1. Introduction
Cardiac arrest is common and may result in significant hypoxic-ischemic injury. According to the American Heart Association, there are more than 356,000 out of hospital cardiac arrests annually in the US [
1,
2,
3]. Although resuscitation practices have greatly improved over the years, the outcome remains poor [
3] with survival from out-of-hospital cardiac arrest remaining under 10% [
1,
2]. Even when patients are resuscitated in the hospital, fewer than 20% patients are discharged home [
4,
5,
6].
By the time cardio-pulmonary resuscitation (CPR) restores adequate perfusion, the brain may have been critically injured [
3] and this risk is greater with longer periods of hypoxia. Neurological recovery sufficient to lead an independent life occurs in only a small proportion of patients, whilst others are left with significant neurological disability. Hypoxic-ischemic encephalopathy may lead to coma, a vegetative state, seizures, myoclonic jerks, or status epilepticus. Patients that awake from coma generally do so within 3 days after CPR, or significant neurologic impairment can be expected [
3,
7]. The prognosis of such patients with post anoxic coma is a challenging task for physicians.
Prediction of neurological outcome after resuscitation from cardiac arrest is an important component of the management of comatose resuscitated patients. Early and accurate identification of patients with an expected favourable neurological recovery is beneficial [
3,
8,
9] and allows discussions with families around withdrawal of life sustaining treatment for those with poor chances of outcome. Clinical examination features such as absent pupillary light response or corneal reflexes, extensor or no motor response to pain after 3 days of assessment, and myoclonic status epilepticus combined with electrophysiological procedures can identify recognised markers of poor outcome after CPR [
3,
7,
10].
Electroencephalography (EEG), a non-invasive technique to monitor neuronal activity, is widely used to assess neurological status after cardiac arrest, both as a diagnostic and prognostic tool, and may reveal subclinical seizures. Both EEG and SSEP [
3,
10,
11,
12,
13,
14,
15] can be highly specific for differentiating between poor versus favourable prognosis if they are applied to appropriate patient populations as there are recognised patterns with prognostic significance. However, they should not be considered in isolation but in combination with other assessments. A multimodal approach to prognostication, that includes the use of EEG, may particularly improve early prediction of clinical evolution after cardiac arrest [
3,
10,
11,
12,
13,
14,
15].
Over the years, many EEG scoring categories or grading have been used. An initial grading system for prognostication after cardiac arrest was published with 5 different grades (I-V) based on the dominant frequency or presences of abnormal patterns [
9,
16,
17,
18]. Subsequently in 1970 [
19] the effects of reactivity of the EEG to stimuli was added increasing the diagnostic accuracy of the EEG grading scale.
More recently the American Society of Clinical Neurophysiology (ACNS) provided standardised critical care EEG terminology to include a classification for the background and other relevant patterns, initially in 2012 [
20] and revised in 2021 [
21]. This new terminology maximises inter-rater reliability [
15,
22,
23,
24,
25].
Three categories to reliably predict outcome in comatose patients following cardiac arrest have been proposed [
26] and validated [
15] based on the standardised interpretation of EEG according to these ACNS guidelines:
Highly malignant
- -
Suppressed background without Discharges
- -
Suppressed background with Continuous Periodic Discharges
- -
Burst-suppression background (with or without discharges).
- -
Malignant EEG
- -
Periodic or rhythmic patterns (abundant periodic discharges; abundant rhythmic polyspike-/spike-/sharp-and-wave; unequivocal electrographic seizure)
- -
Malignant background (discontinuous background; low voltage background; reversed anterior-posterior gradient)
- -
Reactivity (absence of background reactivity or only stimulus-induced discharges).
- -
Benign EEG
- -
Absence of all malignant features stated above.
Subsequent work has shown the importance of these categories and early EEG evaluation for prognosis in patients following cardiac arrest [
25,
27]
The aim of the present study is to retrospectively evaluate the patterns seen on EEG in patients with post-anoxic coma following cardiac arrest in a single centre and their relationship to outcome, evaluating in the context of the existing literature on EEG patterns in this clinical situation. We chose to focus solely on EEG findings but acknowledge this forms only part of a multimodal prognostic approach.
2. Materials and Methods
2.1. Patient Cohort and Outcome Assessment
We retrospectively reviewed medical records and EEG recordings of patients with post-anoxic coma due to cardiac arrest admitted to the Intensive Care Unit (ICU) at Kingston General Hospital, Ontario, Canada. We included all adult patients (18 years or over) from 2017 to 2020 that were referred for EEG. Data extracted included etiology, age, gender, seizures, myoclonus, sedation with propofol, therapeutic hypothermia (TH), imaging findings (cortical and subcortical injuries or both) and outcome.
The targeted body temperature for TH is usually 33-36 degrees Celsius [
28]. Highly Malignant and malignant patterns may occur during the initial period after resuscitation and during the target temperature management among survivors who have shown good outcome [
12]. Thus, EEGs recorded during therapeutic hypothermia were replaced by subsequent EEG after rewarming to a temperature of >36 degrees Celsius where available.
Patient outcome was assessed at a follow up rehabilitation appointment within 3-6 months (for those who did not die during or shortly after admission) using the Cerebral Performance Category where 1 is normal or mild disability, 2 is moderate disability (but independent), 3 is severe disability, 4 is unconscious (coma/vegetative state) and 5 is brain death [
6,
24]. We dichotomised outcome into CPC 1-2 (favourable) and CPC 3-5 (unfavourable) [
27,
29,
30].
Ethics approval for retrospective use of clinically acquired data was obtained from the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board (DMED-2413-20).
2.2. Analysis of EEG Recordings
Patients underwent routine digital EEG recordings for at least 20 minutes with 23 scalp electrodes placed according to the 10–20 International System of electrode placement, reformatted to both bipolar and referential montages. The filter settings were 0.5 Hz and 70 Hz. Sedation was stopped prior to all recordings.
Data extracted from qualitative review of EEG included background activity, presence of myoclonus and/or seizures during EEG, presence of rhythmic patterns or periodic patterns and reactivity of EEG upon stimulation. All EEG recordings were interpreted by board-certified epileptologists.
Reactivity of the EEG was defined as distinct changes in amplitude or frequency or attenuation of background following external stimuli. Stimuli were tactile or nociceptive stimulation, auditory stimuli (clapping, voice sounds) or passive eye opening and suctioning for deep stimulation. Increase in muscle artifacts and or movement during stimulation was not considered reactivity [
8,
20,
25].
We classified recordings using the categories described previously (highly malignant, malignant, benign) based on ACNS terminology [
15], but did not include reactivity as a separate category as there were no recordings demonstrating a lack of reactivity that were not already classified as highly malignant or malignant. We instead quote the proportion in each category that show reactivity. Where several EEG recordings existed in the same patient, we also evaluated the change over time from the initial EEG pattern and its relationship to outcome.
2.3. Neuroimaging Findings
We reviewed the neuroimaging findings based on the reports and classified into 5 categories. Classical findings of hypoxic-ischemic injury include loss of grey/white matter differentiation and edema on CT, or diffusion restriction on MRI [
31]. We divided these findings into diffuse hypoxic injury where changes were widespread (e.g. multiple cortical areas, cortex and basal ganglia) and limited when present in a single cortical or subcortical area. Other findings included acute ischemic changes (e.g. stroke), hemorrhage (both intraparenchymal and subdural) and unremarkable (including incidental findings such as atrophy or microvascular disease). We used MRI findings where available, and if not a CT scan.
3. Results
3.1. Characteristics of Cohort
A total of 81 patients were identified for the study, with a time delay from cardiac arrest to referral for EEG recording varying from 2 to 14 days. The final cohort was 81 patients (57 male, 24 female, aged 18 to 87 years,
Table 1) in whom 18 (22%) of patients had a favourable outcome (CPC 1-2), whilst 63 (78%) had unfavourable outcome (CPC 3-5).
Causes of cardiac arrest included ventricular fibrillation (VF), myocardial infarction (MI), pulseless electrical activity (PEA), respiratory arrest, airway obstruction, pulmonary embolism (PE), sepsis, aortic dissection, and hemorrhagic stroke (
Table 2). The indications given for EEG referral included decreased level of consciousness, myoclonic jerks or twitching post cardiac arrest, possible seizure post cardiac arrest, and agitation/confusion (
Table 3).
Only a single patient who had highly malignant EEG during therapeutic hypothermia and warming did not have a follow up EEG, and who had an outcome of CPC 5 (death).
3.2. General EEG Features and Outcomes
General EEG features and outcomes are noted in
Table 1. None of the patients who had seizures or were categorised as highly malignant on EEG survived. For those with myoclonus, only a single patient survived (1 of 18, 6%) but most of these patients also had a highly malignant EEG pattern.
Reactivity was seen on the EEG in 25/80 (31%) of the patients (one patient was not tested, and one showed Stimulus-Induced Rhythmic, Periodic, or Ictal Discharges (SIRPIDs)). Most patients (72%) with reactive EEG survived, and the majority (62%) had a favourable outcome.
Of the 40 patients who had follow up EEGs, 9 (23%) showed improvement, as detailed below. All patients demonstrating improvement on EEG survived and most (67%) had a favourable outcome.
3.3. Specific EEG Patterns and Outcomes
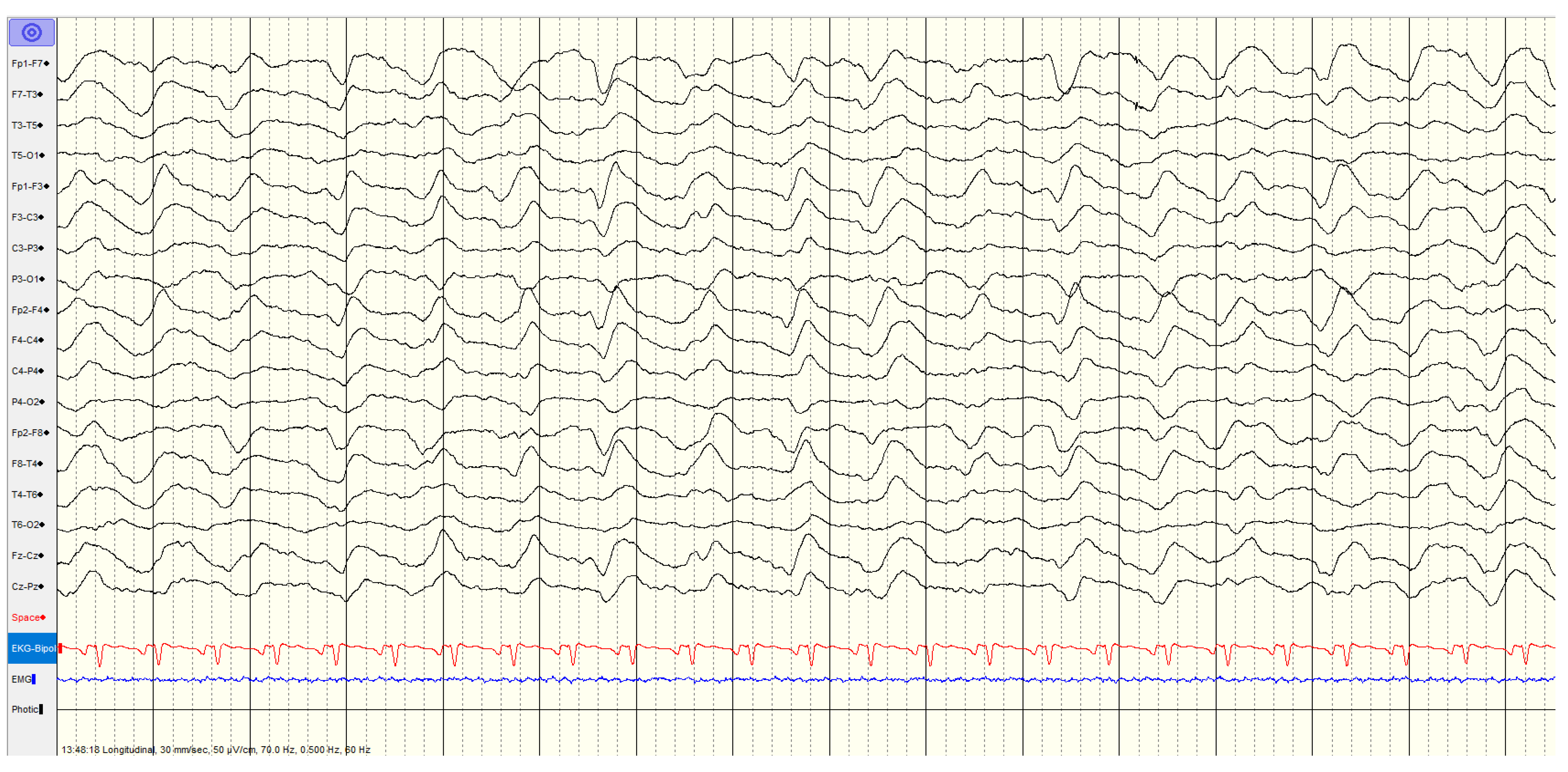
3.3.1. High Malignant EEG Pattern
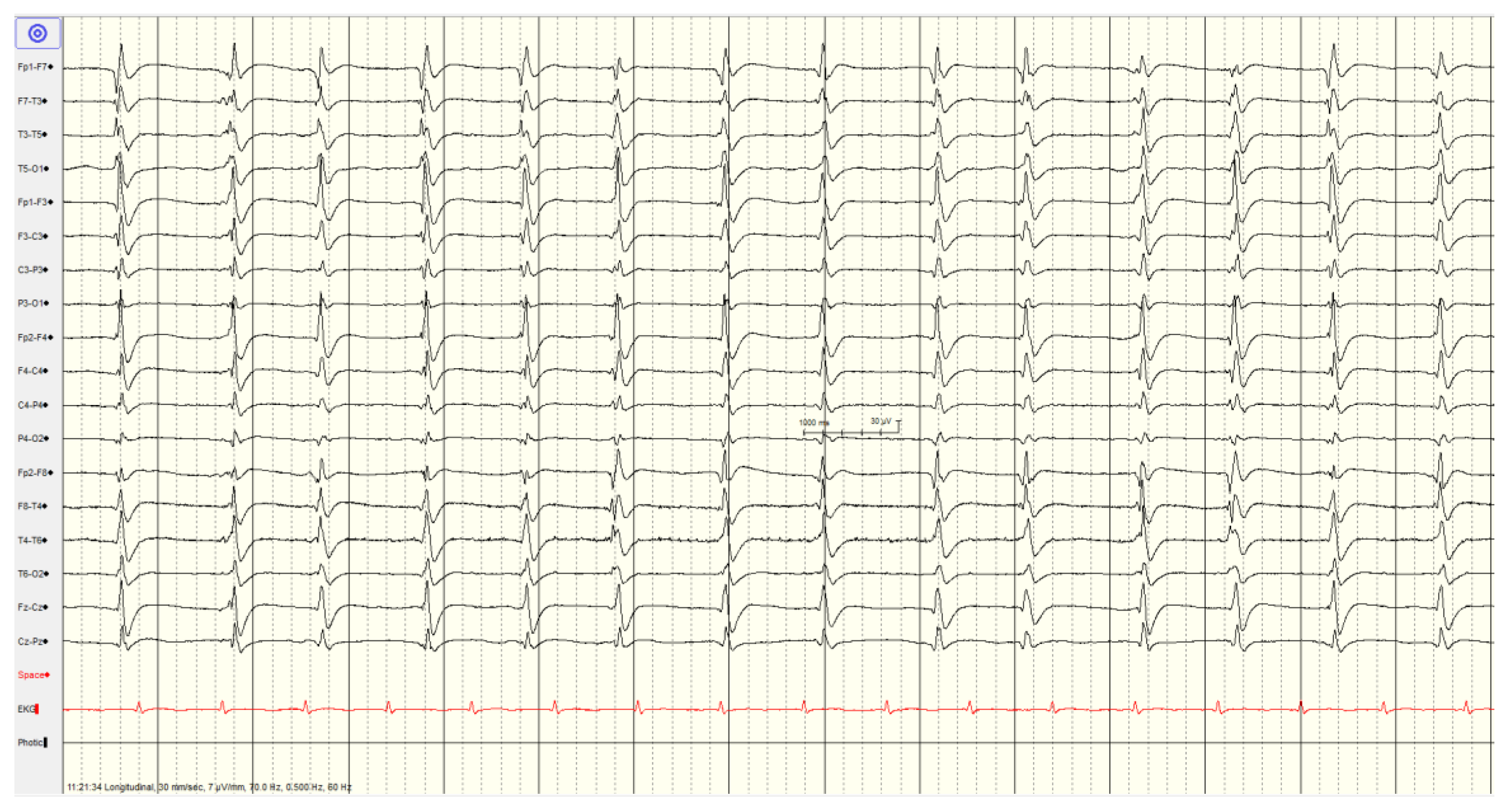
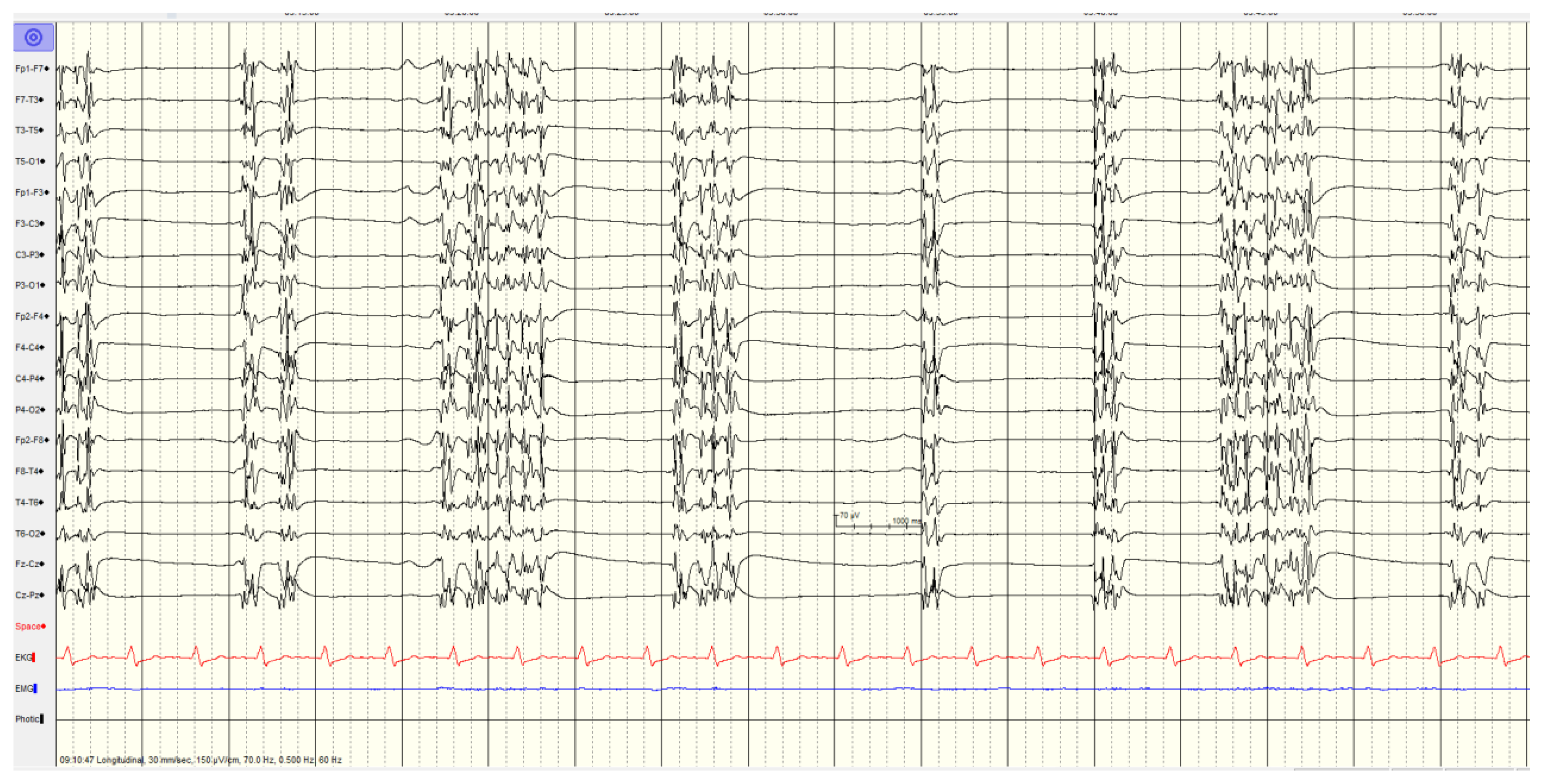
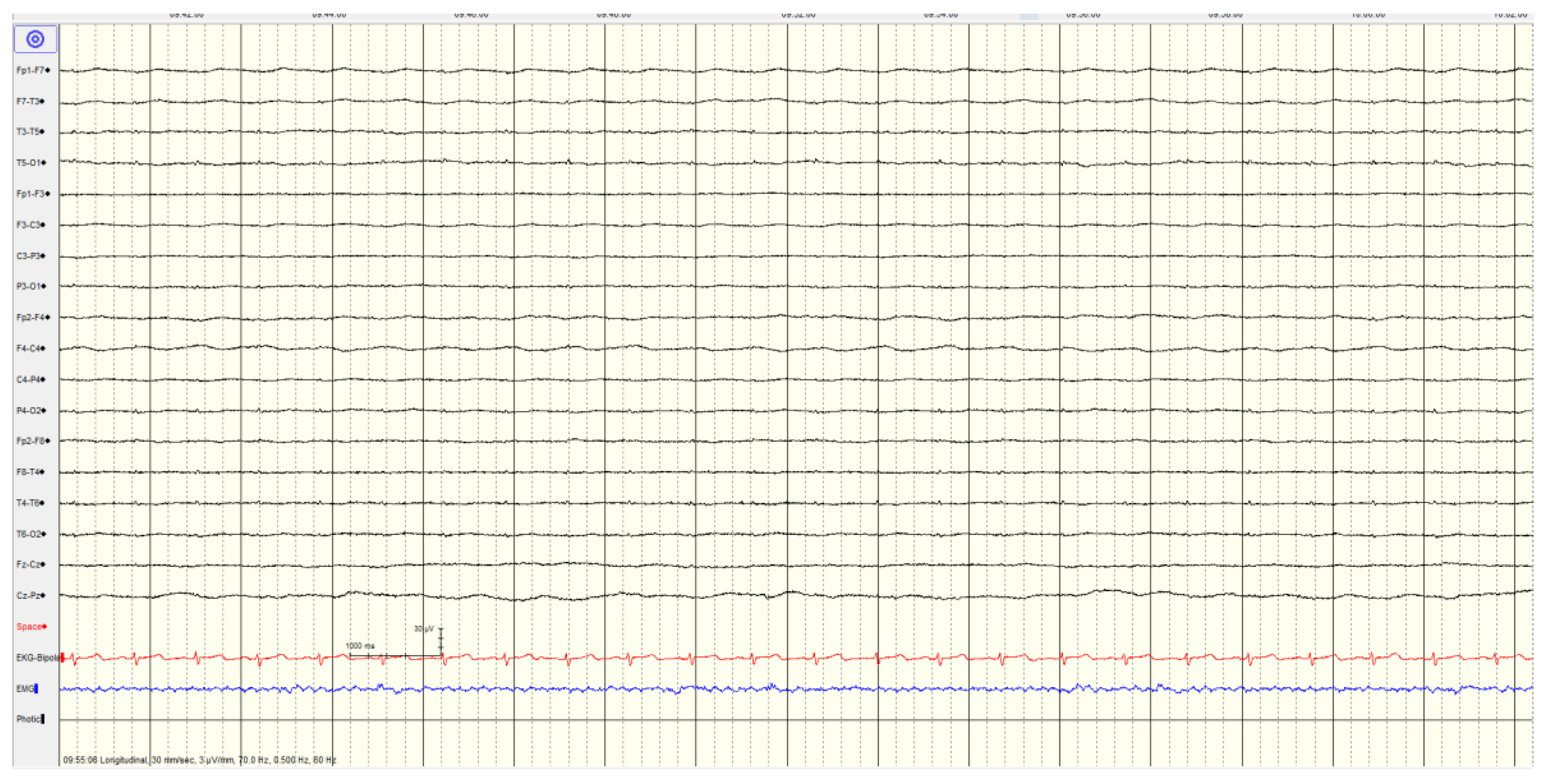
Overall, 46 (57%) of patients showed a
highly malignant EEG pattern (
Figure 1,
Figure 2 and
Figure 3). None of these patients showed reactivity on EEG or improvement on a subsequent EEG when performed, and none in this category survived.
The most common pattern, representing 23 patients, was a suppressed background with continuous periodic discharges, of whom around two thirds also had seizures or myoclonus and one patient demonstrated SIRPIDs (which showed generalized discharges and seizures). Most (12/22, 55%) had diffuse hypoxic changes on imaging whereas the remainder showed more limited hypoxic changes (n=5), acute ischemic changes (n=2) or were unremarkable (n=3).
In category of burst-suppression background with or without generalized spike and slow wave discharges the recordings with a burst-suppression background with generalized spike and slow wave discharges, all patients had seizures or myoclonus, whereas only a single patient without spike wave discharges had seizures. Just over half (5/9, 56%) had diffuse hypoxic changes on imaging with the other 4 scans being unremarkable.
A suppressed background (<10 μV) without discharges was not associated with seizures or myoclonus. Most (9/10, 90%) had diffuse hypoxic changes on imaging with the last having only ischemic changes.
3.3.2. Malignant EEG Pattern
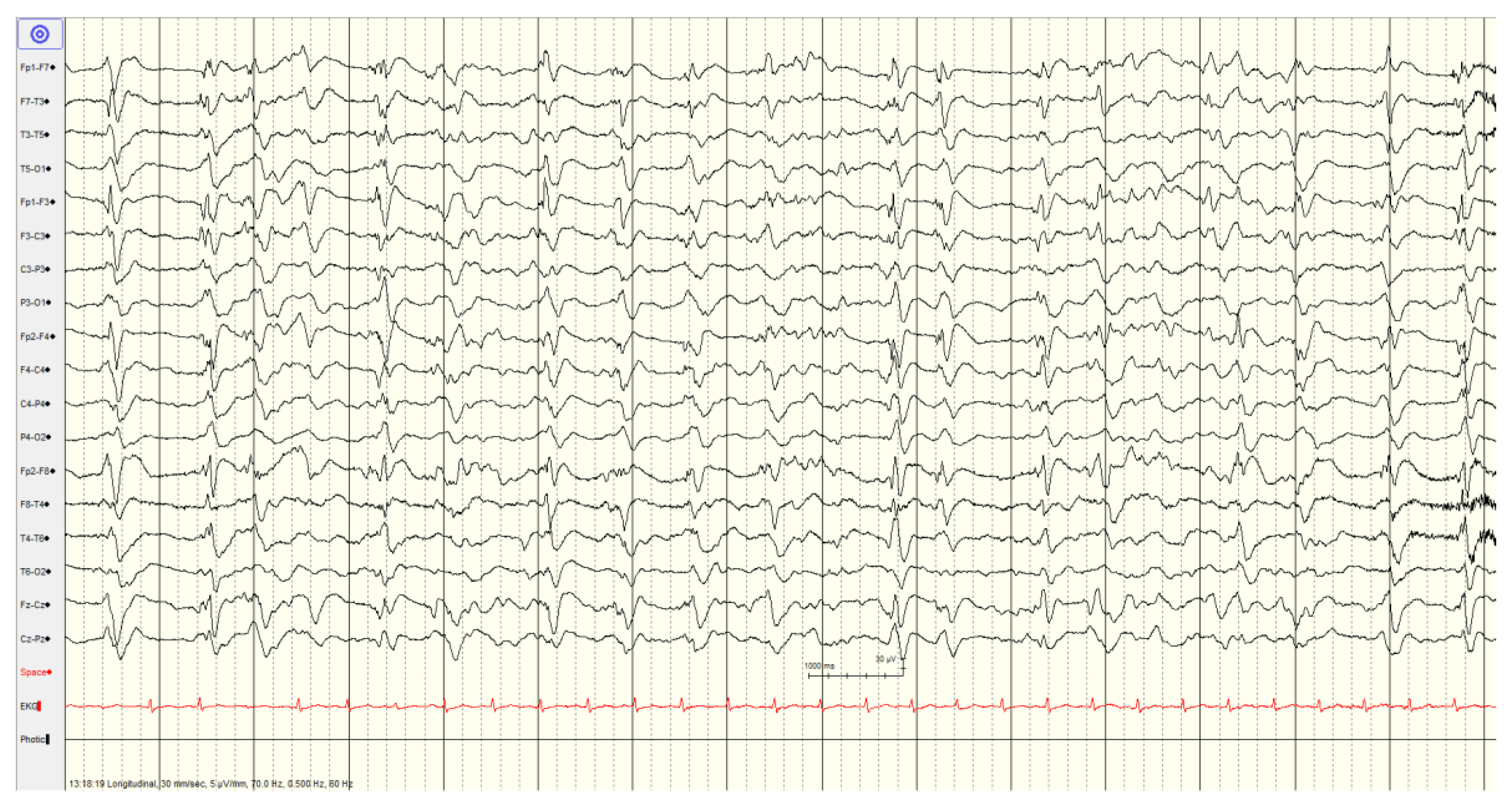
A total of 33 (41%) had a
malignant EEG pattern (
Figure 4 and
Figure 5). Overall survival in this category was 70%, although only 48% had a favourable outcome.
In the first malignant category pattern of abundant periodic discharges or rhythmic patterns (polyspike-/spike-/sharp-and-wave), there were 11 patients. Four of the EEGs showed generalized as well as lateralized discharges, some showed lateralized periodic discharges. A single patient with sharps at the vertex, associated with myoclonus, survived. Most patients in this category demonstrated reactivity (64%) and the majority who had follow-up EEG (time range 1-4 days) demonstrated improvement (71%). Improvements included one patient who developed sleep architecture, 3 patients with improved background activity and a final patient with decreased slowing and sharp activity. Only two patients showed an increase in slowing or burst suppression pattern. Overall favourable outcome in this subcategory was 36%. Only 2/11 (18%) had diffuse hypoxic changes on imaging with the rest demonstrating more limited hypoxic changes (n=1), ischemic changes (n=4), hemorrhage (n=1) or being unremarkable (n=3).
In the second subcategory of the malignant pattern with discontinuous background; low voltage background; reversed anterior-posterior gradient, the majority showed reactivity (73%) and when repeated (after 1-4 days) 60% showed an improvement in background activity. The others showed an increase in slowing or lower amplitude EEG. There was 82% survival in this group with a favourable outcome (CPC1-2) in 55%. The EEG in this group did not show epileptic activity (spike/sharp and slow wave pattern). Few (3/22, 14%) had diffuse hypoxic changes with the rest showing more limited hypoxic changes (n=1), ischemic changes (n=5), hemorrhage (n=3) or being unremarkable (n=10).
3.3.3. Benign EEG Pattern
The remaining 2 patients (2%) showed a benign EEG pattern (absence of all malignant features). All showed reactivity, and all survived with favourable outcome. Neuroimaging studies were unremarkable.
4. Discussion
4.1. Main Findings of the Study
We systematically report the EEG findings and their relationship to outcome in a single centre cohort of patients with post-anoxic coma following cardiac arrest. In line with prior literature, we found that most patients (79%) had an unfavourable outcome. A highly malignant pattern, seizures and myoclonus on EEG recordings were each highly associated with unfavourable outcome whereas patients with EEG reactivity or demonstrating improvement on follow-up EEG were more likely to have a favourable outcome.
4.2. Role of EEG in Prognostication
EEG has developed significantly as an important tool since the initial important observations regarding EEG abnormalities, made by Gibbs, Gibbs and Lennox, in comatose and altered conscious state patients [
28]. Many EEG grading processes have since been suggested including Hockaday et al in 1965 [
16].
The American Society of Clinical Neurophysiology (ACNS) established standardized terminology and criteria for interpreting critical care EEGs in 2012 [
20] that has since been updated in 2021 [
21]. For potential use in prognostication, the consistent use of EEG terminology is essential.
We used the 2012 criteria since the EEGs were recorded and interpreted prior to the publication of the newer guidelines, and the definitions of highly malignant and malignant are based on this. However, the changes made in 2021 do not affect the definitions we use.
4.3. EEG Background
EEG background activity can help to predict favourable outcome in patients after cardiac arrest [
32]. The voltage of background EEG is defined as low when most or all activity is <20 μV (measured from peak to peak) in longitudinal bipolar montage with standard 10–20 electrodes, while suppression is defined as all voltage being <10 μV [
8,
12]. Amplitude of the EEG signal may depend on the effect of drugs, body temperature, and on a variety of technical conditions such as electrode-scalp impedance, inter-electrode distances, type and placement of the electrodes, and type of filters adopted.
The burst suppression EEG pattern is alternate bursts of EEG activity of variable duration and amplitude alternate with periods of suppression or flattening. Bursts consist of high amplitude slow activity, or spike and wave, or poly spike and slow wave complexes that are epileptic [
11]. In cardiac arrest, the physiology is not clear, but it is generally accepted that burst suppression occurs from “dissociation of the cortex from the intrinsic pacemaker of neurons in the reticular thalamus” [
11]. This indicates severe injury to the thalamus, cerebral cortex and the interconnecting relay circuits [
11,
15]. This pattern is usually noted when there is severe cortical damage [
23].
Low amplitude or burst suppression patterns observed after an anoxic episode may be considered reversible in the absence of sedation [
24]. Although EEG may be transiently suppressed early on after cardiac arrest, it may subsequently recover with dynamic changes seen in EEG patterns after cardiac arrest [
11,
13]. Persistence of suppression after 24-72 hours or non-reactivity is associated with unfavourable outcome [
24]. Thus, for predictive purposes the timing of EEG recording is important [
24]. In our study, EEG recordings were primarily recorded beyond this initial period based on the referral pattern.
These EEG patterns are considered part of the highly malignant category, especially burst suppression with identical (monomorphic) burst of spike and wave or sharps which is highly correlated with severe anoxic brain injury and specific for poor outcome [
15,
27]. Overall, most patients with highly malignant EEG patterns (including suppressed background without discharges, and suppressed background with continuous periodic discharges, or burst-suppression background with or without discharges) have poor outcome [
11,
12,
15,
17,
33]. In our study, no patient in this category survived.
4.4. Reactivity
EEG patterns showing reactivity and continuity, seem promising as prognostic indicators [
14,
19,
24,
25,
28]. EEG reactivity was tested by assessing reproducible changes in amplitude or frequency of EEG background following external stimuli, such as tactile or nociceptive stimulation, auditory stimuli (clapping, voice sounds) or visual, passive eye opening in step wise manner and noxious stimuli like sternum pressure or rub. These stimuli should be at short intervals to see the effect of stimuli, if any [
8,
12,
19]. Favourable outcome can still be seen with absence of EEG reactivity, although the outcome is generally worse with status myoclonus [
8]. Importantly, movement and muscle artifacts can not be marked as a reactive EEG [
8,
15,
25]. In our cohort, nearly all patients with reactivity survived and most (64%) had favourable outcomes.
4.5. Interictal Epileptiform Activity
Generalized Periodic Discharges (GPDs) are defined as the occurrence of periodic complexes occupying at least 50% of a standard 20-minute EEG recording, over both hemispheres in a symmetric, diffuse, and synchronized manner [
12] giving the appearance of burst suppression pattern. BIPDs are defined as bilateral independent periodic lateralized epileptiform discharges. Periodic epileptic discharges are defined as repetitive monotonous sharp transients occurring throughout the recording, typically every 1 to 3 seconds with some irregularity in the interval between discharges, without entraining into discrete electrographic seizures. Periodic discharges may be lateralised (LPDs), bilateral independent (BIPDs), generalized (GPDs) or multifocal [
12,
20].
Patients whose EEG shows epileptiform activity with a continuous background may be effectively treated with anti-seizure medications (ASM) [
8]. However, the difficulty is distinguishing generalized periodic discharge (GPDs) that are treatable with anti-seizure drugs from severe ischemic damage. Recommendations are that the EEG in these patients need to be evaluated regularly over time rather than just recorded once. Frequency and timing of follow up EEG would depend on the EEG findings along with clinical correlation.
4.6. Seizures
Seizures and myoclonus are associated with poor outcomes [
24,
34,
35]. When seizures occur early during the first few days, they are usually associated with unfavourable features like unreactive or suppressed EEG background [
14,
33]. EEG is also important for detecting subclinical, non convulsive seizures. Seizures can be missed especially in patients who are sedated [
15,
35]. Timing and duration of EEG monitoring is important, especially if the patient is undergoing TH or is on sedative treatment.
Myoclonus is sudden, brief shock-like muscle contractions, common in patients after cardiac arrest [
33,
35]. Myoclonus that occurs early, as generalised, synchronous and stereotyped and lasting for more than 30 minutes is usually referred to as status myoclonus [
35]. This is usually associated with worse outcome [
24,
33,
35] especially if the EEG is non-reactive. However favourable outcome has been reported in patients with status myoclonus [
36]. Myoclonus can be inhibited by sedation and neuromuscular blocking drugs.
Consistent with literature, all patients with seizures except a single patient with myoclonus and a malignant rather than highly malignant EEG had unfavourable outcomes in the current study. Continuous EEG (cEEG) monitoring can be helpful in active treatment of status epilepticus. cEEG is labour intensive and costly as it requires constant expert monitoring and real time reporting which can be difficult [
35]. It can be used to monitor EEG patterns during administration of ASM [
37]. The benefits are debatable and a viable alternative is prolonged 1-3 or 6 hours of cEEG monitoring or routine intermittent 30-minutes EEGs over a few days. Some centres may routinely use cEEG in all patients following cardiac arrest, but cEEG is rarely performed in our setting.
4.7. Benefits and Limitations of EEG
EEG is now a standard tool used to aid prognostication in patients with post-anoxic coma that provides useful information to influence decision making. However, the evaluation of EEG patterns in resuscitated and comatose patients after anoxic brain injury has its limitations. Just like clinical examination, the EEG is prone to interference from initial treatment of patients including sedation and therapeutic hypothermia.
It is well-known that EEG activity is affected by sedation [
9,
13,
15,
26,
38] and can cause various changes in the EEG. The EEG is either markedly suppressed or will show burst suppression by sedative and anesthetic drugs. But it is not known if or how the sedation affects or helps with the prognostic value of malignant EEG patterns. So, interpreting such EEGs needs caution. Moreover, the neuronal activity would already be affected by the anoxic episode immediately post resuscitation and is not stable. It could show any of the variety of patterns depending on the timing, after the anoxic episode, of the recording [
13,
39]. Early prognostication of neurological recovery is important given the ethical, family, and economic burden.
In the early post anoxic period EEG changes are dynamic, so the timing of recording is important in interpreting EEG [
8,
11] and it emphasises the benefit in repeating this investigation. The effects of sedation and therapeutic hypothermia may combine as decreased body temperature prolongs the metabolism particularly of sedation causing difficulty in knowing if residual sedation is still affecting the patient [
26] and consequently the EEG findings. Further, the interpretation of the EEG is complex and inclined to subjectivity. This is especially true of EEG recordings in ICU because of the variety of periodic and rhythmic patterns of uncertain clinical significance and the poor interrater agreement [
15,
22].
Such issues of subjectivity may be overcome by the more recent development and utilisation of objective quantitative EEG analyses. For example, the application of machine learning models to a combination of quantitative EEG metrics such as entropy and clinical data may be beneficial in predicting outcome of post-anoxic coma [
1,
3,
32]. This highlights that EEG data cannot be considered in isolation and must be considered with other clinical data and patient co-morbidities to best predict outcome.
4.8. EEG in the Context of Multimodal Prognostication
Current European guidelines [
40] suggest incorporating a variety of assessments for neurological prognostication alongside EEG including clinical assessment (Glasgow Motor Score, pupillary, corneal reflexes), SSEP, blood biomarkers (neurone-specific enolase, NSE) and brain imaging (CT or MRI). Our centre does not perform SSEP or have timely access to NSE so we cannot address these in the present study. Hence, we chose to focus solely on EEG for the purposes of this study but recognise this as a limitation. We do however report the number of scans showing diffuse hypoxic injury based on the reports in accordance with these guidelines, and there is a clear association with EEG findings – diffuse hypoxic injury was seen in 57% of those with highly malignant EEG, 15% of those with malignant EEG and none with benign EEG.
4.9. Future Work
Whilst all those with high malignant EEG did not survive, and all those with benign EEG did survive, the malignant EEG category warrants further study to understand how the other prognostic markers included in guidelines can help guide management in this group. Follow up EEG may be particularly beneficial in this group.
A recent sub-study of the Targeted Temperature Management trial 2 suggested that the specificity of the highly malignant EEG patterns in predicting poor neurological outcome may be lower in clinical practice than initially thought [
41]. The authors suggest combining the highly malignant EEG patterns with an unreactive background to improve sensitivity. However, all of patients with highly malignant EEG in our study did also have an unreactive background.
5. Conclusions
Cardiac arrest is common and hypoxic-ischemic injury carries a high mortality. EEG recordings form a key part of a multimodal evaluation for prognosis. We describe the prognostic role of EEG in a well characterised cohort of patients from our centre. Highly malignant EEG, seizures and myoclonus were associated with unfavourable outcome, while reactive EEG and improvement on follow up were associated with better outcomes. Patients with malignant EEG patterns need to be looked at more critically as noted the outcome can be variable and is more likely to be favourable. In our study, an EEG pattern of diffusely slow or with reversed anterior/posterior gradient without clear spike-and-wave discharges was more favourable, a finding which requires further validation. Other prognostic markers including clinical assessment, biomarkers and neuroimaging are essential considerations. Accurate Early prognostication of neurological recovery is important given the ethical, family, and economic implications of treatment.
Author Contributions
Conceptualization: Z.S., G.P.W.; Methodology: Z.S., G.P.W.; Formal analysis: Z.S., G.P.W.; Investigation: Z.S., N.D., C.M., T.W., M.E., L.C., L.B.L., G.S., G.P.W.; Data Curation: Z.S., N.D., C.M., G.P.W.; Writing – Original Draft: Z.S.; Writing – Review & Editing: N.B., C.M., T.W., M.E., L.C., L.B.L., G.S., G.P.W.; Supervision: G.P.W.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board (protocol code DMED-2413-20, date of approval October 14, 2020).
Conflicts of Interest
L.B.L accepts consulting fees from Eisai, UCB, and Paladin. L.B.L. also received honorarium from Eisai, UCB, Sunovion, and Paladin and support for attending meetings from Eisai and UCB. Finally, L.B.L. also has a role in the advisory boards of UCB and the Canadian League Against Epilepsy (CLAE). G.S. holds grants from the Canada Foundation of Innovation (CFI) at the Kingston General Hospital Research Institute and PSI Foundation and has received an honorarium from Paladin Pharmaceuticals for an invited lecture and expert panel discussion as well as an honorarium from Elsevier for editorship of Sleep Medicine. G.P.W. holds grants from the PSI Foundation, Canadian Institutes of Health Research and SEAMO Innovation Fund, has received speaker honoraria from Paladin and Sunovion, travel support from Paladin Pharmaceuticals and has acted on the advisory board for Paladin and Jazz Pharmaceutical. The other authors report there are no competing interests to declare.
References
- Callans, D.J., Out-of-Hospital Cardiac Arrest — The Solution Is Shocking. N Engl J Med 2004. 351: p. 632-634, . [CrossRef]
- Newman, M. Cardiac Arrest Foundation. The American Heart Association has released. Heart and Stroke Statistics 2022; Available from: https://www.sca-aware.org/about-sudden-cardiac-arrest/latest-statistics#:~:text=The%20American%20Heart%20Association%20has,nearly%2090%25%20of%20them%20fatal.
- Wijdicks, E.F., et al., Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology, 2006. 67(2): p. 203-10.
- Huang, Y., et al., Cardiopulmonary resuscitation (CPR) plus delayed defibrillation versus immediate defibrillation for out-of-hospital cardiac arrest. Cochrane Database Syst Rev, 2014. 2014(9): p. CD009803.
- Peberdy, M.A., et al., Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation, 2003. 58(3): p. 297-308., . [CrossRef]
- Safar, P., Cerebral resuscitation after cardiac arrest: a review. Circulation, 1986. 74(6 Pt 2): p. IV138-53.
- Levy, D.E., et al., Predicting outcome from hypoxic-ischemic coma. JAMA, 1985. 253(10): p. 1420-6., . [CrossRef]
- Bronder, J., et al., Revisiting EEG as part of the multidisciplinary approach to post-cardiac arrest care and prognostication: A review. Resusc Plus, 2022. 9: p. 100189., . [CrossRef]
- Kaplan, P.W., Electrophysiological prognostication and brain injury from cardiac arrest. Semin Neurol, 2006. 26(4): p. 403-12., . [CrossRef]
- Chen, R., C.F. Bolton, and B. Young, Prediction of outcome in patients with anoxic coma: a clinical and electrophysiologic study. Crit Care Med, 1996. 24(4): p. 672-8.
- Koenig, M.A., P.W. Kaplan, and N.V. Thakor, Clinical neurophysiologic monitoring and brain injury from cardiac arrest. Neurol Clin, 2006. 24(1): p. 89-106., . [CrossRef]
- San-Juan, O.D., et al., Periodic epileptiform discharges in hypoxic encephalopathy: BiPLEDs and GPEDs as a poor prognosis for survival. Seizure, 2009. 18(5): p. 365-8., . [CrossRef]
- Sandroni, C., et al., Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 1: patients not treated with therapeutic hypothermia. Resuscitation, 2013. 84(10): p. 1310-23.
- Rossetti, A.O., et al., Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol, 2010. 67(3): p. 301-7., . [CrossRef]
- Westhall, E., et al., Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology, 2016. 86(16): p. 1482-90. [CrossRef]
- Hockaday, J.M., et al., Electroencephalographic Changes in Acute Cerebral Anoxia from Cardiac or Respiratory Arrest. Electroencephalogr Clin Neurophysiol, 1965. 18: p. 575-86. [CrossRef]
- Ruijter, B.J., et al., The prognostic value of discontinuous EEG patterns in postanoxic coma. Clin Neurophysiol, 2018. 129(8): p. 1534-1543. [CrossRef]
- Scollo-Lavizzari, G. and C. Bassetti, Prognostic value of EEG in post-anoxic coma after cardiac arrest. Eur Neurol, 1987. 26(3): p. 161-70. [CrossRef]
- Binnie, C.D., et al., Electroencephalographic prediction of fatal anoxic brain damage after resuscitation from cardiac arrest. Br Med J, 1970. 4(5730): p. 265-8. [CrossRef]
- Hirsch, L.J., et al., American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol, 2013. 30(1): p. 1-27.
- Hirsch, L.J., et al., American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol, 2021. 38(1): p. 1-29.
- Gaspard, N., et al., Interrater agreement for Critical Care EEG Terminology. Epilepsia, 2014. 55(9): p. 1366-73. [CrossRef]
- Grindegard, L., et al., Association Between EEG Patterns and Serum Neurofilament Light After Cardiac Arrest: A Post Hoc Analysis of the TTM Trial. Neurology, 2022. 98(24): p. e2487-e2498.
- Sandroni, C., T. Cronberg, and M. Sekhon, Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med, 2021. 47(12): p. 1393-1414. [CrossRef]
- Willems, L.M., et al., EEG patterns and their correlations with short- and long-term mortality in patients with hypoxic encephalopathy. Clin Neurophysiol, 2021. 132(11): p. 2851-2860. [CrossRef]
- Westhall, E., et al., Electroencephalography (EEG) for neurological prognostication after cardiac arrest and targeted temperature management; rationale and study design. BMC Neurol, 2014. 14: p. 159. [CrossRef]
- Guedes, B., et al., Prognostic significance of specific EEG patterns after cardiac arrest in a Lisbon Cohort. Clin Neurophysiol Pract, 2020. 5: p. 147-151. [CrossRef]
- Synek, V.M., EEG abnormality grades and subdivisions of prognostic importance in traumatic and anoxic coma in adults. Clin Electroencephalogr, 1988. 19(3): p. 160-6. [CrossRef]
- Qing, K.Y., P.B. Forgacs, and N.D. Schiff, EEG Pattern With Spectral Analysis Can Prognosticate Good and Poor Neurologic Outcomes After Cardiac Arrest. J Clin Neurophysiol, 2022. [CrossRef]
- Stiell, I.G., et al., Comparison of the Cerebral Performance Category score and the Health Utilities Index for survivors of cardiac arrest. Ann Emerg Med, 2009. 53(2): p. 241-248. [CrossRef]
- Huang, B.Y. and M. Castillo, Hypoxic-Ischemic Brain Injury: Imaging Findings from Birth to Adulthood. RadioGraphics, 2008. 28(2): p. 417-439. [CrossRef]
- Aghaeeaval, M., et al., Prediction of patient survival following postanoxic coma using EEG data and clinical features. Annu Int Conf IEEE Eng Med Biol Soc, 2021. 2021: p. 997-1000.
- Nolan, J.P., et al., European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med, 2021. 47(4): p. 369-421. [CrossRef]
- Al-Said, Y.A., et al., Non-convulsive seizures and electroencephalography findings as predictors of clinical outcomes at a tertiary intensive care unit in Saudi Arabia. Clin Neurol Neurosurg, 2018. 171: p. 95-99. [CrossRef]
- Primary, P., et al., Neuroprognostication in the Post Cardiac Arrest Patient: A Canadian Cardiovascular Society Position Statement. Can J Cardiol, 2023. 39(4): p. 366-380.
- Lybeck, A., et al., Prognostic significance of clinical seizures after cardiac arrest and target temperature management. Resuscitation, 2017. 114: p. 146-151. [CrossRef]
- Kilbride, R.D., D.J. Costello, and K.H. Chiappa, How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol, 2009. 66(6): p. 723-8. [CrossRef]
- Sessler, C.N., M.J. Grap, and M.A. Ramsay, Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care, 2008. 12 Suppl 3(Suppl 3): p. S2. [CrossRef]
- Jorgensen, E.O. and A. Malchow-Moller, Natural history of global and critical brain ischaemia. Part I: EEG and neurological signs during the first year after cardiopulmonary resuscitation in patients subsequently regaining consciousness. Resuscitation, 1981. 9(2): p. 133-53. [CrossRef]
- Nolan, J.P., et al., European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation, 2021. 161: p. 220-269.
- Turella, S., et al., The predictive value of highly malignant EEG patterns after cardiac arrest: evaluation of the ERC-ESICM recommendations. Intensive Care Medicine, 2024. 50(1): p. 90-102. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).