1. Introduction
Understanding how biodiversity affects ecosystem functioning is a fundamental aim in an ecological research but demanding further investigation (Shen, 2016; Yi et al., 2020). There has been increasing interest in the relationship between functional diversity and carbon storage in the context of global climatic change over the past few decades, especially in natural multispecies forest ecosystems (Ding & Nunes, 2014). As both biodiversity and carbon storage are threatened by deforestation and climate change, it is imperative to understand the relationships between species diversity and carbon storage in tropical forest, which helps to inform conservation policies intended to mitigate GHG emissions (IPCC, 2023). Human activities, principally through emissions of GHG, have unequivocally caused global warming, with global surface temperature reaching 1.1 °C above 1850-1900 in 2011-2020 (IPCC, 2023). Adopting the trait-based approach, the relationship between species diversity and AGC, can be examined (Mensah et al., 2021). Studies have linked plant functional traits to carbon storage, since tree species with different characteristics have distinguishing abilities to capture, store, and release biomass carbon (Shen, 2016). Because biomass carbon is an important component of forest stand productivity, the relationship between biomass carbon and biodiversity can also be assimilated to the one of biodiversity and ecosystem function (Lasky et al., 2014).
Two non-mutually exclusive hypotheses have been proposed to explain the relationships between biodiversity and ecosystem functions (Yuan et al., 2018). These are the niche complementarity and mass ratio hypothesis. The concept of niche complementarity is frequently invoked to account for increased biomass yield and productivity within diverse ecosystems. Nonetheless, higher biomass can also arise due to a greater possibility of species, or specific traits, exhibiting robust reactions to resources or exerting significant impacts on the ecosystem (Reich et al., 2004). Some researchers suggest that both theories could influence the impact of diversity on ecosystem functions, particularly through competition and resource partitioning among species (Carroll et al., 2011; Mensah et al. 2018). Research support both the selection effects and niche complementarity hypothesis on forest ecosystems functioning (Rodríguez-Hernández, 2021). Sasaki & Lauenroth (2011) provide support for the selection effects hypothesis, while Michalet et al (2021) and Xu et al (2022) offer evidence in favor of niche complementarity hypothesis. The niche complementarity hypothesis suggests that high species diversity or functional diversity can enhance ecosystem functions, and hence biomass productivity increases through resource-use efficiency by interacting individuals due to the niche partitioning or facilitation (Mensah et al., 2018; Zhang et al., 2021).
Selection effects highlight the implication of dominant species in maintaining ecosystem function and enhancing the relationship between biodiversity and ecosystem function. This relationship is evident when the possibility of dominant species occurring increases with greater tree species diversity, often due to competitive interactions (Mensah et al., 2018). Both species richness (niche complementarity) and the community-weighted mean (CWM) of trait values (selection effects) support the beneficial effects of forest structural diversity on above-ground biomass (AGB); however, the relative importance of these effects differed depending on the type of forest (Noulèkoun et al., 2024). Also, the availability of resources (water and nutrients) limits the relationships between forest diversity and AGB. This knowledge can offer new understandings into the role of functional traits as key determinants of the effects of forest diversity on AGB in tropical forests (Mensah et al. 2018).
There is growing support for the idea that functional traits are indicative of the unique functional roles that species coexist in a given community, and how they are linked to differences in their ecological niches. Thus, functional traits may be more effective at predicting the relationship between biodiversity and productivity compared to taxonomic diversity (Mensah et al., 2016; Weisser et al., 2017; Yi et al., 2020). These indicated that functional diversity may be more helpful to maintaining ecosystem multi-functionality than species diversity (Kong et al., 2023). Therefore, understanding how the relative contributions of these two mechanisms change according to the context may inform about new strategies for the forest management and biodiversity conservation.
Findings shows, AGC had significant positive correlation with species richness and tree density (Mensah et al., 2021). Similarly, Mengistu et al. (2021) found that linking diversity to AGC provides prediction of ecosystem functions and the relationship is direct. The positive effects of species richness on AGC are significantly explained by non-mutually exclusive mechanisms such as complementarity and selection effects where resource availability and environmental filtering play a central role (Mensah et al., 2023). As such, AGC can increase due to a few highly productive and dominant species (selection effects), or a better performance of coexisting species through increased resource use efficiency (niche complementarity) (Mensah et al., 2023). For example, Mensah et al.(2023) found that shifting diversity-carbon relationship at local scale can be explained by the effects of stand structural complexity and large-size tree. On the other hand, there is also negative relationship between diversity and carbon, this is due to the influence of diversity on carbon is scale dependent and environmental variation (Sullivan et al., 2017).
In addition, environmental factors also have a significant influence on the relationship between ecosystem functioning and diversity (Mensah et al., 2023); however, if local environmental factors are ignored in large-scale studies, some significant connections may go unnoticed (Gourlet-Fleury et al., 2011; Mensah et al., 2023; Shen et al., 2016). Because, the shift in species richness-AGC relationships under contrasting environmental conditions suggests that larger scale patterns of diversity and carbon are indeed regulated by environmental conditions, because species distributions, composition and growth performance are governed by adaptations to physical conditions of the environment (Mensah et al., 2023). For example, Ma et al. (2010) found that large-scale environmental variation had significant effects in regulating the relationship between species diversity of grasses and above-ground productivity. From this, environmental variables are an important predictors of carbon and diversity-carbon relationship are scale dependent and environmental variation effects (Sullivan et al., 2017).
Among the various traits used to study the predictive role of species diversity for AGC and to clearly elucidate their relationships, forest ecologists commonly use tree maximum height (Hmax), wood density (WD) and specific leaf area (SLA). Also, functional diversity and community weighted mean of Hmax, WD and SLA are important traits used as predictor of AGC (Mensah et al., 2021). The thing is these attributes of functional traits are linked to hypotheses of complementarity and selection effect in ecological studies, based on the assumption that plant functional traits can explain differences in fitness and niches, resulting in competitive exclusion and coexistence, respectively (Cadotte, 2017; Carroll et al., 2011). This shows that, both complementarity and selection are complementary and can partly result from either trait dissimilarity or community weighted mean (Mensah et at., 2024). Accordingly, it has been shown that both complementarity and selection effects are described as non-mutually exclusive mechanisms interacting to regulate ecosystems functioning (Fargione et al., 2007; Mensah et al., 2018; Noulèkoun et al., 2024). Therefore, examining the relationship between traits and their diversity in different systems, including variations within species, is now essential in understanding function (Cadotte, 2017; Laughlin, 2014; Violle et al., 2012). Because, these traits provides a proxy for the potential tree growth performance, (Yuan et al., 2018), species tolerance to climate change (Poorter et al., 2015) and categorize species into fast growth (acquisitive species) and slow growth (conservative species) (Sainge et al., 2019).
Ecosystem functioning is related to taxonomic, functional diversity and functional dominance (Zhang et al., 2021). However, there is the lack of studies that have examined the relative importance of different metrics of biodiversity in relation to AGC levels in the moist Afromontane forest of Southwest Ethiopia, specifically within the YCFBR. Additionally, the influence of topographic variables (such as slope, aspect, and elevation) and anthropogenic disturbances on these relationships has not been thoroughly investigated at a country level. Furthermore, while it is widely recognized that both biodiversity and carbon storage are threatened by deforestation and climate change, there is limited knowledge about the quantitative and causal relationships between forest biodiversity and AGC (Brockerhoff et al., 2017). Therefore, it is crucial to understand the relationships between species diversity and AGC in the YCFBR, to effectively mitigate GHG emissions resulting from deforestation, primarily driven by anthropogenic impacts in the region. Based on this motivation, the study aimed to explore the response of AGC to species richness, functional diversity, and functional dominance, and to investigate the potential mediating effects of functional diversity and functional dominance on the relationship between species richness and AGC in the YCFBR. By addressing these research objectives, the study seeks to improve our understanding and prediction of the consequences of biodiversity loss on ecosystem functioning through the use of traits-based diversity measurement.
2. Materials and Methods
2.1. Sampling Design
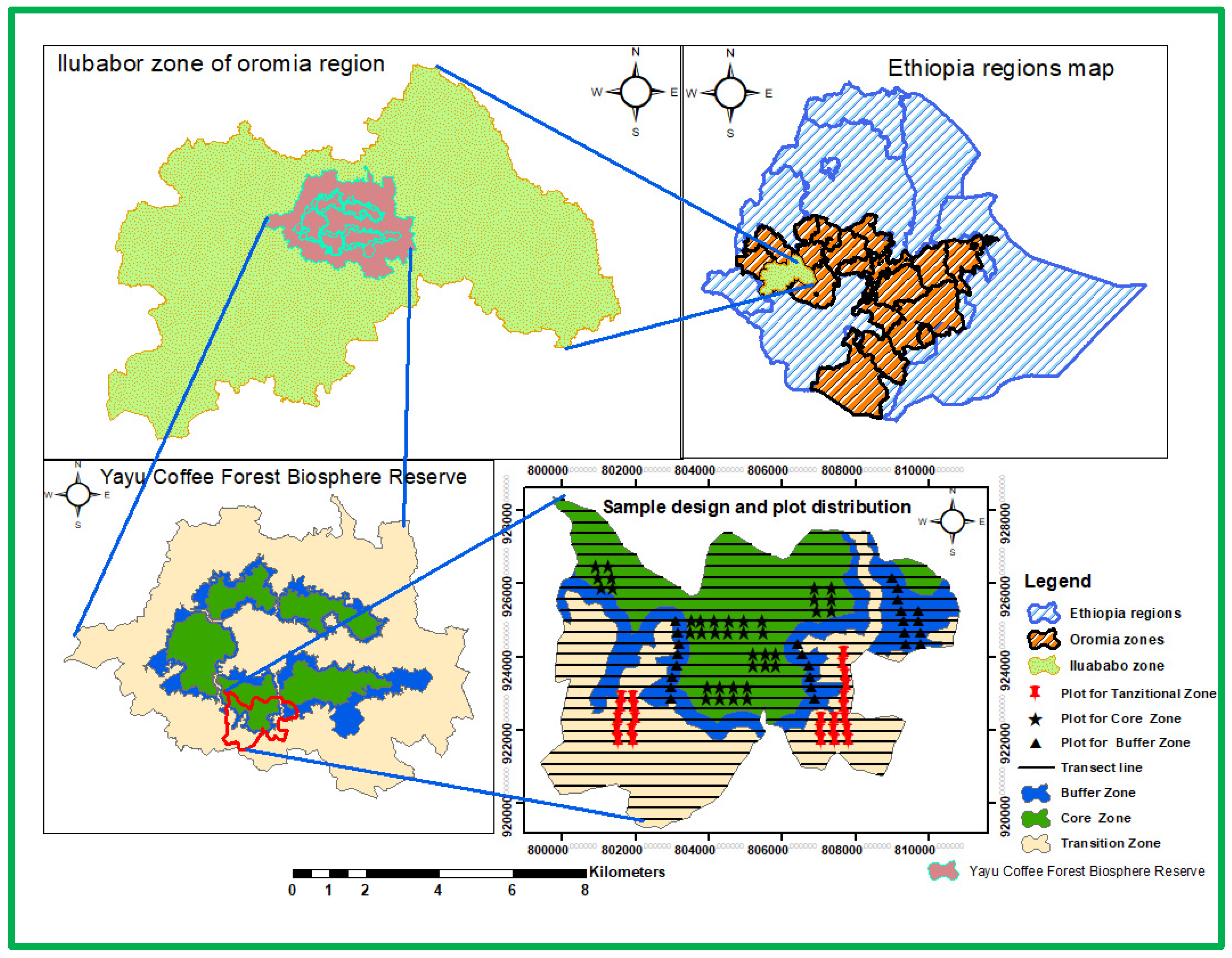
The study was conducted at Yayu Coffee Forest Biosphere Reserve (YCFBR) in Southwest Ethiopia. Multistage sampling techniques were used to select representative samples from the core, buffer, and transitional zones of the reserve following (Jeldu et al., 2023). Yayu and Hurumu districts were purposefully selected because they contain these three zones, unlike other districts. From these districts, Wabo and Gaba kebeles were selected in the final stage using the same criteria as the district selection (
Figure 1).
Transect lines were distributed systematically in the core, buffer, and transitional zones. The lines were oriented northwards and established following a random start. Typical plot sizes used in forest inventory assessments are 200 m2, 400 m2, and 500 m2, but any size could possibly be used (UNFCCC, 2015). Following this, we designed a plot size of 20 m × 20 m (400 m2) to collect vegetation data from the core and buffer zones, while a 30 m × 30 m (900 m2) plot size was designed for the transitional zone (Jeldu et al., 2023). A total of 90 plots were distributed, with a 300 m gap between transect lines and sample plots.
Figure 1.
Map of the study area (Yayu Coffee Forest Biosphere Reserve), sample design and plot distributions within the Zones and transect line. Source: Jeldu et al. (2023)
Figure 1.
Map of the study area (Yayu Coffee Forest Biosphere Reserve), sample design and plot distributions within the Zones and transect line. Source: Jeldu et al. (2023)
2.2. Vegetation and Environmental Data Collection
The diameter at breast height (DBH) and height (H) of trees and shrubs with a DBH greater than 2.5 cm were measured. In two perpendicular directions, the DBH (cm) was measured and its average value was recorded. If multi-stemmed individuals had branching below the DBH, each stem was measured separately. Additionally, all woody lianas with a DBH greater than 1 cm at 1.30 m from the rooting point were recorded to assess diversity (Snowdon et al., 2002). For coffee plants, the diameter at stump height (40 cm height) was measured in two perpendicular directions and the average value was taken. In the case of multi-stemmed coffee plants, each stem in a single plant was measured independently, and its equivalent diameter (d 40 cm) was calculated (Snowdon et al., 2002). Plant identification was done at the National Herbarium (ETH), Addis Ababa University, Ethiopia.
Disturbance intensity levels were measured using indicators such as grazing intensity, number of tree stumps, and coffee dominance. Plots were categorized into four disturbance intensity levels: undisturbed, slightly disturbed, moderately disturbed, and disturbed forest (Wekesa et al., 2016). Data on elevation, aspect, slope, and geographical location of each plot was collected. Elevation and aspect were measured using an altimeter, while slope was measured using a Sunto clinometer and classified into five levels as: flat (0%–3%), gentle (3%–8%), moderate (8%–25%), hilly (25%–40%), and steep (> 40%). Aspect was classified into eight sub-directions as: North (N) for direction readings between (0-22.5°) and Northeast (NE) if it reads between (337.5-360°), Northwest (NW) if it reads from 22.5° to 67.5°, East (E) if it reads between 67.5 and 112.5°, Southeast (SE) if it reads between 112.5 and 157.5°, South (S) if the reads are between 157.5 and 202.5°, Southwest (SW) if it is between 202.5 and 292.5°, and Northwest (NW) if it is located between 292.5 and 337.5° (Hu et al., 2015).
2.3. Quantification of Functional Diversity, Functional Dominance and Taxonomic Diversity
For the functional diversity estimation, traits relevant to the life-history strategies of the species and ecosystem function were selected (McGill et al., 2006). These were WD, SLA and Hmax. The SLA and Hmax were extracted from the TRY database (
www.try-db.org) (Kattge et al., 2020), whereas the WD was obtained from Ethiopia’s Forest Reference Level Submission to the UNFCCC (EFRL, 2017). Average values for the traits was used for multiple values available for a single species. Similarly, the average genus values was used when trait values were missing for a given species (Mendes et al., 2018; Mensah et al., 2016; Montesinos-López et al., 2018). Likewise, the mean values at the family level was used when genus data for the traits was missing. Additionally, a plot-level average values was used for missing family (Jeldu et al., 2023; Mensah et al., 2016).
For each individual plot, functional richness (FRic), functional evenness (FEve), functional divergence (FDiv) and functional dispersion (FDis) were estimated for functional diversity metrics (Zhang et al., 2021). A plot-based community-weighted mean (CWM) of traits was estimated for functional dominance metrics using the mean of the functional trait weighting by species' relative abundance for each plot (Cavanaugh et al., 2014). Then, CWM was estimated for Hmax, SLA and WD. Standardization was performed for multi-trait functional diversity indices, because, this study’s traits data shows differences in orders of magnitude and scale of measurement. Whereas functional dominance (a single functional trait index) was calculated without standardization for CWM (Teshome et al., 2020).
2.4. Estimation of Above-Ground Carbon
The study used a generic multispecies biomass equation developed by Chave et al. (2014) to calculate AGC (
Table 1). For
Diospyros abyssinica trees, a species-specific equation developed by Damena and Teshome (2019) was perfomed (
Table 1). Total AGC was calculated by combining the above-ground biomass (AGB) of individual trees and coffee plants. The carbon fraction of dry biomass was applied to calculate AGC (IPCC, 2006). Then AGC was scaled up from tree to plot level and extrapolated to megagrams of carbon per hectare (Mg C ha
-1).
2.5. Statistical Analysis
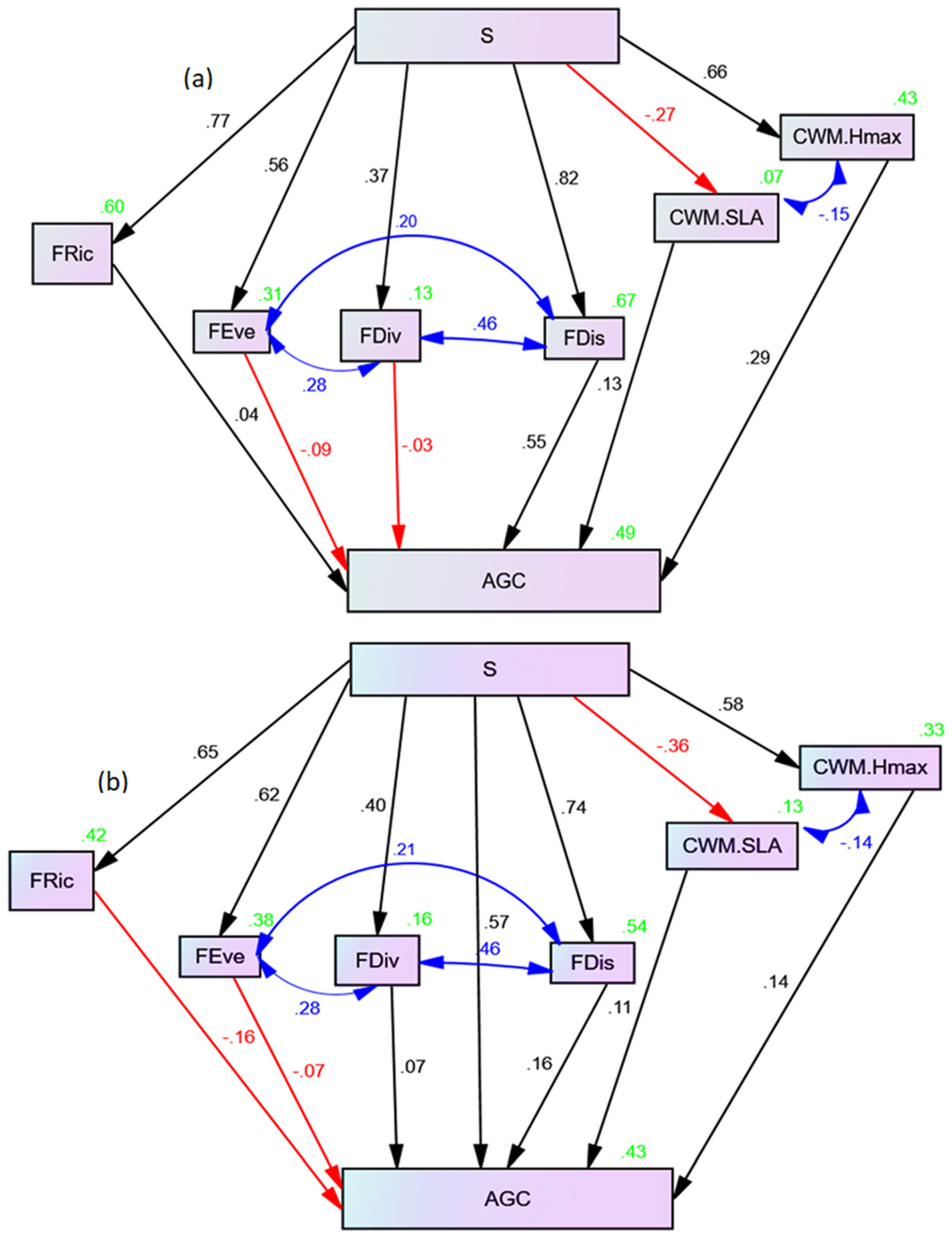
The structural equation models (SEM) was used to test the effects of species diversity, functional diversity and functional dominance on AGC. For this, two separate SEM were constructed: (1) a full mediation model (when diversity effects on AGC are fully transmitted through functional diversity and functional dominance metrics) and (2) a partial mediation model (when both direct and indirect diversity effects on AGC are transmitted through species richness, functional diversity and functional dominance). The SEM was started by fitting a full mediation i.e., all paths minus the direct path between species richness and AGC. Using the path modification indices, the model fitting was improved by performing a step-by-step inclusion of additional significant paths leading to partial mediation of SEM. Independent variables were selected using stepwise procedures to avoid over-fitting the model. The comparison between the full mediation and partial mediation models were performed based on the goodness-of-fit statistics (Zürich et al., 2005). For this, only functional diversity and functional dominance measures that significantly influenced AGC were considered for SEM fitting. All SEM were fitted using the lavaan package (Rosseel, 2012) and the overall fit of the models was evaluated using chi-square (P > 0.05 indicates an absence of significance), the comparative fit index (CFI), the goodness-of-fit index (GFI) and the standardized root-mean-square residual (SRMR) (Mensah et al., 2021). All the selected functional diversity indices was calculated by the FDiversity software (Casanoves et al., 2011).
The mixed effects model (MEM) was fitted with functional diversity and functional dominance as predictors of AGC to test the combined effects of functional diversity, and functional dominance metrics. By using the package ‘lme4’ (Bates et al., 2015), the MEM was run, and selection of variables was done by ‘backward selection’ using ‘cAIC4’package. The significant effect of fixed factors was determined using “lmerTest” package (Kuznetsova et al., 2017). Also, regression model was used to test the effects of disturbance, elevation, aspect, and slope (the fixed factors) on AGC (the response variable). As a result, only the elevation showed slightly significant effects on the AGC and considered for further analysis. For those variables that showed no significant effect on the AGC, further analyses, i.e., a multiple linear regressions model, were used to test their effects on the AGC (Bates et al., 2015). Then, we tested for multicollinearity by means of Breusch-Pagan tests, the Durbin-Watson autocorrelation statistic, and the variance inflation factor. All statistical analyses were performed using R statistical software (v4.1.2; R Core Team, 2021).
4. Discussions
4.1. The Predictive Role of Species Diversity Contributions for the Above-Ground Carbon (AGC)
Full mediation model showed species richness mediated via FRic had a positive and significant effect on AGC as shown under result sections on the
Table 2, whereas, species richness effects mediated via CWM.Hmax on AGC was significant and positive effects. This implied that the relationship between plant diversity and AGC is not independent; there is the functional diversity and dominance is responsible for the observed relationship between the fixed factors (diversity) and response variable (AGC). Furthermore, the findings of this study have important implications for biodiversity conservation and tropical biosphere reserve management by considering the contributions of species richness for AGC enhancement, which is consistence with the previous findings (Gautam and Mandal, 2016; Yuan et al., 2018). Therefore, to effectively manage and enhance AGC stock, it is crucial to consider and understand the role of this mediator (species richness).
This study results further supports that both complementarity and selection effects are not exclusively affecting AGC (Mensah et al., 2020). Species diversity richness boosts AGC stock through effects of both functional diversity and functional dominance, partly because these diversity components are based on specific functional traits, which would reflect functional differences among the species (Fei et al., 2017; Parron et al., 2019). This finding can also be due to the fact that increased species richness indirectly accounted for differences among species, in terms of ecological niche and resource use efficiency. Besides to this, various studies in subtropical forests have reported biomass productivity have the same patterns with increasing species diversity richness i.e., AGC increased with increasing species diversity richness (Gautam and Mandal, 2016; Yuan et al., 2018) and tropical forests (Mensah et al., 2017) have also reported increases in biomass productivity with increasing species diversity richness. This partial mediation model suggest that understanding the mechanisms controlling forest carbon storage is crucial to support “nature-based” solutions for climate change mitigation.
It is usually expected that increasing species diversity would increase AGC storage, because higher taxonomic diversity would enhanced higher stem density and forest ecosystem productivity (Mensah et al., 2020). The positive effect of species diversity can also be explained through the benefits of plant-plant interactions such as facilitation, where by some species could enhance soil fertility (by fixing nitrogen) for the productivity of other species (Mensah et al., 2020). This can be suggested that mixed species communities of forests are more productive than homogeneous species diversity stands. Also this may be increasing species richness increases the probability of inclusion of highly productive and dominant species which had consistence with the previous findings (Mengistu et al., 2021; Teshome et al., 2020).
This study findings of moist Afromontane forest promotes both the positive and negative species richness with AGC relationship, however, there was also inverse effect existed. For example, Mengistu et al., (2021) reported the positive effects of species richness on AGC. Furthermore, various studies have reported null or negative relationships between AGC and species diversity in forest ecosystems (Alfaro et al., 2014). These inconsistencies and provocative results pointed out that the effects of species diversity on AGC might be vary with other factors such as forest sites conditions, topographic factors and anthropogenic factors, as well as the dimension of the diversity metrics performed (Wu et al., 2016; Mengistu et al., 2021).
4.2. Functional Diversity and Functional Dominance Effects on Above-Ground Carbon (AGC)
Conservation efforts aimed at preserving and increasing functional diversity are necessary for the protection and maintenance of the carbon stock in the moist Afromontane forest of the YCFBR, which may help improve biomass carbon storage. This is because factors such as functional richness, evenness, divergence, and dispersion have been found to play a significant role in enhancing biomass carbon storage in this ecosystem. This implies that in order to safeguard and sustain the carbon stock in the biosphere reserve, conservation initiatives must preserve and improve functional diversity. These findings are consistent with similar studies conducted before, indicating the importance of functional diversity in promoting AGC in forest ecosystems. For instances, FRic measures the amount of trait space occupied by the community (Clark et al., 2014); thus increase the carbon storage because species with various traits would more efficiently use the resources for growth, which confirmed the niche complementarity effects on the AGC (Mensah et al., 2020). Policy recommendations can be made based on these findings. For example, if FRic has a significant positive effect on ecosystem services, it might be recommended to prioritize forest management practices that enhance forest resilience. Similarly, if FDiv has a significant positive effect, promoting biodiversity conservation could be encouraged. On the other hand, if FDis has a significant negative effect, it might suggest the need for strategies to reduce disturbances in forest ecosystems.
Also, studies in Dindin dry Afromontane forest (Mengistu et al., 2021), and Mistbelt forests (Mensah et al., 2020) found that functional diversity metrics, such as FRic, FEve, and FDis, significantly predict variation in AGC. Positive relationships between FDis and AGC were found in previous studies (Ziter et al., 2013), consistent with our current findings. Similarly, negative effects of FDiv on biomass carbon were observed in subtropical forests in China (Wong et al., 2015; Xin et al., 2018) and temperate mixed forests (Yuan et al., 2018).
Additionally, the study revealed that, AGC increased as the CWM values increased, which indicates a higher prevalence of species with traits that prioritize acquiring resources (such as taller maximum plant height, lower wood density, and earlier growth initiation), rather than traits that prioritize resource conservation. Although contradicting findings was found as selection effects were more important for the AGC stock in tropical forests (Ruiz-Jaen et al., 2011). Furthermore, Wasof et al. (2018) found that the abundance and traits of the most dominant species matter more than taxonomic, functional or phylogenetic diversity in explaining its biomass, supporting the ‘biomass ratio hypotheses’ and ‘diversity hypothesis’ had a negative impact on biomass. These contrary results was obtained due to the methodology and weighting variables varied among the authors. For instance, Yuan et al. (2018) and Phillips et al. (2019) used basal area and biomass as weighting variables for functional dominance metrics, whereas in this study, the species relative abundance was used as weighting variable. From this, relationship between CWM of traits and the ecosystem function of interest could depend on the weighting variable accounted. Consequently, the magnitudes effects of functional diversity and functional dominance contributions for biomass carbon also varied. This is because of selection effects was strongly transmitted through specific Hmax, which supports the importance of forest vertical stratification for species diversity-AGC relationship. The AGC increased with CWM values that reflect greater dominance of species exhibiting acquisitive traits rather than conservative traits which is consistent with Ariel et al. (2021). For instance, Shen et al. (2016) found that, dominance and diversity explained 34% and 32% of variation in carbon storage, whereas the environments explained 26-44% of variation in dominance and diversity. This showed that the proportions of variation in carbon storage explained by dominance and diversity were improved after considering environments (Mensah et al., 2023).
The CWM.WD combined with CWM.Hmax implied that integrating biodiversity into ecosystem ecology and assessing both traits and diversity improves our mechanistic understanding of biotic effects on ecosystems (Hong et al., 2021). The positive effects of biodiversity were mainly driven by interspecific complementarity and that these effects increased over time in both ambient and manipulated environments (Hong et al., 2021). This showed that biodiversity conservation as a key strategy for sustainable ecosystem management in the face of global environmental change.
Functional dominance effects varied depending on the functional trait used (Sainge et al., 2019; Mensah et al., 2020). For example, WD and SLA are associated with population persistence, species tolerance to climate change, and they are used to categorize species as fast or slow growth and resistance to disturbances (Sainge et al., 2019). This might be because of leaf area is crucial for the radiant energy intercepted by the plant and facilitate the exchange of CO2 and H2o between foliage and atmosphere (Mensah et al., 2020). Thus, mass ratio hypothesis are suggesting ecosystem functions at a given time is mainly determined by the traits of most dominant species (Grime, 1998). Also, it was revealed, Hmax have the greatest impact on forest AGC (Mensah et al., 2020). Because disturbances do not occur in isolation and the effect of multiple drivers on an ecosystem must be considered.
As a new message, understanding the relative contributions and interaction effects of these mechanisms on changes in forest AGC can provide insights for developing new strategies in forest management and conserving plant diversity. This hypothesis proposes that selection effects (mass ratio hypothesis), are more influential than complementarity effects (niche differentiation), in terms of biomass productivity. However, it is important to note that these mechanisms are not mutually exclusive and both play a role in forest ecosystem function. Tree diversity was positively associated with a combination of mass ratio and niche complementarity effects, but the relative importance of these effects depended on the trait and range of environmental conditions (Lammerant et al., 2023).
4.3. Topographic Variables and Site Level Disturbance Effects on Above-Ground Carbon
Integrating biodiversity into ecosystem ecology and assessing both traits and diversity improves our mechanistic understanding of biotic effects on ecosystems. Thus, the results suggest that elevation and species richness are important predictors of the AGC, while aspect and disturbance do not have a significant relationship with AGC in this analysis. Also consistently to previous findings, here slope had significant influence, and contributed for AGC variations, evidencing that differences in carbon stocks can result from topological constraints (Mensah et al., 2016; Mensah et al., 2020). Because, the influence of slope can be seen as prior impacts of environment on resources availability and AGC is intrinsically related to wood and biomass production, which in turn affect forest ecosystem services dynamics (Clark et al., 2014; Durán et al., 2015; Mensah et al., 2016). This informed us, tree growth and biomass production can be potentially reduced at higher slope sites. Because, steeper slope speed up nutrients and water run-off and constrain trees, as consequence of nutrient and moisture stress there, which has consistence with Clark et al. (2014). Whereas, flat and gentle slope sites would allow for more water and relevant resources availability for tree growth and biomass productivity, facilitating species would likely respond positively (Mensah et al., 2016; Wu et al., 2014).
Our findings detected significant influences of elevation on biomass carbon stock, which is in consistence with Cavanaugh et al. (2014), but in contrast with (Mensah et al., 2016), who reported a lack of significant relationships between altitude and forest carbon stock. In addition, previous studies found that, forest biomass carbon increased up to a certain a altitudinal points, as suggested up to 3000 meters above sea levels (m.a.s.l.) and then declined (Ensslin et al., 2015; Singh et al., 1994). The significant influence of altitude on forest biomass was due to altitudinal ranges of our study covered greater ranges, which have been considerable enough to detect substantial variation on growth performance (Teshome et al., 2020) and ultimately AGC (in our cases up to 2600m.a.s.l). However, in contrary to our findings, effects of altitude on forest biomass was unseen due to smaller altitudinal range, which is not considerable enough to detected the significant variation on forest carbon stocks (Mensah et al., 2016).
Regarding the effects of disturbance relations with AGC, the findings showed significant and negative relationship with AGC. Consistently, previous findings also showed AGC in highly disturbed plots was lower than plots with medium and low disturbance levels (Yuan et al., 2018), which is in line with the negative relationship between disturbance and AGC found in the current study. These all detected and visible significant effect of topographic variables and anthropogenic factors confirmed that forest ecosystem productivity in general and AGC in particular, are environment-structured, which is similar with the previous findings (Mensah et al., 2016; Wu et al., 2014). This helps to underscore the importance of considering environmental factors and disturbances in understanding and predicting AGC levels in forest ecosystems.