1. Introduction
Brazil has had one of the highest pediatric COVID-19 mortality rates during the pandemic [
1,
2,
3]. The COVID-19 disease is characterized by symptoms that include high fever, chills, cough and shortness of breath or difficulty breathing, and may also present other symptoms such as diarrhea, myalgia, fatigue, expectoration, and hemoptysis [
4]. After acute respiratory infection (ARI), acute gastroenteritis (AGE) is the second leading cause of morbidity and mortality in children ≤ 5 years of age and is related to ~1.4 million deaths in children ≤ 5 years of age annually [
5,
6,
7]. AGE has been associated with COVID-19, although less common than respiratory symptoms [
8]. Coronavirus 2 (SARS-CoV-2) that causes COVID-19 reaches the gastrointestinal tract through ACE2 receptors present on the epithelial cells of the small intestine, causing gastrointestinal symptoms. Recognizing the gastrointestinal symptoms of COVID-19 is important, especially in patients without typical respiratory symptoms, facilitating early diagnosis and preventing transmission of the disease [
8,
9]. The host’s genetic factors, such as polymorphisms in the genes that code for cell receptors, can alter susceptibility to infection. There is growing evidence of interactions between histo-blood group antigens (HBGA) and enteric viruses, and with respiratory viruses such as SARS-CoV-2 [
10,
11]. The basis of HBGA are carbohydrates expressed in the membranes of red blood cells and other tissues of the respiratory tract, as well as in body secretions (saliva) and plasma, which serve as receptors and co-receptors for viral infections [
12,
13,
14]. Angiotensin converting enzyme 2 (ACE2) is the cell membrane receptor of SARS-CoV-2, mediating viral entry into cells [
15,
16,
17,
18]. The insertion/deletion (I/D) polymorphism of the ACE gene (rs4646994) and the single nucleotide polymorphism (SNP) G8790A of the ACE2 gene (rs2285666) were associated with greater host susceptibility with Sars-CoV-2 [
19].
The risk of new COVID-19 waves will depend on a series of factors but might include the existence of groups of unvaccinated children living in hard-to-reach areas [
20]. This study is an epidemiological survey with children ≤3 years of age living in the Northwest Amazon region (NWAR), where the frequency of SARS-CoV-2 and the presence of polymorphisms in the
ACE I/D and
ACE2 G8780A28 genes were investigated. The profile of HBGA (secretory/non-secretory and Lewis), as a genetic susceptibility factor, was also verified.
2. Materials and Methods
2.1. The Epidemiological Inquiry
This epidemiological inquiry took place at Santo Antonio Children’s Hospital (HCSA) from May to June 2021. The HCSA treated exclusively children ≤12 years old from the city of Boa Vista, capital of the state of Roraima (RR) or Amazonas (AM), bordering Venezuela and Guyana, in villages in indigenous lands, in areas from demarcated indigenous areas in the NWAR, Brazil. For this inquiry, a total of 404 fecal and saliva (collected at least 1h before or after breastfeeding) samples were collected in parallel from 202 children ≤3 years old (two samples/child): 101 presenting acute respiratory infection (ARI); and 101 presenting AGE (inclusion criteria according to the World Health Organization (WHO) [
21].
2.2. SARS-CoV-2 Detection
Total viral nucleic acid was obtained according to what was previously described. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed for detection of SARS-CoV-2
E,
N and
RdRp genes separately as monoplex with primers and probes according to the WHO protocols [
22]. All the reactions were conducted on the Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, California, USA) using 5µL of total viral nucleic acid extracted from feces and saliva and the SuperScript™ III PlatinumTM One-Step qRT-PCR (Invitrogen, California, USA) kit, according to the manufacturer’s recommendations. All samples that showed signals for at least one of the three genes (
E,
N or
RdRp) crossing the threshold line in both replicas up to a threshold cycle (Ct) value of 40.00 and presented a characteristic sigmoid curve were regarded as positive and transformed into viral load in copies per milliliter. Negative and positive control samples were obtained from the biorepository bank of the Regional Rotavirus Reference Laboratory–Laboratory of Comparative and Environmental Virology (RRRL-LVCA), Oswaldo Cruz Institute, Fiocruz.
2.3. Genotyping of Polymorphism in the ACE I/D and ACE2 G8780A Genes
Polymorphism in the
ACE I/D (
rs4646994) gene was verified by SYBR Green Real time PCR according to the previously described protocol modified [
23] using the SYBER™ Green PCR Master Mix (Thermo Fisher Scientific, Waltham, Massachusetts, USA), following the manufacturer’s instructions. Amplification and detection were performed with the ABIPrism 7500 Sequence Detection System (Applied Biosystems, California, USA) using 5µL of total viral nucleic acid extracted from saliva. All reactions were performed in duplicate and the PCR temperature cycling conditions and analysis of melting peaks for I and D alleles were carried out as previously described [
23,
24]. The single nucleotide polymorphism (SNP) of the
ACE2 G8790A (
rs2285666) gene was detected by restriction fragment length polymorphism PCR (PCR-RFLP), as previously described using iTaq™ DNA Polymerase (Bio-Rad Laboratories, California, USA) and
AluI (Invitrogen, California, USA), according to manufacturer instructions. After digestion, the 281 and 185 bp fragments identifying the A allele and 466 base pair (bp) band identifying G were visualized by electrophoresis on SYBR™ green I stained (Invitrogen, California, USA) agarose, LMP, preparative grade for small fragments (Promega, California, USA) [
23].
2.4. Histo Blood Group Antigen Phenotyping and Blood Testing
HBGA phenotyping was performed using the processed saliva samples via Enzyme Linked Immuno Sorbent Assay (ELISA), as previously described [
25]. The lectin-based ELISA, detect A, B, and Lewis antigens. Blood type data collection was performed at the Nossa Senhora de Nazaré Maternity Hospital (HMINSN), located in Boa Vista City, RR State [
16].
2.5. Statistical Analysis of Data and Maps
The Statistica 12.6 software was used for all statistical analysis. The statistical tests were Pearson Chi-Square (differences were considered statistically significant at P > 0.05). Odds ratio (OR) values were calculated according to Hoppe et al. 2008 [
26]. Maps of the, case distribution, and spatial analysis were created using QGIS (version 3.34.3).
3. Results
3.1. Clinical and Epidemiological Features of the Children Living in Northwestern Region in the Amazon
The samples in this inquiry were collected in the months of May (35.6%; 72/202), June (56.5%; 114/202) and July (16/202); with an equal percentage for AGE and ARI. This percentage did not correspond to the frequency of treatments at the HCSA emergency in the months mentioned here. The children were aged 1–3 years; 59.9% (121/202) were boys and 40.1% (81/202) girls. Most of the children in this study lived in RR state, (98.1%, 198/202), but 1.9% (4/202) lived in the Brazilian state of Amazonas (AM), bordering Venezuela and Guyana, in villages in indigenous lands. Most of the children from the state of RR lived in Boa Vista city (73.7%, 146/198). The remaining children live in at least 12 of the 15 municipalities in the state of RR (26.3%, 52/198), in isolated areas in the Amazon rainforest. The number of children presenting AGE (48.6%, 71/146) e ARI (51.4%, 75/146) in the city of Boa Vista presented similar frequencies. All four children admitted to the HCSA coming from the state of Amazon presented AGE.
Table 1 presents the frequency of occurrence of clinical symptoms, which the children in this study presented when treated at the HCSA. Fever >38.5
oC was the most common, with frequencies of over 90% for both AGE and ARI children. Mucus in feces was more frequent in children with AGE (64.3%, 65/101) than those with ARI (1%, 1/101), coughing more frequent in children ARI (87.1%, 88/101) than AGE (21.8%, 22/101). Vomiting was more associated with AGE (59.4%, 60/101) than ARI (29.7%, 30/101) in this epidemiological inquiry, abdominal pain was more associated with AGE (98%, 98/101) than ARI (63.3%, 64/101). Bronchiolitis and pneumonia were only identified in ARI (4,95%, 5/101 and 84.1%, 85/101, respectively). Four children had no vaccine information available in the clinical information form.
3.2. ACE2 G8780A Gene Differed between Male and Female and Males with rs2285666 SNP Were More Susceptible to ARI Symptoms than AGE
Considering all children, with AGE and ARI, with the
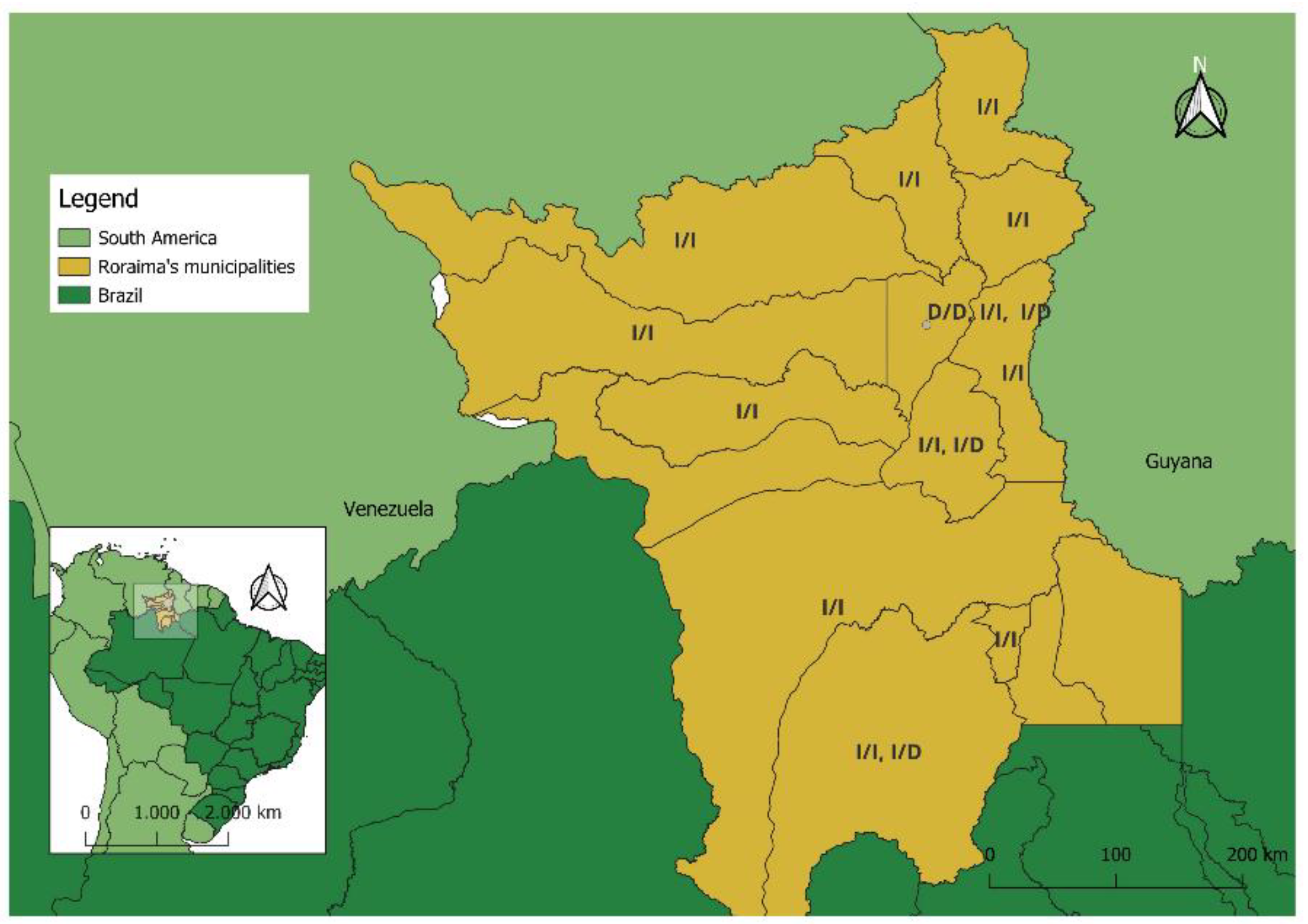
ACE (rs4646994) I/I homozygous polymorphism gene, the frequencies were similar and predominant in relation to I/D and D/D profiles (
Figure 1); these being 89.1% (90/101) and 85.1% (86/101) respectively. Children with the
ACE (rs4646994) I/D heterozygous (AGE 4.95%, 5/101; ARI 9.9%, 10/101) and D/D homozygous (AGE 5.94%, 6/101; ARI 5% 5/101) polymorphisms gene were at a lower frequency. No significant association between
ACE I/D (rs4646994) polymorphisms gene and all children with clinical symptoms of AGE or ARI, whether male or female, was observed.
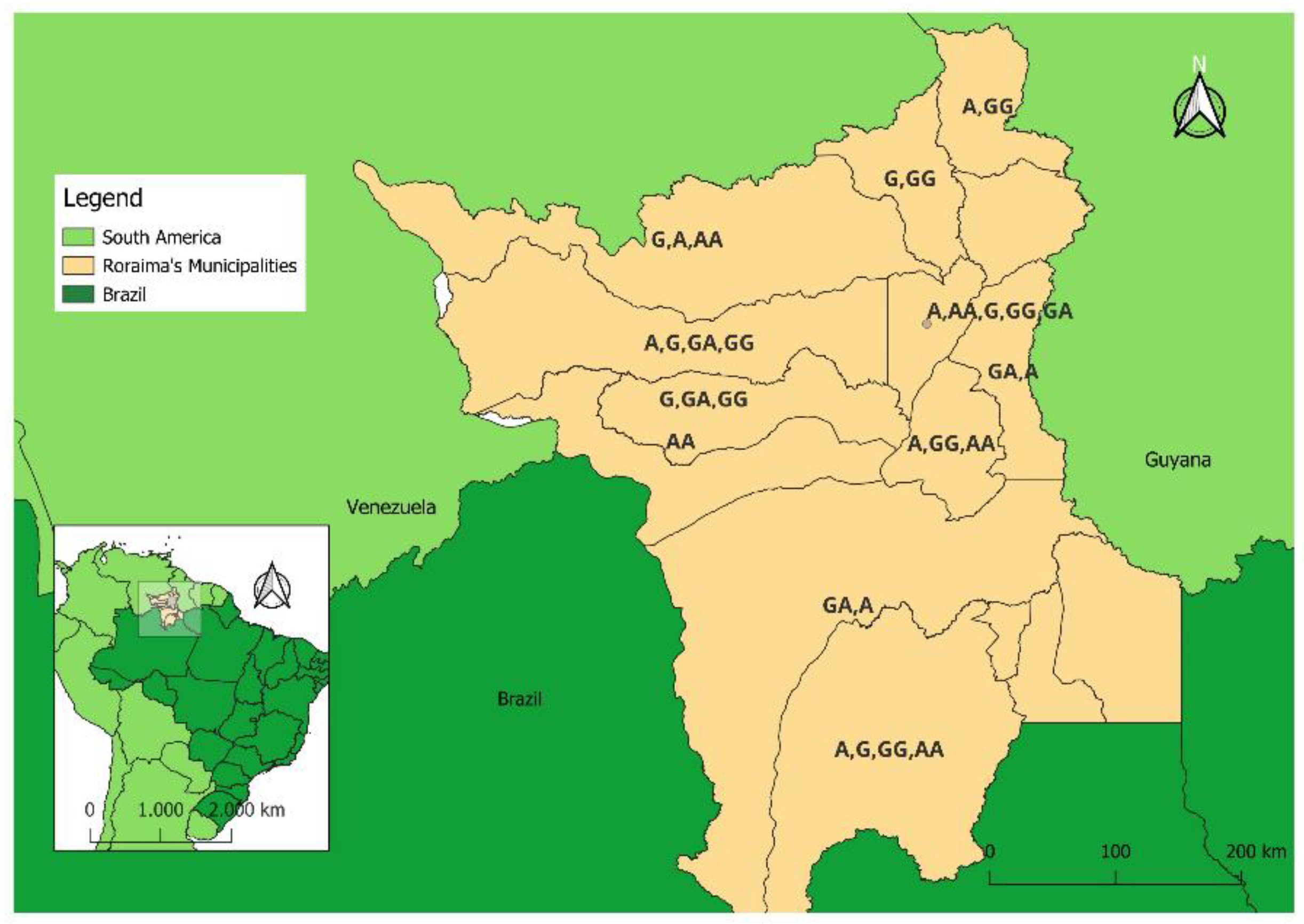
Table 2A shows these results in detail. 28.6% (18/63) of male children with AGE presented an A nucleotide at position 8790 of the
ACE2 gene corresponding to the rs2285666 SNP; 58.7% (37/63) presented a G. The frequencies of A and G for male children with ARI were similar and corresponded to 37.9% (22/58) and 34.5% (20/58) respectively. Female children with AGE or ARI were, respectively, AA homozygous at 15.8% (6/38) and 18.6% (8/43); AG heterozygous at 28.9% (11/38) and 16.3% (7/43); and GG homozygous at 39.5% (15/38) and 28.9% (11/43) (
Figure 2). Males with an A nucleotide at position 8790 of the ACE2 gene were more susceptible to ARI clinical symptoms than AGE, according to the OR = 3.85 (95% CI-1.42-10.38; P 0.007). No significant association was observed considering the ACE2 rs2285666 SNP and clinical symptoms of AGE or ARI (
Table 2A). 13.8% (14/101) and 22.8% (23/101) of DNA samples collected from children with AGE and ARI, respectively, were not of good enough quality to be evaluated for the presence of the ACE2 G8780A SNP. The combination of the ACE ID + ACE2 G8780A polymorphisms was analyzed to investigate its association with the clinical diagnosis of AGE or ARI. No significant association was observed (
Table 2B).
Subtitle: 1 All children (male and female); 2 A+AA; 3 G+AG+GG.
3.3. Prevalence of Secretor Phenotype of Young Children Living in the Northwestern Amazon Region
The secretor phenotype was 92.6% (187/202) of children. Non-secretor children (7.4%; 10/202) had either Le (a-b-) (5%, 10/202) or Le (a+b-) phenotypes (2.5%, 5/202). Blood group O was the most predominantly detected (57.4%, 116/202) followed by A (31.7%, 64/202). Few children were blood typed as B (9.9%, 20/202) or AB (1%, 2/202).
3.4. Characteristics of the SARS-CoV-2 Positive Samples in this Study
Of the 12 samples in which the SARS-CoV-2
E gene were detected, 9 (75%) were from children presenting AGE (5 from saliva samples and 4 from feces). Ten of these samples were detected in the state of RR: 8 in the city of Boa Vista, one in city of Alta Alegre e Caracaraí. Two sample was detected in the state of Amazonas. Three samples were from children presenting ARI (2 feces/1 saliva), these last ones all being from children living in the town of Boa Vista. The children’s ages were varied, and the viral load detected in the qRT-PCR was not lower than 29.65 (
Table 3). All SARS-CoV-2 positive children presented the
ACE I/I homozygous gene polymorphism gene (rs4646994) profile, except for one sample (named 32344) from a female child in the town of Boa Vista with the I/D profile. This child is also the only one from the B blood group and which presented the lowest viral load detected in the samples, from feces in this case (AGE). This child’s
ACE2 gene polymorphism (rs2285666) gene profile (sample 32344) was heterozygous (GA). Of the 12 SARS-CoV-2 positive samples from male children (50%, 6/12), 3 presented the A SNP (
ACE2 gene rs2285666). Two samples were G and one sample’s
ACE2 rs2285666 SNP profile was unidentifiable. Most of the female children infected by SARS-CoV-2 presented the GA profile for the
ACE2 gene SNP (rs2285666). All SARS-CoV-2 children were secretors (Lea-b+/Lea+b+), 58.3% from the O (7/12), 33.4 % from the A group (4/12) and 8.3% (1/12) from the B blood groups (
Table 3).
4. Discussion
In this enquire viral shedding in feces was accompanied by gastrointestinal symptoms such as nausea, vomiting, and diarrhea in approximately 18% (12–25%) of cases. In a cross-sectional study recently presented by Dos Santos et al. 2022 [
27], with data obtained from 677 medical records of patients infected with SARS-CoV-2 treated in hospitals in São Paulo, Brazil, from March to November 2020, diarrhea was more associated with children ≤4 years old (12.7%, 18/142) than those in older age groups (5-19 years old) [
28]. In this study, saliva samples were used instead of the nasopharyngeal swab, the former that has been verified to be more sensitive to the detection of the Delta SARS-CoV-2 variant and of equal sensitivity for the Omicron variant even during the different stages of infection. The Omicron variant started circulating in Brazil in December 2021, after the collection of the samples in this study. Susceptibility in children was found to be around half of the susceptibility in adults, and even lower for very young children (preschoolers) [
29]. Heirstraeten et al. 2022 [40] verified limited transmission of SARS-CoV-2 in Belgian day-care centers among young children aged 6-30 months during May 2020-February 2022 [
30].
In previous studies SARS-CoV-2 detection in asymptomatic children was greater than adults [
31,
32]. Symptomatic individuals had lower Ct values (which corresponded to higher viral RNA levels) than asymptomatic individuals. We could propose therefore that, in our epidemiological inquiry, the detection of only the
E gene in young children might indicate an asymptomatic profile, where the replication of SARS-CoV-2 has not been efficient in these young children. The reasons due to which children mostly do not develop serious conditions have not been completely elucidated. One hypothesis is that the child might present the angiotensin-converting enzyme 2 (ACE2) protein, the main binder for SARS-CoV-2 in human cells - in different configurations or densities. Another possibility is a lower capacity of binding to the virus, or even that the response from the alveolar cells to ACE2 might be less harmful; in other words, the intracellular response induced by ACE2 might differ in children and adults, especially in the elderly. There’s also the hypothesis related to immunological immaturity itself, both from the innate (the inflammatory reaction particularly) and adaptive (antibodies and T-cells) responses [
31,
32].
Pre-existing diseases such as hypertension and diabetes mellitus are associated with increased morbidity and mortality in adults with SARS-CoV-2 infection [
33]. Most of children (84.1%; 85/101) had pneumonia. Garde et al., 2007 [
34], describes that the role of ACE insertion/deletion (I/D) in pneumonia. The disease severity was correlated with lower ACE activity [
30]. Recently was presented that
ACE rs4646994 D/D-genotype and
ACE2 rs2285666 G/G-genotype carriers had a significantly increased risk of developing severe COVID-19 in adults and younger children under 2 years old [
35]. The only study available in Brazil, with samples from the Midwestern region (state of Goiás) demonstrated that ACE rs4646994 D/D-genotype and
ACE2
rs2285666 GG-genotype combined effects may play a role in hypertension [
19]. In this study, the frequencies detected for the
ACE rs4646994 polymorphism gene were distributed among the I/I, I/D and D/D genotypes, so that for AGE it was 89.1%, 4.95% and 5.94% respectively, and for ARI the distribution among the I/I, I/D and D/D genotypes was 85.1%, 9.9% and 5%. The frequency detected for the D/D-genotype in our study was about 5%; the children, both male and female, with AGE and ARI, were predominantly of the I/I genotype. The
ACE I/D polymorphism gene may also be associated with pneumonia because people with the D/D genotype have a lower cough reflex compared to I/I and I/D and carriers of the D/D genotype have higher serum levels of the pro-inflammatory angiotensin-II [
36,
37]. Morimoto et al., 2002 [
38], have already shown that the D allele is an independent risk factor for (fatal) pneumonia in an adult Asian population. This unprecedented data might be a characteristic of the indigenous NWAR population that which predominantly of secretor HBGA profile [
39].
5. Conclusions
The low frequency of positivity for SARS-CoV-2 (around 6%) in this study gives our analysis low statistical power and is a limitation, but it does provide important data on the virus susceptibility profile and epidemiology to help public and private authorities with tools to control and prevent the disease in a socially vulnerable population of children.
Author Contributions
“Conceptualization, J.P.G.L. and M.T.B.M.; methodology, Y.C.P., C.E.S.F., B.L.A., M.F.S., A.I.O.O. and M.T.B.M.; software, F.F.O.B. and M.T.B.M.; validation, Y.C.P and M.T.B.M.; formal analysis, Y.C.P., F.F.O.B. and M.T.B.M.; investigation, Y.C.P. and M.T.B.M.; resources, Y.C.P. , I.F.D. and M.T.B.M.; data curation, Y.C.P., A.I.O.O., J.P.G.L. and M.T.B.M.; writing—original draft preparation, Y.C.P., F.F.O.B. and M.T.B.M.; writing—review and editing; M.T.B.M.; visualization, Y.C.P. and M.T.B.M.; supervision, M.T.B.M.; project administration, M.T.B.M.; funding acquisition, J.P.G.L., I.F.D. and M.T.B.M.;. All authors have read and agreed to the published version of the manuscript.”.
Funding
We are grateful for support from the Coordination for the Improvement of Higher Education Personnel (CAPES) “CAPES/PRINT - Call no. 41/2017, Process number 88887.977276/2024-00”; The National Council for Scientific and Technological Development–CNPq “Chamada CNPq/Decit/SCTIE/MS Nº 49/2022 - Vacinas registradas na ANVISA e/ou incorporadas ao SUS, Process 408463/2022-8”; Foundation for Research Support of the State of Rio de Janeiro-FAPERJ “Edital Carlos Chagas Filho (E26/210.057/2020 and E26/210.701/2024)” and Oswaldo Cruz Institute-IOC (PAEF).
Institutional Review Board Statement
This study was approved by the Federal University of Roraima Ethical Research Committee (CEP No: 1.333.480 from November 23, 2015).
Informed Consent Statement
Written consent was obtained from all subjects involved in the study.
Data Availability Statement
not applicable.
Acknowledgments
The authors acknowledge all children and their parents for making this study possible. Thanks to Isabelle Baroni de Moraes e Souza for the English revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kitano, T.; Kitano, M.; Krueger, C.; Jamal, H.; Al Rawahi, H.; Lee-Krueger, R.; Sun, R.D.; Isabel, S.; García-Ascaso, M.T.; Hibino, H.; et al. The differential impact of pediatric COVID-19 between high-income countries and low- and middle-income countries: A systematic review of fatality and ICU admission in children worldwide. PLOS ONE 2021, 16, e0246326. [Google Scholar] [CrossRef] [PubMed]
- Fink, T.T.; Marques, H.H.; Gualano, B.; Lindoso, L.; Bain, V.; Astley, C.; Martins, F.; Matheus, D.; Matsuo, O.M.; Suguita, P.; et al. Persistent symptoms and decreased health-related quality of life after symptomatic pediatric COVID-19: A prospective study in a Latin American tertiary hospital. Clinics 2021, 76, e3511. [Google Scholar] [CrossRef] [PubMed]
- Hallal, M. and B. Luiz, [homepage on the Internet]. Brasil é o 2º país com mais mortes de crianças por covid. Estadão [cited 2021 Oct 19].. 2021: Available from: https://saude.estadao. com.br/noticias/geral,sem-escolas-e-sem-controle-dapandemia-brasil-e-o-2-pais-que-mais-perdeu-criancas-paraa-covid,70003738573.
- Pal, M. , et al., Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus, 2020. 12(3): p. e7423.
- Collaborators, G.D.D. , Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis, 2017. 17(9): p. 909-948.
- Collaborators, G.D.a.I. , Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. 2020.
- Saúde, M.d. , Situação epidemiológica. 2024, Disponíel em:https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/d/dda/situacao-epidemiologica Acesso no dia 16 de abril de 2024.
- Mehta, D.; Kelkar, R.; Patel, N.; Trivedi, P.D.; Dawoodi, S.; Patel, D.; Solanki, D.; Hussain, A.; Nagaraj, S.; Khayat, A.; et al. Gastrointestinal Manifestations and Outcomes of COVID-19: A Comprehensive Systematic Review and Meta-analysis. Cureus 2023, 15, e47028. [Google Scholar] [CrossRef] [PubMed]
- Vernia, F.; Ashktorab, H.; Cesaro, N.; Monaco, S.; Faenza, S.; Sgamma, E.; Viscido, A.; Latella, G. COVID-19 and Gastrointestinal Tract: From Pathophysiology to Clinical Manifestations. Medicina 2023, 59, 1709. [Google Scholar] [CrossRef] [PubMed]
- Molani, S.; Hernandez, P.V.; Roper, R.T.; Duvvuri, V.R.; Baumgartner, A.M.; Goldman, J.D.; Ertekin-Taner, N.; Funk, C.C.; Price, N.D.; Rappaport, N.; et al. Risk factors for severe COVID-19 differ by age for hospitalized adults. Sci. Rep. 2022, 12, 6568. [Google Scholar] [CrossRef] [PubMed]
- van de Garde, E.M. , et al., Angiotensin-converting enzyme (ACE) I/D corrected serum ACE activity and severity assessment of community-acquired pneumonia. Clin Chem Lab Med, 2007. 45(10): p. 1326-31.
- Pendu, J.L. , et al., ABO Blood Types and COVID-19: Spurious, Anecdotal, or Truly Important Relationships? A Reasoned Review of Available Data. Viruses, 2021. 13(2).
- Ramani, S.; Giri, S. Influence of histo blood group antigen expression on susceptibility to enteric viruses and vaccines. Curr. Opin. Infect. Dis. 2019, 32, 445–452. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, M.T.B. , et al., Phenotyping of Lewis and secretor HBGA from saliva and detection of new FUT2 gene SNPs from young children from the Amazon presenting acute gastroenteritis and respiratory infection. Infect Genet Evol, 2019. 70: p. 61-66.
- Hoffmann, M. , et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, 2020. 181(2): p. 271-280.e8.
- Djomkam, A.L.Z. , et al., Commentary: SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Front Oncol, 2020. 10: p. 1448.
- Shang, J. , et al., Structural basis of receptor recognition by SARS-CoV-2. Nature, 2020. 581(7807): p. 221-224.
- Lan, J. , et al., Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature, 2020. 581(7807): p. 215-220.
- Pinheiro, D.S.; Santos, R.S.; Jardim, P.C.B.V.; Silva, E.G.; Reis, A.A.S.; Pedrino, G.R.; Ulhoa, C.J. The combination of ACE I/D and ACE2 G8790A polymorphisms revels susceptibility to hypertension: A genetic association study in Brazilian patients. PLOS ONE 2019, 14, e0221248. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P. and N. Curtis, Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child, 2020.
- Organization, W.H. , Acute respiratory infection in children: case manage-ment in small hospitals in developing countries – a manual for physicians and other senior health workers (WHO/ARI/90.5). 1990: Geneva: World Health Organization.
- Organization, W.H. , Diagnostic detection of Wuhan coronavirus 2019 by real-time RTPCR. Protocol and preliminary evaluation as of Jan 13, 2020. 2020.
- Benjafield, A.V.; Wang, W.Y.; Morris, B.J. No association of Angiotensin-Converting enzyme 2 gene (ACE2) polymorphisms with essential hypertension*1. Am. J. Hypertens. 2004, 17, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Tseng, C.-C.; Huang, C.-H.; Chong, C.-K. Real-time PCR for rapid genotyping of angiotensin-converting enzyme insertion/deletion polymorphism. Clin. Biochem. 2001, 34, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, J.; Sharma, S.; Bucardo, F.; Nasir, W.; Günaydın, G.; Ouermi, D.; Nitiema, L.W.; Becker-Dreps, S.; Simpore, J.; Hammarström, L.; et al. Both Lewis and Secretor Status Mediate Susceptibility to Rotavirus Infections in a Rotavirus Genotype–Dependent Manner. Clin. Infect. Dis. 2014, 59, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, F.M.; Hoppe, D.J.; Walter, S.D. Explaining odds ratios as conditional risk ratios. J. Clin. Epidemiology 2017, 97, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.O.R.D. , et al., SARS-CoV-2 infection in children and adolescents: a Brazilian experience. Rev Paul Pediatr, 2022. 40: p. e2021172.
- Jone, P.N. , et al., SARS-CoV-2 Infection and Associated Cardiovascular Manifestations and Complications in Children and Young Adults: A Scientific Statement From the American Heart Association. Circulation, 2022. 145(19): p. e1037-e1052.
- Franco, N. , et al., Inferring age-specific differences in susceptibility to and infectiousness upon SARS-CoV-2 infection based on Belgian social contact data. PLoS Comput Biol, 2022. 18(3): p. e1009965.
- Van Heirstraeten, L. , et al., Detection of SARS-CoV-2 in young children attending day-care centres in Belgium, May 2020 to February 2022. Euro Surveill, 2022. 27(21).
- Chung, E. , et al., Comparison of Symptoms and RNA Levels in Children and Adults With SARS-CoV-2 Infection in the Community Setting. JAMA Pediatr, 2021. 175(10): p. e212025.
- Chung, M.K. , et al., COVID-19 and Cardiovascular Disease: From Bench to Bedside. Circ Res, 2021. 128(8): p. 1214-1236.
- Palmeira, P. , et al., Why is SARS-CoV-2 infection milder among children? Clinics (Sao Paulo), 2020. v. 75, p. e1947.
- van de Garde, E.M. , et al., Angiotensin-converting enzyme (ACE) I/D corrected serum ACE activity and severity assessment of community-acquired pneumonia. Clin Chem Lab Med, 2007. 45(10):1326-31.
- Saengsiwaritt, W. , et al., Genetic polymorphisms of ACE1, ACE2, and TMPRSS2 associated with COVID-19 severity: A systematic review with meta-analysis. Rev Med Virol, 2022. 32(4):e2323.
- Brown, N.J.; Blais, C.; Gandhi, S.K.; Adam, A. ACE Insertion/Deletion Genotype Affects Bradykinin Metabolism. J. Cardiovasc. Pharmacol. 1998, 32, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Balachandra, K.; Na Ayuthaya, P.I.; Auwanit, W.; Jayavasu, C.; Okuno, T.; Yamanishi, K.; Takahashi, M. Prevalence of Antibody to Human Herpesvirus 6 in Women and Children. Microbiol. Immunol. 1989, 33, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Okaishi, K.; Onishi, M.; Katsuya, T.; Yang, J.; Okuro, M.; Sakurai, S.; Onishi, T.; Ogihara, T. Deletion allele of the angiotensin-converting enzyme gene as a risk factor for pneumonia in elderly patients. Am. J. Med. 2002, 112, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Olivares, A.I.O.; Leitão, G.A.A.; Pimenta, Y.C.; Cantelli, C.P.; Fumian, T.M.; Fialho, A.M.; Mouta, S.d.S.e.; Delgado, I.F.; Nordgren, J.; Svensson, L.; et al. Epidemiology of enteric virus infections in children living in the Amazon region. Int. J. Infect. Dis. 2021, 108, 494–502. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).