sectional study.
Use of Artificial Intelligence:
AI or AI-assisted tools were not used in drafting any aspect of this manuscript.
Acknowledgments
This work was supported by the Ministry of Health, Rome, Italy, in collaboration with the Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste, Italy. The authors thank E. Grisan for supervising the machine learning analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cohen N, Rosenberg T, Rimon A, Friedman S. Early removal of a permanent catheter during the acute management of the unstable pediatric hemato-oncology patient with suspected catheter-related bloodstream infection: a multi-disciplinary survey and review of the literature. Eur J Pediatr. 2023;182(2):795-802.
- Hord JD, Lawlor J, Werner E, et al. Central line associated blood stream infections in pediatric hematology/oncology patients with different types of central lines. Pediatr Blood Cancer. 2016; 63(9):1603–1607.
- Cellini M, Bergadano A, Crocoli A, et al. Guidelines of the Italian Association of Pediatric Hematology and Oncology for the management of the central venous access devices in pediatric patients with onco-hematological disease. J Vasc Access. 2022;23(1):3-17.
- Baskin KM, Mermel LA, Saad TF, et al. Evidence-based strategies and recommendations for preservation of central venous access in children. J Parenter Enter Nutr. 2019; 43: 591–614.
- Ullman AJ, Marsh N, Mihala G, et al. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136:e1331–e1344.
- Joo EJ, Kang CI, Ha YE, et al. Clinical outcome of catheter salvage in neutropenic cancer patients with catheter-related infection. Scand J Infect Dis. 2011;43(4):258-63.
- Garaventa A, Castagnola E, Dallorso S et al. Sepsis in children with malignant neoplasia, equipped with a Broviac-type venous catheter. Pediatr Med Chir. 1995;17(2):147–150.
- Pinon M, Bezzio S, Tovo PA, et al. A prospective 7-year survey on central venous catheter-related complications at a single pediatric hospital. Eur J Pediatr. 2009; 168: 1505– 1512.
- O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011; 52: e162–e193.
- Fratino G, Molinari AC, Parodi S, et al. Central venous catheter-related complications in children with oncological/hematological diseases: an observational study of 418 devices. Ann Oncol. 2005; 16: 648–654.
- Scientific, The Italian Data Protection Authority. Authorization no. 9/2014 - General Authorisation to Process Personal Data for.
- 12. O'Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter-related infections. https://www.cdc.gov/infectioncontrol/guidelines/bsi/index.html. Accessed January 22, 2024.
- van den Bosch CH, Frakking FNJ, Loeffen YGT, et al. The applicability of the central line-associated bloodstream infection (CLABSI) criteria for the evaluation of bacteremia episodes in pediatric oncology patients. Eur J Haematol. 2024;112(5):832-839.
- Bell T, O'Grady NP. Prevention of Central Line-Associated Bloodstream Infections. Infect Dis Clin North Am. 2017;31(3):551-559.
- Cesaro S, Cavaliere M, Pegoraro A, Gamba P, Zadra N, Tridello G. A comprehensive approach to the prevention of central venous catheter complications: results of 10-year prospective surveillance in pediatric hematology-oncology patients. Ann Hematol. 2016;95(5):817-25.
- R Core Team (2023). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Sjoberg DD, Whiting K, Curry M, Lavery JA, Larmarange J. Reproducible summary tables with the gtsummary package. The R Journal 2021 e 13:570–80. [CrossRef]
- Therneau T (2023). _A Package for Survival Analysis in R_. R package version 3.5-7, https://CRAN.R-project.org/package=survival.
- Carraro F, Cicalese MP, Cesaro S, et al. Guidelines for the use of long-term central venous catheter in children with hemato-oncological disorders. On behalf of supportive therapy working group of Italian Association of Pediatric Hematology and Oncology (AIEOP). Ann Hematol. 2013;92(10):1405-12.
- GaVeCeLT, "Visual Exit Site Score" (2014) https://gavecelt.it/nuovo/sites/default/files/uploads/visual_exit_site_score.pdf.
- Lafuente Cabrero E, Terradas Robledo R, Civit Cuñado A, et al. Risk factors of catheter-associated bloodstream infection: Systematic review and meta-analysis. PLoS One. 2023;18(3):e0282290.
- Alwazzeh MJ, Alnimr A, Al Nassri SA, et al. Microbiological trends and mortality risk factors of central line-associated bloodstream infections in an academic medical center 2015-2020. Antimicrob Resist Infect Control. 2023;12(1):128.
- Dandoy CE, Hausfeld J, Flesch L, et al. Rapid cycle development of a multifactorial intervention achieved sustained reductions in central line-associated bloodstream infections in haematology oncology units at a children's hospital: a time series analysis. BMJ Qual Saf. 2016 Aug;25(8):633-43.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).