Submitted:
29 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
Systemic Inflammation in Cirrhosis
Evidence of Systemic Inflammation
Mechanisms of Systemic Inflammation
- High-grade Systemic Inflammation
Immunological Role of the Resident Cells in the Liver
Consequences of Systemic Inflammation
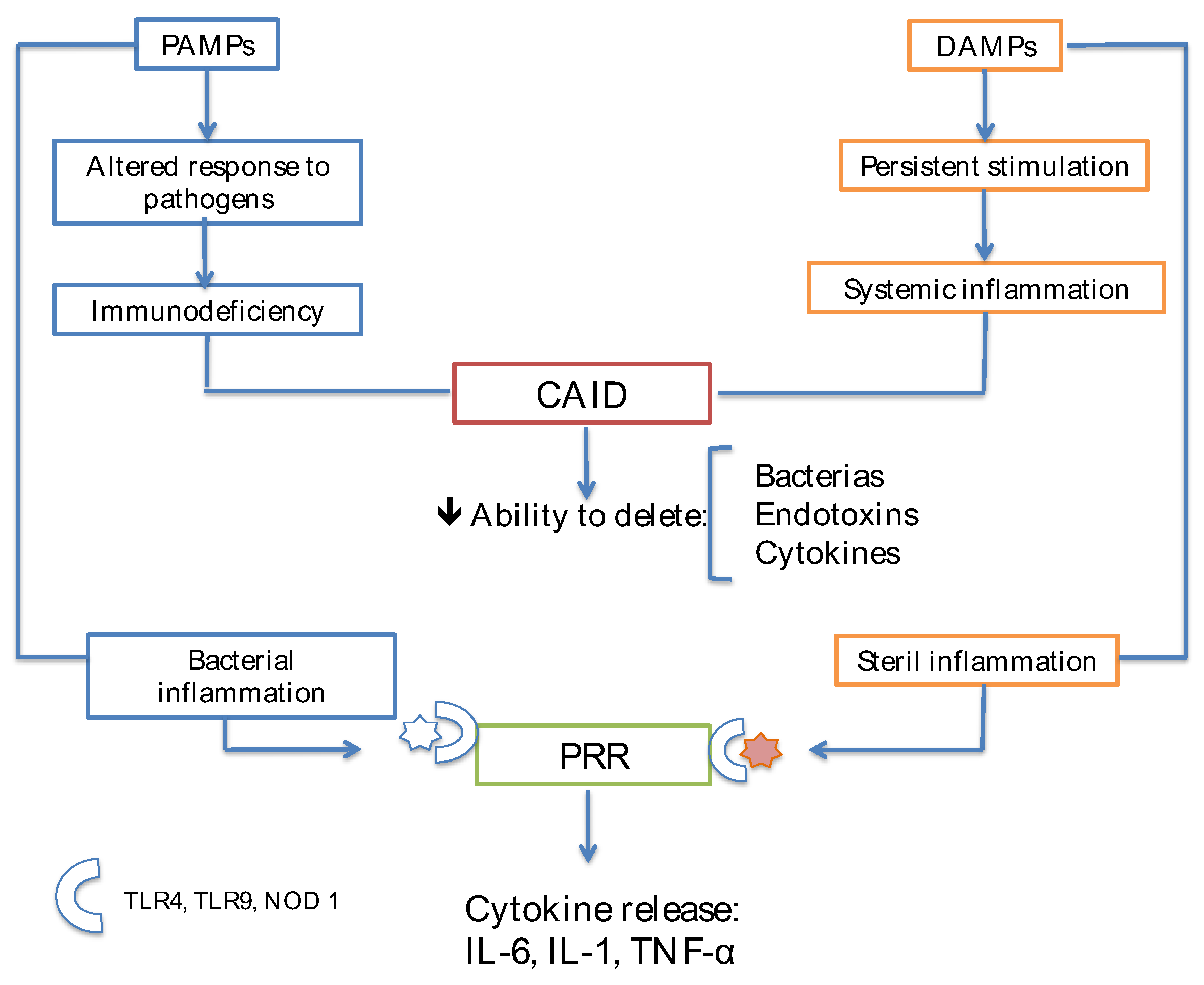
Immunodeficiency and Cirrhosis
Progressive Loss of Tolerance in Cirrhosis
Increased Release of DAMPs from Injured Hepatocytes
Structural Distortion in Cirrhosis
Damage to the Circulating Immune Cells
| Immune cells | Decompensated cirrhosis | ACLF |
| Monocytes | ⇑ HLA-DR expression ⇑ TNF production ⇑ CD14+CD16+ ⇓ capacity of the APC Altered phagocytes Altered chemiotaxis Production of OH+ Defective production of Fc |
⇓ HLA-DR ⇑ producción IL-10 ⇓ producción TNF Altered phagocytosis ⇑MERKT inhibits the activity of TLR and the proinflammatory cytokines inhibit the activity of NF-kB |
| Neutrophils | Altered phagocytosis Reduced chemiotaxis Re⇑ ROS |
⇑Expression of CXCR1 and CXCR2 that favor apoptosis and necrosis |
| Lymphocytes |
TCD4+ ⇓cytolytic activity of the NK cells |
Compensatory Anti-Inflammatory Response Syndrome (CARS)
Dysfunction of Immune Cells Due to Metabolic Anomalies of Cirrhosis
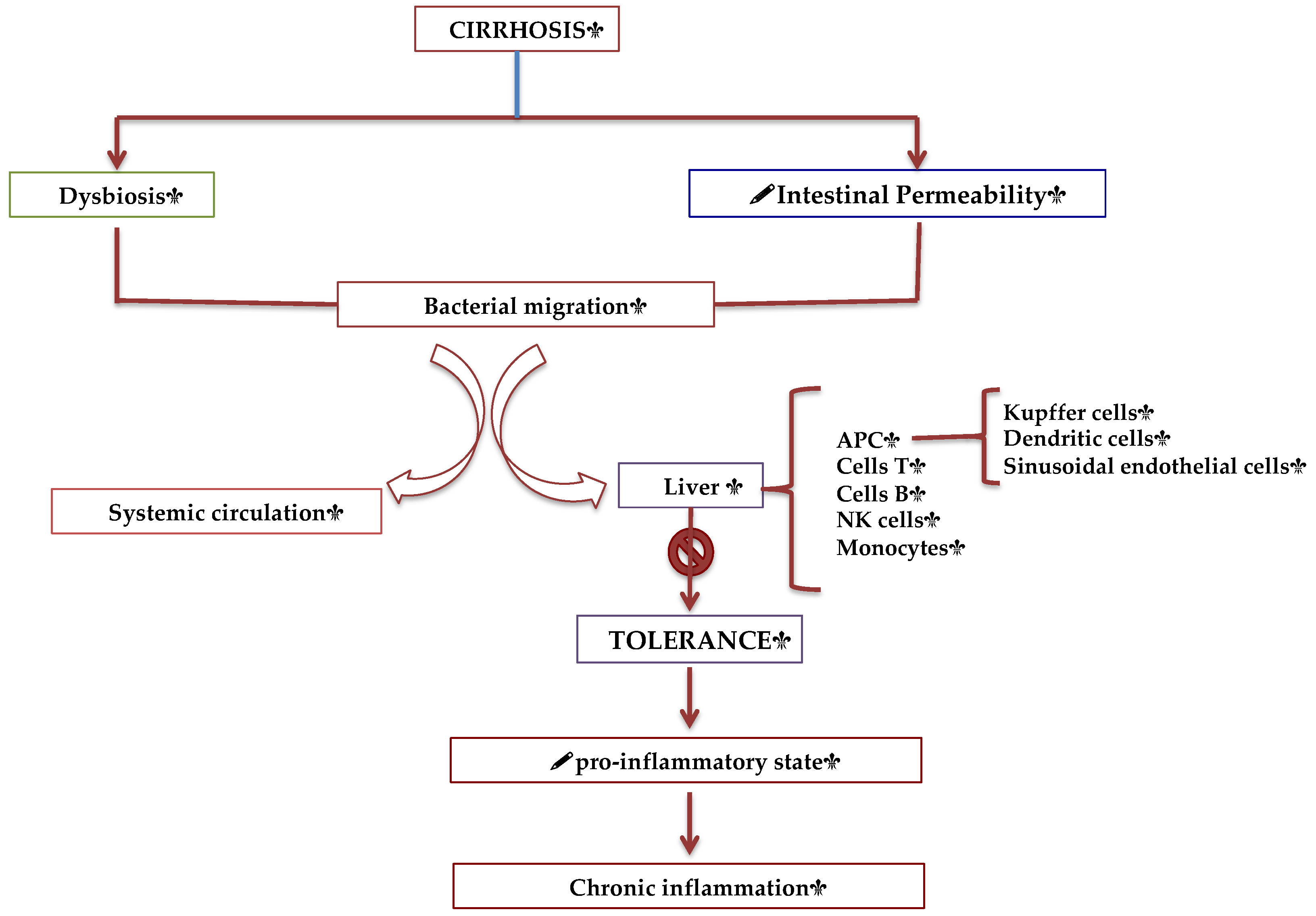
Intestinal—Liver Axis and the Intestinal Immune System
Humoral Factors in Circulation
Infections in Cirrhosis
SI Is the Common Mechanism for Major Complications and Organ Failure in AD
Immunoparesis
Liver Failure and Acute Kidney Injury (AKI) in AD
Albumin Treatment Negatively Regulates Systemic Inflammation in Decompensated Patients with Cirrhosis
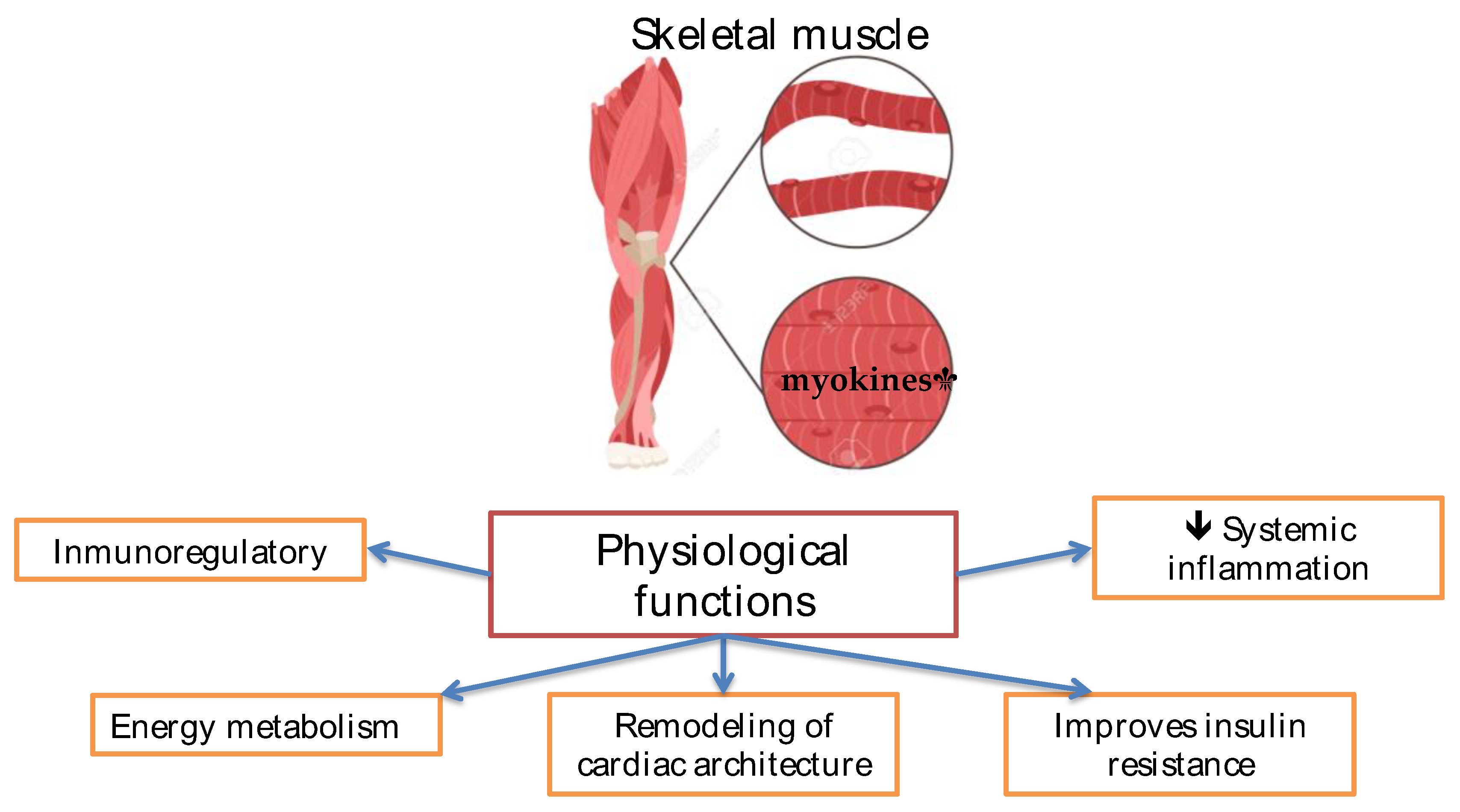
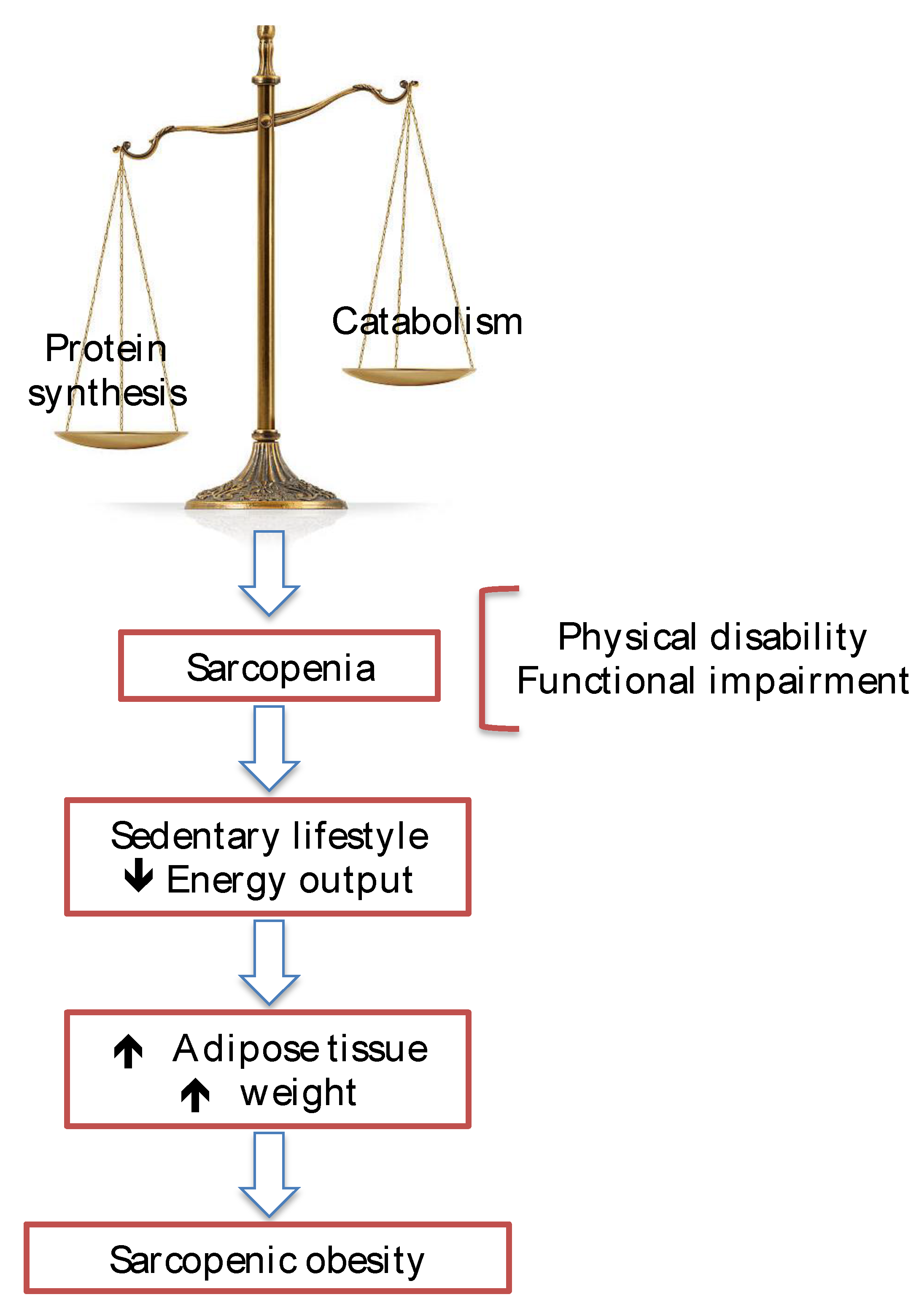
Sarcopenia in Cirrhosis
Cytokines and Their Correlation with Hepatic Encephalopathy (HE)
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren A, He W, Rao J, Ye D, Cheng P, Jian Q, Fu Z, Zhang X, Deng R, Gao Y, Ma Y. Dysregulation of innate cell types in the hepatic immune microenvironment of alcoholic liver cirrhosis. Front Immunol. 2023; 14: 1034356. [CrossRef]
- Albillos A, Martin-Mateos R, Van der Merwe S, Wiest R, Jalan R, Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022; 19(2): 112-134. [CrossRef]
- Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011; 9(9): 727-38. [CrossRef]
- Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010; 139(4): 1246-56. [CrossRef]
- Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, Boccia S, Colloredo-Mels G, Corigliano P, Fornaciari G, Marenco G, Pistarà R, Salvagnini M, Sangiovanni A. Bacterial infection in patients with advanced cirrhosis: A multicentre prospective study. Dig Liver Dis. 2001; 33(1): 41-8. [CrossRef]
- Pauwels A, Mostefa-Kara N, Debenes B, Degoutte E, Lévy VG. Systemic antibiotic prophylaxis after gastrointestinal hemorrhage in cirrhotic patients with a high risk of infection. Hepatology. 1996; 24(4): 802-6. [CrossRef]
- Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J Hepatol. 2014; 61(6): 1385-96. [CrossRef]
- Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013; 144(7): 1426-37, 1437.e1-9. [CrossRef]
- Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jimenez C, Mookerjee R; PREDICT STUDY group of the EASL-CLIF Consortium. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020; 73(4): 842-854. [CrossRef]
- Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020; 72(3): 558-577. [CrossRef]
- Franco A, Barnaba V, Natali P, Balsano C, Musca A, Balsano F. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology. 1988; 8(3): 449-54. [CrossRef]
- Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008; 47(2): 729-36. [CrossRef]
- Mörbe UM, Jørgensen PB, Fenton TM, von Burg N, Riis LB, Spencer J, Agace WW. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021; 14(4): 793-802. [CrossRef]
- MacPherson G, Milling S, Yrlid U, Cousins L, Turnbull E, Huang FP. Uptake of antigens from the intestine by dendritic cells. Ann N Y Acad Sci. 2004; 1029: 75-82. [CrossRef]
- Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015; 63(5): 1272-84. [CrossRef]
- Bernardi M., Sriskandan S, Altmann DM. The immunology of sepsis. J Pathol. 2008; 214(2): 211-23. [CrossRef]
- Solé C, Solá E, Morales-Ruiz M, Fernández G, Huelin P, Graupera I, Moreira R, de Prada G, Ariza X, Pose E, Fabrellas N, Kalko SG, Jiménez W, Ginés P. Characterization of Inflammatory Response in Acute-on-Chronic Liver Failure and Relationship with Prognosis. Sci Rep. 2016; 6: 32341. [CrossRef]
- Monteiro S, Grandt J, Uschner FE, Kimer N, Madsen JL, Schierwagen R, Klein S, Welsch C, Schäfer L, Jansen C, Claria J, Alcaraz-Quiles J, Arroyo V, Moreau R, Fernandez J, Bendtsen F, Mehta G, Gluud LL, Møller S, Praktiknjo M, Trebicka J. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut. 2021; 70(2): 379-387. [CrossRef]
- Arroyo V, Angeli P, Moreau R, Jalan R, Clària J, Trebicka J, Fernández J, Gustot T, Caraceni P, Bernardi M; investigators from the EASL-CLIF Consortium, Grifols Chair and European Foundation for the Study of Chronic Liver Failure (EF-Clif). The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021; 74(3): 670-685. [CrossRef]
- Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol. 2017; 67(5): 1084-1103. [CrossRef]
- Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, Nagy L, Szabo G, Zakhari S. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011; 300(4): G516-25. [CrossRef]
- Coant N, Simon-Rudler M, Gustot T, Fasseu M, Gandoura S, Ragot K, Abdel-Razek W, Thabut D, Lettéron P, Ogier-Denis E. Glycogen synthase kinase 3 involvement in the excessive proinflammatory response to LPS in patients with decompensated cirrhosis. J Hepatol. 2011; 55(4): 784-93. [CrossRef]
- Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012; 143(5): 1158-1172. [CrossRef]
- Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000; 32(5): 742-7. [CrossRef]
- Bellot P, García-Pagán JC, Francés R, Abraldes JG, Navasa M, Pérez-Mateo M, Such J, Bosch J. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology. 2010; 52(6): 2044-52. [CrossRef]
- Ayres JS. Immunometabolism of infections. Nat Rev Immunol. 2020; 20(2): 79-80. [CrossRef]
- Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015; 62(2): 437-47. [CrossRef]
- Martin-Mateos R, Alvarez-Mon M, Albillos A. Dysfunctional Immune Response in Acute-on-Chronic Liver Failure: It Takes Two to Tango. Front Immunol. 2019; 10: 1-10. [CrossRef]
- Laleman W, Claria J, Van der Merwe S, Moreau R, Trebicka J. Systemic Inflammation and Acute-on-Chronic Liver Failure: Too Much, Not Enough. Can J Gastroenterol Hepatol. 2018; 2018: 1-10. [CrossRef]
- Papp M, Vitalis Z, Altorjay I, Tornai I, Udvardy M, Harsfalvi J, Vida A, Kappelmayer J, Lakatos PL, Antal-Szalmas P. Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections. Liver Int. 2012; 32(4): 603-11. [CrossRef]
- Homann C, Varming K, Høgåsen K, Mollnes TE, Graudal N, Thomsen AC, Garred P. Acquired C3 deficiency in patients with alcoholic cirrhosis predisposes to infection and increased mortality. Gut. 1997; 40(4): 544-9. [CrossRef]
- Tritto G, Bechlis Z, Stadlbauer V, Davies N, Francés R, Shah N, Mookerjee RP, Such J, Jalan R. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol. 2011; 55(3): 574-581. [CrossRef]
- Ward NS, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med. 2008; 29(4): 617-25. [CrossRef]
- Berry PA, Antoniades CG, Hussain MJ, McPhail MJ, Bernal W, Vergani D, Wendon JA. Admission levels and early changes in serum interleukin-10 are predictive of poor outcome in acute liver failure and decompensated cirrhosis. Liver Int. 2010; 30(5): 733-40. [CrossRef]
- Bone RC, Grodzin CJ, Balk RA. Sepsis: A new hypothesis for pathogenesis of the disease process. Chest. 1997; 112(1): 235-43. [CrossRef]
- Jalan R, Schnurr K, Mookerjee RP, Sen S, Cheshire L, Hodges S, Muravsky V, Williams R, Matthes G, Davies NA. Alterations in the functional capacity of albumin in patients with decompensated cirrhosis are associated with increased mortality. Hepatology. 2009; 50(2): 555-64. [CrossRef]
- O'Brien AJ, Fullerton JN, Massey KA, Auld G, Sewell G, James S, Newson J, Karra E, Winstanley A, Alazawi W, Garcia-Martinez R, Cordoba J, Nicolaou A, Gilroy DW. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med. 2014; 20(5): 518-23. [CrossRef]
- Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010; 139(4): 1246-56. [CrossRef]
- Gustot T, Felleiter P, Pickkers P, Sakr Y, Rello J, Velissaris D, Pierrakos C, Taccone FS, Sevcik P, Moreno C, Vincent JL; EPIC II Group of Investigators. Impact of infection on the prognosis of critically ill cirrhotic patients: Results from a large worldwide study. Liver Int. 2014; 34(10): 1496-503. [CrossRef]
- Muñoz L, José Borrero M, Ubeda M, Lario M, Díaz D, Francés R, Monserrat J, Pastor O, Aguado-Fraile E, Such J, Alvarez-Mon M, Albillos A. Interaction between intestinal dendritic cells and bacteria translocated from the gut in rats with cirrhosis. Hepatology. 2012; 56(5): 1861-9. [CrossRef]
- Lozano-Ruiz B, Bachiller V, García-Martínez I, Zapater P, Gómez-Hurtado I, Moratalla A, Giménez P, Bellot P, Francés R, Such J, González-Navajas JM. Absent in melanoma 2 triggers a heightened inflammasome response in ascitic fluid macrophages of patients with cirrhosis. J Hepatol. 2015; 62(1): 64-71. [CrossRef]
- Navasa M, Follo A, Filella X, Jiménez W, Francitorra A, Planas R, Rimola A, Arroyo V, Rodés J. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: Relationship with the development of renal impairment and mortality. Hepatology. 1998; 27(5): 1227-32. [CrossRef]
- Stengel S, Quickert S, Lutz P, Ibidapo-Obe O, Steube A, Köse-Vogel N, Yarbakht M, Reuken PA, Busch M, Brandt A, Bergheim I, Deshmukh SD, Stallmach A, Bruns T. Peritoneal Level of CD206 Associates With Mortality and an Inflammatory Macrophage Phenotype in Patients With Decompensated Cirrhosis and Spontaneous Bacterial Peritonitis. Gastroenterology. 2020; 158(6): 1745-1761. [CrossRef]
- Rasaratnam B, Kaye D, Jennings G, Dudley F, Chin-Dusting J. The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis. A randomized trial. Ann Intern Med. 2003; 139(3): 186-93. [CrossRef]
- Mehta G, Mookerjee RP, Sharma V, Jalan R. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int. 2015; 35(3): 724-34. [CrossRef]
- McGovern BH, Golan Y, Lopez M, Pratt D, Lawton A, Moore G, Epstein M, Knox TA. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007; 44(3): 431-7. [CrossRef]
- Lario M, Muñoz L, Ubeda M, Borrero MJ, Martínez J, Monserrat J, Díaz D, Alvarez-Mon M, Albillos A. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013; 59(4): 723-30. [CrossRef]
- Wiest R, Das S, Cadelina G, Garcia-Tsao G, Milstien S, Groszmann RJ. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest. 1999; 104(9): 1223-33. [CrossRef]
- Opal SM, Scannon PJ, Vincent JL, White M, Carroll SF, Palardy JE, Parejo NA, Pribble JP, Lemke JH. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999; 180(5): 1584-9. [CrossRef]
- Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003; 37(1): 208-17. [CrossRef]
- Le Roy D, Di Padova F, Adachi Y, Glauser MP, Calandra T, Heumann D. Critical role of lipopolysaccharide-binding protein and CD14 in immune responses against gram-negative bacteria. J Immunol. 2001; 167(5): 2759-65. [CrossRef]
- Ghassemi S, Garcia-Tsao G. Prevention and treatment of infections in patients with cirrhosis. Best Pract Res Clin Gastroenterol. 2007; 21(1): 77-93. [CrossRef]
- Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis. 2000; 182(2): 526-33. [CrossRef]
- Runyon BA, Morrissey RL, Hoefs JC, Wyle FA. Opsonic activity of human ascitic fluid: A potentially important protective mechanism against spontaneous bacterial peritonitis. Hepatology. 1985; 5(4): 634-7. [CrossRef]
- Bruce R, Richard M., John H., Frederic W. Opsonic Activity of Human Ascitic Fluid: A Potentially Important Protective Mechanism Against Spontaneous Bacterial Peritonitis. Hepatology. 1985; 5(4): 634-637.
- Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005; 41(3): 422-33. [CrossRef]
- Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009; 50(6): 2022-33. Erratum in: Hepatology. 2010; 51(2): 725. [CrossRef]
- Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: Relationship with their in-hospital outcome. J Hepatol. 2009; 51(3): 475-82. [CrossRef]
- Chan CC, Hwang SJ, Lee FY, Wang SS, Chang FY, Li CP, Chu CJ, Lu RH, Lee SD. Prognostic value of plasma endotoxin levels in patients with cirrhosis. Scand J Gastroenterol. 1997; 32(9): 942-6. [CrossRef]
- Guarner C, Soriano G, Tomas A, Bulbena O, Novella MT, Balanzo J, Vilardell F, Mourelle M, Moncada S. Increased serum nitrite and nitrate levels in patients with cirrhosis: Relationship to endotoxemia. Hepatology. 1993; 18(5): 1139-43.
- Bernard B, Grangé JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: A meta-analysis. Hepatology. 1999; 29(6): 1655-61. [CrossRef]
- Sandhu BS, Gupta R, Sharma J, Singh J, Murthy NS, Sarin SK. Norfloxacin and cisapride combination decreases the incidence of spontaneous bacterial peritonitis in cirrhotic ascites. J Gastroenterol Hepatol. 2005; 20(4): 599-605. [CrossRef]
- Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: A consensus document. International Ascites Club. J Hepatol. 2000; 32(1): 142-53. [CrossRef]
- Follo A, Llovet JM, Navasa M, Planas R, Forns X, Francitorra A, Rimola A, Gassull MA, Arroyo V, Rodés J. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: Incidence, clinical course, predictive factors and prognosis. Hepatology. 1994; 20(6): 1495-501. [CrossRef]
- Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Ginès P, Rodés J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999; 341(6): 403-9. [CrossRef]
- Ginés P, Rimola A, Planas R, Vargas V, Marco F, Almela M, Forné M, Miranda ML, Llach J, Salmerón JM; et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: Results of a double-blind, placebo-controlled trial. Hepatology. 1990; 12(4): 716-24. [CrossRef]
- Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, Bach J, Geier A, Purucker EA, Gressner AM, Matern S, Lammert F. Patients with acute on chronic liver failure display "sepsis-like" immune paralysis. J Hepatol. 2005; 42(2): 195-201. [CrossRef]
- Úbeda M, Muñoz L, Borrero MJ, Díaz D, Francés R, Monserrat J, Lario M, Lledó L, Such J, Álvarez-Mon M, Albillos A. Critical role of the liver in the induction of systemic inflammation in rats with preascitic cirrhosis. Hepatology. 2010; 52(6): 2086-95. [CrossRef]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008; 454(7203): 428-35. [CrossRef]
- Arroyo V, Moreau R, Jalan R, Ginès P; EASL-CLIF Consortium CANONIC Study. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J Hepatol. 2015; 62(1 Suppl): S131-43. [CrossRef]
- Piano S, Tonon M, Vettore E, Stanco M, Pilutti C, Romano A, Mareso S, Gambino C, Brocca A, Sticca A, Fasolato S, Angeli P. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. J Hepatol. 2017; 67(6): 1177-1184. [CrossRef]
- Heneka MT, McManus RM, Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci. 2018; 19(10): 610-621. [CrossRef]
- Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014; 146: 1513-1524. [CrossRef]
- Pascual S, Such J, Esteban A, Zapater P, Casellas JA, Aparicio JR; et al. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology 2003; 50: 1482-1486.
- Gandoura S, Weiss E, Ratou PE, Fassen M, Gustot T, Lemoine F, Hurtado-Nedelec M, Hego C, Vadrot N, Elkrief L; et al. Gene and exon-expression profiling reveals an extensive LPS-induced response in immune cells in patients with cirrhosis. J Hepatol 2013; 58: 936-948. [CrossRef]
- Michelena J, Altamirano J, Abraldes JG, Affò S, Morales-Ibanez O, Sancho-Bru P; et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology 2015; 62(3): 762-72. [CrossRef]
- Lozano-Ruiz B, Bachiller V, García-Martinez I, Zapater P, Gomez-Hurtado I, Moratalla A, Giménez P, Bellot P, Francés R, Such J; et al. Absent in melanoma 2 triggers a heightened inflammasome response in ascitic fluid macrophages of patients with cirrhosis. J Hepatol 2015; 62: 64-71. [CrossRef]
- Gottardi A, Gronbaek H, Saliba F, Trautwein C, Özdogan OC, Francque S, Ryder S, Nahon P, Romero-Gomez M, Van Vlierberghe H; PREDICT STUDY group of the EASL-CLIF Consortium. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020; 73(4): 842-854. [CrossRef]
- Clària J; Stauber R; Coenraad M; Moreau R; Jalan R; Pavesi M; Amorós À; Titos E; Alcaraz J; Oettl K. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute on chronic liver failure. Hepatology. 2016; 64(4): 1249-1264. [CrossRef]
- Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jansen C, Jimenez C. PREDICT STUDY group of the EASL-CLIF CONSORTIUM. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021; 74(5): 1097-1108. [CrossRef]
- Moreau R, Clària J, Aguilar F, Fenaille F, Lozano JJ, Junot C, Colsch B, Caraceni P, Trebicka J, Pavesi M; et al. Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. Journal of Hepatology. 2020; 72(4): 688-701. [CrossRef]
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002; 420(6917): 885-91. [CrossRef]
- Fernández J, Acevedo J, Wiest R, Gustot T, Amorós A, Deulofeu C; et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut 2018; 67(10): 1870–1880. [CrossRef]
- Clària J, Moreau R, Fenaille F, Amorós A, Junot C, Gronbaek H, Coenraad MJ, Pruvost A, Ghettas A, Chu-Van E; CANONIC Study Investigators of the EASL Clif Consortium, Grifols Chair and the European Foundation for the Study of Chronic Liver Failure (EF Clif). Orchestration of Tryptophan-Kynurenine Pathway, Acute Decompensation, and Acute-on-Chronic Liver Failure in Cirrhosis. Hepatology. 2019; 69(4): 1686-1701. [CrossRef]
- Bernsmeier C, van der Merwe S, Périanin A. Innate immune cells in cirrhosis. J Hepatol. 2020; 73(1): 186-201. [CrossRef]
- Piano S, Schmidt HH, Ariza X, Amoros A, Romano A, Hüsing-Kabar A, Solà E, Gerbes A, Bernardi M, Alessandria C; EASL CLIF Consortium, European Foundation for the Study of Chronic Liver Failure (EF Clif). Association Between Grade of Acute on Chronic Liver Failure and Response to Terlipressin and Albumin in Patients With Hepatorenal Syndrome. Clin Gastroenterol Hepatol. 2018; 16(11): 1792-1800. [CrossRef]
- Fernández J, Clària J, Amorós A, Aguilar F, Castro M, Casulleras M, Acevedo J, Duran-Güell M, Nuñez L, Costa M. Effects of Albumin Treatment on Systemic and Portal Hemodynamics and Systemic Inflammation in Patients With Decompensated Cirrhosis. Gastroenterology. 2019; 157(1): 149-162. [CrossRef]
- Bhanji R, Montaño-Loza A., Watt K. Sarcopenia in Cirrhosis: Loking Beyond the Skeletal Muscle Loss to See the Systemic Disease. Hepatology. 2019; 70(6): 2193-2203. [CrossRef]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010; 39(4): 412-23. [CrossRef]
- Kazemi-Bajestani SM, Becher H, Ghosh S, Montano-Loza AJ, Baracos VE. Concurrent depletion of skeletal muscle, fat, and left ventricular mass in patients with cirrhosis of the liver. J Cachexia Sarcopenia Muscle. 2016; 7(1): 97-9. [CrossRef]
- Iizuka K, Machida T, Hirafuji M. Skeletal muscle is an endocrine organ. J Pharmacol Sci. 2014; 125(2): 125-31. [CrossRef]
- Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016; 65(6): 1232-1244. [CrossRef]
- Kim TN, Park MS, Lim KI, Choi HY, Yang SJ, Yoo HJ, Kang HJ, Song W, Choi H, Baik SH, Choi DS, Choi KM. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: The Korean Sarcopenic Obesity Study. Clin Endocrinol (Oxf). 2013; 78(4): 525-32. [CrossRef]
- Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, de Vos M, Papadimitriou K, Thorburn D, O'Beirne J; et al. Malnutrition and sarcopenia predict post– liver transplantation outcomes independently of the Model for End-Stage Liver Disease score. J Cachexia Sarcopenia Muscle 2017; 8: 113-121. [CrossRef]
- Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, Rodés J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999; 30(5): 890-5. [CrossRef]
- Hui AY, Chan HL, Leung NW, Hung LC, Chan FK, Sung JJ. Survival and prognostic indicators in patients with hepatitis B virus-related cirrhosis after onset of hepatic decompensation. J Clin Gastroenterol. 2002; 34(5): 569-72. [CrossRef]
- Odeh M, Sabo E, Srugo I, Oliven A. Relationship between tumor necrosis factor-alpha and ammonia in patients with hepatic encephalopathy due to chronic liver failure. Ann Med. 2005; 37(8): 603-12. [CrossRef]
- Li W, Li N, Wang R, Li Q, Wu H. Interferon gamma, interleukin-6, and -17a levels were correlated with minimal hepatic encephalopathy in HBV patients. Hepatol Int. 2015; 9(2): 218-23. [CrossRef]
- Shawcross DL, Wright G, Olde Damink SW, Jalan R. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis. 2007; 22(1): 125-38. [CrossRef]
- Montoliu C, Piedrafita B, Serra MA, del Olmo JA, Urios A, Rodrigo JM, Felipo V. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J Clin Gastroenterol. 2009; 43(3): 272-9. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





