Submitted:
29 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
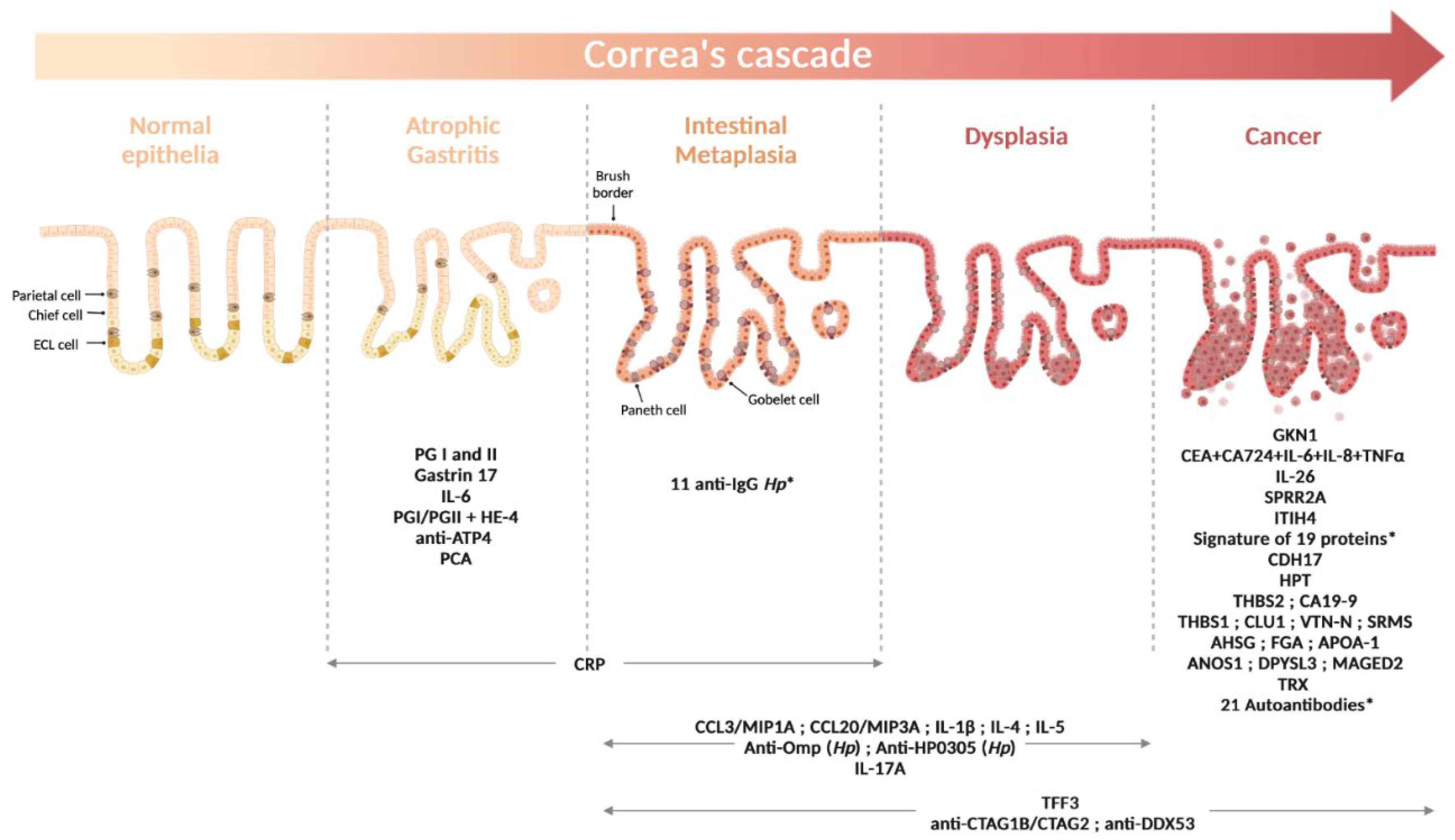
1. Introduction
2. Detection of Gastric Pre-Neoplastic Lesions by Endoscopy
3. Emerging Serum/Plasma Protein Biomarkers for the Detection of Gastric Pre-Neoplasia and Early Cancer Lesions
3.1. Atrophic Gastritis (AG)
3.1.1. Gastric Physiology-Related Biomarkers
3.1.2. Immunity and Related Autoantibodies
3.2. Intestinal Metaplasia (IM)
3.2.1. Inflammation and Immunity-Related Proteins
3.2.2. Immunity and Related Autoantibodies
3.2.3. Antimicrobial Defense
3.3. Dysplasia (DYS)
3.4. Early Gastric Cancer (EGC)
3.4.1. Gastric Physiology-Related Proteins
3.4.2. Inflammation and Immunity-Related Biomarkers
3.4.3. Immunity and Related Autoantibodies
3.4.4. Cellular Physiology and Metabolism Related Proteins
4. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abraham, S.C.; Park, S.J.; Lee, J.-H.; Mugartegui, L.; Wu, T.-T. Genetic Alterations in Gastric Adenomas of Intestinal and Foveolar Phenotypes. Mod. Pathol. 2003, 16, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Aihara, E.; Engevik, K.A.; Montrose, M.H. Trefoil Factor Peptides and Gastrointestinal Function. Annu. Rev. Physiol. 2017, 79, 357–380. [Google Scholar] [CrossRef]
- Aikou, S.; Ohmoto, Y.; Gunji, T.; Matsuhashi, N.; Ohtsu, H.; Miura, H.; Kubota, K.; Yamagata, Y.; Seto, Y.; Nakajima, A.; et al. Tests for Serum Levels of Trefoil Factor Family Proteins Can Improve Gastric Cancer Screening. Gastroenterology 2011, 141, 837–845. [Google Scholar] [CrossRef]
- AJCC Cancer Staging Manual. n.d. Accessed 15 March 2023. https://link.springer.com/book/9783319406176.
- Aleksandrova, K.; Chuang, S.-C.; Boeing, H.; Zuo, H.; Tell, G.S.; Pischon, T.; Jenab, M.; Bueno-De-Mesquita, B.; Vollset, S.E.; Midttun. ; et al. A Prospective Study of the Immune System Activation Biomarker Neopterin and Colorectal Cancer Risk. JNCI J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Amedei, A.; Bergman, M.P.; Appelmelk, B.J.; Azzurri, A.; Benagiano, M.; Tamburini, C.; van der Zee, R.; Telford, J.L.; Vandenbroucke-Grauls, C.M.; D'Elios, M.M.; et al. Molecular Mimicry between Helicobacter pylori Antigens and H+,K+–Adenosine Triphosphatase in Human Gastric Autoimmunity. J. Exp. Med. 2003, 198, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.F.; Rabkin, C.S.; Turner, N.; Fraumeni, J.F.; Rosenberg, P.S.; Camargo, M.C. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. JNCI J. Natl. Cancer Inst. 2018, 110, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Antico, A.; Tampoia, M.; Villalta, D.; Tonutti, E.; Tozzoli, R.; Bizzaro, N. Clinical Usefulness of the Serological Gastric Biopsy for the Diagnosis of Chronic Autoimmune Gastritis. J. Immunol. Res. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Beck, M.; Bringeland, E.A.; Qvigstad, G.; Fossmark, R. Gastric Cancers Missed at Upper Endoscopy in Central Norway 2007 to 2016—A Population-Based Study. Cancers 2021, 13, 5628. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, N.; Antico, A.; Villalta, D. Autoimmunity and Gastric Cancer. Int. J. Mol. Sci. 2018, 19, 377. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Capelle, L.G.; de Vries, A.C.; Haringsma, J.; Ter Borg, F.; de Vries, R.A.; Bruno, M.J.; van Dekken, H.; Meijer, J.; van Grieken, N.C.; Kuipers, E.J. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest. Endosc. 2010, 71, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Capelle, L.G.; de Vries, A.C.; Haringsma, J.; Ter Borg, F.; de Vries, R.A.; Bruno, M.J.; van Dekken, H.; Meijer, J.; van Grieken, N.C.; Kuipers, E.J. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest. Endosc. 2010, 71, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Carmack, S.W.; Genta, R.M.; Graham, D.Y.; Lauwers, G.Y. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, N.; Osmola, M.; Martin, J.; Blin, J.; Leroy, M.; Jirka, I.; Moussata, D.; Lamarque, D.; Olivier, R.; Tougeron, D.; et al. Serum Pepsinogens Combined with New Biomarkers Testing Using Chemiluminescent Enzyme Immunoassay for Non-Invasive Diagnosis of Atrophic Gastritis: A Prospective, Multicenter Study. Diagnostics 2022, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, N.; Petryszyn, P.; Blin, J.; Leroy, M.; Le Berre-Scoul, C.; Jirka, I.; Neunlist, M.; Moussata, D.; Lamarque, D.; Olivier, R.; et al. A panel of stomach-specific biomarkers (GastroPanel®) for the diagnosis of atrophic gastritis: A prospective, multicenter study in a low gastric cancer incidence area. Helicobacter 2020, 25, e12727. [Google Scholar] [CrossRef]
- Choi, B.; Lee, H.-J.; Min, J.; Choe, H.-N.; Choi, Y.-S.; Son, Y.-G.; Ahn, H.-S.; Suh, Y.-S.; Goldenring, J.R.; Yang, H.-K. Plasma expression of the intestinal metaplasia markers CDH17 and TFF3 in patients with gastric cancer. Cancer Biomarkers 2017, 19, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. . 1992, 52, 6735–40. [Google Scholar] [PubMed]
- Dan, Y.Y.; So, J.; Yeoh, K.G. Endoscopic Screening for Gastric Cancer. Clin. Gastroenterol. Hepatol. 2006, 4, 709–716. [Google Scholar] [CrossRef]
- Dayon, L.; Cominetti, O.; Affolter, M. Proteomics of human biological fluids for biomarker discoveries: technical advances and recent applications. Expert Rev. Proteom. 2022, 19, 131–151. [Google Scholar] [CrossRef]
- D'Elios, M.M.; Appelmelk, B.J.; Amedei, A.; Bergman, M.P.; Del Prete, G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol. Med. 2004, 10, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Della Bella, C.; D’elios, S.; Coletta, S.; Benagiano, M.; Azzurri, A.; Cianchi, F.; de Bernard, M.; D’elios, M.M. Increased IL-17A Serum Levels and Gastric Th17 Cells in Helicobacter pylori-Infected Patients with Gastric Premalignant Lesions. Cancers 2023, 15, 1662. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Ribeiro, M.; da Costa-Pereira, A.; Lopes, C.; Barbosa, J.; Guilherme, M.; Moreira-Dias, L.; Lomba-Viana, H.; Silva, R.; Abreu, N.; Lomba-Viana, R. Validity of Serum Pepsinogen I/II Ratio for the Diagnosis of Gastric Epithelial Dysplasia and Intestinal Metaplasia during the Follow-Up of Patients at Risk for Intestinal-Type Gastric Adenocarcinoma. Neoplasia 2004, 6, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F., R. M. Genta, J.H. Yardley, and P. Correa. 1994. ‘Classification and Grading of Gastritis. The Updated Sydney System’. International Workshop on the Histopathology of Gastritis, Houston 20:1161–81.
- Dong, Z.; Zhang, X.; Chen, X.; Zhang, J. Significance of Serological Gastric Biopsy in Different Gastric Mucosal Lesions: an Observational Study. Clin. Lab. 2019, 65, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Epplein, M.; Butt, J.; Zhang, Y.; Hendrix, L.H.; Abnet, C.C.; Murphy, G.; Zheng, W.; Shu, X.-O.; Tsugane, S.; Qiao, Y.-L.; et al. Validation of a Blood Biomarker for Identification of Individuals at High Risk for Gastric Cancer. Cancer Epidemiology Biomarkers Prev. 2018, 27, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Espinel, J.; Uacute, J.; Espinel, E.P.S. Treatment modalities for early gastric cancer. World J. Gastrointest. Endosc. 2015, 7, 1062–1069. [Google Scholar] [CrossRef]
- Ezoe, Y.; Muto, M.; Uedo, N.; Doyama, H.; Yao, K.; Oda, I.; Kaneko, K.; Kawahara, Y.; Yokoi, C.; Sugiura, Y.; et al. Magnifying Narrowband Imaging Is More Accurate Than Conventional White-Light Imaging in Diagnosis of Gastric Mucosal Cancer. Gastroenterology 2011, 141, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Gawron, A.J.; Shah, S.C.; Altayar, O.; Davitkov, P.; Morgan, D.; Turner, K.; Mustafa, R.A. AGA Technical Review on Gastric Intestinal Metaplasia—Natural History and Clinical Outcomes. Gastroenterology 2019, 158, 705–731. [Google Scholar] [CrossRef]
- Genta, R. M. 1998. ‘Review Article: Gastric Atrophy and Atrophic Gastritis--Nebulous Concepts in Search of a Definition’. Alimentary Pharmacology & Therapeutics 12 Suppl 1 (February):17–23.
- Genta, R.M.; Turner, K.O.; Robiou, C.; Singhal, A.; Rugge, M. Incomplete Intestinal Metaplasia Is Rare in Autoimmune Gastritis. Dig. Dis. 2023, 41, 369–376. [Google Scholar] [CrossRef]
- Shabgah, A.G.; Abdelbasset, W.K.; Rahman, H.S.; Bokov, D.O.; Suksatan, W.; Thangavelu, L.; Ahmadi, M.; Malekahmadi, M.; Gheibihayat, S.M.; Navashenaq, J.G. A comprehensive review of IL-26 to pave a new way for a profound understanding of the pathobiology of cancer, inflammatory diseases and infections. Immunology 2021, 165, 44–60. [Google Scholar] [CrossRef]
- Grozinsky-Glasberg, S.; Alexandraki, K.I.; Angelousi, A.; Chatzellis, E.; Sougioultzis, S.; Kaltsas, G. Gastric Carcinoids. Endocrinol. Metab. Clin. North Am. 2018, 47, 645–660. [Google Scholar] [CrossRef]
- Hartl, J.; Kurth, F.; Kappert, K.; Horst, D.; Mülleder, M.; Hartmann, G.; Ralser, M. Quantitative protein biomarker panels: a path to improved clinical practice through proteomics. EMBO Mol. Med. 2023, 15. [Google Scholar] [CrossRef]
- Hoed, C.M.D.; Holster, I.L.; Capelle, L.G.; de Vries, A.C.; Hartog, B.D.; ter Borg, F.; Biermann, K.; Kuipers, E.J. Follow-up of premalignant lesions in patients at risk for progression to gastric cancer. Endoscopy 2013, 45, 249–256. [Google Scholar] [CrossRef]
- Hollander, W.J.D.; Holster, I.L.; Hoed, C.M.D.; Capelle, L.G.; Tang, T.J.; Anten, M.-P.; Prytz-Berset, I.; Witteman, E.M.; ter Borg, F.; Hartog, G.D.; et al. Surveillance of premalignant gastric lesions: a multicentre prospective cohort study from low incidence regions. Gut 2018, 68, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.J.; Choi, A.Y.; Truong, C.D.; Yeh, M.M.; Hwang, J.H. Diagnosis and Management of Gastric Intestinal Metaplasia: Current Status and Future Directions. Gut Liver 2019, 13, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-B.; Zhang, H.-T.; Yu, B.; Yu, D.-H. Cell-free DNA as a liquid biopsy for early detection of gastric cancer (Review). Oncol. Lett. 2020, 21, 1–1. [Google Scholar] [CrossRef]
- Isajevs, S.; Liepniece-Karele, I.; Janciauskas, D.; Moisejevs, G.; Putnins, V.; Funka, K.; Kikuste, I.; Vanags, A.; Tolmanis, I.; Leja, M. Gastritis staging: interobserver agreement by applying OLGA and OLGIM systems. Virchows Arch. 2014, 464, 403–407. [Google Scholar] [CrossRef]
- Ishida, Y.; Suzuki, K.; Taki, K.; Niwa, T.; Kurotsuchi, S.; Ando, H.; Iwase, A.; Nishio, K.; Wakai, K.; Ito, Y.; et al. Significant association between Helicobacter pylori infection and serum C-reactive protein. Int. J. Med Sci. 2008, 5, 224–229. [Google Scholar] [CrossRef]
- Jeong, S.; Oh, M.J.; Kim, U.; Lee, J.; Kim, J.-H.; An, H.J. Glycosylation of serum haptoglobin as a marker of gastric cancer: an overview for clinicians. Expert Rev. Proteom. 2020, 17, 109–117. [Google Scholar] [CrossRef]
- Kanda, M.; Suh, Y.-S.; Park, D.J.; Tanaka, C.; Ahn, S.-H.; Kong, S.-H.; Lee, H.-J.; Kobayashi, D.; Fujiwara, M.; Shimada, H.; et al. Serum levels of ANOS1 serve as a diagnostic biomarker of gastric cancer: a prospective multicenter observational study. Gastric Cancer 2019, 23, 203–211. [Google Scholar] [CrossRef]
- Kanemitsu, T.; Yao, K.; Nagahama, T.; Imamura, K.; Fujiwara, S.; Ueki, T.; Chuman, K.; Tanabe, H.; Atsuko, O.; Iwashita, A.; et al. Extending magnifying NBI diagnosis of intestinal metaplasia in the stomach: the white opaque substance marker. Endoscopy 2017, 49, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Park, J.Y.; Kim, B.J.; Hwang, H.W.; Hong, S.A.; Kim, J.G. Risk of metachronous gastric neoplasm occurrence during intermediate-term follow-up period after endoscopic submucosal dissection for gastric dysplasia. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Koivurova, O.-P.; Koskela, R.; Blomster, T.; Ala-Rämi, A.; Lumme, H.; Kettunen, O.; Hukkanen, J.; Karttunen, T.J.; Mäkinen, M.; Ronkainen, J.; et al. Serological Biomarker Panel in Diagnosis of Atrophic Gastritis andHelicobacter pyloriInfection in Gastroscopy Referral Patients: Clinical Validation of the New-Generation GastroPanel®Test. Anticancer. Res. 2021, 41, 5527–5537. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, D.; Sun, W.; Chen, M.; Chen, J.; Shi, J.; Zhang, J.; Chen, X. Small Proline-Rich Protein 2A and 2D Are Regulated by the RBM38-p73 Axis and Associated with p73-Dependent Suppression of Chronic Inflammation. Cancers 2021, 13, 2829. [Google Scholar] [CrossRef] [PubMed]
- Kutluana, U.; Kilciler, A.G.; Mizrak, S.; Dilli, U. Can neopterin be a useful immune biomarker for differentiating gastric intestinal metaplasia and gastric atrophy from non-atrophic non-metaplastic chronic gastritis? Gastroenterol. Y Hepatol. 2019, 42, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Lage, J.; Pimentel-Nunes, P.; Figueiredo, P.C.; Libanio, D.; Ribeiro, I.; Jacome, M.; Afonso, L.; Dinis-Ribeiro, M. Light-NBI to identify high-risk phenotypes for gastric adenocarcinoma: do we still need biopsies? Scand. J. Gastroenterol. 2015, 51, 501–506. [Google Scholar] [CrossRef]
- Lahner, E.; Brigatti, C.; Marzinotto, I.; Carabotti, M.; Scalese, G.; Davidson, H.W.; Wenzlau, J.M.; Bosi, E.; Piemonti, L.; Annibale, B.; et al. Luminescent Immunoprecipitation System (LIPS) for Detection of Autoantibodies Against ATP4A and ATP4B Subunits of Gastric Proton Pump H+,K+-ATPase in Atrophic Body Gastritis Patients. Clin. Transl. Gastroenterol. 2017, 8, e215. [Google Scholar] [CrossRef] [PubMed]
- Lahner, E.; Marzinotto, I.; Lampasona, V.; Dottori, L.; Bazzigaluppi, E.; Brigatti, C.; Secchi, M.; Piemonti, L.; Conti, L.; Pilozzi, E.; et al. Autoantibodies Toward ATP4A and ATP4B Subunits of Gastric Proton Pump H plus,K plus -ATPase Are Reliable Serological Pre-endoscopic Markers of Corpus Atrophic Gastritis. Clin. Transl. Gastroenterol. 2020, 11, e00240. [Google Scholar] [CrossRef]
- Lahner, E.; Norman, G.L.; Severi, C.; Encabo, S.; Shums, Z.; Vannella, L.; Fave, G.D.; Annibale, B. Reassessment of Intrinsic Factor and Parietal Cell Autoantibodies in Atrophic Gastritis With Respect to Cobalamin Deficiency. Am. J. Gastroenterol. 2009, 104, 2071–2079. [Google Scholar] [CrossRef]
- Lahner, E.; Zagari, R.M.; Zullo, A.; Di Sabatino, A.; Meggio, A.; Cesaro, P.; Lenti, M.V.; Annibale, B.; Corazza, G.R. Chronic atrophic gastritis: Natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Dig. Liver Dis. 2019, 51, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Latorre, G.; Pizarro, M.; Ford, J.S.; Gándara, V.; Muñoz, G.; Araya, J.C.; Bellolio, E.; Villaseca, M.; Fuentes-López, E.; Cortés, P.; et al. Evaluation of trefoil factor 3 as a non-invasive biomarker of gastric intestinal metaplasia and gastric cancer in a high-risk population. Gastroenterol. Y Hepatol. 2023, 46, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Laurén, P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and so-called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Lavery, D.L.; Nicholson, A.M.; Poulsom, R.; Jeffery, R.; Hussain, A.; Gay, L.J.; A Jankowski, J.; Zeki, S.S.; Barr, H.; Harrison, R.; et al. The stem cell organisation, and the proliferative and gene expression profile of Barrett's epithelium, replicates pyloric-type gastric glands. Gut 2014, 63, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.J.; Zhu, F.; Srivastava, S.; Tsao, S.K.; Khor, C.; Ho, K.Y.; Fock, K.M.; Lim, W.C.; Ang, T.L.; Chow, W.C.; et al. Severity of gastric intestinal metaplasia predicts the risk of gastric cancer: a prospective multicentre cohort study (GCEP). Gut 2021, 71, 854–863. [Google Scholar] [CrossRef]
- Lee, J.; Hua, S.; Lee, S.H.; Oh, M.J.; Yun, J.; Kim, J.Y.; Kim, J.-H.; Kim, J.H.; An, H.J. Designation of fingerprint glycopeptides for targeted glycoproteomic analysis of serum haptoglobin: insights into gastric cancer biomarker discovery. Anal. Bioanal. Chem. 2018, 410, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Linē, A. Early detection of gastric cancer beyond endoscopy - new methods. Best Pr. Res. Clin. Gastroenterol. 2021, 50-51, 101731. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.K.; Yu, J.; Chan, F.K.L.; To, K.F.; Chan, M.W.Y.; Ebert, M.P.A.; Ng, E.K.W.; Chung, S.C.S.; Malfertheiner, P.; Sung, J.J.Y. Expression of trefoil peptides (TFF1, TFF2, and TFF3) in gastric carcinomas, intestinal metaplasia, and non-neoplastic gastric tissues. J. Pathol. 2002, 197, 582–588. [Google Scholar] [CrossRef]
- Lewin, K.J. Nomenclature Problems of Gastrointestinal Epithelial Neoplasia. Am. J. Surg. Pathol. 1998, 22, 1043–1047. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Run, Z.-C.; Feng, W.; Liu, W.; Zhang, P.-J.; Li, Z. Multiple cytokine profiling in serum for early detection of gastric cancer. World J. Gastroenterol. 2018, 24, 2269–2278. [Google Scholar] [CrossRef]
- Li, L.; Dong, J.; Fu, L.; Xia, X.; Pan, F.; Ning, Y. Clinical Value of Serum Thrombospondin-2 Combined with CA19-9 in Early Diagnosis of Gastric Cancer. J. Oncol. 2021, 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.-M.; Malfertheiner, P.; Lee, Y.-C.; Sheu, B.-S.; Sugano, K.; Cheng, H.-C.; Yeoh, K.-G.; Hsu, P.-I.; Goh, K.-L.; Mahachai, V.; et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut 2020, 69, 2093–2112. [Google Scholar] [CrossRef] [PubMed]
- Loong, T.H.; Soon, N.C.; Mahmud, N.R.K.N.; Naidu, J.; Rani, R.A.; Hamid, N.A.; Elias, M.H.; Rose, I.M.; Tamil, A.; Mokhtar, N.M.; et al. Serum pepsinogen and gastrin-17 as potential biomarkers for pre-malignant lesions in the gastric corpus. Biomed. Rep. 2017, 7, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Yashiro, M. Biomarkers of gastric cancer: Current topics and future perspective. World J. Gastroenterol. 2018, 24, 2818–2832. [Google Scholar] [CrossRef] [PubMed]
- Matsusaka, K.; Ushiku, T.; Urabe, M.; Fukuyo, M.; Abe, H.; Ishikawa, S.; Seto, Y.; Aburatani, H.; Hamakubo, T.; Kaneda, A.; et al. Coupling CDH17 and CLDN18 markers for comprehensive membrane-targeted detection of human gastric cancer. Oncotarget 2016, 7, 64168–64181. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Tsugawa, H.; Suzuki, H. Precision Medicine Approaches to Prevent Gastric Cancer. Gut Liver 2021, 15, 3–12. [Google Scholar] [CrossRef]
- Meistere, I.; Werner, S.; Zayakin, P.; Siliņa, K.; Rulle, U.; Pismennaja, A.; Šantare, D.; Kikuste, I.; Isajevs, S.; Leja, M.; et al. The Prevalence of Cancer-Associated Autoantibodies in Patients with Gastric Cancer and Progressive Grades of Premalignant Lesions. Cancer Epidemiology Biomarkers Prev. 2017, 26, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Michel, A.; Waterboer, T.; Kist, M.; Pawlita, M. Helicobacter pylori Multiplex Serology. Helicobacter 2009, 14, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Rajagopalan, P.; Jain, A.P.; Khan, A.A.; Datta, K.; Mohan, S.V.; Lateef, S.S.; Sahasrabuddhe, N.; Somani, B.; Prasad, T.K.; et al. LC–MS-based serum metabolomic analysis reveals dysregulation of phosphatidylcholines in esophageal squamous cell carcinoma. J. Proteom. 2015, 127, 96–102. [Google Scholar] [CrossRef]
- Miyaoka, M.; Yao, K.; Tanabe, H.; Kanemitsu, T.; Otsu, K.; Imamura, K.; Ono, Y.; Ishikawa, S.; Yasaka, T.; Ueki, T.; et al. Diagnosis of early gastric cancer using image enhanced endoscopy: a systematic approach. Transl. Gastroenterol. Hepatol. 2020, 5, 50–50. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: A population-based modelling study. eClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Morson, B.C.; Sobin, L.H.; Grundmann, E.; Johansen, A.; Nagayo, T.; Serck-Hanssen, A. Precancerous conditions and epithelial dysplasia in the stomach. J. Clin. Pathol. 1980, 33, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Formichella, L.; Zhang, L.; Zhang, Y.; Ma, J.; Li, Z.; Liu, C.; Wang, Y.; Goettner, G.; Ulm, K.; et al. Helicobacter pylori antibody responses and evolution of precancerous gastric lesions in a Chinese population. Int. J. Cancer 2013, 134, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, X.; He, Y.; Lin, S.; Zhu, M.; Li, Y.; Wang, J.; Wang, J.; Ma, X.; Xu, J.; et al. Tumor suppressor ATP4B serve as a promising biomarker for worsening of gastric atrophy and poor differentiation. Gastric Cancer 2021, 24, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhou, Z.; Zhong, Y.; Sun, Y.; Wang, Y.; Zhu, Z.; Jiao, W.; Bai, M.; Sun, J.; Lu, J.; et al. Plasma activity of Thioredoxin Reductase as a Novel Biomarker in Gastric Cancer. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Petryszyn, P.; Chapelle, N.; Matysiak-Budnik, T. Gastric Cancer: Where Are We Heading? Dig. Dis. 2020, 38, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Libânio, D.; Lage, J.; Abrantes, D.; Coimbra, M.; Esposito, G.; Hormozdi, D.; Pepper, M.; Drasovean, S.; White, J.R.; et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy 2016, 48, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; de Martel, C. Global burden of gastric cancer attributable toHelicobacterpylori. Int. J. Cancer 2014, 136, 487–490. [Google Scholar] [CrossRef]
- Qin, J.; Wang, S.; Shi, J.; Ma, Y.; Wang, K.; Ye, H.; Zhang, X.; Wang, P.; Wang, X.; Song, C.; et al. Using recursive partitioning approach to select tumor-associated antigens in immunodiagnosis of gastric adenocarcinoma. Cancer Sci. 2019, 110, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Romańczyk, M.; Osmola, M.; Link, A.; Druet, A.; Hémont, C.; Martin, J.; Chapelle, N.; Matysiak-Budnik, T. Non-Invasive Markers for the Detection of Gastric Precancerous Conditions. Cancers 2024, 16, 2254. [Google Scholar] [CrossRef]
- Rusak, E.; Chobot, A.; Krzywicka, A.; Wenzlau, J. Anti-parietal cell antibodies – diagnostic significance. Adv. Med Sci. 2016, 61, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Saka, A.; Yagi, K.; Nimura, S. OLGA- and OLGIM-based staging of gastritis using narrow-band imaging magnifying endoscopy. Dig. Endosc. 2015, 27, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021, 161, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Shan, Y.-S.; Hu, H.-M.; Price, T.J.; Sirohi, B.; Yeh, K.-H.; Yang, Y.-H.; Sano, T.; Yang, H.-K.; Zhang, X.; et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013, 14, e535–e547. [Google Scholar] [CrossRef]
- Shen, Q.; Polom, K.; Williams, C.; de Oliveira, F.M.S.; Guergova-Kuras, M.; Lisacek, F.; Karlsson, N.G.; Roviello, F.; Kamali-Moghaddam, M. A targeted proteomics approach reveals a serum protein signature as diagnostic biomarker for resectable gastric cancer. EBioMedicine 2019, 44, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wu, H.; Qu, K.; Sun, Q.; Li, F.; Shi, C.; Li, Y.; Xiong, X.; Qin, Q.; Yu, T.; et al. Identification of serum proteins AHSG, FGA and APOA-I as diagnostic biomarkers for gastric cancer. Clin. Proteom. 2018, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA: A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Song, L.; Song, M.; Rabkin, C.S.; Chung, Y.; Williams, S.; Torres, J.; Corvalan, A.H.; Gonzalez, R.; Bellolio, E.; Shome, M.; et al. Identification of anti-Helicobacter pylori antibody signatures in gastric intestinal metaplasia. J. Gastroenterol. 2022, 58, 112–124. [Google Scholar] [CrossRef]
- Song, M.; Rabkin, C.S.; Torres, J.; Kemp, T.J.; Zabaleta, J.; A Pinto, L.; Hildesheim, A.; Sánchez-Figueroa, L.; Guarner, J.; Herrera-Goepfert, R.; et al. Circulating inflammation-related markers and advanced gastric premalignant lesions. J. Gastroenterol. Hepatol. 2018, 34, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Storskrubb, T.; Aro, P.; Ronkainen, J.; Sipponen, P.; Nyhlin, H.; Talley, N.J.; Engstrand, L.; Stolte, M.; Vieth, M.; Walker, M.; et al. Serum biomarkers provide an accurate method for diagnosis of atrophic gastritis in a general population: The Kalixanda study. Scand. J. Gastroenterol. 2008, 43, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, J.; Jing, H.; Lu, Y.; Zhu, Q.; Shu, C.; Zhang, Q.; Jing, D. ITIH4 is a novel serum biomarker for early gastric cancer diagnosis. Clin. Chim. Acta 2021, 523, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.K. Diagnosis and management of gastric dysplasia. Korean J. Intern. Med. 2016, 31, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2019, 18, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Toh, B.-H. Diagnosis and classification of autoimmune gastritis. Autoimmun. Rev. 2014, 13, 459–462. [Google Scholar] [CrossRef]
- Valente, P.; Garrido, M.; Gullo, I.; Baldaia, H.; Marques, M.; Baldaque-Silva, F.; Lopes, J.; Carneiro, F. Epithelial dysplasia of the stomach with gastric immunophenotype shows features of biological aggressiveness. Gastric Cancer 2014, 18, 720–728. [Google Scholar] [CrossRef]
- de Vries, A.C.; van Grieken, N.C.; Looman, C.W.; Casparie, M.K.; de Vries, E.; Meijer, G.A.; Kuipers, E.J. Gastric Cancer Risk in Patients With Premalignant Gastric Lesions: A Nationwide Cohort Study in the Netherlands. Gastroenterology 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Wang, H.; Jin, W.; Wan, C.; Zhu, C. Diagnostic value of combined detection of CA72-4, CA19-9, and carcinoembryonic antigen comparing to CA72-4 alone in gastric cancer: a systematic review and meta-analysis. Transl. Cancer Res. 2022, 11, 848–856. [Google Scholar] [CrossRef]
- Wang, X.; Lu, B.; Meng, L.; Fan, Y.; Zhang, S.; Li, M. The correlation between histological gastritis staging- ‘OLGA/OLGIM’ and serum pepsinogen test in assessment of gastric atrophy/intestinal metaplasia in China. Scand. J. Gastroenterol. 2017, 52, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; El-Serag, H.B.; Thrift, A.P. Increasing Incidence of Advanced Non-cardia Gastric Cancers Among Younger Hispanics in the USA. Dig. Dis. Sci. 2020, 66, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wei, S.; Chen, Y.; Yu, D.; Wang, X.; Dong, X. Serum Small Proline-Rich Protein 2A (SPRR2A) Is a Noninvasive Biomarker in Gastric Cancer. Dis. Markers 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Yang, J.; Song, P.; Zhou, G. Investigation on correlations of serum IL-26 with diagnosis and staging of gastric cancer. . 2019, 24, 215–220. [Google Scholar]
- Yanan, Z.; Juan, W.; Xin, M.; Kejian, W.; Fangyu, W. Application of serum gastric function markers and digestive tumor indices to the diagnosis of early gastric cancer and precancerous lesions. SciVee 2023, 44, 795–800. [Google Scholar] [CrossRef]
- Yang, Q.; Qin, J.; Sun, G.; Qiu, C.; Jiang, D.; Ye, H.; Wang, X.; Dai, L.; Zhu, J.; Wang, P.; et al. Discovery and Validation of Serum Autoantibodies Against Tumor-Associated Antigens as Biomarkers in Gastric Adenocarcinoma Based on the Focused Protein Arrays. Clin. Transl. Gastroenterol. 2020, 12, e00284. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Anagnostopoulos, G.; Ragunath, K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy 2009, 41, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Doyama, H.; Gotoda, T.; Ishikawa, H.; Nagahama, T.; Yokoi, C.; Oda, I.; Machida, H.; Uchita, K.; Tabuchi, M. Diagnostic performance and limitations of magnifying narrow-band imaging in screening endoscopy of early gastric cancer: a prospective multicenter feasibility study. Gastric Cancer 2014, 17, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.; Park, J.; Han, H.; Yun, Y.; Kang, J.W.; Choi, D.; Lee, J.W.; Jung, J.H.; Lee, K.; Kim, K.P. Discovery of gastric cancer specific biomarkers by the application of serum proteomics. Proteomics 2017, 17. [Google Scholar] [CrossRef]
- Yoon, J.H.; La Cho, M.; Choi, Y.J.; Back, J.Y.; Park, M.K.; Lee, S.W.; Choi, B.J.; Ashktorab, H.; Smoot, D.T.; Nam, S.W.; et al. Gastrokine 1 regulates NF-κB signaling pathway and cytokine expression in gastric cancers. J. Cell. Biochem. 2013, 114, 1800–1809. [Google Scholar] [CrossRef]
- Yoon, J.H.; Park, Y.G.; Nam, S.W.; Park, W.S. The diagnostic value of serum gastrokine 1 (GKN1) protein in gastric cancer. Cancer Med. 2019, 8, 5507–5514. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Philpott, H.; Singh, R. Endoscopic diagnosis and treatment of gastric dysplasia and early cancer: Current evidence and what the future may hold. World J. Gastroenterol. 2021, 27, 5126–5151. [Google Scholar] [CrossRef] [PubMed]
- Zaanan, A.; Bouché, O.; Benhaim, L.; Buecher, B.; Chapelle, N.; Dubreuil, O.; Fares, N.; Granger, V.; Lefort, C.; Gagniere, J.; et al. Gastric cancer: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig. Liver Dis. 2018, 50, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Zagari, R.M.; Rabitti, S.; Greenwood, D.C.; Eusebi, L.H.; Vestito, A.; Bazzoli, F. Systematic review with meta-analysis: diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment. Pharmacol. Ther. 2017, 46, 657–667. [Google Scholar] [CrossRef]
- Zan, X.; Chen, Z.; Guo, Q.; Zhang, Z.; Ji, R.; Zheng, Y.; Zhang, J.; Wu, Z.; Wang, X.; Ye, Y.; et al. The Association of Trefoil Factors with Gastric Cancer and Premalignant Lesions: A Cross-Sectional Population-Based Cohort Study. Cancer Epidemiology Biomarkers Prev. 2022, 31, 625–632. [Google Scholar] [CrossRef]
- Zhu, Q.; He, P.; Zheng, C.; Chen, Z.; Qi, S.; Zhou, D.; Li, Y.; Ouyang, Q.; Zi, H.; Tang, H.; et al. Identification and evaluation of novel serum autoantibody biomarkers for early diagnosis of gastric cancer and precancerous lesion. J. Cancer Res. Clin. Oncol. 2023, 149, 8369–8378. [Google Scholar] [CrossRef]
| Protein biomarker (cut-off values). | Studied cohort | Comparison conditions | AUC | Se % | Sp % | OR (95% CI) | Reference |
|---|---|---|---|---|---|---|---|
| Atrophic Gastritis (AG) | |||||||
| G-17 | 519 (446 H ; 35 Hp-gastritis ; 38 AG) | *AGA vs H | 0.402 | 10.7 | 69.5 | NA | Koivurova et al., 2021 |
| AGA2+ vs H | 0.429 | 15.4 | 70.4 | ||||
| PGI (15 ng/ml) | **AGC vs H | 0.777 | 55.6 | 99.8 | |||
| AGC2+ vs H | 0.878 | 76.0 | 99.6 | ||||
| PGI (30 ng/ml) | **AGC vs H | 0.858 | 72.2 | 99.4 | |||
| AGC2+ vs H | 0.954 | 92.0 | 98.8 | ||||
| PGI/PGII (3) | **AGC vs H | 0.885 | 77.8 | 99.2 | |||
| AGC2+ vs H | 0.993 | 100 | 98.6 | ||||
|
Gastropanel® : PGI/PGII ; G-17 ; Anti-Hp |
344 (196 H ; 148 AG) | AG vs H | NA | 39.9 | 93.4 | NA | Chapelle et al., 2020 |
| AGC vs H | 61.0 | 98.5 | |||||
| PGI (≤30 ng/ml) | 356 (113 H ; 91 NAG ; 152 AG) | AGC2+/AGAC2+ vs H/NAG | 0.856 | 77.8 | 83.8 | NA | Chapelle et al., 2022 |
| PGI (≤20.2 ng/ml) | AGC2+/AGAC2+ vs H/NAG | 0.856 | 77.8 | 95.6 | |||
| PGI/PGII (≤3) | AGC2+/AGAC2+ vs H/NAG | 0.859 | 75 | 92.6 | |||
| PGI/PGII (≤0.96) | AGC2+/AGAC2+ vs H/NAG | 0.859 | 72.2 | 98 | |||
| IL-6 | AGA2+ vs H/NAG | 0.588 | 72.2 | 41.2 | |||
| PGI/PGII + HE-4 | AG2+ vs H/NAG | 0.684 | 40.7 | 96.1 | |||
| PGI / PGII | 72 (48 H ; 12 CAG ; 9 IM ; 3 GC) | CAG/IM vs H | 0.902 | 83.3 | 77.9 | NA | Loong et al., 2017 |
| Anti-ATP4A | 218 (111 H ; 107 AGC) | AGC vs H | 0.826 | 75 | 88 | NA | Lahner et al., 2020 |
| Anti-ATP4B | 0.838 | 77 | 88 | ||||
| PCA | 0.805 | 69 | 91 | ||||
| PGI | 0.775 | 73 | 80 | ||||
| Intestinal Metaplasia (IM) | |||||||
| CCL3/MIP1A | 174 (75 NAG ; 95 IM ; 4 IM/DYS) | IM/DYS vs NAG | NA | NA | NA | 3.08 (1.23-7.68) p=0.027 |
Song et al., 2019 |
| CCL20/MIP3A | 2.69 (1.10-6.57) p=0.049 |
||||||
| IL-1ß | 2.39 (1.02-5.60) p=0.047 |
||||||
| IL-4 | 3.02 (1.29-7.12) p=0.009 |
||||||
| IL-5 | 3.07 (1.32-7.14) p=0.007 |
||||||
| IL-17A | 135 (45 H ; 45 NAG ; 45 IM/DYS) | IM/DYS vs NAG IM/DYS vs H NAG vs H |
0.62 | NA | NA | NA | Della Bella et al., 2023 |
| 0.67 | |||||||
| 0.64 | |||||||
| CRP (>1.95 mg/l) | 236 (70 H ; 68 GAG ; 98 GIMG) | GAG/GIMG vs H | 0.77 | 66.3 | 77.6 | NA | Kutluana et al., 2019 |
| TFF3 | 288 (164 H ; 110 GIM ; 14 GC) | GIM vs H | 0.58 | 55.5 | 58.5 | 1.2 (0.87-1.65) p=0.273 |
Latorre et al., 2022 |
| 2,980 (1,659 H ; 1,321 IM) | IM (OLGIM III-IV) vs IM (OLGIM 0-II) | 0.749 | NA | NA | NA | Lee et al., 2022 | |
| 3,986 (773 H ; 746 CAG ; 1,002 IM ; 1,334 LGD ; 131 GC) | IM vs H | NA | NA | NA | 1.92 (1.64-2.25) p<0.001 |
Zan et al., 2022 | |
| Anti-Omp | 1,402 (512 H ; 890 PGL) | PGL vs H | NA | NA | NA | 5.37 (4.20-6.89) p<0.0001 |
Epplein et al., 2018 |
| Anti-HP0305 | NA | 3.85 (3.04-4.88) p<0.0001 |
|||||
| Anti-Omp+Anti-HP0305 | 0.751 | 7.43 (5.59-9.88) p<0.001 |
|||||
| anti-HP1177/Omp27 |
Validation, 200 (100 NAG ; 100 IM) |
IM vs NAG | 0.73 | NA | NA | 8.08 ; p< 0.001 | Song et al., 2023 |
| anti-HP0547/CagA | 0.77 | 4.64 ; p< 0.001 | |||||
| anti-HP0596/Tipa | 0.66 | 3.97 ; p=0.002 | |||||
| anti-HP0103/TlpB | 0.68 | 3.83 ; p=0.001 | |||||
| anti-HP1125/PalA/Omp18 | 0.65 | 3.08 ; p=0.001 | |||||
| anti-HP0153/RecA | 0.55 | 0.48 ; p=0.030 | |||||
| anti-HP0385 | 0.57 | 0.41 ; p=0.006 | |||||
| anti-HP0243/NapA | 0.63 | 0.39 ; p=0.016 | |||||
| anti-HP0371/AccB /FabE | 0.65 | 0.37 ; p=0.017 | |||||
| anti-HP0900/HypB | 0.50 | 0.35 ; p= 0.048 | |||||
| anti-HP0709 | 0.61 | 0.30 ; p= 0.003 | |||||
| Panel of the 11 anti-HP | 0.81 | NA | |||||
| Early Gastric Cancer (EGC) | |||||||
| GKN1 (4.94 ng/ml) | 700 (200 H ; 140 EGC ; 360 AGC†) | GC vs H | 0.995 | 91.2 | 96 | NA | Yoon et al., 2019 |
| EGC vs H | 1.000 | 79.3 | 96 | ||||
| AGC† vs H | 1.000 | 95.8 | 96 | ||||
| CEA+CA724+IL-6+IL-8+TNFa | Discovery: 497 (204 H ; 117 AH ; 63 EGC ; 113 AGC†) | GC vs H | 0.95 | NA | NA | NA | Li et al., 2018 |
| EGC vs H | 0.95 | ||||||
| AGC† vs H | 0.95 | ||||||
| CA724+IL-6+IL-8+TNFa | GC vs AH | 0.97 | NA | NA | NA | ||
| EGC vs AH | 0.98 | ||||||
| AGC† vs AH | 0.96 | ||||||
| CEA+CA724+IL-6+IL-8+TNFa | Validation: 165 (66 H ; 41 AH ; 19 EGC ; 39 AGC†) | GC vs H | NA | 89.66 | 92.42 | NA | |
| EGC vs H | 84.21 | 90.91 | |||||
| AGC†vs H | 92.31 | 90.91 | |||||
| CA724+IL-6+IL-8+TNFa | GC vs AH | NA | 87.93 | 87.80 | NA | ||
| EGC vs AH | 78.95 | 85.37 | |||||
| AGC†vs AH | 92.31 | 90.24 | |||||
| SPRR2A (80.7 pg/ml) | 490 (100 H ; 100 CG ; 200 GC (I+II n=122, III+IV n=78) ; 40 RC ; 50 CC) | GC (I+II) vs H | 0.78 | 69.6 | 68.1 | NA | Xu et al., 2020 |
| ITIH4 (171.2 ng/ml) | 400 (178 H ; 37 Hpi ; 28 LGN ; 38 EGC ; 70 AGC† ; 49 OST) | EGC vs H | 0.8394 | 73.08 | 94.44 | NA | Sun et al., 2021 |
| Signature of 19 proteins: CEACAM5+CA9+MSLN+CCL20+SCF+TGFa+MMP-1+MMP-10 +IGF-1 +CDCP1+PPIA+DDAH-1 +HMOX-1 +FLI1+IL-7+ZBTB-17 +APBB1IP+KAZALD-1+ADAMTS-15 |
150 (50 H ; 100 GC (I n=8, II n=20, III n=57, IV n=14, missing n=1)) | GC (I+II) vs H | 0.99 | 89 | 100 | NA | Shen et al., 2019 |
| TFF3 | 155 (44 H ; 111 GC (I n=42, II n=39, III n=27, IV n=3)) | GC (I) vs H | 0.703 | 83.3 | 54.5 | NA | Choi et al., 2017 |
| CDH17 | GC (II+III) vs H | 0.667 | 77.3 | 61.4 | |||
|
HPT Asn-211: Hex6HexNAc5Fuc1NeuAc1 HPT Asn-241: Hex6HexNAc5Fuc1NeuAc1Hex7HexNAc6Fuc1 |
25 (15 H ; 10 GC (I n=5, III-IV n=5)) | GC (I) vs H | 1 | 100 | 100 | NA | Lee et al., 2018 |
| THBS2 | 120 (41 H ; 33 BGT ; 46 EGC) | EGC vs H | 0.816 | NA | NA | NA | Li et al., 2021 |
| EGC vs BGT | 0.840 | ||||||
| CA19-9 | EGC vs H | 0.901 | |||||
| EGC vs BGT | 0.847 | ||||||
| THBS2 / CA19-9 | EGC vs H | 0.951 | |||||
| EGC vs BGT | 0.928 | ||||||
| THBS1 | 89 (29 H ; 31 EGC ; 29 AGC†) | EGC vs H | 0.646 | NA | NA | NA | Yoo et al., 2017 |
| AGC† vs H | 0.656 | ||||||
| Clusterin isoform 1 | EGC vs H | 0.878 | |||||
| AGC† vs H | 0.937 | ||||||
| Vitronectin | EGC vs H | 0.756 | |||||
| AGC† vs H | 0.833 | ||||||
| Tyrosine protein kinase SRMS | EGC vs H | 0.887 | |||||
| AGC† vs H | 0.856 | ||||||
| AHSG | Validation: 130 (28 H ; 42 GC (I n=5, II n=11, III n=21, IV n=5) ; 30 CRC ; 30 HCC) | GC vs H | 0.93 | NA | NA | NA | Shi et al., 2018 |
| GC (I+II) vs H | 0.82 | ||||||
| FGA | GC vs H | 0.98 | |||||
| GC (I+II) vs H | 0.98 | ||||||
| APOA-1 | GC vs H | 0.83 | |||||
| GC (I+II) vs H | 0.96 | ||||||
| 9 TAA-panel: c-Myc+p16+HSPD1+PTEN+p53+NPM1+ ENO1+p62+HCC1.4 |
814 (407 H ; 407 GAC (I n=67, II n=87, III n=142, IV n=40, unknown n=71)) | GC vs H | 0.857 | 71.5 | 71.3 | NA | Qin et al., 2019 |
| GC (I+II) vs H | 0.737 | 64.9 | 70.5 | ||||
|
Training: 410 (205 H ; 205 GAC (I n=38, II n=42, III n=75, IV n=33 ; NA n=17)) Validation: 252 (126 H ; 126 GAC (I n=15, II n=15, III n=27, IV n=7 ; NA n=62)) |
GC (I+II) vs H | Yang et al., 2020 | |||||
| TAA-panel I: p53+COPB1+GNAS+PBRM1+ACVR1B |
|||||||
| Panel I, | |||||||
| Training | 0.885 | 66.7 | 94.6 | NA | |||
| Validation | 0.821 | 76.7 | 83.3 | ||||
| TAA-panel II: p53+SMARCB1+COPB1+SRSF2+GNAS |
|||||||
| Panel II, | |||||||
| Training | 0.869 | 74.7 | 90.3 | NA | |||
| Validation | 0.876 | 76.7 | 80.9 | ||||
| RAE1 aAbs | 364 (122 H ; 51 PL ; 78 EGC ; 113 AGC†) | EGC vs H | 0.745 | 94.9 | 47.1 | NA | Zhu et al., 2023 |
| PGK1 aAbs | 0.648 | 41 | 87.6 | ||||
| NPM1 aAbs | 0.611 | 20.5 | 100 | ||||
| PRDX3 aAbs | 0.613 | 88.5 | 65.2 | ||||
| UBE2N aAbs | 0.585 | 24.4 | 95 | ||||
| ARF4 aAbs | 0.488 | 11.5 | 98.3 | ||||
| ANXA2 aAbs | 0.563 | 19 | 86.9 | ||||
| ANOS1 | 367 (66 H ; 301 GC (I n=225, II n=47, III n=26, IV n=3)) | GC vs H | 0.7058 | 36 | 85 | NA | Kanda et al., 2020 |
| GC (I) vs H | 0.7131 | 36 | 85 | ||||
| DPYSL3 | GC vs H | 0.6188 | 48 | 82 | |||
| GC (I) vs H | 0.5948 | 45 | 82 | ||||
| MAGED2 | GC vs H | 0.5031 | 28 | 92 | |||
| GC (I) vs H | 0.5113 | 27 | 92 | ||||
| TrxR activity |
Before clinical intervention: 261 (130 H ; 131 GC (I n=25, II n=39, III n=46, IV n=21) |
GC (I) vs H | 0.948 | 80.00 | 97.69 | NA | Peng et al., 2019 |
| GC (II) vs H | 0.955 | 84.62 | 97.69 | ||||
| GC (III) vs H | 0.971 | 89.13 | 96.15 | ||||
| GC (IV) vs H | 0.974 | 90.48 | 97.69 | ||||
| GC vs H | 0.963 | 85.5 | 97.69 | ||||
| CEA+CA19-9+CA72-4 | GC vs H | 0.834 | 78.41 | 96.92 | |||
| CEA+CA19-9+CA72-4+TrxR | GC vs H | 0.982 | 91.60 | 94.62 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





