Submitted:
29 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
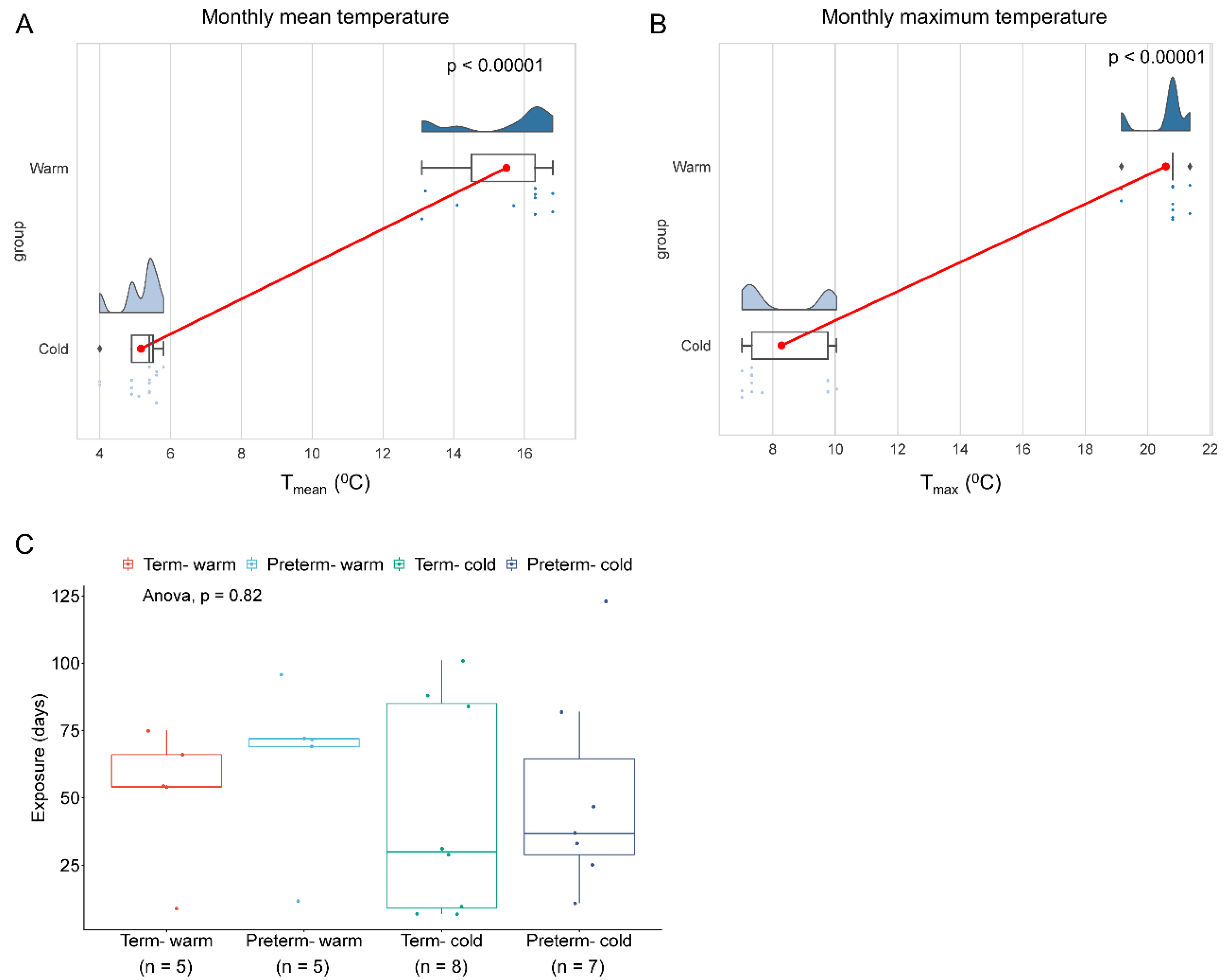
2.1. Study Subjects and Temperature Exposures
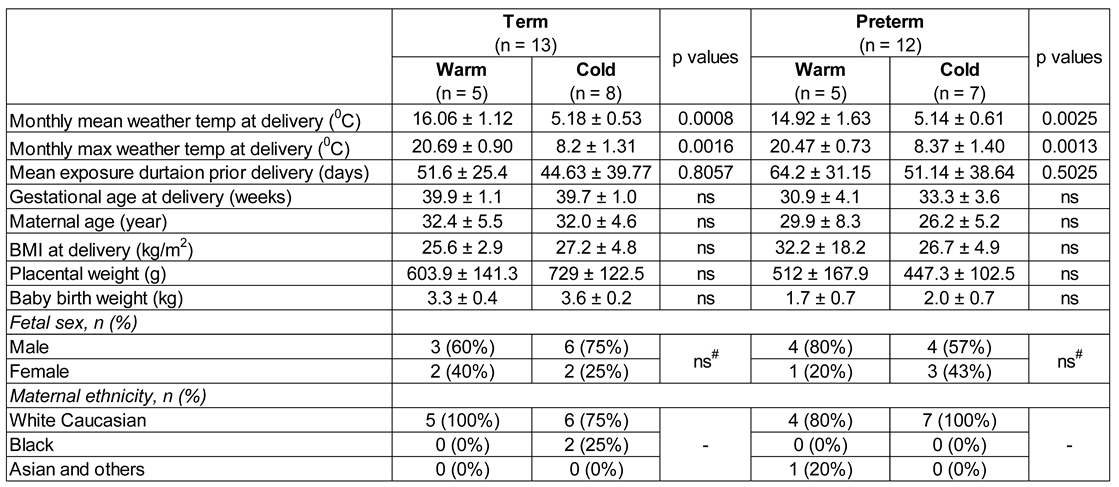 |
2.2. Warm Environmental Temperature Alters Placental Transcriptome in Preterm Birth
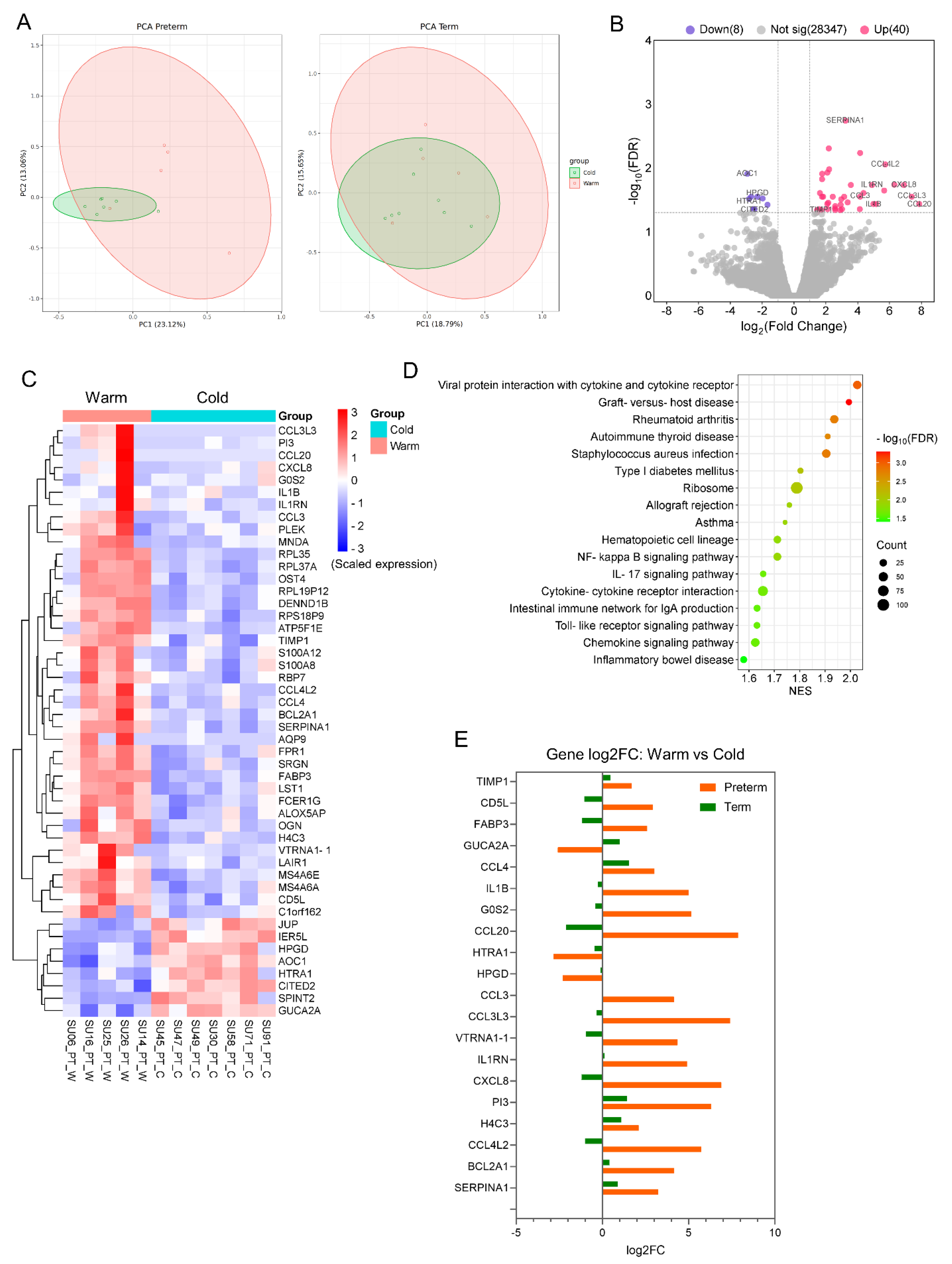
2.3. Warm Environmental Temperature Enriches Inflammatory and Immune Cells and Their Functions in Preterm Placenta
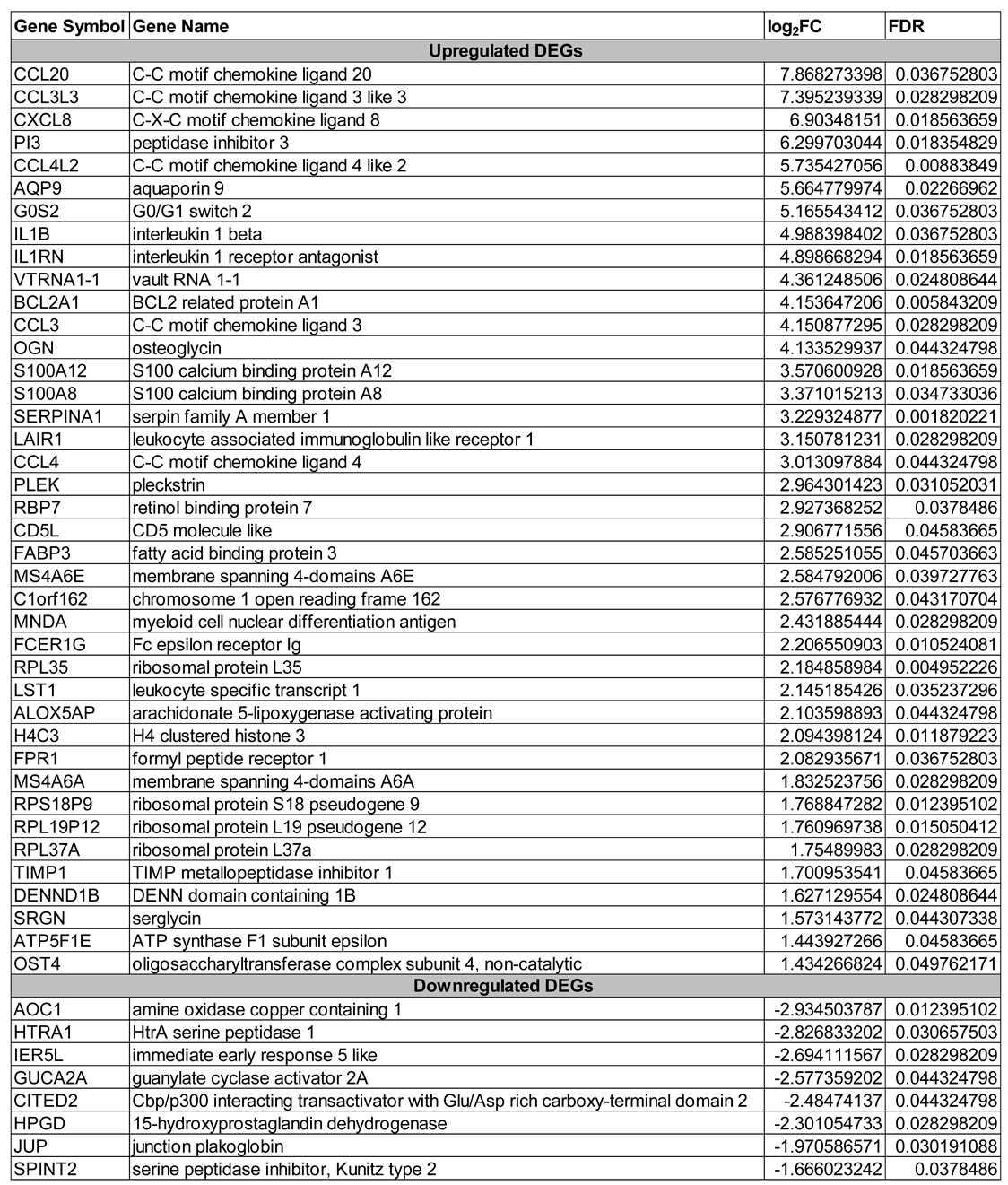 |
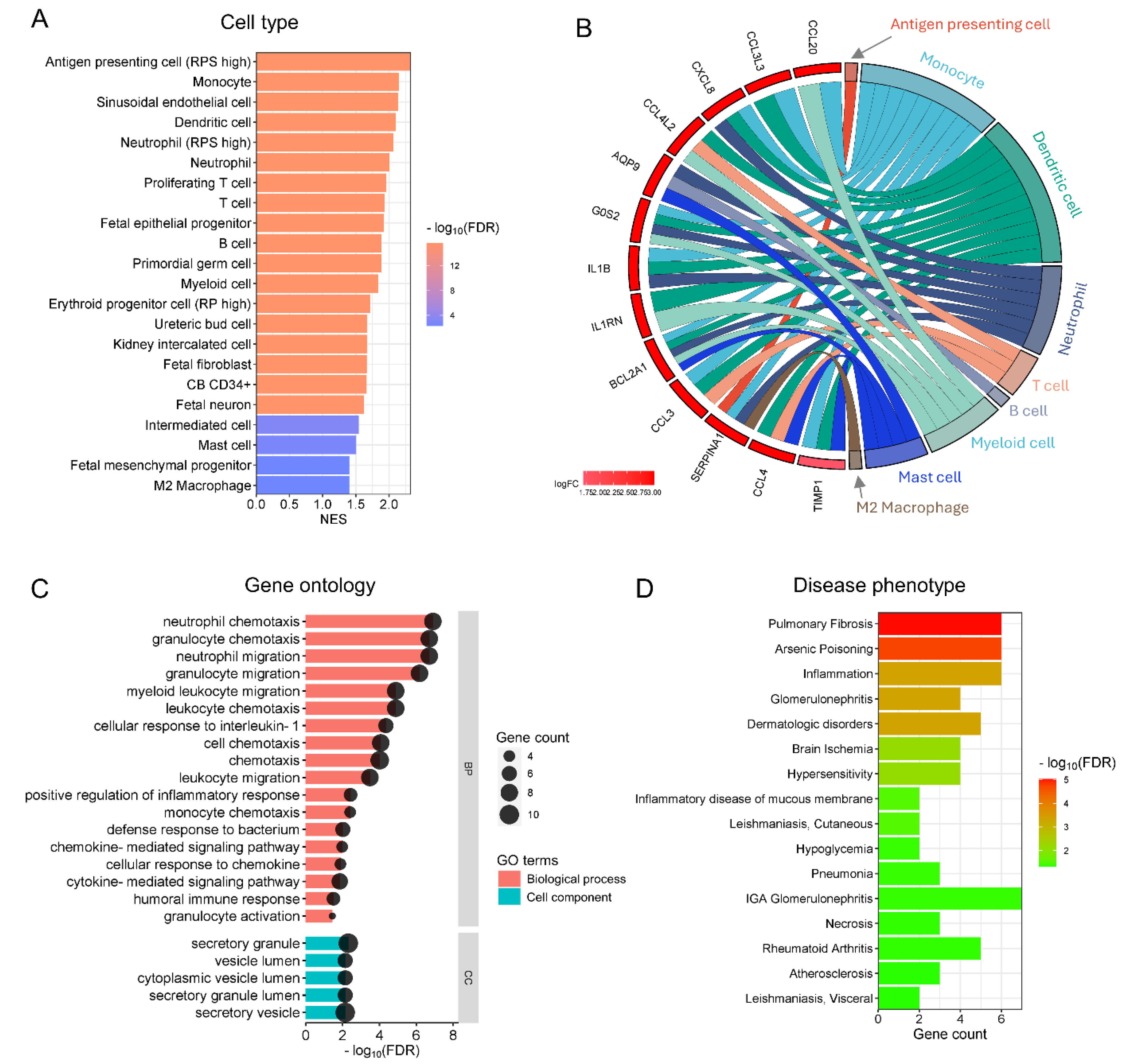
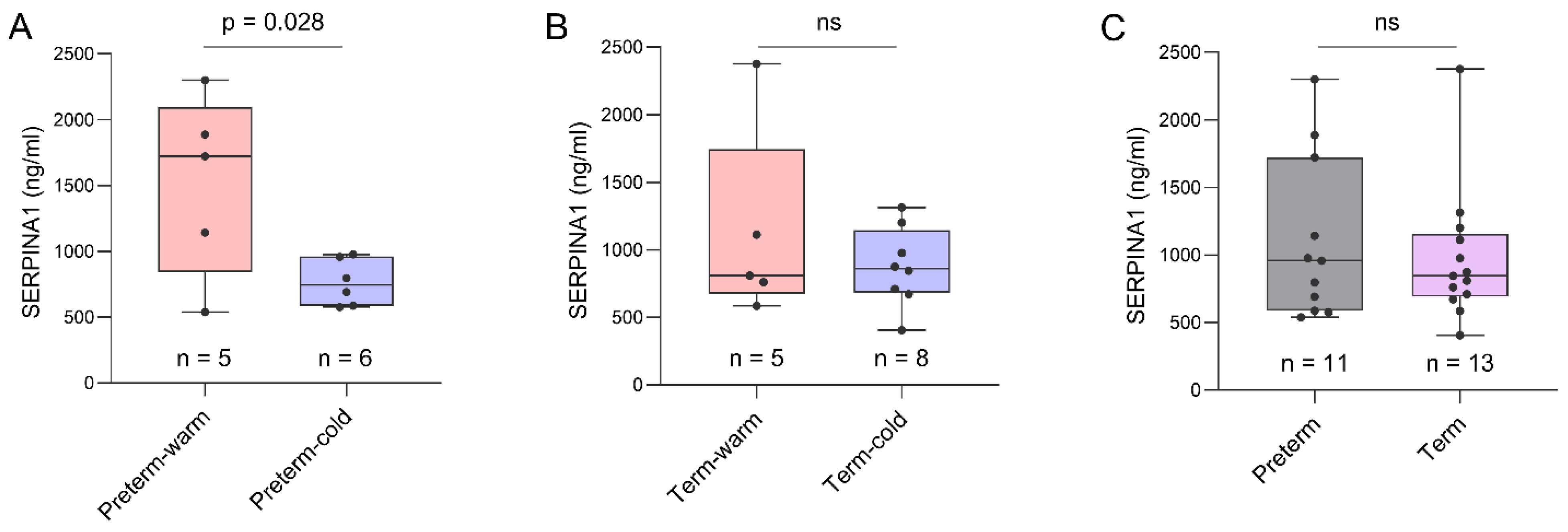
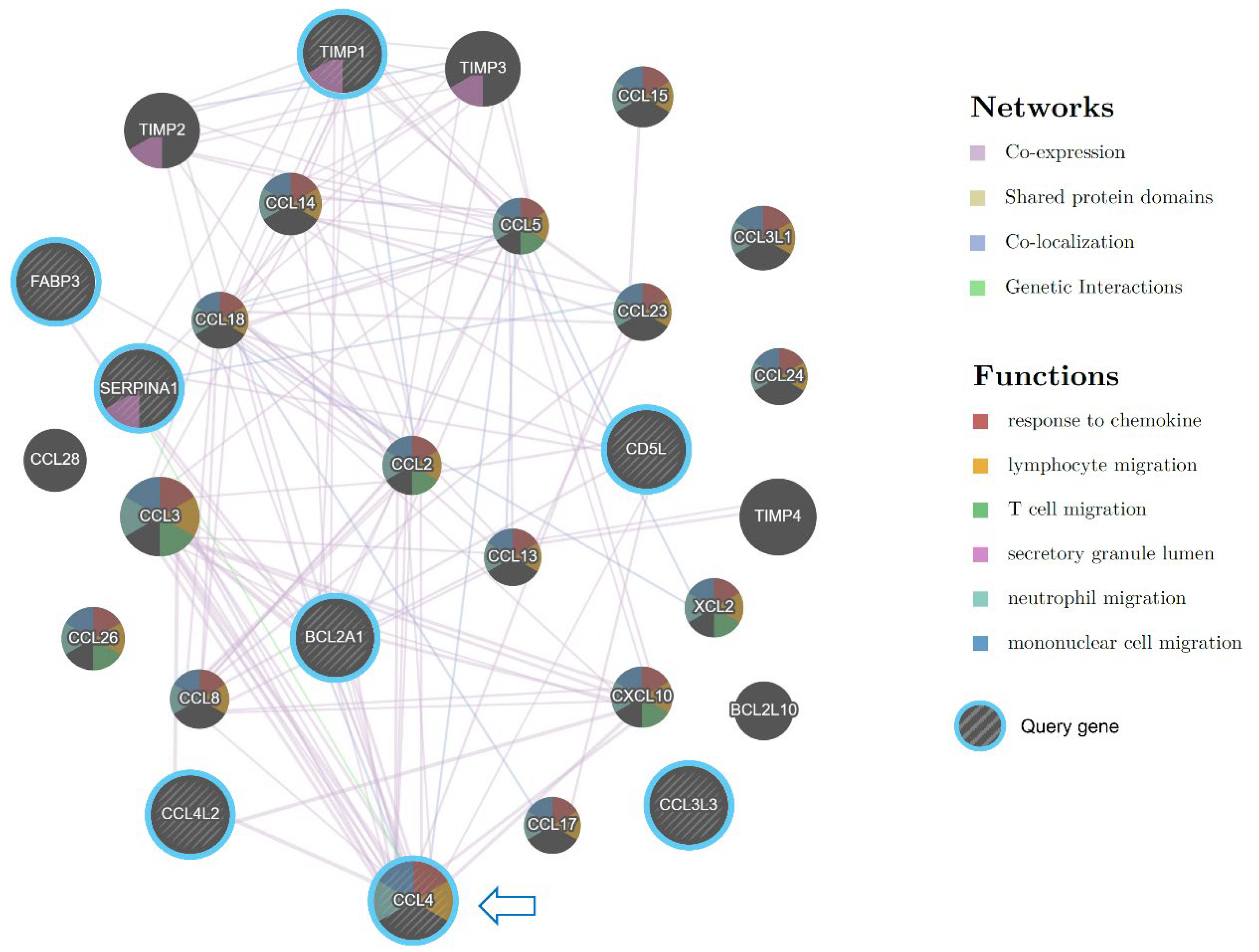
2.4. Key Genes Linked to Placental Inflammatory and Immune Responses Secondary to Increased Environmental Temperature
3. Discussion
4. Conclusion
5. Methods
5.1. Study Participants and Thermal Exposure Groups Stratification
5.2. Differential Gene Expression (DE) Analysis
5.3. Gene Set Enrichment Analysis (GSEA) and Gene Ontology (GO) Analysis
5.4. ELISA Assay
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Ethical Approval
Data Availability Statement
Acknowledgement
Conflicts of Interest
References
- Watts, N.; Amann, M.; Arnell, N.; Ayeb-Karlsson, S.; Beagley, J.; Belesova, K.; Boykoff, M.; Byass, P.; Cai, W.; Campbell-Lendrum, D.; et al. The 2020 report of the Lancet Countdown on health and climate change: responding to converging crises. Lancet 2021, 397, 129–170. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Bolaños, T.G.; Bindi, M.; Brown, S.; Camilloni, I.A.; Diedhiou, A.; Djalante, R.; Ebi, K.; et al. The human imperative of stabilizing global climate change at 1.5°C. Science 2019, 365, aaw6974. [Google Scholar] [CrossRef] [PubMed]
- Judge, C.M.; Chasan-Taber, L.; Gensburg, L.; Nasca, P.C.; Marshall, E.G. Physical exposures during pregnancy and congenital cardiovascular malformations. Paediatr. Périnat. Epidemiology 2004, 18, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Suarez, L.; Felkner, M.; Hendricks, K. The effect of fever, febrile illnesses, and heat exposures on the risk of neural tube defects in a Texas-Mexico border population. Birth Defects Res. A Clin. Mol. Teratol. 2004, 70, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Chersich, M.F.; Pham, M.D.; Areal, A.; Haghighi, M.M.; Manyuchi, A.; Swift, C.P.; Wernecke, B.; Robinson, M.; Hetem, R.; Boeckmann, M.; et al. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. BMJ 2020, 371, m3811. [Google Scholar] [CrossRef] [PubMed]
- Bekkar, B.; Pacheco, S.; Basu, R.; DeNicola, N. Association of Air Pollution and Heat Exposure With Preterm Birth, Low Birth Weight, and Stillbirth in the US. JAMA Netw. Open 2020, 3, e208243–e208243. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chai, J.; Yang, M.; Sun, P.; Sun, R.; Dong, W.; Li, Q.; Zhou, D.; Yu, F.; Wang, Y.; et al. Effects of ambient temperature on the risk of preterm birth in offspring of adolescent mothers in rural henan, China. Environ. Res. 2021, 201, 111545. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.; O’sullivan, T.L.; Phillips, K.P. Extreme Heat and Pregnancy Outcomes: A Scoping Review of the Epidemiological Evidence. Int. J. Environ. Res. Public Heal. 2022, 19, 2412. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Wang, Q.; Zhao, W.; Ren, Z.; Zhang, H.; Jalaludin, B.; Benmarhnia, T.; Di, J.; Hu, H.; Wang, Y.; et al. Effects of extreme temperature on the risk of preterm birth in China: A population-based multi-center cohort study. Lancet Reg. Heal. - West. Pac. 2022, 24, 100496. [Google Scholar] [CrossRef]
- Wang, Q.; Yin, L.; Wu, H.; Ren, Z.; He, S.; Huang, A.; Huang, C. Effects of gestational ambient extreme temperature exposures on the risk of preterm birth in China: A sibling-matched study based on a multi-center prospective cohort. Sci. Total. Environ. 2023, 887, 164135. [Google Scholar] [CrossRef]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob. Heal. 2018, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Bonell, A.; Sonko, B.; Badjie, J.; Samateh, T.; Saidy, T.; Sosseh, F.; Sallah, Y.; Bajo, K.; A Murray, K.; Hirst, J.; et al. Environmental heat stress on maternal physiology and fetal blood flow in pregnant subsistence farmers in The Gambia, west Africa: an observational cohort study. Lancet Planet. Heal. 2022, 6, e968–e976. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Dong, M.; Sun, X.; Xiao, J.; Zeng, W.; Hu, J.; Li, X.; Guo, L.; Rong, Z.; et al. Associations of maternal ambient temperature exposures during pregnancy with the placental weight, volume and PFR: A birth cohort study in Guangzhou, China. Environ. Int. 2020, 139, 105682. [Google Scholar] [CrossRef] [PubMed]
- Galan, H.L.; Hussey, M.J.; Barbera, A.; Ferrazzi, E.; Chung, M.; Hobbins, J.C.; Battaglia, F.C. Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am. J. Obstet. Gynecol. 1999, 180, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Limesand, S.; Regnault, T.; Hay, W. Characterization of Glucose Transporter 8 (GLUT8) in the Ovine Placenta of Normal and Growth Restricted Fetuses. Placenta 2004, 25, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Akram, K.M.; Kulkarni, N.S.; Brook, A.; Wyles, M.D.; Anumba, D.O.C. Transcriptomic analysis of the human placenta reveals trophoblast dysfunction and augmented Wnt signalling associated with spontaneous preterm birth. Front. Cell Dev. Biol. 2022, 10, 987740. [Google Scholar] [CrossRef] [PubMed]
- Elizarraras, J.M.; Liao, Y.; Shi, Z.; Zhu, Q.; Pico, A.R.; Zhang, B. WebGestalt 2024: faster gene set analysis and new support for metabolomics and multi-omics. Nucleic Acids Res. 2024, 52, W415–W421. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.M.; Marciniak, S.J.; Teckman, J.; Ferrarotti, I.; Brantly, M.L.; Lomas, D.A.; Stoller, J.K.; McElvaney, N.G. α1-Antitrypsin deficiency. Nat. Rev. Dis. Primers 2016, 2, 6051. [Google Scholar] [CrossRef]
- Sindhu, S.; Kochumon, S.; Shenouda, S.; Wilson, A.; Al-Mulla, F.; Ahmad, R. The Cooperative Induction of CCL4 in Human Monocytic Cells by TNF-α and Palmitate Requires MyD88 and Involves MAPK/NF-κB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 4658. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Bystry, R.S.; Aluvihare, V.; Welch, K.A.; Kallikourdis, M.; Betz, A.G. B cells and professional APCs recruit regulatory T cells via CCL4. Nat. Immunol. 2001, 2, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moral, L.; Paul, T.; Martori, C.; Font-Díaz, J.; Sanjurjo, L.; Aran, G.; Téllez, T.; Blanco, J.; Carrillo, J.; Ito, M.; et al. Macrophage CD5L is a target for cancer immunotherapy. EBioMedicine 2023, 91, 104555. [Google Scholar] [CrossRef] [PubMed]
- Sanjurjo, L.; Aran, G.; Téllez, É.; Amézaga, N.; Armengol, C.; López, D.; Prats, C.; Sarrias, M.-R. CD5L Promotes M2 Macrophage Polarization through Autophagy-Mediated Upregulation of ID3. Front. Immunol. 2018, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Schoeps, B.; Frädrich, J.; Krüger, A. Cut loose TIMP-1: an emerging cytokine in inflammation. Trends Cell Biol. 2022, 33, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Harris, J.; Rosario, F.J.; Powell, T.L.; Jansson, T.; Mohan, R.; Baumann, D.; Alejandro, E.U.; Gandhi, K.; Li, C.; et al. Increased placental fatty acid transporter 6 and binding protein 3 expression and fetal liver lipid accumulation in a mouse model of obesity in pregnancy. Am. J. Physiol. Integr. Comp. Physiol. 2015, 309, R1569–R1577. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, M.-C.; O'Byrne, K.J.; Reynolds, J.V.; O'Sullivan, J.; Pidgeon, G.P. COX-derived prostanoid pathways in gastrointestinal cancer development and progression: Novel targets for prevention and intervention. Biochim. Biophys. Acta (BBA) Rev. Cancer 2012, 1825, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.M. The role of prostaglandins in the initiation of parturition. Best Pr. Res. Clin. Obstet. Gynaecol. 2003, 17, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C. Thermal Environment and Human Birth Weight. J. Theor. Biol. 2002, 214, 413–425. [Google Scholar] [CrossRef]
- Basu, R.; Malig, B.; Ostro, B. High Ambient Temperature and the Risk of Preterm Delivery. Am. J. Epidemiology 2010, 172, 1108–1117. [Google Scholar] [CrossRef]
- Yackerson, N.; Piura, B.; Sheiner, E. The influence of meteorological factors on the emergence of preterm delivery and preterm premature rupture of membrane. J. Perinatol. 2008, 28, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Sun, Y.; Avila, C.; Chiu, V.; Slezak, J.; Sacks, D.A.; Abatzoglou, J.T.; Molitor, J.; Chen, J.-C.; Benmarhnia, T.; et al. Analysis of Heat Exposure During Pregnancy and Severe Maternal Morbidity. JAMA Netw. Open 2023, 6, e2332780–e2332780. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146. [Google Scholar] [CrossRef]
- Akram, K.M.; Frost, L.I.; Anumba, D.O. Impaired autophagy with augmented apoptosis in a Th1/Th2-imbalanced placental micromilieu is associated with spontaneous preterm birth. Front. Mol. Biosci. 2022, 9, 897228. [Google Scholar] [CrossRef] [PubMed]
- Buthmann, J.; Huang, D.; Casaccia, P.; O’neill, S.; Nomura, Y.; Liu, J. Prenatal Exposure to a Climate-Related Disaster Results in Changes of the Placental Transcriptome and Infant Temperament. Front. Genet. 2022, 13, 887619. [Google Scholar] [CrossRef]
- Ghosh, S.; Park, C.-H.; Lee, J.; Lee, N.; Zhang, R.; Huesing, C.; Reijnders, D.; Sones, J.; Münzberg, H.; Redman, L.; et al. Maternal cold exposure induces distinct transcriptome changes in the placenta and fetal brown adipose tissue in mice. BMC Genom. 2021, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.J.; Telfer, J.F.; Young, A.; Campbell, S.; Stewart, C.J.; Cameron, I.T.; Greer, I.A.; Norman, J.E. Leukocytes infiltrate the myometrium during human parturition: Further evidence that labour is an inflammatory process. Hum. Reprod. 1999, 14, 229–236. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; StLouis, D.; Lehr, M.A.; Sanchez-Rodriguez, E.N.; Arenas-Hernandez, M. Immune cells in term and preterm labor. Cell Mol. Immunol. 2014, 11, 571–581. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Gómez-Chávez, F.; Correa, D.; Navarrete-Meneses, P.; Cancino-Diaz, J.C.; Cancino-Diaz, M.E.; Rodríguez-Martínez, S. NF-κB and Its Regulators During Pregnancy. Front. Immunol. 2021, 12, 679106. [Google Scholar] [CrossRef] [PubMed]
- Elliott, C.; Allport, V.; Loudon, J.; Wu, G.; Bennett, P. Nuclear factor-kappa B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Mol. Hum. Reprod. 2001, 7, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Lindström, T.M.; Bennett, P.R. The role of nuclear factor kappa B in human labour. Reproduction 2005, 130, 569–581. [Google Scholar] [CrossRef]
- Noguchi, T.; Sado, T.; Naruse, K.; Shigetomi, H.; Onogi, A.; Haruta, S.; Kawaguchi, R.; Nagai, A.; Tanase, Y.; Yoshida, S.; et al. Evidence for Activation of Toll-Like Receptor and Receptor for Advanced Glycation End Products in Preterm Birth. Mediat. Inflamm. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Zarember, K.A.; Godowski, P.J. Tissue Expression of Human Toll-Like Receptors and Differential Regulation of Toll-Like Receptor mRNAs in Leukocytes in Response to Microbes, Their Products, and Cytokines. J. Immunol. 2002, 168, 554–561. [Google Scholar] [CrossRef]
- Schaefer, T.M.; Fahey, J.V.; Wright, J.A.; Wira, C.R. Innate Immunity in the Human Female Reproductive Tract: Antiviral Response of Uterine Epithelial Cells to the TLR3 Agonist Poly(I:C). 2005, 174, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Nakashima, A.; Hidaka, T.; Okabe, M.; Bac, N.D.; Ina, S.; Yoneda, S.; Shiozaki, A.; Sumi, S.; Tsuneyama, K.; et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J. Reprod. Immunol. 2010, 84, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Starodubtseva, N.; Nizyaeva, N.; Baev, O.; Bugrova, A.; Gapaeva, M.; Muminova, K.; Kononikhin, A.; Frankevich, V.; Nikolaev, E.; Sukhikh, G. SERPINA1 Peptides in Urine as A Potential Marker of Preeclampsia Severity. Int. J. Mol. Sci. 2020, 21, 914. [Google Scholar] [CrossRef]
- Tiensuu, H.; Haapalainen, A.M.; Tissarinen, P.; Pasanen, A.; Määttä, T.A.; Huusko, J.M.; Ohlmeier, S.; Bergmann, U.; Ojaniemi, M.; Muglia, L.J.; et al. Human placental proteomics and exon variant studies link AAT/SERPINA1 with spontaneous preterm birth. BMC Med. 2022, 20, 1–23. [Google Scholar] [CrossRef]
- Tissarinen, P.; Tiensuu, H.; Haapalainen, A.M.; Ronkainen, E.; Laatio, L.; Vääräsmäki, M.; Öhman, H.; Hallman, M.; Rämet, M. Maternal serum alpha-1 antitrypsin levels in spontaneous preterm and term pregnancies. Sci. Rep. 2024, 14, 1–9. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, W.; Li, C. Recent Advances in Genetic and Epigenetic Modulation of Animal Exposure to High Temperature. Front. Genet. 2020, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Tessadori, F.; Duran, K.; Knapp, K.; Fellner, M.; Smithson, S.; Meireles, A.B.; Elting, M.W.; Waisfisz, Q.; O’donnell-Luria, A.; Nowak, C.; et al. Recurrent de novo missense variants across multiple histone H4 genes underlie a neurodevelopmental syndrome. Am. J. Hum. Genet. 2022, 109, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.M.; Hornaday, K.K.; Slater, D.M. Prostaglandins in biofluids in pregnancy and labour: A systematic review. PLOS ONE 2021, 16, e0260115. [Google Scholar] [CrossRef] [PubMed]
- Palliser, H.K.; Kelleher, M.A.; Welsh, T.N.; Zakar, T.; Hirst, J.J. Mechanisms Leading to Increased Risk of Preterm Birth in Growth-Restricted Guinea Pig Pregnancies. Reprod. Sci. 2014, 21, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; A Fortier, M.; Bernal, A.L. Prostaglandin pathway gene expression in human placenta, amnion and choriodecidua is differentially affected by preterm and term labour and by uterine inflammation. BMC Pregnancy Childbirth 2014, 14, 241–241. [Google Scholar] [CrossRef] [PubMed]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxyplatform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene Set Analysis Toolkit with Revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, B.; Kirov, S.; Snoddy, J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005, 33, W741–W748. [Google Scholar] [CrossRef] [PubMed]
- Montojo, J.; Zuberi, K.; Rodriguez, H.; Kazi, F.; Wright, G.; Donaldson, S.L.; Morris, Q.; Bader, G.D. GeneMANIA Cytoscape plugin: fast gene function predictions on the desktop. Bioinformatics 2010, 26, 2927–2928. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLOS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




