1. Introduction
Cancer is a complex disease with a broad group of disorders. It has a major impact on the human population and complicates the healthcare industry and public policy. In 2022, there were 9.7 million people died from cancer, with nearly 20 million new cases reported worldwide [
1]. This has led to the extensive screening of synthetic and natural compounds, intending to develop new chemotherapeutic treatments that are more effective, selective, and less hazardous. Medicinal plants contain a wide array of bioactive molecules with potential anticancer properties. Some of them were approved as cancer treatment by the Food and Drug Administration (FDA). The prominent examples of plant-derived anticancer compounds are vincristine, vinblastine, and taxanes, which are secondary metabolites with effectiveness in combating cancer cells by targeting the cellular cytoskeleton [
2].
Apoptosis is a controlled process that helps eliminate unwanted cells via programmed cell death, which is crucial for tissue homeostasis. The abnormalities of apoptosis can lead to diseases like cancer and autoimmune disorders. Cancer is caused by mutations in tumor suppressors or oncogenes, leading to resistance against apoptosis and requiring higher drug doses for treatment [
3]. Inducing apoptosis in malignant cells plays a crucial role in cancer therapy, however, the nonspecific initiation of apoptosis may be harmful to normal cells as well. Therefore, understanding apoptotic pathways and developing pro-apoptotic agents with negligible side effects are essential for novel cancer treatments and apoptosis resistance [
4]. There were reports of plant-derived compounds with potential pro-apoptotic activities. Terpenoids such as ursolic acid, isolated from different herbs like rosemary, oregano, lavender, and thyme, were shown to inhibit breast cancer stem cells by activating Akt [
5]. Betulinic acid from evergreen plant of the Rhamnaceae family upregulated Bax, and inhibited Bcl-2, thereby activating caspase-9 and downstream caspase-3, -7 leading to the apoptosis in leukemia stem cells [
6]. Polyphenols such as resveratrol from grapes, peanuts, and mulberry could also induce apoptosis of osteoma and glioma stem cells by activating the p53 gene [
7]. Curcumin from the rhizome of
Curcuma longa Linn. (Zingiberaceae) was shown to regulate the apoptotic pathway by blocking the Akt/FoxM1 pathway, thus exhibiting the anti-proliferative activity of gastric cancer stem cells [
8]. Alkaloids are also among the natural compounds that were reported to induce apoptosis. Berberine isolated from Rhizoma coptidis has been shown to inhibit various tumors such as breast cancer, pancreatic cancer, lung cancer, and other tumors by regulating the Hedgehog pathway [
9].
Vernonia amygdalina, commonly known as bitter leaf, is a small shrub or tree belonging to the Asteraceae family native to tropical regions of Africa and Asia [
10]. The leaf extract from
V. amygdalina was reported to be composed of alkaloids, tannins, flavonoids, saponins, triterpenoids, steroids, cardiac glycosides, and reducing sugars making it renowned for its extensive pharmacological activities [
11]. It exhibited significant antidiabetic effects, displayed strong antioxidant and anti-inflammatory properties, demonstrated antimalarial activity, and possessed broad-spectrum antimicrobial abilities against bacteria, fungi, and viruses [
12]. In terms of anti-cancer properties,
V. amygdalina extract was reported to be a potential cancer treatment. It was suggested to destroy cancer cells by inducing apoptosis in hepatoma [
13] and prostate cancer cells [
14]. It also displayed cardioprotective effects in rats when given concomitantly with the anticancer, doxorubicin [
15].
The omics approach has been developed to understand the complexity of biological systems at different levels of biological molecules. This technology engages in screening and analyzing large amounts of data demonstrating the structure and function of a given entity [
16]. Metabolomics involves small molecules, commonly known as metabolites whereas proteomics focuses on a set of proteins available within the biological system. Both metabolomics and proteomics play crucial roles in the discovery of new drugs from plants by providing comprehensive insights into the pharmacological actions of plant-derived substances. Metabolomics enables the detailed profiling of metabolites and the identification of bioactive compounds. Proteomics complements this by revealing interactions and changes in pathways which validates the pharmacological effects [
17]. By integrating metabolomics and proteomics, we can understand a holistic view of how the particular substances from plants exert their effects, consequently accelerating the development of effective and safe therapeutic agents.
Although V. amygdalina leaf extract was reported to have apoptotic effects in various cells. The comparative safety profile to normal cells has not been verified. Moreover, the link between the composition of the extract and the comprehensive molecular pathways was not established. In this study, we aimed to identify the composition of V. amygdalina leaf extract that possesses cytotoxic effects on the cancerous HeLa cells focusing on the apoptotic activation. The study utilized the approaches of metabolomics to obtain the profile of substances in the extract that might be crucial for the cytotoxic effects, as well as proteomics for achieving comprehensive information on molecular mechanistic pathways and potential markers for the apoptosis induced by V. amygdalina extract to establish its potential for the development as chemotherapy.
2. Results and Discussion
3.1. Cell Cytotoxicity
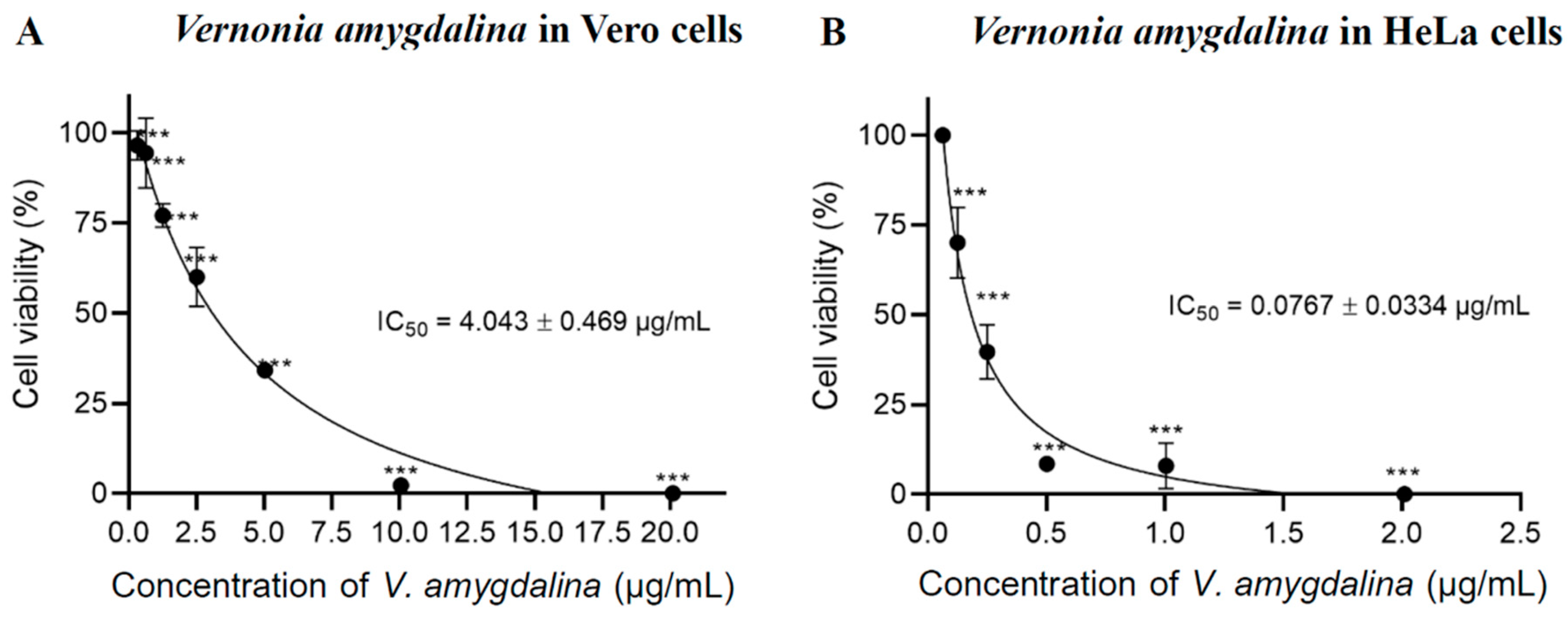
The cytotoxicity effect of
V. amygdalina leaf extract demonstrated the anti-cancer properties. The extract exhibited a dose-dependent inhibitory effect as the concentration increased in both HeLa and Vero cells. However, the half-maximal inhibitory concentration (IC
50) to kill Vero cells was higher than the cytotoxic concentration in HeLa cells after being treated for 24 h. The IC
50 values of V. amygdalina leaf extract in Vero cells and HeLa cells were determined to be 4.043 ± 0.469 µg/mL (
Figure 1A) and 0.0767 ± 0.0334 µg/mL (
Figure 1B), respectively. These findings indicated that the
V. amygdalina leaf extract exhibited selective cytotoxicity against cancer cells as it required a higher dose to kill normal cells. The leaf extract of
V. amygdalina has been reported for its cytotoxic effects against cancer cells in various studies. Harefa and colleagues (2022) established the IC
50 of ethyl acetate extract of
V. amygdalina leaf in human hepatoma HepG2 cells to be 19.91 ± 0.24 µg/mL, however, its toxicity against normal cells was not demonstrated in this paper [
19]. Nkono et al. (2022) also demonstrated the antiproliferative effects of aqueous alcoholic extract of
V. amygdalina leaf on human osteosarcoma MG-63 cells at concentrations of 125 and 250 μg/mL, without cytotoxicity on rabbit primary dermal fibroblasts (RPDF) at these concentrations [
20].
3.2. The Metabolomics Profile of V. amygdalina Leaf Extract
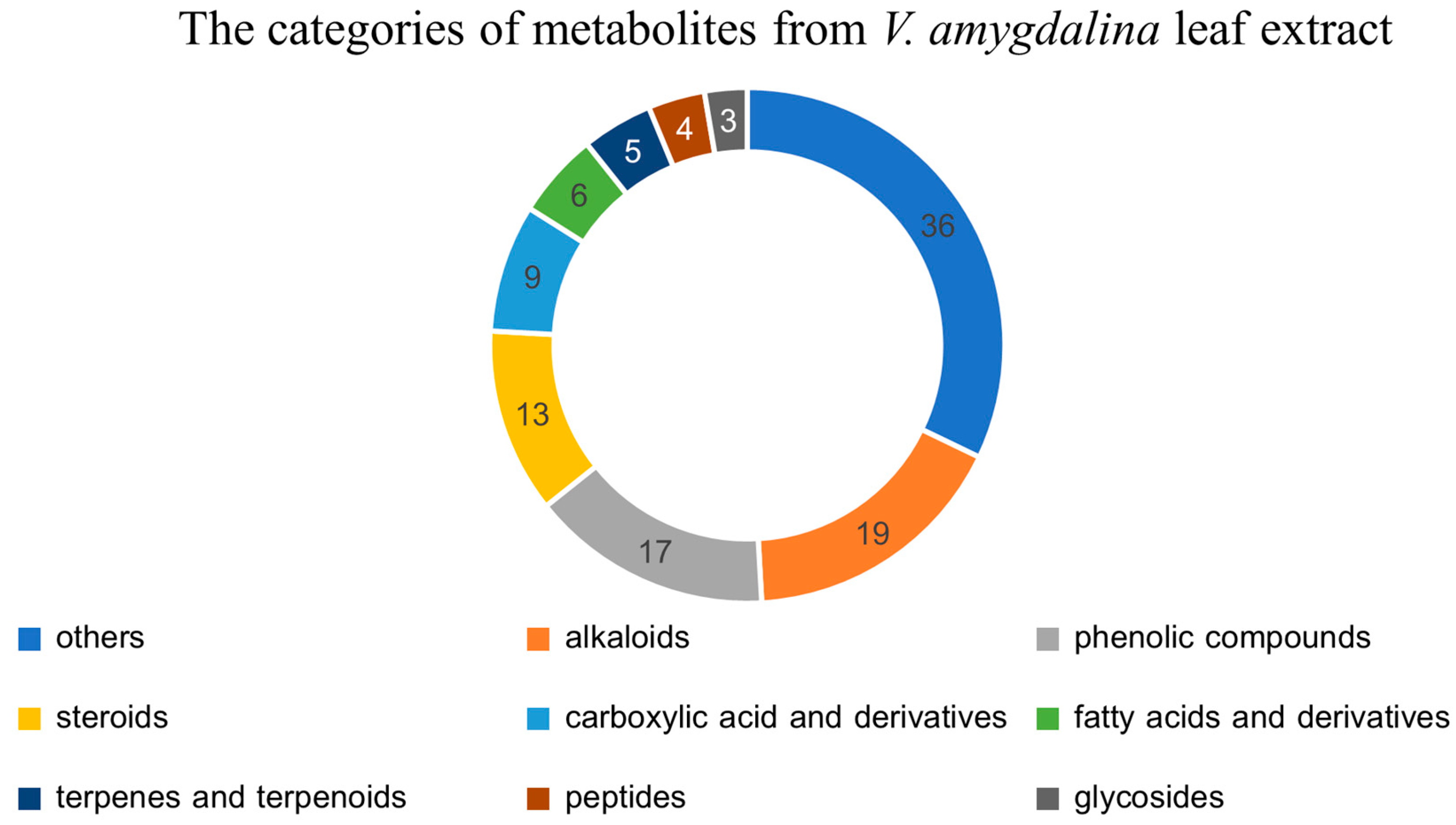
The LC-MS/MS identified 112 known metabolites from the leaf extract of
V. amygdalina. These metabolites were categorized based on their chemical structures into alkaloids, phenolic compounds, steroids, carboxylic acid and derivatives, fatty acids and derivatives, terpenes and terpenoids, peptides, glycosides, and others. The metabolomic profile of
V. amygdalina leaf extract was depicted as a pie chart in
Figure 2. The top three most abundant categories of metabolites were alkaloids with 19 identified substances, 17 phenolic compounds, and 13 steroids. Although there were 36 metabolites in the “others” category, they were not recognized as the major secondary metabolites in plants. Based on the phytochemical screening, it was also previously reported that alkaloids, tannins, flavonoids, saponins, triterpenoids, steroids, and glycosides were the major composition of the extract from the variation of
V. amygdalina [
11,
21]. The FT-IR spectroscopy also identified the chemicals with functional groups OH, C-H, C=C, and C=O, indicating the presence of alkanes (C-H stretching), alkenes (C=C stretching), carboxylic acids (C=O stretching), and alcohols (OH stretching) in the methanol extract of
V. amygdalina [
22].
Since the majority of secondary metabolites found in
V. amygdalina extract were alkaloids which were established as substances that induce apoptosis and some have been developed into current cancer treatment [
2]. One of the most abundant alkaloids identified in the extract was deacetylvindoline, as shown in
Table 1. Vindoline, an indole alkaloid, is a member of the Vinca alkaloids that was initially extracted from
Catharanthus roseus and is the synthetic precursor of the anticancer drugs, vinblastine, and vincristine [
23,
24]. The discovery of this alkaloid may explain the cytotoxic effect in cancer cells. However, other compositions in
V. amygdalina extract may help mitigate the severity of cell death and may even exhibit a cytoprotective effect as the higher concentration of IC
50 was required to kill normal cells. The cytoprotective substances that were established to mitigate oxidative stress and inflammation are flavonoids and phenolic compounds. Licochalcone B was one of the highest amounts of phenolic compounds in the leaf extract of
V. amygdalina. Licochalcone B is a bioactive chalcone compound with anti-inflammatory and antioxidative effects [
25]. Moreover, licochalcone B was also reported to have a cytotoxic effect on hepatoma HepG2 cells via apoptotic activation [
26]. Therefore, it was likely that these compounds from
V. amygdalina leaf extract were involved with the apoptotic process.
3.3. Protein Pathway Analysis
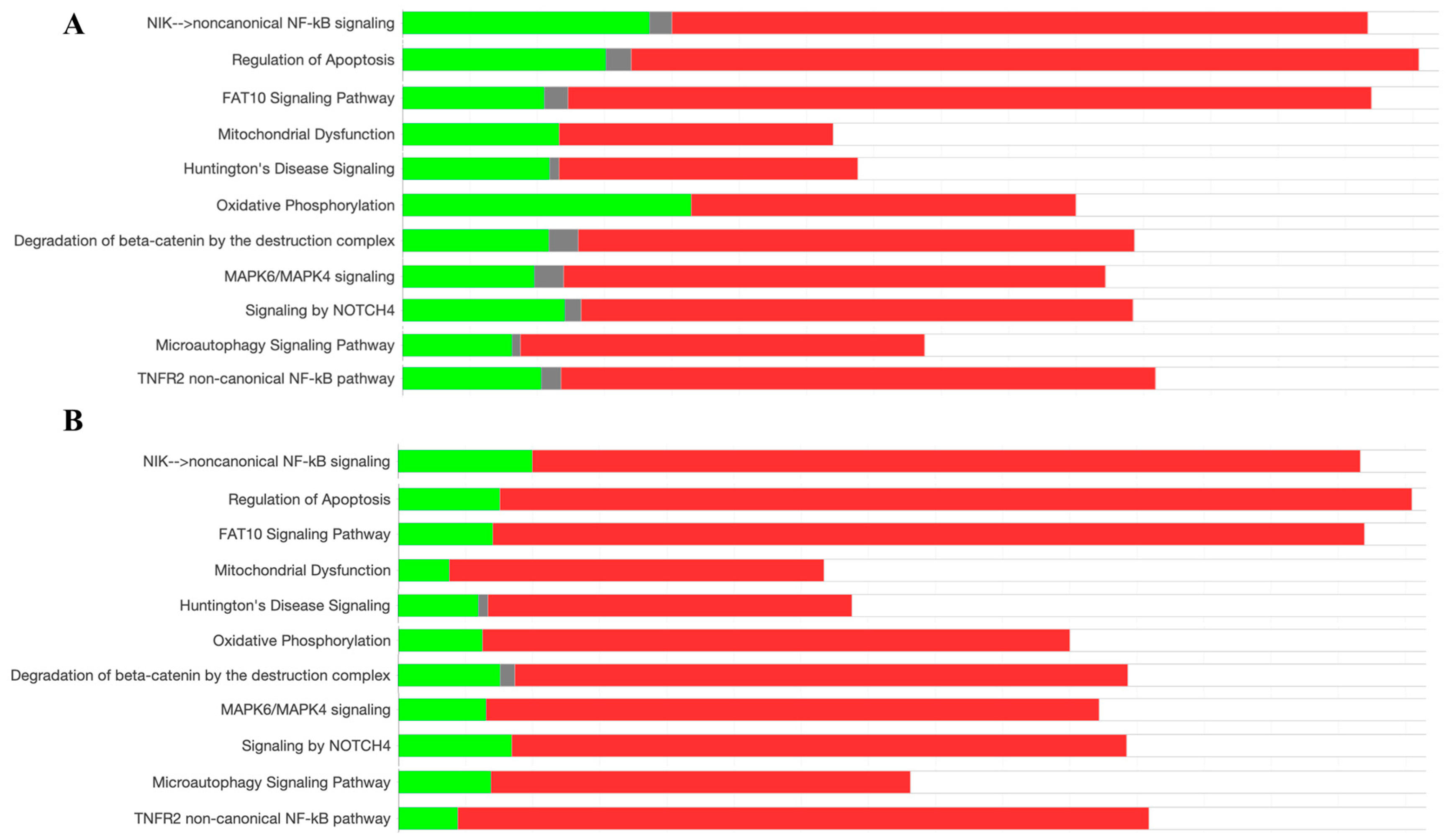
The analysis of signaling pathways from the entire proteome of HeLa cells was performed using an IPA. Differential proteome was compared between the
V. amygdalina leaf extract exposed group versus the control non-treated group. Various pathways were identified to be activated and inhibited. The pathways involved in cell survival including the NF-κB pathway, the regulation of apoptosis, MAPK6/MAPK4 signaling, degradation of beta-catenin by the destruction complex, NOTCH4 signaling, and microautophagy signaling were triggered along with the pathways involved with the response to stress, i.e., the FAT10 signaling pathway, mitochondrial dysfunction, Huntington’s disease signaling, and oxidative phosphorylation as shown in
Figure 3A. These patterns were also depicted in a higher intensity as the proteins working in these pathways were greatly regulated in HeLa cells treated with the anti-cancer, doxorubicin, compared with the control group as illustrated in
Figure 3B. Doxorubicin is an anti-cancer therapy in which the concluding mechanism of killing tumors is apoptosis [
27]. It could be suggested that the composition in the leaf extract of
V. amygdalina activated the proteins functioning in apoptosis and stress response in a similar pattern as doxorubicin, although at a lower intensity.
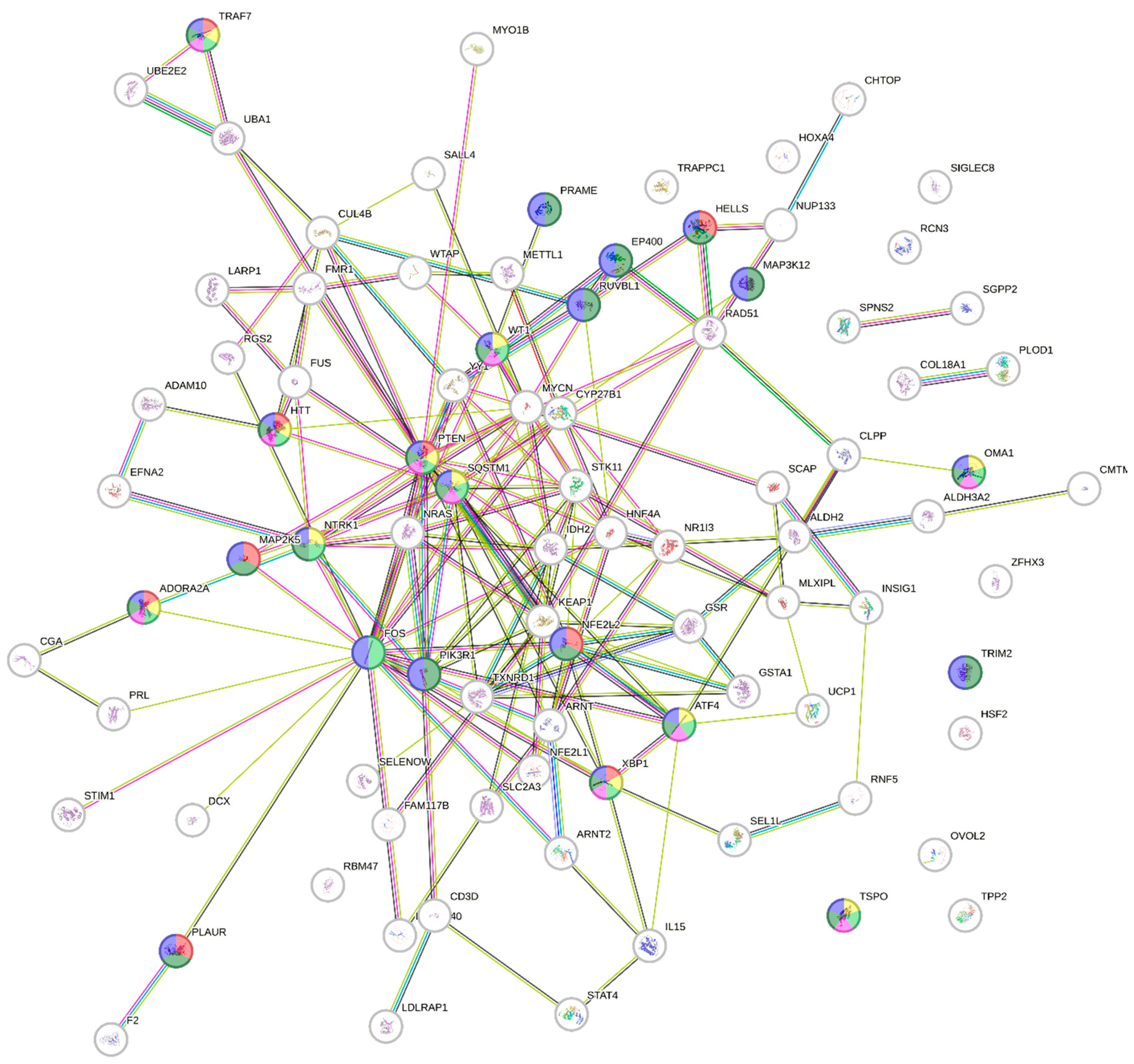
In terms of the prediction of upstream regulatory pathways in response to
V. amygdalina leaf extract in HeLa cells, there were significant changes in 95 upstream regulators with
z-scores ranging from -4.433 to +5.229 (the negative values referred to the inhibition, and the positive activation) with top ten highest changed upstream regulators depicted in
Table 2. All significant upstream regulators were input into the protein-protein interaction mapping using the online database, STRING (string-db.org) to identify the connection between these regulators (
Figure 4) and establish the potential Gene Ontology (GO) biological process. The GO biological processes involved with cell death and apoptosis were altered in the exposure to
V. amygdalina leaf extract (
Table 3) with the related upstream regulators highlighted in each pathway as illustrated in
Figure 4. Among these pathways, we discovered that PTEN and XBP1 were crucial and functioned in all GO biological processes regarding cell death and apoptosis.
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is a phosphatidylinositol-3,4,5-triphosphate (PIP3) phospholipid phosphatase, an enzyme that primarily inhibits the PI3K/Akt pathway. This enzyme is a critical tumor suppressor that is frequently mutated in various human cancers. It plays a significant role in regulating apoptosis through various mechanisms, such as antagonizing the PI3K/Akt signaling pathway, thus promoting apoptosis [
28]. Additionally, PTEN regulates Ca
2+ release from the endoplasmic reticulum (ER), influencing mitochondrial Ca
2+ overload and inducing apoptosis [
29]. Although the interaction between PTEN and X-box binding protein 1 (XBP1) was not specifically addressed, the role of XBP1 in regulating apoptosis was reported in the different molecular mechanisms. XBP1 was reported to regulate apoptosis by modulating the unfolded protein response (UPR) signaling pathway in response to endoplasmic reticulum (ER) stress [
30]. However, XRP1 had a distinct effect on different cell types. XBP1 overexpression in β-cell impaired insulin secretion and enhanced apoptosis, whereas it protected fibroblasts against cell death induced by ER stress [
31]. Therefore, the cytotoxic effects of
V. amygdalina leaf extract might involve apoptosis via the activation of upstream regulators, PTEN and XBP1.
3.4. Apoptotic Cell Imaging and Apoptotic Protein Level Quantification
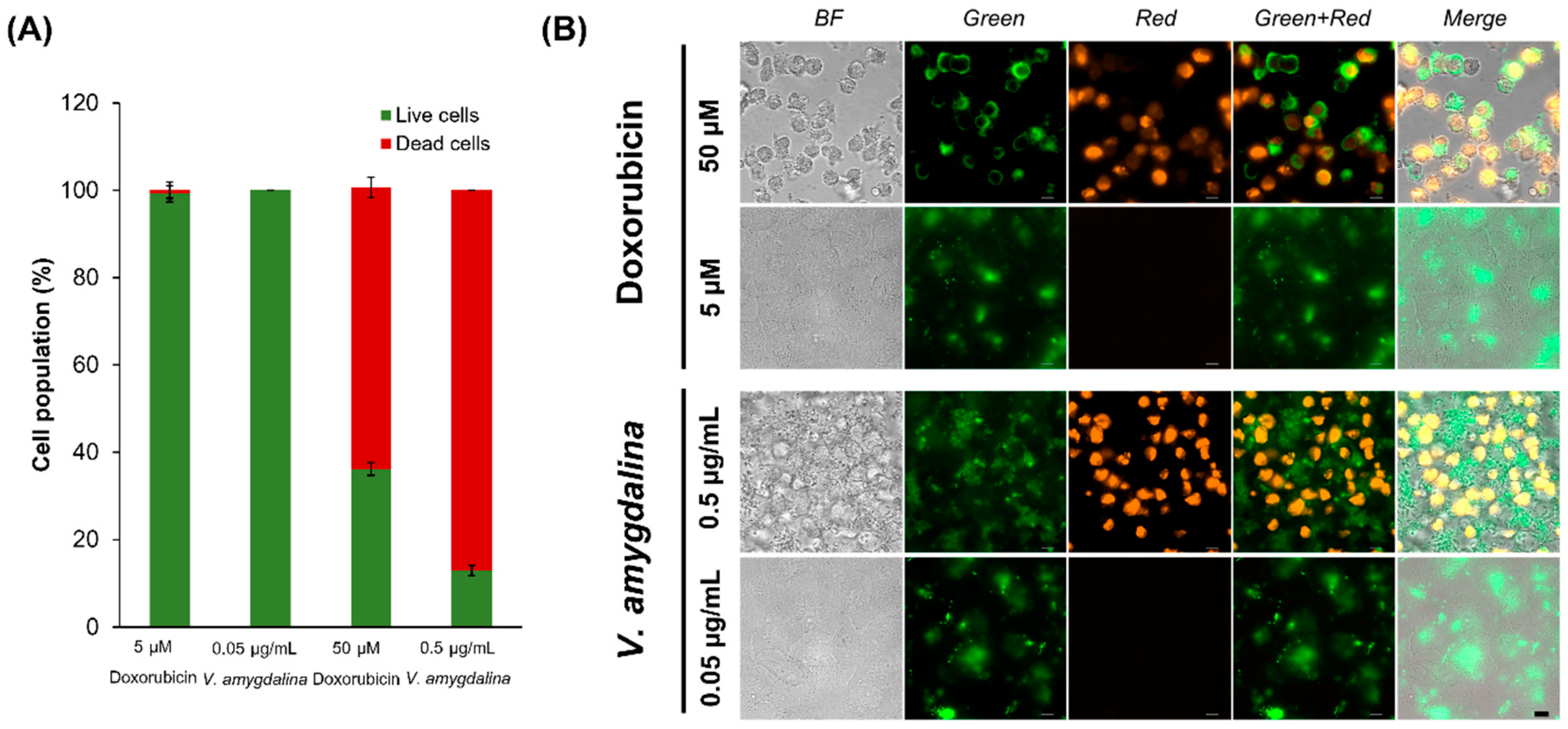
The percentage of dead cells calculated by the fluorescence intensity from the image showed that 0.5 µg/mL concentration of
V. amygdalina leaf extract induced apoptosis slightly better than in the 50 µM doxorubicin, while 5 µM doxorubicin and 0.05 µg/mL
V. amygdalina extract did not induce the apoptosis in HeLa cells (
Figure 5A). The green channel represents the detection of annexin V conjugated with the green fluorescent dye, FITC, the stain that interacts specifically with phosphatidyl serine, a target for the loss of plasma membrane asymmetry as presented in apoptosis. In addition, PI staining in the red channel indicated the dead cells as PI only crosses compromised membranes that lose integrity and bind with the DNA of those cells. We discovered the dead cells stained with both annexin V and PI, as depicted in
Figure 5B.
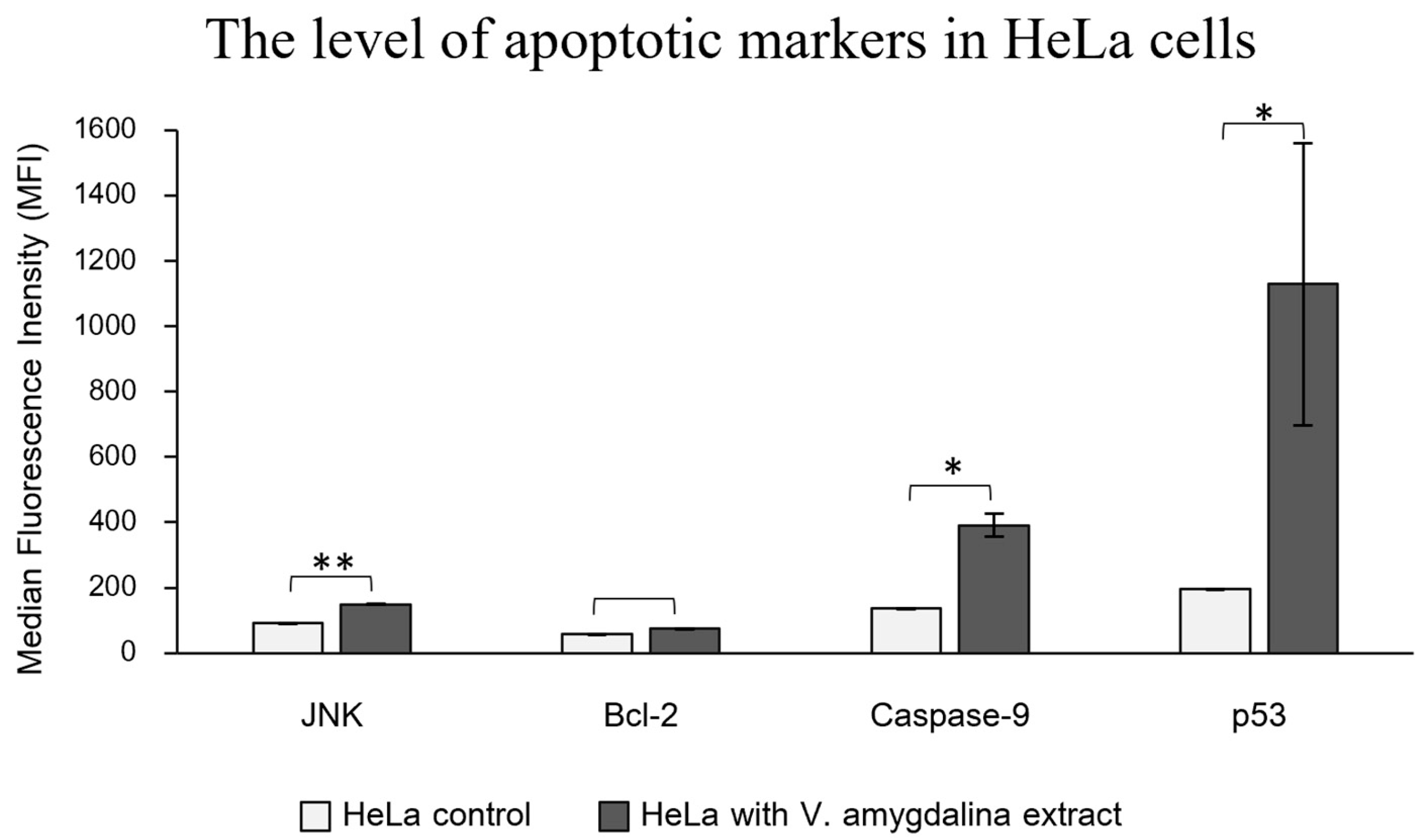
To verify the activation of apoptotic-related protein expression induced by
V. amygdalina leaf extract, the immuno-based Luminex
® assay was performed to detect the apoptotic markers that are involved in the apoptotic signaling pathways. We detected significantly higher levels of 3 apoptotic proteins; JNK, caspase-9, and p53, in HeLa cells treated with the extract, while the increase of anti-apoptotic marker, Bcl-2 was insignificant, as shown in
Figure 6. The molecular pathway involving JNK, p53, and caspase-9 in the process of apoptosis is intricate and crucial for cell fate determination. The c-Jun N-terminal kinases (JNKs) are part of the MAP kinases superfamily, which plays an essential role in controlling cellular development including cell growth, cell differentiation, and cell death [
32]. The mediation of apoptosis by JNK relates to the phosphorylation of the p53 protein family, which consequently elevates the expression of several pro-apoptotic genes such as Bax (Bcl2-associated X protein) and PUMA (p53 up-regulated modulator of apoptosis) [
33]. Moreover, JNKs have an important role in regulating apoptotic proteins in mitochondria [
34]. The translocation of JNKs into mitochondria initiates apoptotic signaling by releasing cytochrome C from the mitochondrial inner membrane along with the secretion of Apaf-1 and caspase-9 from the apoptosomes, which in turn activates the caspase-9 cascade [
35]. Since there was an established connection between the apoptotic proteins, JNK, p53, and caspase-9, which features a molecular cascade of apoptosis, it is suggested that the apoptosis could induce the cytotoxicity of HeLa cells treated with
V. amygdalina extract.
4. Materials and Methods
2.1. The Extraction of V. amygdalina Leaves
The cleaned V. amygdalina leaves were dried at 60 ̊C in the oven for 16 h, and then ground into a coarse powder, followed by maceration in hexane (Analytical grade, Fisher) to remove non-polar compounds. The maceration was stirred every 8 h for 24 h. After that, hexane was removed by filtering through filter papers (Whatman No.4) leaving a coarse powder of V. amydalina leaf, that was further extracted in ethyl acetate (EtOAc, Analytical grade, Fisher) and stirred periodically every 8 h for 24 h, repeated 3 times. The combined extracts was filtered using filter papers (Whatman No.4) and the EtOAc was removed using the rotary evaporator, Rotavapor® R-300 (Buchi, Flawil, Switzerland). The resultant dark green crude powder was stored in an amber glass bottle at 4 ̊C until use.
2.2. Cell Cultures and Cytotoxicity Test
HeLa cells (CRM CCL-2™) representing cancerous cells and Vero cells (CRM CCL-81™) representing normal cells were cultured in RPMI 1640 (Elabscience®, USA) medium containing 10% (v/v) fetal bovine (Gibco®, USA) serum at 37°C of a humidified atmosphere with 5% CO2 in a T-75 cm2 flask until they were grown about 60-70% confluent.
The cytotoxicity was investigated using the MTT assay. The cell lines (1 × 104 cells/mL) were seeded in 96-well plates and incubated for 24 h at 37 ̊C in 5% CO2 atmosphere. The extract from V. amygdalina leaves at concentrations of 0–2.5 µg/mL for HeLa cells and 0-20.0 µg/mL for Vero cells were fixed in 0.1% DMSO in culture medium and incubated for 24 h. After the removal of the culture medium and test substances, cells were washed with phosphate-buffered saline (PBS, pH 7.4). A 5 mg/mL aliquot of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution (MTT) was added, and incubated for 4 h at 37 ̊C in 5% CO2 atmosphere. The reading was performed using a SpectraMax i3x multi-mode microplate reader at 540 and 620 nm. The percentages of cell viability were calculated using GraphPad Prism version 8.0. Finally, data were analyzed for statistical significance by one-way ANOVA for potential cell viability. This experiment was conducted in triplicate.
2.3. The Metabolomics Profiling of V. amygdalina Leaf Extract
The profiling of metabolomics from V. amygdalina leaf extract was performed using a Q-Exactive Quadrupole Orbitrap Mass Spectrometer coupled with an UltiMate 3000 LC system. The sample injection of 5 µL was introduced into the system at a flow rate of 0.3 mL/min. The temperature in the Hypersil GOLD™ column and auto-sampler were maintained at 60 ̊C and 6 ̊C, respectively. Methanol/water (1:1) with 0.1% formic acid (MP: A) and acetonitrile with 0.1% formic acid (MP: B) (LC-MS grade, Sigma) were used as the mobile phase. Gradient starting conditions were 99% MP: A and 5% MP: B for 1min then rose to 95% MP: B over 23 min. The column was flushed with 100% MP: B for 5 min before returning to the starting conditions. A blank sample (0.1% formic acid in methanol) was administered after every injection. MS was operated in a positive mode with a spray voltage of 3.8 kV using flow rates of sheath gas and auxiliary gas flow rates of 48 and 11 arbitrary units (AU), respectively. The capillary temperature was set at 350 ̊C. The MS analysis interchanged between MS full scans and data-dependent MS/MS scans with dynamic exclusion. For full MS, a scan range of 75–700 m/z with a resolution of 120,000 and AGC target 3e6 with max IT 30 ms was operated. Full MS/MS was performed at a resolution of 15,000 with AGC target 1e5 at max IT 50 ms. The top five ions with the most intense signal were fragmented. All runs were acquired using the Xcalibur 3.1 software (Thermo Scientific).
2.4. Sample Preparation for Label-Free Proteomics Analysis
The HeLa cells in the treatment group were exposed to 0.1 µg/mL (IC50 of cytotoxic concentration) of V. amygdalina leaf extract for 24 h before the protein extraction process. After that, the cells were lysed on-ice using a probe tip sonication at a frequency of 20 kHz and 75% amplitude for 2 seconds on, and 3 seconds off at a total of 15 seconds in 200 µL lysis buffer (0.2% Triton X-100, 2 mM TCEP, 5 mM NaCl, 10 mM HEPES-KOH, pH 8.0) with protease inhibitor cocktail. The protein solution was collected by centrifugation at 15,000g for 10 minutes after 24-h ice-cold acetone precipitation (1:4 v/v). The protein pellet was reconstituted in 0.5% RapiGest SF (Waters, UK), 5 mM NaCl in 15 mM ammonium bicarbonate. A total of 40 µg of protein was subjected to a gel-free based digestion. Reduction of disulfide bonds was conducted using 1 mM TCEP in 15 mM ammonium bicarbonate at 50 ̊C for 1 hour and alkylation of sulfhydryl groups was performed using 4 mM iodoacetamide (IAA) in 15 mM ammonium bicarbonate at room temperature for 45 minutes in the dark. The solution was cleaned up by a desalting column (Zeba™ Spin Desalting Columns, 7K MWCO, 0.5 mL, ThermoFisher). The flow-through solution was enzymatically digested by Trypsin (Promega, Germany) at ratio 1:40 (enzyme: protein) ratio and incubated at 37 ̊C for 4 hours. The tryptic peptides were dried and subjected to MS/MS analysis immediately.
2.5. LC-MS/MS Settings and Configurations for Proteomics Analysis
The tryptic peptides were analyzed using an Orbitrap HF hybrid mass spectrometer, integrated with an UltiMate 3000 LC system. Initially, peptides were desalted using a reverse-phase C18 PepMap 100 trapping column and then separated on a C18 PepMapTM 100 capillary column. The dried tryptic peptides were reconstituted with 0.1% formic acid and 1.2 μg of protonated peptides were subjected to the nanoLC system. The mobile phases consisted of A) 0.1% formic acid in water and B) 95% acetonitrile with 0.1% formic acid. The mass spectrum was acquired in data-dependent acquisition mode, with full scans over a mass range of 400-1600 m/z. The top 15 most abundant peptide ions with charge states ranging from 2 to 5 were selected for fragmentation. The dynamic exclusion duration was set at 18 seconds. Full scan mass spectra were acquired from m/z 400 to 1600 with an AGC target set at 3×106 ions and a resolution of 120,000. MS/MS scan was initiated when the ACG target reached 105 ions. on selection was performed applying a dynamic exclusion window of 15 seconds.
2.6. Biological Pathway Analysis
To reduce the variability in the protein dataset, The normalization of protein intensity was performed using the NormalyzerDE, with quantile normalization applied to the relative expression data analysis after adding “1” to all expression values. To ensure high-confidence data, only proteins identified with an FDR ≤1% were considered for the confidential protein list. The effect of V. amygdalina leaf extract on the pathway signaling cascade in HeLa cells was analyzed using Ingenuity Pathway Analysis (IPA). All differentially expressed proteins were imported to the core analysis and analyzed to define the significantly changed protein signaling pathways and upstream regulators. The detailed procedures for IPA analysis and its parameters are described in the previous report [
18]. The analysis was performed by comparing all changed proteins against known canonical pathways within the IPA database (accessed on 29 February 2024). The activation and deactivation state of pathways and upstream regulator (any protein that can affect the expression of another protein) was analyzed based on all differentially expressed proteins and adj. p-value (z-score). Major signal transduction pathways were reconstructed according to IPA results. Acceptable upstream regulators were required to have a z-score ≥ 1.5 and a p-value < 0.01. The biological pathway analysis was also conducted in the comparison of doxorubicin and the control group.
2.7. Imaging of Apoptotic Cells and Immuno-Based Early Apoptosis Protein Quantification
Live HeLa cells were treated with V. amygdalina leaf extract or doxorubicin for 24 h prior to the fluorescein (FITC) conjugated annexin V (Thermo Fischer, USA) and propidium iodide (PI) staining (Thermo Fischer, USA). The imaging of apoptotic cells was captured by using a fluorescence microscope (ZEISS Axio Observer with optical sectioning and deconvolution algorithm) using the green channel for annexin V with an excitation wavelength of 495 nm and an emission wavelength of 519 nm. In addition, the dead cells stained by PI were taken in the red channel with an excitation wavelength of 553 nm and an emission wavelength of 568 nm.
The level of apoptotic protein markers was measured using the MILLIPLEX® early apoptosis magnetic bead kit ((Merck, USA). The levels of JNK, Bcl-2, Caspase-9, and p53 were quantitated based on Luminex® xMAP® technology. Following treatment with V. amygdalina leaf extract and a negative control group at IC50 for 72 hours, the HeLa cells were washed with ice-cold buffered saline and disrupted with 0.3 mL of 1× MILLIPLEX® Lysis Buffer containing a protease inhibitor cocktail. To obtain lysed cells, the reaction was incubated at 50 ̊C for 10 min with manual mixing. The supernatant was collected by centrifugation at 14,000g at 16 ̊C for 30 min. The protein concentration was measured using the BCA protein assay and adjusted with PBS to a concentration of 2.5 μg/μL. Before the experiment, the protein solution was further diluted in PBS at a 1:5 (v/v) ratio, resulting in a final concentration of 0.5 μg/μL. A total of 20 μL (10 μg) of the protein solution was subjected to the assay. For the magnetic beads, biotin-labeled detection antibody, streptavidin-PE, normalizing control proteins, and MILLIPLEX® cell lysates were prepared according to the manufacturer’s instructions without any modifications. The quantification of protein levels was reported as the median fluorescence intensity (MFI) value and the standard deviation, based on two biological replicate experiments and two replicate wells.
5. Conclusions
We have found that alkaloids, phenolic compounds, and steroids were the major secondary metabolites in V. amygdalina leaf extract. This composition of metabolites exhibited potential cytotoxic effects on cancerous HeLa cells at a lower concentration than that of the normal Vero cells. The proteomics profile of HeLa cells treated with the extract revealed the proteins functioning in apoptosis and stress response were upregulated in a similar pattern to the group of doxorubicin, the anti-cancer treatment. The upstream regulator proteins with the most significant changes, i.e., PTEN, XBP1, and ADORA2A, were involved in the regulation of apoptosis and cell death. Apoptosis was verified in HeLa cells treated with V. amygdalina leaf extract using annexin V/PI staining along with the immuno-based Luminex® assay, which confirmed the significant increase in expression levels of apoptotic markers, JNK, p53, and caspase-9. These suggested that the leaf extract of V. amygdalina induced the cytotoxicity of cancer cells via apoptosis and supported its potential for being a cancer treatment. However, further research on certain active compounds and their effects on specific biological targets is required for the development of an anti-cancer medication.
Supplementary Materials
The following supporting information can be downloaded at website of this paper posted on Preprints.org, Figure S1: The unique fragment ions spectrum (MS2) of nandrolene. Figure S2: The unique fragment ions spectrum (MS2) of deacetylvindoline.
Author Contributions
Conceptualization, P.S.; Data curation, P.S. and S.K.; Formal analysis, P.S. and Y.Y.; Funding acquisition, P.S.; Investigation, Y.Y., K.F., T.V., V.C., J.C., S.K. and C.A.; Methodology, P.S., K.F., T.V., V.C., J.C., S.K. and C.A.; Project administration, P.S.; Resources, S.K. and C.A.; Software, S.K.; Supervision, S.K. and C.A.; Validation, C.A.; Visualization, S.K.; Writing – original draft, P. S.; Writing – review & editing, P.S., T.V., J.C. and C.A.
Funding
This work is supported by the CMU Junior Research Fellowship Program of Chiang Mai University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Upon a reasonable request, the corresponding author is willing to provide the data and materials supporting the results of this study.
Acknowledgments
We wish to thank Assoc.Prof.Dr. Jakkapan Sirithunyalug for the V. amygdalina leaves sample.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; nor in the decision to publish the results.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R. L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, K.; Aktar, A.; Roy, P.; Biswas, B.; Hossain, Md. E.; Sarkar, K. K.; Bachar, S. C.; Ahmed, F.; Monjur-Al-Hossain, A. S. M.; Fukase, K. A Review on Mechanistic Insight of Plant Derived Anticancer Bioactive Phytocompounds and Their Structure-Activity Relationship. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R. A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, H. The Program Cell Death (Apoptosis) and the Therapy of Cancer. In Regulation and Dysfunction of Apoptosis; Tutar, Y., Ed.; IntechOpen, 2022. [CrossRef]
- Kim, G. D. Ursolic Acid Decreases the Proliferation of MCF-7 Cell-Derived Breast Cancer Stem-Like Cells by Modulating the ERK and PI3K/AKT Signaling Pathways. Prev Nutr Food Sci 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Leong, K. H.; Mahdzir, M. A.; Din, M. F. M.; Awang, K.; Tanaka, Y.; Kulkeaw, K.; Ishitani, T.; Sugiyama, D. Induction of Intrinsic Apoptosis in Leukaemia Stem Cells and in Vivo Zebrafish Model by Betulonic Acid Isolated from Walsura Pinnata Hassk (Meliaceae). Phytomedicine 2017, 26. [Google Scholar] [CrossRef] [PubMed]
- Clark, P. A.; Bhattacharya, S.; Elmayan, A.; Darjatmoko, S. R.; Thuro, B. A.; Yan, M. B.; Van Ginkel, P. R.; Polans, A. S.; Kuo, J. S. Resveratrol Targeting of AKT and P53 in Glioblastoma and Glioblastoma Stem-like Cells to Suppress Growth and Infiltration. J Neurosurg 2017, 126. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Wang, C.; Yang, D.; Wei, Z.; Xu, J.; Hu, Z.; Zhang, Y.; Wang, W.; Yan, R.; Cai, Q. Curcumin Regulates Proliferation, Autophagy, and Apoptosis in Gastric Cancer Cells by Affecting PI3K and P53 Signaling. J Cell Physiol 2018, 233. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. Z.; Li, Z.; Yuan, Z. Effect of Berberine on the Proliferation and Apoptosis of Lung Cancer Stem Cells and the Possible Mechanism. Chinese Journal of Tissue Engineering Research 2017, 21. [Google Scholar] [CrossRef]
- Patathananone, S.; Pothiwan, M.; Uapipatanakul, B.; Kunu, W. Inhibitory Effects of Vernonia Amygdalina Leaf Extracts on Free Radical Scavenging, Tyrosinase, and Amylase Activities. Prev Nutr Food Sci 2023, 28. [Google Scholar] [CrossRef]
- Usunobun, U.; Ngozi, O. Phytochemical Analysis and Proximate Composition of Vernonia Amygdalina. International Journal of Scientific World 2016, 4. [Google Scholar] [CrossRef]
- Ugbogu, E. A.; Emmanuel, O.; Dike, E. D.; Agi, G. O.; Ugbogu, O. C.; Ibe, C.; Iweala, E. J. The Phytochemistry, Ethnobotanical, and Pharmacological Potentials of the Medicinal Plant-Vernonia Amygdalina L. (Bitter Leaf). Clinical Complementary Medicine and Pharmacology 2021, 1. [Google Scholar] [CrossRef]
- Lai, C. C.; Zhou, X.; Wang, H. K.; Lin, Y. C.; Lin, H. Y.; Way, T. Der; Liu, B. L. Vernonia Amygdalina Extract Induces Apoptosis and Inhibits Epithelial-Mesenchymal Transition in Hep 3B Cells through the Inhibition of PI3k/Akt Signaling Pathway. International Journal of Applied Science and Engineering 2022, 19. [Google Scholar] [CrossRef]
- G Yedjou, C.; Johnson, W.; Tchounwou, S. S.; Dasari, S.; Njiki, S.; Tchounwou, P. B. Vernonia Amygdalina Delile Induces Apoptotic Effects of PC3 Cells: Implication in the Prevention of Prostate Cancer. Journal of Biomedical Research & Environmental Sciences 2022, 3. [Google Scholar] [CrossRef]
- Syahputra, R. A.; Harahap, U.; Harahap, Y.; Gani, A. P.; Dalimunthe, A.; Ahmed, A.; Zainalabidin, S. Vernonia Amygdalina Ethanol Extract Protects against Doxorubicin-Induced Cardiotoxicity via TGFβ, Cytochrome c, and Apoptosis. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Shen, L. Advances and Trends in Omics Technology Development. Frontiers in Medicine. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Ivanisevic, T.; Sewduth, R. N. Multi-Omics Integration for the Design of Novel Therapies and the Identification of Novel Biomarkers. Proteomes. [CrossRef]
- Degryse, S.; De Bock, C. E.; Demeyer, S.; Govaerts, I.; Bornschein, S.; Verbeke, D.; Jacobs, K.; Binos, S.; Skerrett-Byrne, D. A.; Murray, H. C.; Verrills, N. M.; Van Vlierberghe, P.; Cools, J.; Dun, M. D. Mutant JAK3 Phosphoproteomic Profiling Predicts Synergism between JAK3 Inhibitors and MEK/BCL2 Inhibitors for the Treatment of T-Cell Acute Lymphoblastic Leukemia. Leukemia 2018, 32. [Google Scholar] [CrossRef]
- Harefa, H. S.; Hasibuan, P. A. Z.; Harahap, U. Cytotoxic and Apoptotic Activities of Vernonia Amygdalina Extract in HepG2 Cell Line. Indonesian Journal of Pharmaceutical and Clinical Research 2022, 5. [Google Scholar] [CrossRef]
- Nkono, B. L. N. Y.; Rouamba, A.; Duceac, I. A.; Verestiuc, L. Antihyperglycemic Effect of Vernonia Amygdalina and in Vitro Evaluation of Its Antiproliferative Activity on Human Osteosarcoma MG-63. Pan African Medical Journal 2022, 42. [Google Scholar] [CrossRef]
- Dumas, N. G. E.; Anderson, N. T. Y.; Godswill, N. N.; Thiruvengadam, M.; Ana-Maria, G.; Ramona, P.; Crisan, G. C.; Laurian, V.; Shariati, M. A.; Tokhtarov, Z.; Emmanuel, Y. Secondary Metabolite Contents and Antimicrobial Activity of Leaf Extracts Reveal Genetic Variability of Vernonia Amygdalina and Vernonia Calvoana Morphotypes. Biotechnol Appl Biochem 2021, 68. [Google Scholar] [CrossRef]
- Mukhtar, Y.; Chimbekujwo, I. B. ; Aisha; Fatima; Salamatu, S. U. Phytochemical Screening and Fourier Transform Infrared Spectroscopy (FT-IR) Analysis of Vernonia Amygdalina Del. (Bitter Leaf) Methanol Leaf Extract. FUTY J Environ.
- Sasaki, Y.; Kato, D.; Boger, D. L. Asymmetric Total Synthesis of Vindorosine, Vindoline, and Key Vinblastine Analogues. J Am Chem Soc 2010, 132. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, M.; Wen, W.; Yu, R. Biosynthesis and Regulation of Terpenoid Indole Alkaloids in Catharanthus Roseus. Pharmacognosy Reviews. [CrossRef]
- Huang, J.; Zhu, Y.; Li, S.; Jiang, H.; Chen, N.; Xiao, H.; Liu, J.; Liang, D.; Zheng, Q.; Tang, J.; Meng, X. Licochalcone B Confers Protective Effects against LPS-Induced Acute Lung Injury in Cells and Mice through the Keap1/Nrf2 Pathway. Redox Report 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, A. M.; Thakur, K.; Zhang, J. G.; Huang, J. H.; Wei, Z. J. Licochalcone B Extracted from Glycyrrhiza Uralensis Fisch Induces Apoptotic Effects in Human Hepatoma Cell HepG2. J Agric Food Chem 2019, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Konorev, E. A.; Kotamraju, S.; Joseph, J.; Kalivendi, S.; Kalyanaraman, B. Doxorubicin Induces Apoptosis in Normal and Tumor Cells Via distinctly different mechanisms. intermediacy of H2O2- and p53-dependent pathways. Journal of Biological Chemistry 2004, 279. [Google Scholar] [CrossRef]
- Naderali, E.; Khaki, A. A.; Rad, J. S.; Ali-Hemmati, A.; Rahmati, M.; Charoudeh, H. N. Regulation and Modulation of PTEN Activity. Molecular Biology Reports, 1007. [Google Scholar]
- Bononi, A.; Bonora, M.; Marchi, S.; Missiroli, S.; Poletti, F.; Giorgi, C.; Pandolfi, P. P.; Pinton, P. Identification of PTEN at the ER and MAMs and Its Regulation of Ca 2+ Signaling and Apoptosis in a Protein Phosphatase-Dependent Manner. Cell Death Differ 2013, 20. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Li, Y.; Liang, S.; Chen, Z. XBP1 Negatively Regulates CENPF Expression via Recruiting ATF6α to the Promoter during ER Stress. Cancer Cell Int 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Allagnat, F.; Christulia, F.; Ortis, F.; Pirot, P.; Lortz, S.; Lenzen, S.; Eizirik, D. L.; Cardozo, A. K. Sustained Production of Spliced X-Box Binding Protein 1 (XBP1) Induces Pancreatic Beta Cell Dysfunction and Apoptosis. Diabetologia 2010, 53. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, A. Role of JNK Activation in Apoptosis: A Double-Edged Sword. Cell Research. [CrossRef]
- Oleinik, N. V.; Krupenko, N. I.; Krupenko, S. A. Cooperation between JNK1 and JNK2 in Activation of P53 Apoptotic Pathway. Oncogene 2007, 26. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Boyd, C. S.; Ahmed, R.; Spencer, J. P. E.; Duncan, R. F.; Rice-Evans, C.; Cadenas, E. C-Jun N-Terminal Kinase (JNK)-Mediated Modulation of Brain Mitochondria Function: New Target Proteins for JNK Signalling in Mitochondrion-Dependent Apoptosis. Biochemical Journal 2003, 372. [Google Scholar] [CrossRef]
- Chinnaiyan, A. M. The Apoptosome: Heart and Soul of the Cell Death Machine. Neoplasia (New York, N.Y.). [CrossRef]
Figure 1.
Cell viability and IC50 using MTT assay of V. amygdalina in (A) Vero cells and (B) HeLa cells. The data shown are the mean from three independent experiments ± S.D. (n = 3). Statistical significance was determined using One–way ANOVA and presented as *** p < 0.001 vs. control (0.1% DMSO).
Figure 1.
Cell viability and IC50 using MTT assay of V. amygdalina in (A) Vero cells and (B) HeLa cells. The data shown are the mean from three independent experiments ± S.D. (n = 3). Statistical significance was determined using One–way ANOVA and presented as *** p < 0.001 vs. control (0.1% DMSO).
Figure 2.
The metabolomics profile of the extract from V. amygdalina was categorized into 9 groups; alkaloids, phenolic compounds, steroids, carboxylic acid and derivatives, fatty acids and derivatives, terpenes and terpenoids, peptides, glycosides, and others. The most identified metabolites were alkaloids, phenolic compounds, and steroids.
Figure 2.
The metabolomics profile of the extract from V. amygdalina was categorized into 9 groups; alkaloids, phenolic compounds, steroids, carboxylic acid and derivatives, fatty acids and derivatives, terpenes and terpenoids, peptides, glycosides, and others. The most identified metabolites were alkaloids, phenolic compounds, and steroids.
Figure 3.
The percentage of upregulated (red), downregulated (green), and unchanged (gray) proteins functioning in the biological pathways of cell survival and response to stress shown as the comparison between HeLa cells exposed to V. amygdalina leaf extract and the non-treated control group (A), and HeLa cells exposed to doxorubicin compared with the control group (B).
Figure 3.
The percentage of upregulated (red), downregulated (green), and unchanged (gray) proteins functioning in the biological pathways of cell survival and response to stress shown as the comparison between HeLa cells exposed to V. amygdalina leaf extract and the non-treated control group (A), and HeLa cells exposed to doxorubicin compared with the control group (B).
Figure 4.
The protein-protein interaction map of the upstream regulators that significantly altered after the exposure to V. amygdalina leaf extract interaction prediction. The proteins functioning in GO biological processes are highlighted according to each process, i.e., GO:0042981 (regulation of apoptotic process) in dark green, GO:0010941 (regulation of cell death) in blue, GO:0010942 (positive regulation of cell death) in light green, GO:0043068 (positive regulation of programmed cell death) in yellow, GO:2001233 (regulation of apoptotic signaling pathway) in red, and GO:0043065 (positive regulation of apoptotic process) in pink. The upstream protein regulators that were involved with all cell death and apoptosis pathways were PTEN, XBP1, and ADORA2A.
Figure 4.
The protein-protein interaction map of the upstream regulators that significantly altered after the exposure to V. amygdalina leaf extract interaction prediction. The proteins functioning in GO biological processes are highlighted according to each process, i.e., GO:0042981 (regulation of apoptotic process) in dark green, GO:0010941 (regulation of cell death) in blue, GO:0010942 (positive regulation of cell death) in light green, GO:0043068 (positive regulation of programmed cell death) in yellow, GO:2001233 (regulation of apoptotic signaling pathway) in red, and GO:0043065 (positive regulation of apoptotic process) in pink. The upstream protein regulators that were involved with all cell death and apoptosis pathways were PTEN, XBP1, and ADORA2A.
Figure 5.
The intensity and images of apoptotic HeLa cells treated with 5 and 50 µM doxorubicin and V. amygdalina in the concentration of 0.05 and 0.5 µg/mL. A: the intensity of fluorescence of dead and live cells and B: the images of green and red channels representing cells stained with annexin V and PI, respectively. (Scale bar 10 µm).
Figure 5.
The intensity and images of apoptotic HeLa cells treated with 5 and 50 µM doxorubicin and V. amygdalina in the concentration of 0.05 and 0.5 µg/mL. A: the intensity of fluorescence of dead and live cells and B: the images of green and red channels representing cells stained with annexin V and PI, respectively. (Scale bar 10 µm).
Figure 6.
The levels of apoptotic markers, JNK, caspase-9, and p53 in the treatment of the V. amygdalina group (black bars), were significantly higher than in the control group (white bars) (** represents the p-value of 0.01 and * p-value of 0.05) whereas the level of anti-apoptotic protein, Bcl-2 increase insignificantly between two groups.
Figure 6.
The levels of apoptotic markers, JNK, caspase-9, and p53 in the treatment of the V. amygdalina group (black bars), were significantly higher than in the control group (white bars) (** represents the p-value of 0.01 and * p-value of 0.05) whereas the level of anti-apoptotic protein, Bcl-2 increase insignificantly between two groups.
Table 1.
The most abundant 15 annotated compounds identified in the V. amygdalina leaf extract. The examples of unique fragment ions mass spectra (MS2) that elucidate the structure of nandrolene and deacetylvindoline are illustrated in the supplementary.
Table 1.
The most abundant 15 annotated compounds identified in the V. amygdalina leaf extract. The examples of unique fragment ions mass spectra (MS2) that elucidate the structure of nandrolene and deacetylvindoline are illustrated in the supplementary.
| Name |
Formula |
Molecular Weight |
Peak area |
Categories |
| Nandrolone decanoate |
C28H44O3
|
428.3216 |
8.07E+08 |
Steroids |
| Deacetylvindoline |
C23H30N2O5
|
414.2143 |
7.28E+08 |
Alkaloids |
| Rubiarbonone C |
C32H50O5
|
536.3454 |
5.03E+08 |
Terpenes and terpenoids |
| Glycyrrhetinic acid |
C30H46O4
|
492.3183 |
4.49E+08 |
Terpenes and terpenoids |
| Testosterone decanoate |
C29H46O3
|
442.3378 |
2.23E+08 |
Steroids |
| Psoralidin |
C20H16O5
|
336.1055 |
1.82E+08 |
Steroids |
| Ursolic acid |
C30H48O3
|
456.3540 |
1.65E+08 |
Terpenes and terpenoids |
| 3-Hydroxybupivicaine |
C18H28N2O2
|
304.2111 |
1.64E+08 |
Alkaloids |
| Phenylglyoxylic acid |
C8H6O3
|
150.0316 |
1.53E+08 |
Phenolic compounds |
| 2-Hydroxyoctanoic acid |
C8H16O3
|
182.0888 |
1.17E+08 |
Fatty acids and derivatives |
| Deidaclin |
C12H17NO6
|
271.1056 |
7.48E+07 |
Glycosides |
| Prostaglandin F2 |
C21H36O5
|
368.2553 |
5.63E+07 |
Steroids |
| Licochalcone B |
C16H14O5
|
308.0693 |
5.09E+07 |
Phenolic compounds |
| Prostaglandin E2-biotin |
C35H58N4O6S |
684.4016 |
3.75E+07 |
Steroids |
| Cafestol |
C20H28O3
|
316.2089 |
2.44E+07 |
Terpenes and terpenoids |
Table 2.
Top ten remarkable upstream protein regulators predicted to be activated (positive z-score) or inhibited (negative z-score) in HeLa cells after the treatment of V. amygdalina leaf extract.
Table 2.
Top ten remarkable upstream protein regulators predicted to be activated (positive z-score) or inhibited (negative z-score) in HeLa cells after the treatment of V. amygdalina leaf extract.
| Upstream Regulator |
Molecule Type |
z-score |
p-value |
| HNF4A |
Transcription regulator |
2.67 |
2.44E-108 |
| LARP1 |
Translation regulator |
5.229 |
1.34E-68 |
| MYCN |
Transcription regulator |
-3.682 |
1.76E-56 |
| NFE2L2 |
Transcription regulator |
5.012 |
3.11E-41 |
| MLXIPL |
Transcription regulator |
-4.433 |
4.52E-37 |
| XBP1 |
Transcription regulator |
3.704 |
3.06E-28 |
| STK11 |
Kinase |
2.964 |
1.22E-27 |
| CLPP |
Peptidase |
-2.411 |
1.31E-27 |
| PTEN |
Phosphatase |
2.149 |
4.7E-16 |
| IL15 |
Cytokine |
3.184 |
1.03E-14 |
Table 3.
Biological processes from GO annotation using 95 upstream regulators established six significant pathways involving apoptosis with ≤0.05 false discovery rate.
Table 3.
Biological processes from GO annotation using 95 upstream regulators established six significant pathways involving apoptosis with ≤0.05 false discovery rate.
| GO term |
Biological process |
False discovery rate |
| GO:0042981 |
Regulation of apoptotic process |
0.00046 |
| GO:0010941 |
Regulation of cell death |
0.00073 |
| GO:0010942 |
Positive regulation of cell death |
0.0025 |
| GO:0043068 |
Positive regulation of programmed cell death |
0.0037 |
| GO:2001233 |
Regulation of apoptotic signaling pathway |
0.0062 |
| GO:0043065 |
Positive regulation of apoptotic process |
0.0119 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).