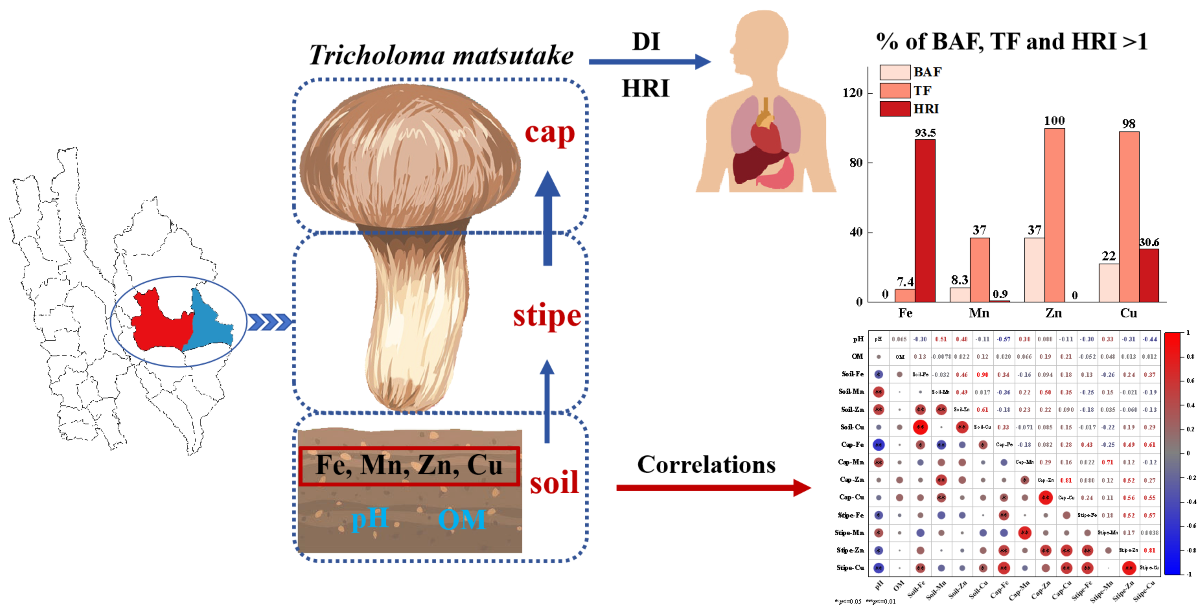
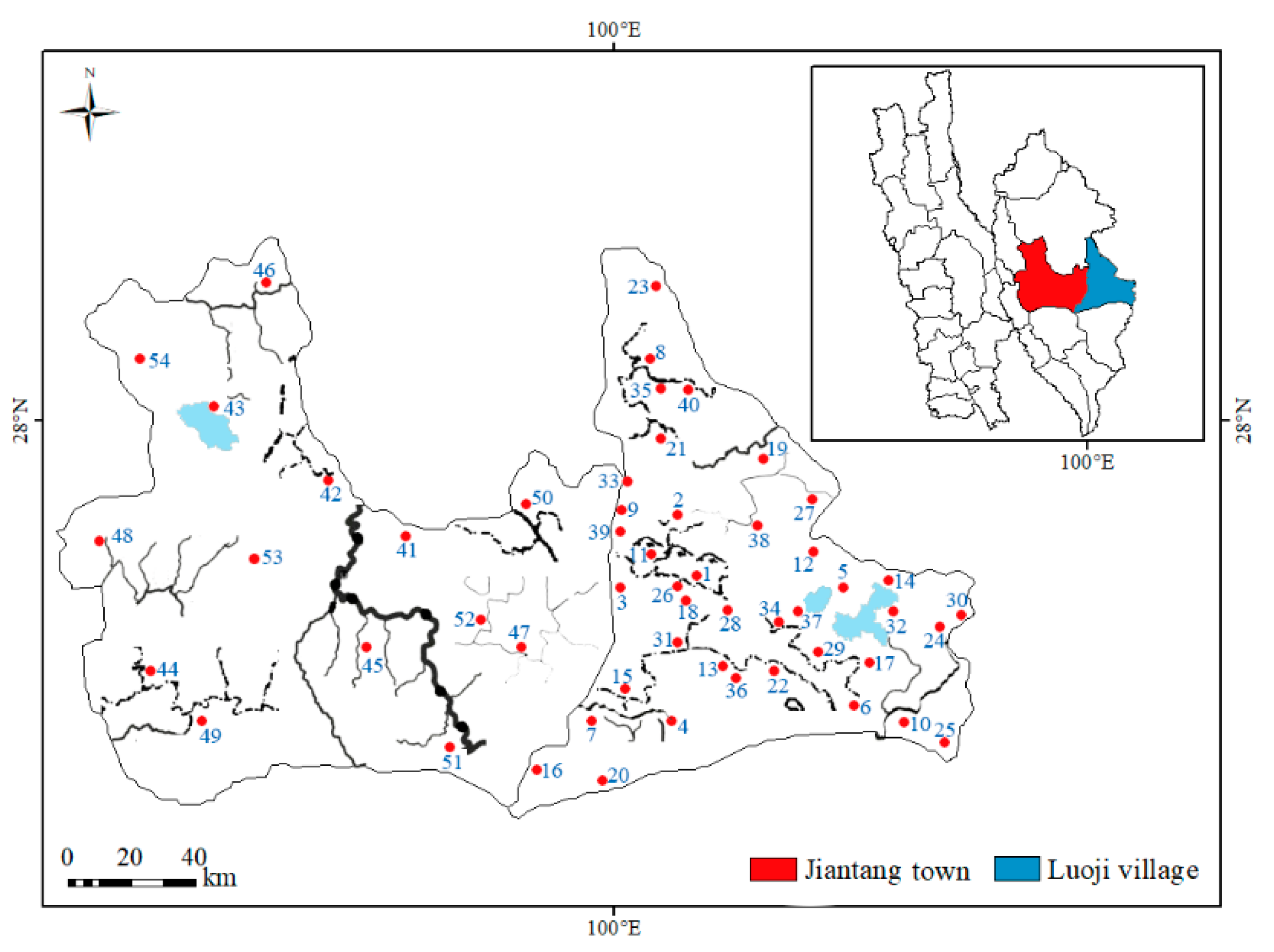
1. Introduction
Generally, mushroom is potentially source of bioactive compounds like vitamin B2, minerals and antioxidants (ergothioneine and glutathione), which have pro-health properties of anticancer, antidiabetic and immunomodulating. Typically, wild edible mushrooms play an important role in world diet, with the consumption being steadily increased for their texture, flavor and nutritional and medicinal values, especially in Asia and Europe (Brzezicha-Cirocka et al., 2019; Wagner et al., 2021). Yunnan province located in southwest China, is rich in wild edible mushrooms and is the major distribution and production region (Zhu et al., 2011). It accounts 91% of the known abundance of wild edible mushroom in China and 43% of the world (Liu et al., 2015). Specifically, the local consumption reaching 24 kg per head annually (Falandysz et al., 2017). Tricholoma matsutake is the most valuable, frequently consumed and economically important species (Xu et al., 2010; Guo et al., 2017), whose terpenoids and polysaccharides extracts showed antitumor and antioxidant values (Li et al., 2016a, 2016b). Besides, the export rate of T. matsutake from Yunnan accounts for 80% of total export in China (Wang et al., 2014).
In addition to the abundance of mushrooms, Yunnan is characterized by diverse polymetallic bedrocks and geochemically high background concentrations of metals. Besides, Yunnan has abundant mineral resources and is known as “the kingdom of nonferrous metals”. The high background metals together with the intensive mining activities lead to soils being heavily polluted, thereby potential transfer via food chain thus cause risk to humans. Moreover, wild mushrooms are efficient metal accumulators, which showed greater metal accumulations than common agricultural crop plants and vegetables (Liu et al., 2015). Typically, T. matsutake can accumulate toxic metal(loid)s like cadmium (Cd), arsenic (As) and lead (Pb) to 2.88, 7.12 and 8.63 mg kg–1 (Falandysz and Borovička, 2013; Liu et al., 2015). Indeed, hazardous metals and nutritional components (proteins, vitamins and antioxidants) in T. matsutake are most studied (Dong et al., 2024; Li et al., 2019; Ronda et al., 2022). However, there are limited studies on mineral metals accumulation and potential health risk in T. matsutake from areas with elevated geogenic metals.
Metallic elements (Fe, Mn, Zn and Cu) are essential mineral components for human health (Brzezicha-Cirocka et al., 2019), which however, exerting toxic effects when exceeding the amount required for physiological functions (Gharibzahedi and Jafari, 2017). Wild mushroom consumption is an important dietary source of mineral metals, which may exert risk to human health. Generally, metals transfer and accumulation in mushrooms depend on the total concentration and soil physicochemical properties. Among soil properties, pH and organic matter (OM) are predominant in affecting metals bioavailability thereby mushroom accumulation (Wang et al., 2024). However, studies mainly focused on mineral concentration (Li et al., 2011; Sarikurkcu et al., 2015; Szymańska et al., 2020), composition and nutritional values, limited information is available about the process of transfer, distribution, potential health risk and the underline influencing factors.
Therefore, the aims of this study are to: (1) analyze mineral metals (Fe, Mn, Zn and Cu) concentration and distribution (cap and stipe) in paired soils and T. matsutake (n=54) from Yunnan province, China; 2) evaluate metals soil-to-fruiting body accumulation and stipe-to-cap transfer efficiency, and clarify the correlation of soil metals concentration, pH and OM with T. matsutake; and 3) assess the edible safety by calculating metals daily intake (DI) via T. matsutake ingestion and the associated health risk index (HRI). This study helps to better understand the uptake, transfer, accumulation, potential health risk and influencing factors of mineral metals in wild mushrooms, and provides hazard level indications and requirements for exposure control awareness.
2. Materials and Methods
2.1. Sample Collection and Pretreatment
Paired soil (0–10 cm) and
T.
matsutake (n=54) were collected from two geographic villages in Diqing state, Yunnan province, China (
Figure 1). Each soil or
T.
matsutake sample was made up by five well-mixed subsamples. Soils were air-dried, ground, well mixed and passed through a 100-mesh (0.15 mm) nylon sieve. Fresh
T.
matsutake fruiting bodies were rinsed with deionized (DI) water to remove surface adsorbed soils and elements, then separated into caps and stipes and lyophilized at −80°C to constant weights (FreezZone 12, LabConco, Kansas City, USA). Freeze-dried mushrooms were ground under liquid nitrogen to obtain homogeneous powders and stored at −20°C before further analyses.
2.2. Chemical Analysis
Soil pH was determined by mixing soil with 0.01 M CaCl2 solution at 1:5 (m/v), shaking at 180 rpm and 25℃ for 1 h, then the supernatant was analyzed with a pH meter (Mettler–Toldo) (George et al., 2005). Soil organic matter (OM) content was determined gravimetrically after combustion at 550℃ for 16 h in a furnace horn (Select–Horn, SELECTA) (Melgar et al., 2009).
Metal concentrations in soils and T. matsutake were analyzed with X-ray fluorescence (XRF; E-max500) under normal detection mode. The radio frequency power was 1050 W and measuring time was 600 s (Ju et al., 2024). Standard reference materials including mushroom Lentinus edodes (GBW10197) and soil (GSS1) were used for concentration assays for quality assurance and quality control. Fe, Mn, Zn and Cu concentrations obtained via XRF for GBW10197 were 141±0.61, 26.2±0.16, 53±0.17 and 6.23±0.05 mg kg–1 (mean±SD, n=3), which were in good agreement with the certified values at 152±21, 25±0.8, 51±3.8 and 5.73±0.18 mg kg–1. Mn, Zn and Cu concentrations obtained via XRF for GSS1 were 1644±5.86, 630±0.7 and 20.9±0.46 mg kg–1, which were in good agreement with the certified values at 1760±63, 680±25 and 21±2 mg kg–1. The average recoveries were 92.6–109%. All analyses were performed in triplicates.
2.3. Bioaccumulation and Translocation Analysis
To evaluate metals accumulation from soil to
T.
matsutake cap and stipe, bioaccumulation factor (BAF) was calculated via Eq. 1 (Wang et al., 2020):
where C
mushroom is the concentration of individual metal in
T.
matsutake cap or stipe (mg kg
–1) and C
soil is the corresponding metal concentration in soils (mg kg
–1). BAF>1 indicates that the organism is an accumulator towards given element.
To estimate metals transfer and distribution from
T.
matsutake stipe to cap, translocation factor (TF) was calculated via Eq. 2 (Dimitrijevic et al., 2021):
where C
C is the concentration of individual metal in the cap and C
S is the concentration in the stipe.
2.4. Health Risk Analysis
To assess the potential health risk of humans exposure to metals-contaminated
T.
matsutake, health risk index (HRI) was analyzed via Eq. 3 (Cui et al., 2004):
where DI is the daily intake of metal via
T.
matsutake consumption (µg kg
–1 bw day
–1; Eq. 4). R
fD
i is the reference dose of oral intake of metal (i) (µg kg
–1 bw day
–1) that proposed by the Joint FAO/WHO Expert Committee on Food Additives (JECEFA) and US Environmental Protection Agency (USEPA). R
fD values established for Fe (JECEFA), Mn, Cu and Zn (USEPA) were 300, 140, 300 and 40 µg kg
–1 bw d
–1 (
Table 1) (Zhang et al., 2020).
Daily intake (DI; µg kg
–1 bw day
–1) of metals was calculated via Eq. 4 (Liu et al., 2015):
where SM is daily serving amount (0.03 kg dried
T.
matsutake), MCM is metal concentration in mushroom (mg kg
–1 dw), and ABW is the average human body weight (70 kg for adults) (Kalač and Svoboda, 2000). The provisional tolerable maximum daily intake (PTMDI) values for Cu and Zn were 300–1000 and 5000 µg kg
–1 bw day
–1 (JECEFA) (
Table 1).
2.5. Statistical Analysis
Results are presented as the mean of triplicate analyses and standard deviation. Statistical differences and variance was evaluated by one-way ANOVA and Duncan’s multiple range tests at P<0.05 (SPSS 20.0, SPSS Corporation). Pearson correlation analysis was established by SPSS 25.0 at P<0.05 or P<0.01. The figures were drawn using Origin 2022 (Origin Lab Corporation, USA).
3. Results and Discussion
3.1. Soil pH, OM and Metals Concentration
Among soil characteristics, soil pH, OM content and metal total concentration are critical in mediating metals uptake and accumulation in mushrooms (García et al., 2009). Soil pH values were 3.95–6.56, indicating
T.
matsutake prefers to grow in acidic environment (
Table 1). Normally, southwest China is prevalent with karst soils with pH at 6.07–8.53 (Qi et al., 2018). Given that mushroom usually grow in forest, the acidic to weak acidic soils may be attributed to litter decomposition, which produce organic acids to contribute protons (Tanikawa et al., 2018). Soil acidification rendered metals being mobilized and released into soil solution, which are readily being uptake by mushrooms (Rasalanavho et al., 2020). This was in consistent with the finding that forest soils growing wild mushrooms are acidic in Poland, with pH values low at 3.35 in pine understorey soils (Mleczek et al., 2021b).
Soil OM is the organic fraction originated from plant and animal decomposition and microbial activities (Galicia-Andres et al., 2021). OM is a strong sorbent of metals in organic forest soils because it is rich in carboxyl and hydroxyl groups, which can complex with and are retain metal cations, thus affecting their mobility and bioavailability (Sarwar et al., 2010; Saqib Rashid et al., 2022). In this study, soil OM showed strong heterogeneity ranging in 1.29–44.5% (
Table 1), which was higher than the reported values in agricultural soils of Yunnan (1.67–9.78%) and South Africa (1.5–13.7%) (Rasalanavho et al., 2020). It is generally recognized that metals bioavailability will decrease with the increasing OM due to the strong adsorption, complexation and chelation (Hu et al., 2014; Xu et al., 2016). However, growing evidence suggested that metals bioavailability can be increased with increasing OM, attributing to that OM chelate metals to form soluble organo-mineral complexes (Su et al., 2021). This was supported by the finding that soil Cu and Zn bioavailability was significantly (
P<0.05) positively correlated with OM content (R=0.73 and R=0.86) (Hernandez-Soriano and Jimenez-Lopez, 2012).
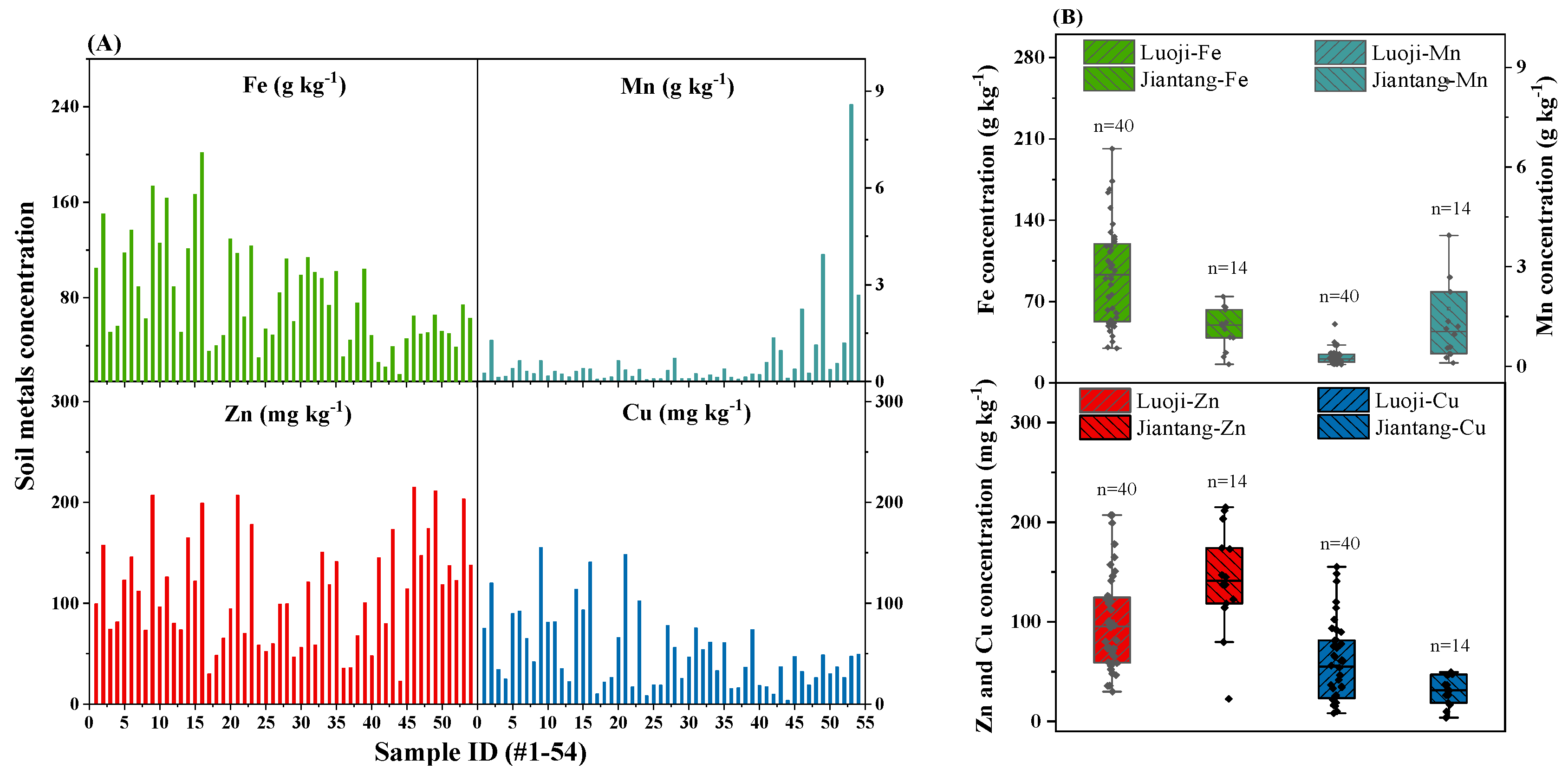
Metals concentration in
T.
matsutake growing soils showed strong heterogeneity, with Fe and Mn (16–201 and 0.046–8.58 g kg
–1) were great higher than Zn and Cu (22.6–215 and 3.7–155 mg kg
–1) (
Figure 2). Soil metals concentrations in the present study were higher than soils growing wild mushrooms (
Macrolepiota procera,
Imleria badia,
Leccinum scabrum and
Boletus edulis) at Fe (0.12–5.36 g kg
–1), Mn (0.014–0.12 g kg
–1), Zn (3.75–31 mg kg
–1) and Cu (0.24–21 mg kg
–1) (Mleczek et al., 2021b). In forested areas, soil metals often origin from parent material and atmospheric deposition (busy roads and emitters). The high concentration of metals in soils may be transferred to
T.
matsutake, so metals concentration and bioaccumulation in
T.
matsutake were examined.
3.2. Metals Concentration and Distribution in T. matsutake
3.2.1. High Metals Concentration in T. matsutake
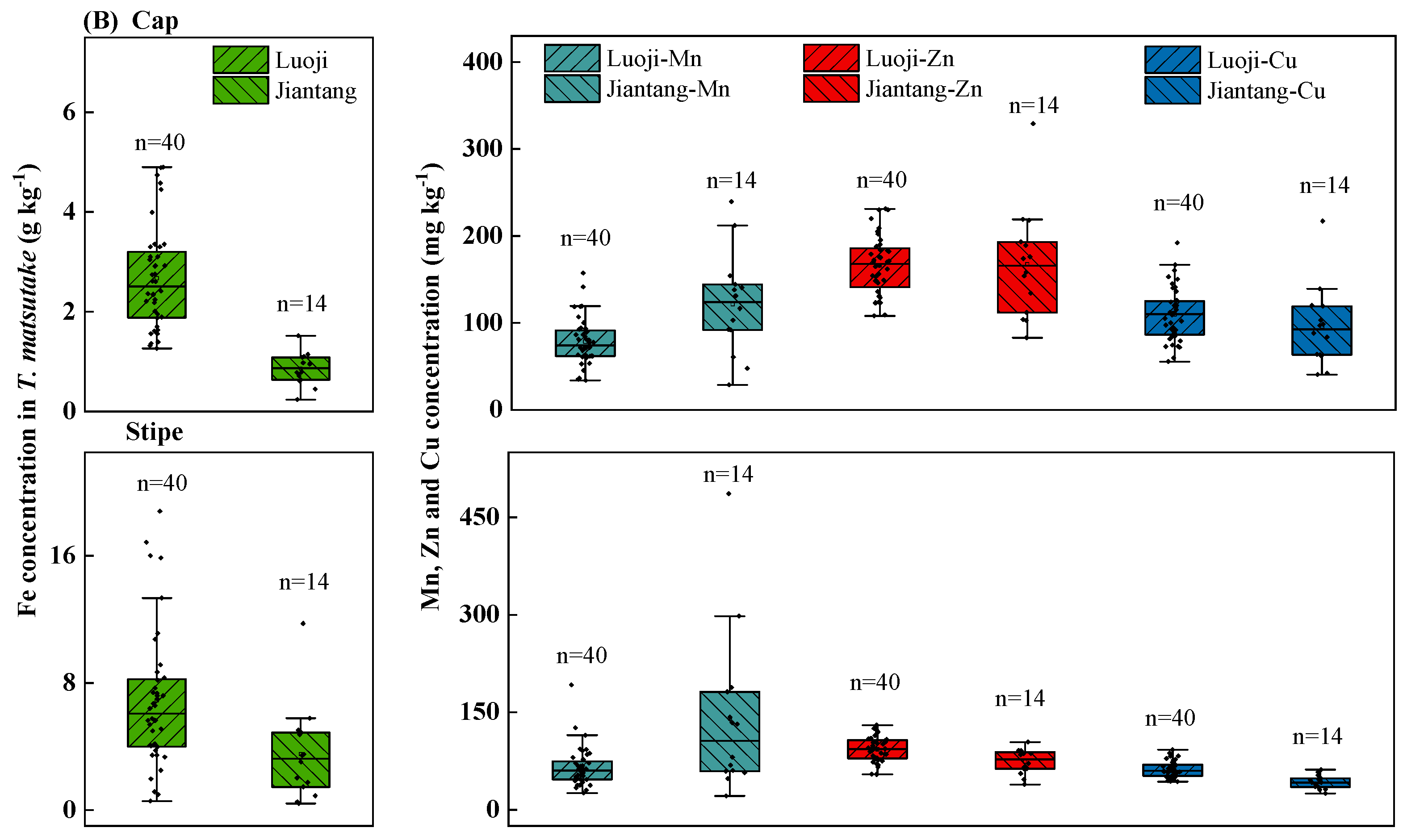
Given the high concentration of Fe in soils, Fe concentration in
T.
matsutake was the highest among the four metals (0.24–18.8 g kg
–1 vs. 21.1–487 mg kg
–1) especially in Luoji village (
Figure 2B). However, with great higher of Mn than Zn and Cu in soils (0.046–8.58 g kg
–1 vs. 22.6–215 and 3.7–155 mg kg
–1) (
Figure 2A), their concentrations in
T.
matsutake were comparable (21.1–487 vs. 38.7–329 and 24.9–217 mg kg
–1) (
Figure 3). This indicated that
T.
matsutake prefers to accumulated Zn and Cu than Mn.
Generally, Fe is a major mineral element in mushrooms (Gharibzahedi and Jafari, 2017). Fe concentration in
T.
matsutake (0.24–18.8 g kg
–1;
Figure 3) was higher than that in
T.
matsutake collected from Sichuan (0.01–0.08 g kg
–1) (Li et al., 2013). Besides, it was great higher than a wide range of wild mushroom species (
Lactarius deliciosus,
Clitocybe houghtonii,
T. argyraceum and
B. chrysenteron) at 0.16–0.43 g kg
–1 (Mleczek et al., 2021a) and a large sample size (
Amanita rubescens,
Suillus granulatus,
Bovista plumbea and
Lycoperdon perlatum) at 0.02–0.17 g kg
–1 (n=102) from unpolluted areas with soil Fe at 14.4–27 g kg
–1 (Zsigmond et al., 2023). This suggested that soil Fe is an important source for its accumulation in wild mushrooms. Still, compared within Yunnan province,
T.
matsutake Fe concentration in the present studied areas Luoji (0.57–18.8 g kg
–1) and Jiantang (0.24–11.7 g kg
–1) was higher than that from Lijiang, Nanhua, Zhongshan and Deqin (0.046–0.42 g kg
–1) with lower soil Fe content at 0.29–3.07 g kg
–1 (Liu et al., 2015).
Though soil Mn concentration was order of magnitudes higher than Zn and Cu, their concentrations in
T.
matsutake were comparable. Specifically, Mn concentration in
T.
matsutake was 21.1–487 mg kg
–1 (
Figure 3), which was higher than that in
T.
matsutake (1.54–29.4 mg kg
–1) from Lijiang, Nanhua, Zhongshan and Deqin, Yunnan province (Liu et al., 2015) and other species including
Coprinus comatus,
Voluariella volvacea and
Pleurotus nebrodensis at 13.5–113 mg kg
–1 (Zhu et al., 2011). Similarly, Zn concentration in the present study (38.7–329 mg kg
–1) was higher than that in
T.
matsutake (8.71–46.9 mg kg
–1) (Liu et al., 2015) and
M.
procera (22–240 mg kg
–1) (Gucia et al., 2012), but was comparable with that in
Agaricus bisporus,
B.
edulis and
T. columbetta (30–310 mg kg
–1) (Tuzen et al., 2007).
Similarly to Mn and Zn, Cu concentration (24.9–217 mg kg
–1) was great higher than the reported values in
T.
matsutake from four regions in Yunnan province at 1.53–12.6 mg kg
–1, which may be attributed to the difference in soil concentrations at 3.7–155 (
Figure 2) vs. 26.5–51.9 mg kg
–1 (Liu et al., 2015). Besides, it was higher than Cu concentrations (7.3–123 mg kg
–1) in 20 wild mushroom species grown in “green lung region” of Poland without urbanization or industry (Mirończuk-Chodakowska et al., 2019). However, it was also higher than wild mushrooms (
L.
scabrum,
B. reticulatus and
L. griseum) at 17.1–162 mg kg
–1 collected in a highly contaminated area of eastern Slovakia (Svoboda et al., 2000).
As such, the data indicated that metals concentration in the studied T. matsutake was great higher other mushroom species from other regions and the same species in the same province. Therefore, the underling influencing factors and potential health risk to humans should be studied.
3.2.2. Metals Distribution in Cap and Stipe
Fe were mainly stored in the stipe (69.1%), while Mn, Zn and Cu were transferred to the cap (54.1%, 65.3% and 64.1%) (
Figure 3), indicating that
T.
matsutake was efficient in transferring Mn, Zn and Cu than Fe. Specifically, Fe contents in cap and stipe were 0.24–4.9 and 0.41–18.8 g kg
–1, with the average value at 2.2 and 6.02 g kg
–1. Greater Fe content in stipe (0.26 g kg
–1) than cap (0.08 g kg
–1) was also found in
M.
procera (n=15) (Gucia et al., 2012).
In contrary, Mn, Zn and Cu content in the cap (21.1–487, 82.8–329 and 40.5–218 mg kg–1) was great higher than the stipe (28.7–239, 38.7–130 and 24.9–92.1 mg kg–1). A similar result was found in Amanita muscaria, showing greater Zn content in cap (150–250 mg kg–1) than stipe (110–240 mg kg–1) (Falandysz et al., 2020). Similarly, greater cap Cu was found in wild mushrooms (B. edulis, B. reticulatus, L. scabrum and L. griseum) at 35.2–162 vs. 17.1–72.4 mg kg–1 (Svoboda et al., 2000). This suggested that greater stipe-to-cap transfer of Zn and Cu may be common in wild mushrooms including T. matsutake.
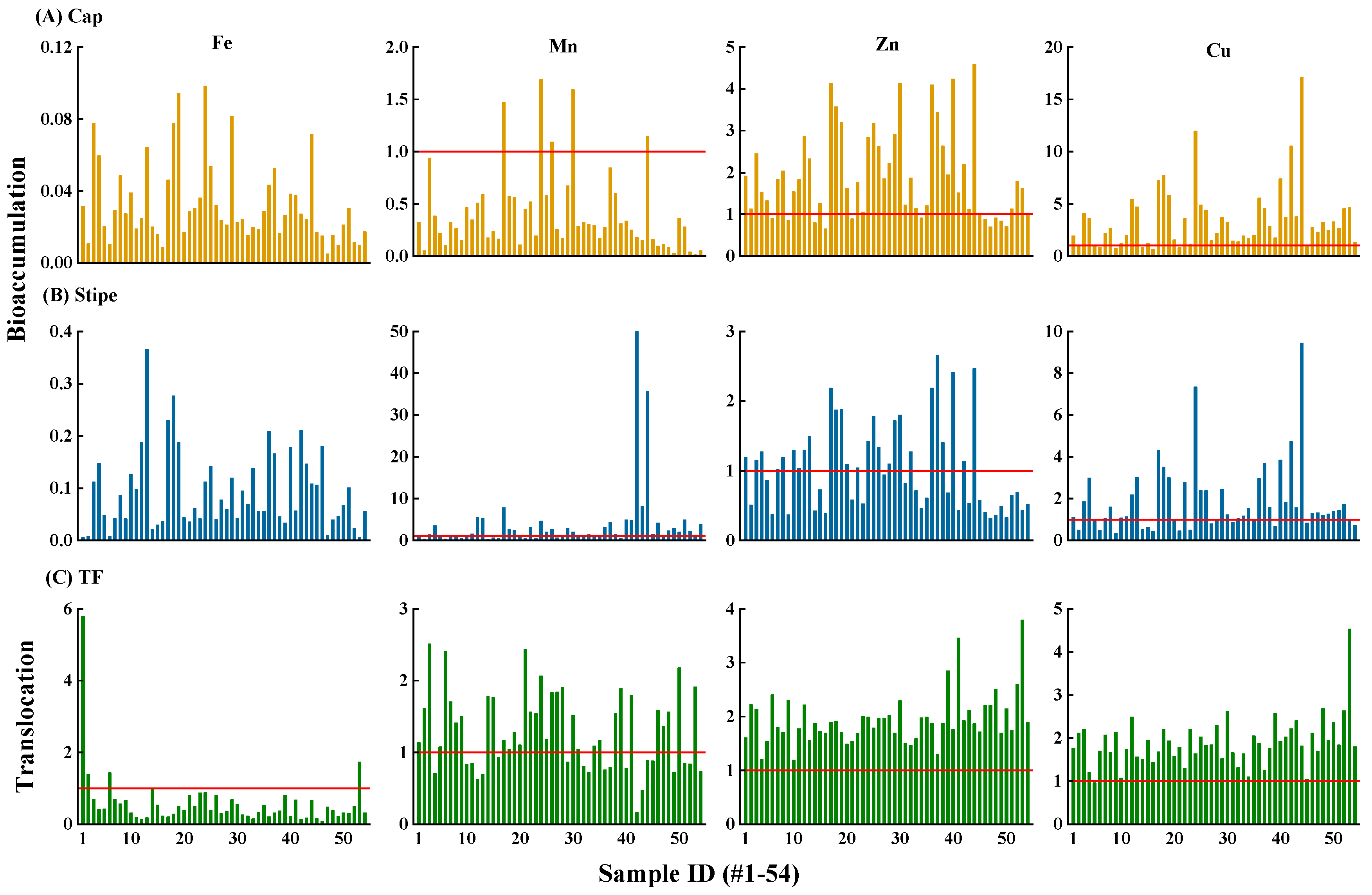
3.3. Metals Bioaccumulation and Transfer in T. matsutake
Though
T.
matsutake accumulated high concentrations of Fe (
Figure 3), the bioaccumulation factor (BAF=0.005–0.1) suggested that it is not a hyperaccumulator (BAF<1) towards Fe (
Figure 4AB). Similarly,
T.
matsutake was also not a Mn hyperaccumulator with 91.7% of the samples showing BAF<1. Instead,
T.
matsutake can hyperaccumulate Zn and Cu, with 63% and 77.8% of samples showing BAF>1 and can reach 4.59 and 17.1. The ability of wild mushrooms to accumulate metallic elements is related to the network of hyphae located in the upper soil horizon (Demirbaş, 2001; Gupta et al., 2016). Hyphae consisting of elongated tubular cells enveloped by a chitin wall, are widely spread over the bioavailable areas to accumulate metal ions (Damodaran et al., 2014). In addition, this process is influenced by environmental factors (soil metal concentration, pH and OM) and intrinsic properties (size and mycelial age) (Kokkoris et al., 2019).
In consistent with metals distribution (
Figure 3), average stipe-to-cap translocation factor (TF) of Zn and Cu were higher than Fe and Mn (1.94 and 1.89 vs. 0.58 and 1.28) (
Figure 4C). Specifically, the percentage of TF>1 was 100% and 98% for Zn and Cu, while that for Fe and Mn was 7.4% and 63%. The greater translocation of Zn and Cu was in consistent with but higher than the reported values in
M.
procera (TF=1.22–2.07 and 0.55–1.76) (Barea-Sepúlveda et al., 2022). This may be due to the different nature and concentration of proteins between cap and stipe, which was evidenced by the various carpophore structure showing more complex electrophoretic spectrum in the cap than the stipe (Gadd, 1993; Barea-Sepúlveda et al., 2022).
3.4. Potential Risk to Human Health
Mineral metals are essential components for human health, which however, exerting toxic effects when exceeding the amount required for physiological functions (Brzezicha-Cirocka et al., 2019). To evaluate the potential health risk associated with T. matsutake consumption, daily intake (DI) of metals and health risk index (HRI) were analyzed.
3.4.1. Metals Daily Intake Estimate
Daily intake (DI) of metals was calculated and compared with certificated values proposed by JECEFA and USEPA. The reference dose (R
fD) values established for Fe (JECEFA), Mn, Zn and Cu (USEPA) were 300, 140, 300 and 40 µg kg
–1 bw day
–1 (
Table 1). The provisional tolerable maximum daily intake (PTMDI) values for Zn and Cu were 300–1000 and 5000 µg kg
–1 bw day
–1 (JECEFA).
Among the four metals, Fe showed the highest DI values (102–8058 µg kg
–1 bw day
–1), especially for Luoji region at 244–8058 µg kg
–1 bw day
–1 (
Table 1). Typically, 93.5% of DI values for Fe in
T.
matsutake exceeded the R
fD limit (300 µg kg
–1 bw day
–1). In contrary, DI values for Mn (99.1%) and Zn (100%) were generally within the R
fD limits. In terms of Cu, 30.6% of DI values exceeded the R
fD limit established by USEPA (40 µg kg
–1 bw day
–1), but all were within the PTMDI limit established by JECEFA (5000 µg kg
–1 bw day
–1). This suggested that the daily intake of Fe via
T.
matsutake consumption may cause risk to human health.
DI values in the present study were generally higher than reported values in wild mushrooms Amanitaceae, Lactarius and Russulaceae but lower than Agaricaceae from different regions including Spain and Morocco (Barea-Sepúlveda et al., 2021, 2022a). For example, the average DI value for Mn in this study was 5-fold that of 13 wild mushroom species from Belgrad forest at 41.2 vs. 8.16 µg kg–1 bw day–1 (Keskin et al., 2021). The difference can be attributed to regional soil geochemical characteristics and physiology and genetic characteristics of individual mushroom species (Brzezicha-Cirocka et al., 2019).
Further, thermal cooking processes were reported can increase metals concentration in mushrooms. Frying increased Fe and Mn content in A. bisporus (n=540) from 66.2 to 69.5 and from 5.77 to 7.0 mg kg–1 dw. Boiling and frying increased Zn and Cu content from 126 to 153–156 and from 56.4 to 59.4–60 mg kg–1 dw (Ziarati and Rabizadeh, 2013). This addressed that high-temperature processing may increase the risk of metals via wild mushroom ingestion, so the detail effects and toxic mechanisms deserve further investigation for risk control during food preparation process.
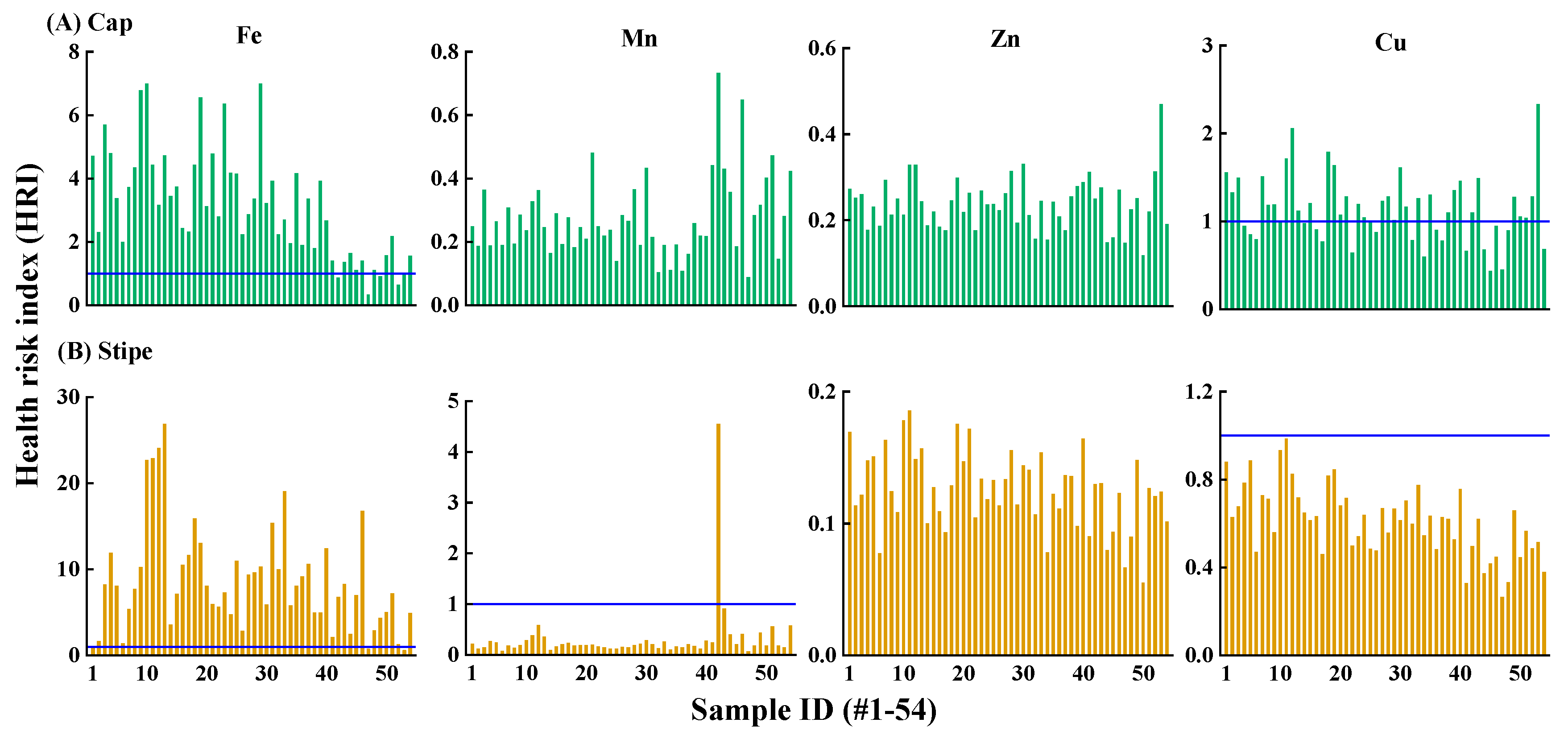
3.4.2. Health Risk Assessment
Health risk index (HRI) >1 for a given metal indicates there is potential risk for human health (Liu et al., 2015; Sarikurkcu et al., 2020). In consistent with the high concentration and high DI value in
T.
matsutake, Fe showed the highest potential risk with 93.5% of HRI>1 (
Figure 5). Especially, since Fe was mainly stored in the stipe (
Figure 3), stipe Fe showed greater risk than cap with HRI values were 0.59–26.9 vs. 0.34–7.0 (
Figure 5). Fe-HRI value in this study was lower that wild mushrooms (
Amanita mellea,
Hygrophorus pudorinus,
Polyporus squamosus, and
Russula vinosa) from Turkey, Spain and Morocco at 21.4–97 (Sarikurkcu et al., 2020). HRI values suggested that Fe in wild mushrooms from specific geographical locations may exert health risk to humans.
Compared to Fe, Mn (99.1%) and Zn (100%) in
T.
matsutake showed no potential health risk (
Figure 5). Cu is an essential element occurring in enzymes that important in immune and nervous systems (Chen et al., 2022). Nonetheless, it may still pose risk to human health at elevated levels of exposure. The results indicated that 61.1% of Cu in the cap showed risk with HRI value at 1.01–2.33. The higher risk of Cu than Zn was in consistent with
M.
procera from southern Spain and northern Morocco, showing HRI of Cu was >1 while Zn was <1 (Barea-Sepúlveda et al., 2022). As such, Fe in
T.
matsutake showed the greatest risk, followed by cap Cu, while Mn and Zn were considered risk-free.
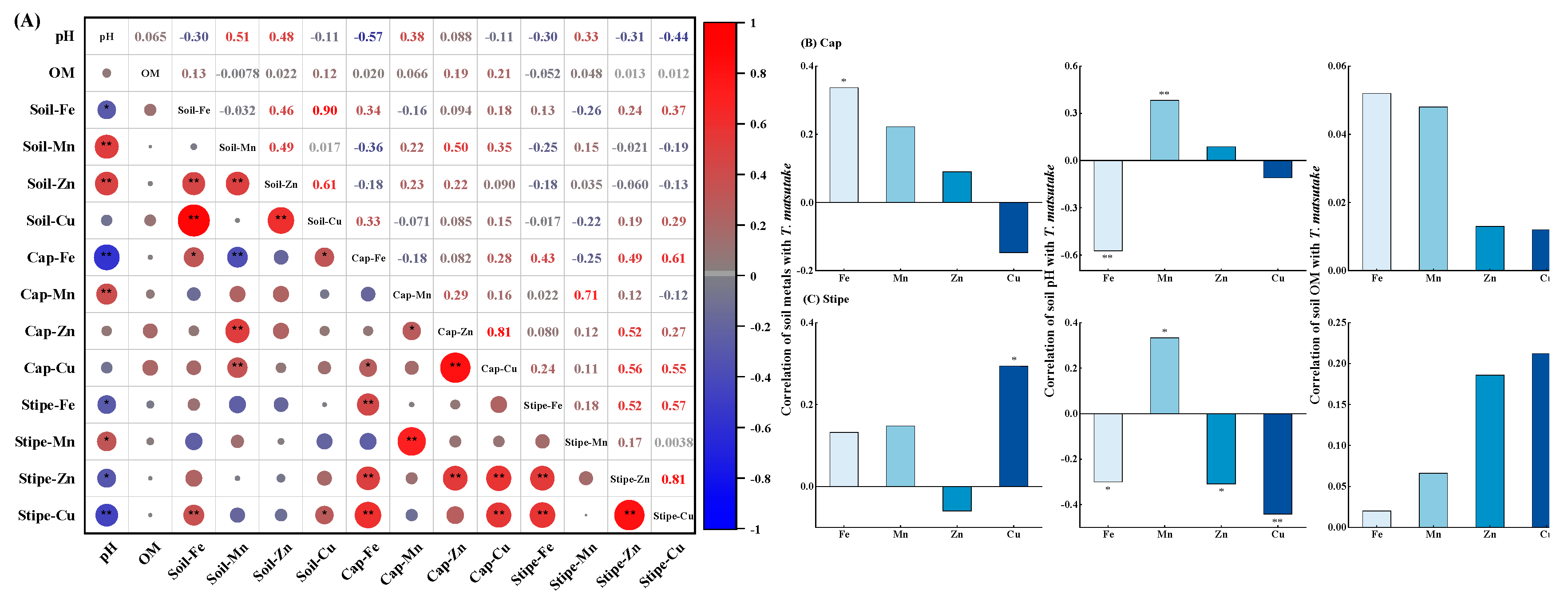
3.5. Correlation between Soil Properties and T. matsutake Metals Accumulation
Metals bioavailability in soils and accumulation in
T.
matsutake depend on soil metals total concentration, pH and OM, therefore correlations between soil and
T.
matsutake were analyzed (
Figure 6A).
The results showed that soil metals total concentration and pH showed significant effects on metals accumulation in
T.
matsutake, especially on cap Fe (
Figure 6B) and stipe Cu (
Figure 6C). Specifically, cap Fe was significantly positively affected by soil Fe content (R=0.34,
P<0.05) while negatively affected by soil pH (R=–0.57,
P<0.01). Similar to cap Fe, stipe Cu was significantly positively correlated with soil Cu content (R=0.29,
P<0.05) while negatively correlated with soil pH (R=–0.44,
P<0.01). Besides, soil Cu content showing greater effects on its accumulation in the stipe than cap (R=0.29 vs. –0.15). This was in consistent with Su et al. (2018), finding that the correlation of soil Cu concentration with
B.
edulis stipe and cap was R=0.65 and –0.13. This again suggested that soil is an important source for Fe and Cu accumulation in
T.
matsutake cap and stipe respectively, and acidic soils (pH=3.95–6.56;
Table 1) further increase their mobility thus accumulation.
In contrary to Fe and Cu, both cap (R=0.38,
P<0.01) and stipe Mn (R=0.33,
P<0.05) were significantly positively correlated with soil pH. Therefore, acidic conditions may decrease Mn accumulation in
T.
matsutake. This was supported by the great high Mn content in soils (0.046–8.58 g kg
–1;
Figure 2A) while low accumulation in
T.
matsutake (21.1–487 mg kg
–1;
Figure 3). Besides, cap and stipe Mn were positively correlated with soil Mn content (R=0.22 and 0.15), which were lower than that in
B.
badius (R=0.34 and 0.43) (Proskura et al., 2017). In terms of Zn, cap was positively correlated with soil concentration (R=0.09) and pH (R=0.09), while stipe showed significant negative correlation with soil pH (R=–0.31,
P<0.05). This indicated that soil Mn and Zn concentration pose relatively low effects on their accumulation in
T.
matsutake.
4. Conclusions
This study investigated four mineral elements (Fe, Mn, Zn and Cu) concentration, translocation and accumulation from soils to T. matsutake, and evaluated the potential health risk of metallic elements via T. matsutake ingestion. The results showed that metals concentrations in T. matsutake growing soils were strongly heterogenous. Fe and Mn (16–201 and 0.046–8.58 g kg–1) concentrations were great higher than Zn and Cu (22.6–215 and 3.7–155 mg kg–1). The highest Fe concentration in T. matsutake cap (0.24–18.8 g kg–1) and significant positive correlation with soils (R=0.34, P<0.05) suggested that soil Fe is an important source for its accumulation in T. matsutake. In contrary to Fe, high concentration of Mn in soils is not necessarily leading to high accumulation in T. matsutake, with BAF at 0.006–1.69. Besides, T. matsutake showed accumulation and transfer ability towards Zn and Cu, with BAF and TF were 0.32–17.1 and 0.96–4.53. Correspondingly, Fe showed the highest health risk with 92.6–94.4% of samples showing HRI>1. In addition to soil Fe concentration, the great Fe accumulation in T. matsutake and the high potential risk was related to low soil pH (3.95–6.56), which were significantly negatively correlated (R=–0.57, P<0.01).
Acknowledgments
This work was supported by Yunnan Agricultural Joint Research Foundation (202301BD070001-154, 202101BD070001-043), Yunnan Xingdian Talent Project (YNQR-QNRC-2019-027), Scientific Research Foundation of Yunnan Education Department (2024Y599), and National Natural Science Foundation of China (41867066, 41907129).
References
- Barea-Sepúlveda, M.; Espada-Bellido, E.; Ferreiro-González, M.; Benítez-Rodríguez, A.; López-Castillo, J.G.; Palma, M.; Barbero, G.F. Metal concentrations in Lactarius mushroom species collected from Southern Spain and Northern Morocco: Evaluation of health risks and benefits. Journal of Food Composition and Analysis 2021, 99, 103859–103868. [Google Scholar] [CrossRef]
- Barea-Sepúlveda, M.; Espada-Bellido, E.; Ferreiro-González, M.; Bouziane, H.; López-Castillo, J.G.; Palma, M.; F. Barbero, G. Exposure to Essential and Toxic Elements via Consumption of Agaricaceae, Amanitaceae, Boletaceae, and Russulaceae Mushrooms from Southern Spain and Northern Morocco. Journal of Fungi 2022, 8, 545–562. [Google Scholar] [CrossRef]
- Barea-Sepulveda, M.; Espada-Bellido, E.; Ferreiro-Gonzalez, M.; Bouziane, H.; Lopez-Castillo, J.G.; Palma, M.F.; Barbero, G.F. Toxic elements and trace elements in Macrolepiota procera mushrooms from southern Spain and northern Morocco. Journal of Food Composition and Analysis 2022, 108, 104419–104430. [Google Scholar] [CrossRef]
- Brzezicha-Cirocka, J.; Grembecka, M.; Grochowska, I.; Falandysz, J.; Szefer, P. Elemental composition of selected species of mushrooms based on a chemometric evaluation. Ecotoxicology and Environmental Safety 2019, 173, 353–365. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduction and Targeted Therapy 2022, 7, 378–394. [Google Scholar] [CrossRef]
- Cui, Y.J.; Zhu, Y.G.; Zhai, R.H.; Chen, D.Y.; Huang, Y.Z.; Qiu, Y.; Liang, J.Z. Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environment International 2004, 30, 785–791. [Google Scholar] [CrossRef]
- Damodaran, D.; Vidya Shetty, K.; Raj Mohan, B. Uptake of certain heavy metals from contaminated soil by mushroom–Galerina vittiformis. Ecotoxicology and Environmental Safety 2014, 104, 414–422. [Google Scholar] [CrossRef]
- Demirbaş, A. Heavy metal bioaccumulation by mushrooms from artificially fortified soils. Food Chemistry 2001, 74, 293–301. [Google Scholar] [CrossRef]
- Dimitrijevic, M.; Mitic, V.; Dordevic, D.; Popovic, G.; Krstic, N.; Nikolic, J.; Jovanovic, V.S. Macroelements versus toxic elements in selected wild edible mushrooms of the Russulacea family from Serbia. Journal of the Serbian Chemical Society 2021, 86, 927–940. [Google Scholar] [CrossRef]
- Dong, W.J.; He, S.X.; Li, X.Y.; Zeng, J.Y.; Li, M.Y.; Guan, D.X.; Ma, L.Q. Chromium contents, distribution and bioaccessibility in cultivated mushrooms from market: Health implications for human consumption. Journal of Hazardous Materials 2024, 461, 132643. [Google Scholar] [CrossRef]
- Falandysz, J.; Borovička, J. ; Macro and trace mineral constituents and radionuclides in mushrooms: health benefits and risks. Applied Microbiology and Biotechnology 2013, 97, 477–501. [Google Scholar] [CrossRef]
- Falandysz, J.; Hanć, A.; Barałkiewicz, D.; Zhang, J.; Treu, R. Metallic and metalloid elements in various developmental stages of Amanita muscaria (L. ) Lam. Fungal Biology 2020, 124, 174–182. [Google Scholar] [CrossRef]
- Falandysz, J.; Sapkota, A.; Mędyk, M.; Feng, X. Rare earth elements in parasol mushroom Macrolepiota procera. Food Chemistry 2017, 221, 24–28. [Google Scholar] [CrossRef]
- Gadd, G.M. Interactions of fungi with toxic metals. New Phytologist 1993, 124, 25–60. [Google Scholar] [CrossRef]
- Galicia-Andres, E.; Escalona, Y.; Oostenbrink, C.; Tunega, D.; Gerzabek, M.H. Soil organic matter stabilization at molecular scale: The role of metal cations and hydrogen bonds. Geoderma 2021, 401, 115237–115249. [Google Scholar] [CrossRef]
- García, M.Á.; Alonso, J.; Melgar, M.J. Lead in edible mushrooms: Levels and bioaccumulation factors. Journal of Hazardous Materials 2009, 167, 777–783. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends in Food Science & Technology 2017, 62, 119–132. [Google Scholar]
- Gucia, M.; Jarzynska, G.; Rafal, E.; Roszak, M.; Kojta, A.K.; Osiej, I.; Falandysz, J. Multivariate analysis of mineral constituents of edible Parasol Mushroom (Macrolepiota procera) and soils beneath fruiting bodies collected from Northern Poland. Environmental Science and Pollution Research 2012, 19, 416–431. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Zhao, Z.; Wei, H.; Gao, B.; Gu, W. Prediction of the potential geographic distribution of the ectomycorrhizal mushroom Tricholoma matsutake under multiple climate change scenarios. Scientific Reports 2017, 7, 46221–46231. [Google Scholar] [CrossRef]
- Lalotra, P.; Gupta, D.; Yangdol, R.; Sharma, Y.P.; Gupta, S.K. Bioaccumulation of heavy metals in the sporocarps of some wild mushrooms. Current Research in Environmental & Applied Mycology 2016, 6, 159–165. [Google Scholar]
- Hu, X.; Yuan, X.; Dong, L. Coal fly ash and straw immobilize Cu, Cd and Zn from mining wasteland. Environmental Chemistry Letters 2014, 12, 289–295. [Google Scholar] [CrossRef]
- Ju, Y.; Luo, Z.; Bi, J.; Liu, C.; Liu, X. Transfer of heavy metals from soil to tea and the potential human health risk in a regional high geochemical background area in southwest China. Science of the Total Environment 2024, 908, 168122–168134. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P.; Svoboda, L. A review of trace element concentrations in edible mushrooms. Food Chemistry 2000, 69, 273–281. [Google Scholar] [CrossRef]
- Keskin, F.; Sarikurkcu, C.; Akata, I.; Tepe, B. Metal concentrations of wild mushroom species collected from Belgrad forest (Istanbul, Turkey) with their health risk assessments. Environmental Science and Pollution Research 2021, 28, 36193–36204. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Science of the Total Environment 2019, 685, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Wang, P.; Wang, J.Y.; Chen, X.Q.; Zhao, D.; Yin, D.X.; Luo, J.; Juhasz, A.L.; Li, H.B.; Ma, L.Q. Arsenic concentrations, speciation, and localization in 141 cultivated market mushrooms: Implications for arsenic exposure to humans. Environmental Science & Technology 2019, 53, 503–511. [Google Scholar]
- Li, Q.; Li, X.L.; Chen, C.; Li, S.H.; Huang, W.L.; Xiong, C.; Jin, X.; Zheng, L.Y. Analysis of bacterial diversity and communities associated with Tricholoma matsutake fruiting bodies by barcoded pyrosequencing in Sichuan Province, Southwest China. Journal of Microbiology and Biotechnology 2016, 26, 89–98. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Li, W.H.; Li, X.L.; Huang, W.L.; Yang, H.; Zheng, L.Y. Chemical compositions and volatile compounds of Tricholoma matsutake from different geographical areas at different stages of maturity. Food Science and Biotechnology 2016, 25, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Y.; Zhang, J.; Zhao, Y.; Liu, H. Trace element content of Boletus tomentipes mushroom collected from Yunnan, China. Food Chemistry 2011, 127, 1828–1830. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Shen, T.; Shi, Y.D.; Yang, S.B.; Zhang, T.; Li, J.Q.; Wang, Y.Z.; Liu, H.G. Mineral element content in prized matsutake mushroom (Tricholoma matsutake) collected in China. Chemical Papers 2013, 67, 672–676. [Google Scholar] [CrossRef]
- Liu, B.R.; Huang, Q.; Cai, H.J.; Guo, X.; Wang, T.T.; Gui, M.Y. Study of heavy metal concentrations in wild edible mushrooms in Yunnan Province, China. Food Chemistry 2015, 188, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, R.; Kowalska, J.; Gąsiorek, M.; Zadrożny, P.; Józefowska, A.; Zaleski, T.; Kępka, W.; Tymczuk, M.; Orłowska, K. Assessment of heavy metals contamination in surface layers of Roztocze National Park forest soils (SE Poland) by indices of pollution. Chemosphere 2017, 168, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.J.; Alonso, J.; Garcia, M.A. Mercury in edible mushrooms and underlying soil: Bioconcentration factors and toxicological risk. Science of the Total Environment 2009, 407, 5328–5334. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Socha, K.; Zujko, M.E.; Terlikowska, K.M.; Borawska, M.H.; Witkowska, A.M. Copper, manganese, selenium and zinc in wild-growing edible mushrooms from the eastern territory of “Green Lungs of Poland”: Nutritional and toxicological implications. International Journal of Environmental Research and Public Health 2019, 16, 3614–3635. [Google Scholar] [CrossRef]
- Mleczek, M.; Budka, A.; Kalac, P.; Siwulski, M.; Niedzielski, P. Family and species as determinants modulating mineral composition of selected wild-growing mushroom species. Environmental Science and Pollution Research 2021, 28, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Mleczek, M.; Siwulski, M.; Budka, A.; Mleczek, P.; Budzynska, S.; Szostek, M.; Kuczynska-Kippen, N.; Kalac, P.; Niedzielski, P.; Gasecka, M.; Golinski, P.; Magdziak, Z.; Rzymski, P. Toxicological risks and nutritional value of wild edible mushroom species -a half-century monitoring study. Chemosphere 2021, 263, 128095–128106. [Google Scholar] [CrossRef] [PubMed]
- Proskura, N.; Podlasinska, J.; Skopicz-Radkiewicz, L. Chemical composition and bioaccumulation ability of Boletus badius (Fr.) Fr. collected in western Poland. Chemosphere 2017, 168, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Wieneke, X.; Tao, J.; Zhou, X.; Desilva, U. Soil pH is the primary factor correlating with soil microbiome in karst rocky desertification regions in the Wushan County, Chongqing, China. Frontiers in Microbiology 2018, 9, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Rasalanavho, M.; Moodley, R.; Jonnalagadda, S.B. Elemental bioaccumulation and nutritional value of five species of wild growing mushrooms from South Africa. Food Chemistry 2020, 319, 126596–126606. [Google Scholar] [CrossRef]
- Ronda, O.; Grządka, E.; Ostolska, I.; Orzeł, J.; Cieślik, B.M. Accumulation of radioisotopes and heavy metals in selected species of mushrooms. Food Chemistry 2022, 367, 130670. [Google Scholar] [CrossRef]
- Saqib Rashid, M.; Liu, G.; Yousaf, B.; Song, Y.; Ahmed, R.; Rehman, A.; Arif, M.; Irshad, S.; Cheema, A.I. Efficacy of rice husk biochar and compost amendments on the translocation, bioavailability, and heavy metals speciation in contaminated soil: Role of free radical production in maize (Zea mays L.). Journal of Cleaner Production 2022, 330, 129805–129820. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Popović-Djordjević, J.; Solak, M.H. Wild edible mushrooms from Mediterranean region: Metal concentrations and health risk assessment. Ecotoxicology and Environmental Safety 2020, 190, 110058–110064. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Tepe, B.; Kocak, M.S.; Uren, M.C. Metal concentration and antioxidant activity of edible mushrooms from Turkey. Food Chemistry 2015, 175, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Saifullah, Malhi, S. S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. Journal of the Science of Food and Agriculture 2010, 90, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, J.; Li, J.; Li, T.; Liu, H.; Wang, Y. Determination of mineral contents of wild Boletus edulis mushroom and its edible safety assessment. Journal of Environmental Science and Health Part B-Pesticides Food Contaminants and Agricultural Wastes 2018, 53, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Kwong, R.W.M.; Tang, W.; Yang, Y.; Zhong, H. Straw return enhances the risks of metals in soil? Ecotoxicology and Environmental Safety 2021, 207, 111201–111210. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, L.; Zimmermannová, K.; Kalač, P. Concentrations of mercury, cadmium, lead and copper in fruiting bodies of edible mushrooms in an emission area of a copper smelter and a mercury smelter. Science of the Total Environment 2000, 246, 61–67. [Google Scholar] [CrossRef]
- Szymańska K, Strumińska-Parulska, D. ; Falandysz, J. Uranium (234U, 238U) and thorium (230Th, 232Th) in mushrooms of genus Leccinum and Leccinellum and the potential effective ionizing radiation dose assessment for human. Chemosphere 2020, 250, 126242. [Google Scholar] [CrossRef]
- Tanikawa, T.; Fujii, S.; Sun, L.; Hirano, Y.; Matsuda, Y.; Miyatani, K.; Doi, R.; Mizoguchi, T.; Maie, N. Leachate from fine root litter is more acidic than leaf litter leachate: A 2.5-year laboratory incubation. Science of the Total Environment 2018, 645, 179–191. [Google Scholar] [CrossRef]
- Tuzen, M.; Sesli, E.; Soylak, M. Trace element levels of mushroom species from East Black Sea region of Turkey. Food Control 2007, 18, 806–810. [Google Scholar] [CrossRef]
- USEPA. A review of the reference dose and reference concentration processes. Washington, DC: U.S. Environmental Protection Agency RAF. 2002.
- Wagner, D.; Heider, D.; Hattab, G. Mushroom data creation, curation, and simulation to support classification tasks. Scientific Reports 2021, 11, 8134–8145. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Chen, Y.; Li, Z.; Hedding, D.W.; Nel, W.; Ji, J.; Chen, J. Geochemical behavior and potential health risk of heavy metals in basalt-derived agricultural soil and crops: A case study from Xuyi County, eastern China. Science of the Total Environment 2020, 729, 139058–139067. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, J.; Wu, L.H.; Zhao, Y.L.; Li, T.; Li, J.Q.; Wang, Y.Z.; Liu, H.G. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chemistry 2014, 151, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Li, Y.; Liang, C.; Huang, H.; Wang, S. Available heavy metals concentrations in agricultural soils: Relationship with soil properties and total heavy metals concentrations in different industries. Journal of Hazardous Materials 2024, 471, 134410. [Google Scholar] [CrossRef]
- Xu, J.; Cadorin, M.; Liang, Y.J.; Yang, Z.L. DNA-based geographic typing of the gourmet mushroom Tricholoma matsutake traded in China. Mycoscience 2010, 51, 248–251. [Google Scholar] [CrossRef]
- Xu, P.; Sun, C.X.; Ye, X.Z.; Xiao, W.D.; Zhang, Q.; Wang, Q. The effect of biochar and crop straws on heavy metal bioavailability and plant accumulation in a Cd and Pb polluted soil. Ecotoxicology and Environmental Safety 2016, 132, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tao, C.; Zhang, Y.; Hou, Y.; Chang, Q. Health risk assessment of heavy metals in agricultural soils and identification of main influencing factors in a typical industrial park in northwest China. Chemosphere 2020, 252, 126591–126600. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Qu, L.; Fan, W.; Qiao, M.; Hao, H.; Wang, X. Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province, China. Environmental Monitoring and Assessment 2011, 179, 191–199. [Google Scholar] [CrossRef]
- Ziarati, P.; Rabizadeh, H. The effect of thermal and non thermal food processes and cooking method in some essential mineral contents in mushroom (Agaricus bisporus) in Iran. Journal of Novel Applied Sciences 2013, 2, 954–959. [Google Scholar]
- Ziarati, P.; Rabizadeh, H. Safety and nutritional comparison of fresh, cooked and frozen mushroom (Agaricus bisporus). International Journal of Farming and Allied Sciences 2013, 2, 1141–1147. [Google Scholar]
- Zsigmond, A.R.; Fejer, I.R.; Kantor, I.; May, Z.; Urak, I. Influence of the urban environment on four mushroom species in the light of their elemental composition. Chemosphere 2023, 335, 139052–139060. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Distribution of 54 sampling sits in Luoji (n=40) and Jiantang (n=14), Yunnan province, southwest China.
Figure 1.
Distribution of 54 sampling sits in Luoji (n=40) and Jiantang (n=14), Yunnan province, southwest China.
Figure 2.
Metals (Fe, Mn, Zn, Cu) concentration in soils (A) and variations between regions of Luoji (n=40) and Jiantang (n=14) (B). The bottom and top of the box represent the 25th and 75th percentiles and error bars represent minimum and maximum values within the normal range. The solid lines inside the box represent the median value.
Figure 2.
Metals (Fe, Mn, Zn, Cu) concentration in soils (A) and variations between regions of Luoji (n=40) and Jiantang (n=14) (B). The bottom and top of the box represent the 25th and 75th percentiles and error bars represent minimum and maximum values within the normal range. The solid lines inside the box represent the median value.
Figure 3.
Metals (Fe, Mn, Zn, Cu) concentration in T. matsutake cap and stipe (A) and comparisons between regions of Luoji (n=40) and Jiantang (n=14) (B). The bottom and top of the box represent the 25th and 75th percentiles and error bars represent minimum and maximum values within the normal range. The solid lines inside the box represent the median value.
Figure 3.
Metals (Fe, Mn, Zn, Cu) concentration in T. matsutake cap and stipe (A) and comparisons between regions of Luoji (n=40) and Jiantang (n=14) (B). The bottom and top of the box represent the 25th and 75th percentiles and error bars represent minimum and maximum values within the normal range. The solid lines inside the box represent the median value.
Figure 4.
Bioaccumulation factor (BAF) of Fe, Mn, Zn and Cu in cap (A) and stipe (B) and translocation factor (TF) (C) in T. matsutake (n=54). BAF>1 and TF>1 indicates that T. matsutake possesses accumulating or stipe-to-cap translocating ability towards the given element, respectively.
Figure 4.
Bioaccumulation factor (BAF) of Fe, Mn, Zn and Cu in cap (A) and stipe (B) and translocation factor (TF) (C) in T. matsutake (n=54). BAF>1 and TF>1 indicates that T. matsutake possesses accumulating or stipe-to-cap translocating ability towards the given element, respectively.
Figure 5.
Health risk index (HRI) of Fe, Mn, Zn and Cu via ingestion of T. matsutake cap (A) and stipe (B) (n=54). HRI>1 indicates there is a potential health risk of the element via consumption of T. matsutake cap or stipe.
Figure 5.
Health risk index (HRI) of Fe, Mn, Zn and Cu via ingestion of T. matsutake cap (A) and stipe (B) (n=54). HRI>1 indicates there is a potential health risk of the element via consumption of T. matsutake cap or stipe.
Figure 6.
Correlations among metals (Fe, Mn, Zn and Cu) between soil and T. matsutake (A) and correlations of metals concentration in T. matsutake cap (B) and stipe (C) with soil metals concentration, pH and organic matter content (OM) with significance at P<0.05 (*) or P<0.01 (**).
Figure 6.
Correlations among metals (Fe, Mn, Zn and Cu) between soil and T. matsutake (A) and correlations of metals concentration in T. matsutake cap (B) and stipe (C) with soil metals concentration, pH and organic matter content (OM) with significance at P<0.05 (*) or P<0.01 (**).
Table 1.
Soil pH and organic matter content, and daily intake (DI) of Fe, Mn, Zn and Cu via ingestion of T. matsutake cap and stipe from Yunnan province, southwest China.
Table 1.
Soil pH and organic matter content, and daily intake (DI) of Fe, Mn, Zn and Cu via ingestion of T. matsutake cap and stipe from Yunnan province, southwest China.
| Sample ID |
Soil pH |
Soil organic matter content (%) |
Fe |
Mn |
Zn |
Cu |
| cap |
stipe |
cap |
stipe |
cap |
stipe |
cap |
stipe |
| 1 |
4.57 |
6.96 |
1413 |
244 |
34.7 |
30.5 |
81.6 |
50.9 |
62.1 |
35.2 |
| 2 |
5.33 |
11.9 |
689 |
493 |
26.0 |
16.2 |
75.6 |
34.1 |
53.1 |
25.2 |
| 3 |
4.32 |
8.79 |
1708 |
2464 |
50.9 |
20.3 |
77.8 |
36.5 |
59.9 |
27.1 |
| 4 |
4.39 |
10.6 |
1437 |
3564 |
26.4 |
37.2 |
53.2 |
44.3 |
37.8 |
31.4 |
| 5 |
4.64 |
13.7 |
1013 |
2412 |
37.0 |
34.4 |
69.3 |
45.2 |
34.0 |
35.5 |
| 6 |
4.58 |
9.63 |
596 |
416 |
26.5 |
11.0 |
55.8 |
23.3 |
31.8 |
18.8 |
| 7 |
4.32 |
9.58 |
1115 |
1613 |
43.0 |
25.3 |
88.0 |
49.0 |
60.3 |
29.1 |
| 8 |
4.99 |
18 |
1303 |
2301 |
27.0 |
19.2 |
63.7 |
37.3 |
47.4 |
28.5 |
| 9 |
5.02 |
27.8 |
2033 |
3077 |
39.9 |
26.6 |
74.8 |
32.5 |
47.6 |
22.4 |
| 10 |
4.19 |
3.86 |
2097 |
6799 |
33.0 |
39.8 |
63.5 |
53.4 |
39.8 |
37.4 |
| 11 |
5.06 |
11.4 |
1329 |
6857 |
45.8 |
54.0 |
98.6 |
55.6 |
68.5 |
39.5 |
| 12 |
4.06 |
7.71 |
948 |
7216 |
50.8 |
82.2 |
98.7 |
44.6 |
82.2 |
33.0 |
| 13 |
4.32 |
7.87 |
1416 |
8058 |
34.3 |
49.2 |
73.0 |
47.1 |
44.8 |
28.7 |
| 14 |
4.74 |
10.6 |
1033 |
1073 |
22.8 |
12.9 |
56.2 |
30.0 |
39.1 |
26.0 |
| 15 |
4.23 |
1.29 |
1119 |
2132 |
40.4 |
22.9 |
65.9 |
38.2 |
48.1 |
24.6 |
| 16 |
4.84 |
5.07 |
727 |
3145 |
26.8 |
29.0 |
55.2 |
32.7 |
36.3 |
25.3 |
| 17 |
4.68 |
6.84 |
697 |
3495 |
38.6 |
33.1 |
52.9 |
28.0 |
30.8 |
18.4 |
| 18 |
4.83 |
15.1 |
1330 |
4760 |
25.5 |
24.4 |
73.7 |
38.7 |
71.6 |
32.7 |
| 19 |
4.8 |
6.23 |
1964 |
3912 |
34.3 |
26.9 |
89.4 |
52.6 |
65.4 |
33.8 |
| 20 |
4.31 |
17.3 |
935 |
2419 |
29.2 |
26.4 |
65.5 |
44.1 |
43.0 |
27.2 |
| 21 |
4.81 |
3.77 |
1434 |
1784 |
67.4 |
27.7 |
79.0 |
51.5 |
51.2 |
28.7 |
| 22 |
4.13 |
6.21 |
836 |
1695 |
34.8 |
22.3 |
52.7 |
31.4 |
25.6 |
19.9 |
| 23 |
4.52 |
11.1 |
1907 |
2185 |
30.6 |
19.9 |
80.4 |
40.1 |
47.7 |
21.7 |
| 24 |
4.26 |
2.78 |
1253 |
1427 |
33.3 |
16.1 |
70.7 |
35.5 |
41.7 |
25.6 |
| 25 |
4.12 |
7.55 |
1246 |
3285 |
19.4 |
16.4 |
71.1 |
39.9 |
39.3 |
19.4 |
| 26 |
4.1 |
3.85 |
667 |
840 |
39.7 |
21.6 |
67.0 |
34.1 |
34.9 |
19.1 |
| 27 |
4.7 |
6.2 |
860 |
2811 |
37.1 |
20.1 |
78.5 |
40.1 |
49.2 |
26.8 |
| 28 |
4.48 |
6.68 |
1008 |
2877 |
51.1 |
26.8 |
94.2 |
46.7 |
51.3 |
22.3 |
| 29 |
4.44 |
3.09 |
2099 |
3081 |
26.5 |
30.7 |
58.1 |
34.3 |
40.5 |
26.7 |
| 30 |
4.19 |
44.5 |
962 |
1764 |
60.7 |
40.0 |
99.1 |
43.2 |
64.4 |
24.6 |
| 31 |
4.57 |
8.4 |
1178 |
4609 |
29.9 |
28.7 |
63.3 |
42.2 |
46.6 |
28.2 |
| 32 |
4.2 |
8.61 |
669 |
2996 |
14.5 |
18.1 |
46.9 |
32.0 |
31.4 |
23.9 |
| 33 |
4.55 |
10.3 |
810 |
5716 |
26.4 |
36.5 |
73.3 |
46.1 |
50.5 |
31.0 |
| 34 |
4.74 |
8.76 |
584 |
1733 |
15.6 |
14.3 |
46.2 |
23.4 |
23.8 |
21.8 |
| 35 |
3.95 |
12.2 |
1250 |
2414 |
26.7 |
22.8 |
72.8 |
36.7 |
52.0 |
25.4 |
| 36 |
4.89 |
10.6 |
566 |
2739 |
15.2 |
20.0 |
62.5 |
33.4 |
36.1 |
19.3 |
| 37 |
4.66 |
8.77 |
1005 |
3172 |
22.5 |
28.7 |
52.9 |
41.0 |
31.2 |
25.1 |
| 38 |
4.68 |
5.59 |
539 |
1485 |
36.0 |
23.3 |
76.5 |
40.8 |
43.8 |
24.8 |
| 39 |
4.55 |
7.71 |
1175 |
1479 |
30.5 |
16.2 |
83.5 |
29.4 |
54.1 |
21.1 |
| 40 |
4.68 |
10.6 |
800 |
3722 |
30.4 |
39.0 |
86.6 |
49.3 |
58.3 |
30.3 |
| 41 |
6.37 |
5.53 |
417 |
627 |
61.8 |
34.5 |
93.6 |
27.1 |
26.6 |
13.1 |
| 42 |
5.52 |
10.3 |
261 |
2027 |
103 |
637 |
74.7 |
38.9 |
44.0 |
19.8 |
| 43 |
5.94 |
13.4 |
408 |
2473 |
60.1 |
128 |
82.7 |
39.2 |
59.6 |
24.8 |
| 44 |
5.08 |
7.12 |
490 |
744 |
49.9 |
56.5 |
44.5 |
23.9 |
27.1 |
15.0 |
| 45 |
6.29 |
4.48 |
335 |
2092 |
26.0 |
29.4 |
48.0 |
28.0 |
17.4 |
16.7 |
| 46 |
6.11 |
8.74 |
418 |
5031 |
90.8 |
57.3 |
81.1 |
36.9 |
37.8 |
17.9 |
| 47 |
4.79 |
7.49 |
102 |
217 |
12.3 |
9.0 |
43.9 |
20.0 |
18.0 |
10.7 |
| 48 |
5.96 |
10.6 |
334 |
861 |
39.6 |
25.3 |
67.5 |
27.0 |
35.8 |
13.3 |
| 49 |
6.3 |
6.51 |
273 |
1293 |
44.1 |
61.0 |
75.3 |
44.5 |
51.1 |
26.4 |
| 50 |
5.96 |
12.9 |
472 |
1502 |
56.2 |
25.8 |
35.5 |
16.6 |
42.2 |
17.9 |
| 51 |
5.38 |
3.94 |
650 |
2156 |
66.1 |
77.7 |
66.0 |
38.1 |
41.6 |
22.6 |
| 52 |
6.25 |
21.3 |
192 |
385 |
20.5 |
24.5 |
93.7 |
36.2 |
51.3 |
19.5 |
| 53 |
5.79 |
7.12 |
306 |
177 |
39.3 |
20.6 |
141 |
37.1 |
93.2 |
20.6 |
| 54 |
6.56 |
15.3 |
465 |
1476 |
59.1 |
80.6 |
57.2 |
30.4 |
27.3 |
15.2 |
| RfDa (µg kg–1 body weight day–1) |
300 (JECEFA)c
|
140 (USEPA)d
|
300d
|
40d
|
| PTMDIb (µg kg–1 body weight day–1) |
– |
– |
300–1000c |
5000c |
| Percentage of samples exceeding RfD (%) |
92.6% |
94.4% |
0 |
1.85% |
0 |
0 |
61.1% |
0 |
| Percentage of samples exceeding PTMDI (%) |
– |
– |
0 |
0 |
0 |
0 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).