1. Introduction
Immune alterations in End-Stage Kidney Disease (ESKD) have been widely described and involve both innate and acquired immunity [
1]. These disturbances are closely related to persistent and chronic activation of inflammatory response, and are implicated in several clinical complications of ESKD, such as susceptibility to serious infections, inadequate response to vaccination, increased malignancy incidence, frailty, and nutritional disturbances, and above all increased cardiovascular risk and mortality [
2,
3,
4,
5].
In the setting of ESKD, acquired immunity alterations have been more extensively studied. Several studies show that lymphocytes from patients with ESKD exhibit phenotypic and functional characteristics of older individuals, thus resembling prematurely senescent cells. These include lymphopenia with inverted CD4+/CD8+ ratio, elimination of naïve and low differentiation subpopulations, increased cytotoxicity, decrease of regulatory T cells and imbalance among Th responses [
6,
7,
8].
Several pathophysiological mechanisms have been proposed to elicit these alterations. Premature thymic involution and cytokine dysregulation, particularly decrease of IL-7 and increase of TNF-α have been associated with naïve T cells elimination and senescent CD28- T cells accumulation [
9,
10]. Moreover, dialysis related factors including dialyzers biocompatibility, water contamination, frequent catheter infection and volume and sodium overload appear to play a major role [
11,
12]. Most importantly, increased levels of uremic toxins, mainly produced by impaired intestinal flora are poorly removed by dialysis and contribute to inflammatory activation [
13].
Conventional hemodialysis (HD) is the most widespread method of kidney replacement therapy and relies on solute clearance with diffusion. However, its capacity to remove larger molecules is limited due to the relatively low speed of diffusion of these molecules through the dialyzer membrane. Online hemodiafiltration (HDF) provides an additional solute clearance with convection, which is achieved by the removal of large fluid volumes through a membrane of high hydraulic permeability (“high flux” membrane). The fluid removed is substituted with sterile dialysate of optimal electrolyte composition, infused directly into the bloodstream of the patient, hence, ultra-pure water is needed [
14]. Online HDF improves removal of middle molecular weight solutes. Most importantly, appears to have a beneficial effect in all-cause and cardiovascular mortality, in oxidative stress and hospitalization rates and, also, improve the patients’ quality of life [
15,
16]. Moreover, a reduction of inflammatory markers has been associated to HDF [
17].
Despite the wide range of clinical associations that immune alterations have on ESKD patients, there are no data available to the impact of dialysis prescription on immune phenotype. The present study aims to investigate for potential discrepancies in lymphocytic phenotypes, between ESKD patients, on two main dialysis modalities, HD and HDF, examine the role of hemofiltration volume (HFV), and explore the impact of immune phenotype to mortality.
2. Results
2.1. Patients’ Characteristics
Patients’ demographics are shown in table 1. Patients were equally distributed within the three age groups. Sex distribution did not differ among the three age groups (P=0.26), however, patients in the 20-40 year age group had a significantly lower body weight and BMI (P=0.03) . A 30% of the patients had a history immunosuppressive treatment, either due to their primary cause of ESKD, or after kidney transplantation. However, the vast majority of the patients had ceased any immunosuppressive treatment for a considerable time period before study initiation (
Table 1). Dialysis prescription differed among the groups, as more patients were on conventional HD in the older group 61-80 years (P=0.02), without significant difference in the mean duration of each dialysis session between the groups (P=0.14). Vintage of dialysis differed significantly among the groups, as patients in the middle age group 41-60 years, had a mean vintage of 101±81months, P=0.01 in comparison to the young patients group (45±48 months) or the older patients group (67±44 months).
2.2. Comparison of Immune Phenotype between HD and HDF Patients
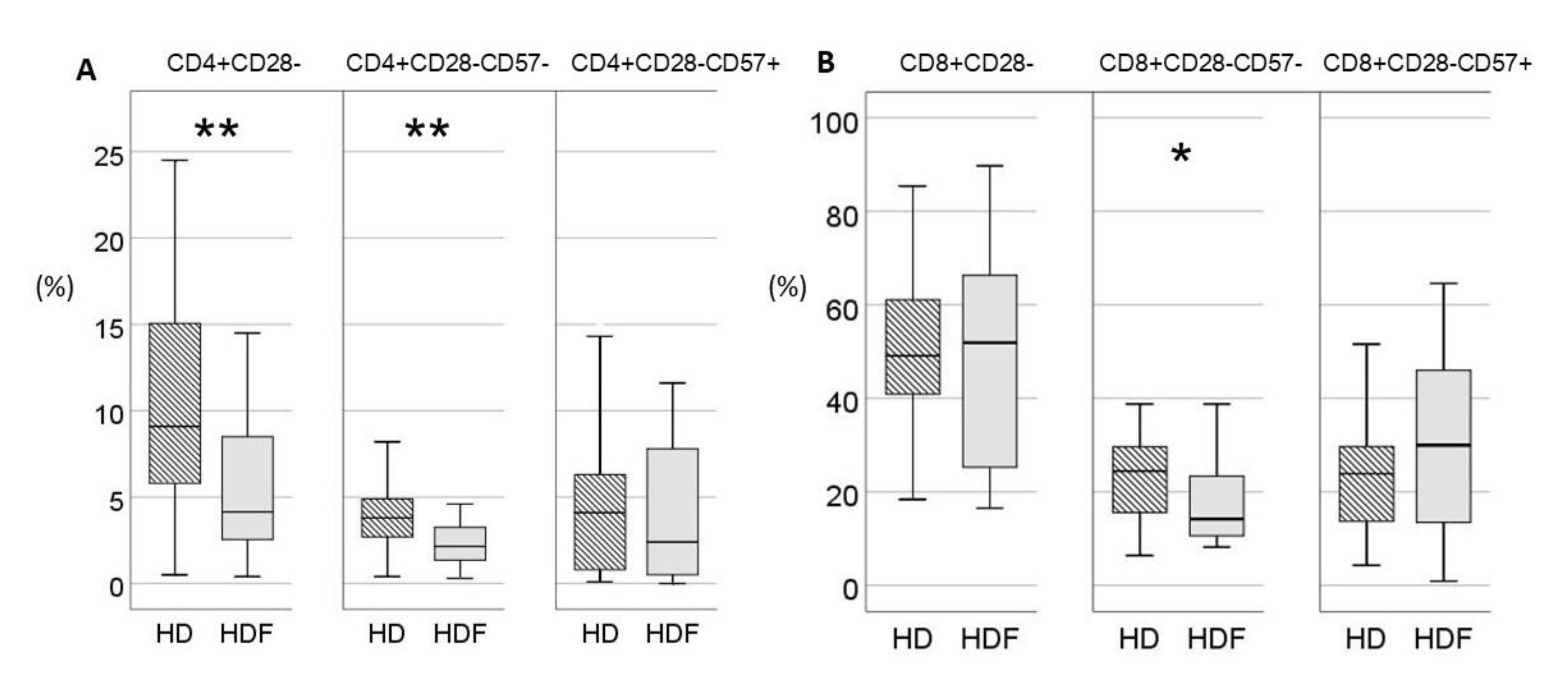
Online HDF was applied in 26/62(42%) patients. Age of patients did not differ significantly between the two groups [57(38-71) vs 49(31-58)years for HD and HDF respectively, P=0.08]. Patients on HDF had significantly higher proportions of low differentiation CD4+CD28+CD57- and decreased proportions of highly differentiated CD4+CD28- lymphocytes. However, within the compartment of CD28- T cells, the difference between the two patient groups was restrained only in CD28-CD57- subset which was increased in HD patients, while terminally differentiated CD28-CD57+ T cells did not differ between the two groups (
Figure 1). In the CD8+ compartment, proportions of CD28+CD57- T cells and total CD28- T cells did not differ between the two subgroups, whereas CD8+CD28-CD57- T cells were significantly reduced in the HDF group. Absolute numbers and proportions of T cells according to expression of CD28 and CD57 are shown in Supplemental
Table 1.
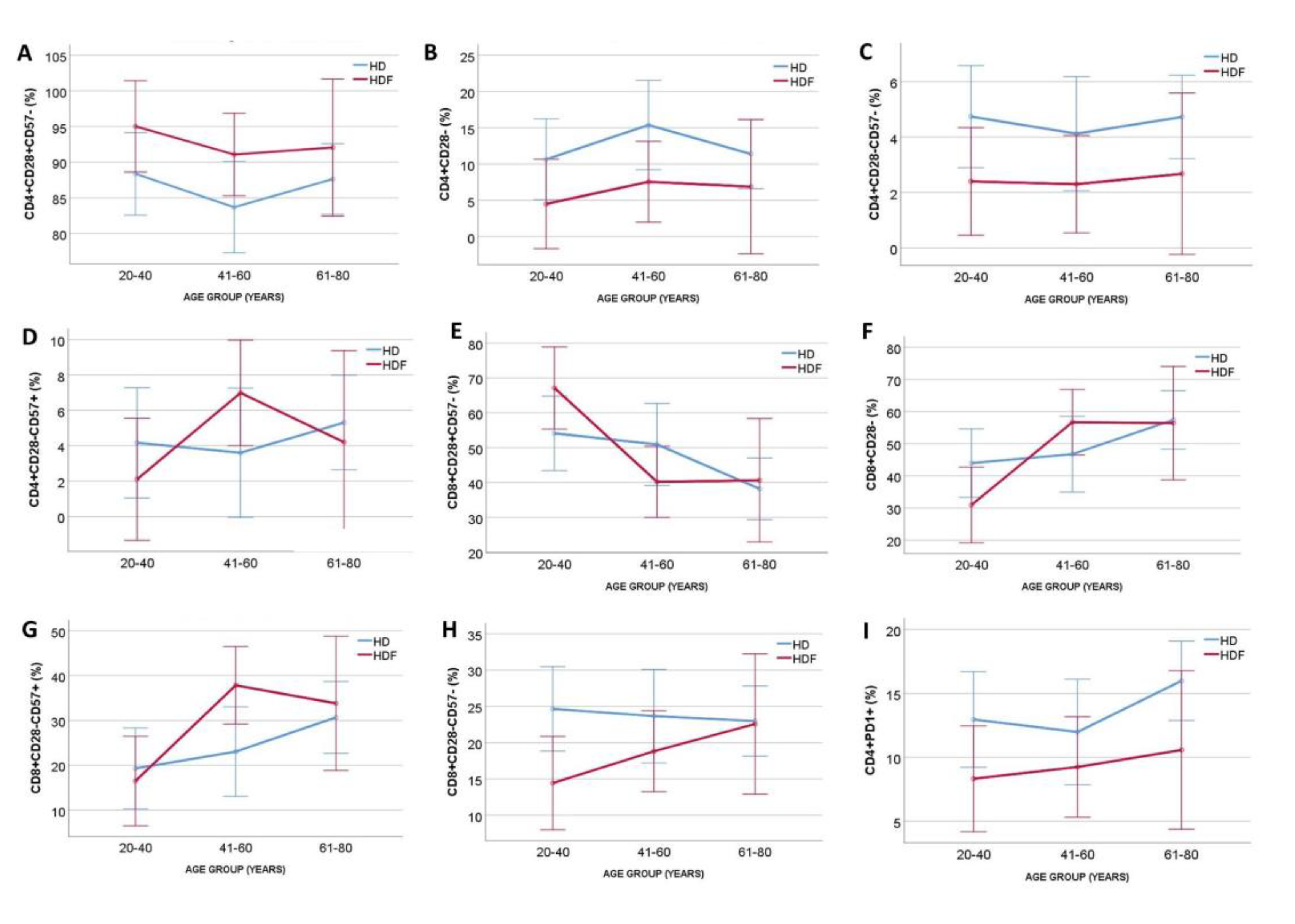
To adjust for age, the two patient cohorts were divided into three age groups and ANCOVA was performed. Patients on HDF had increased proportions of CD4+CD28+CD57- (P=0.03, for modality), and decreased proportions of CD4+CD28- (P=0.02, for modality) and CD4+CD28-CD57- (P=0.02, for modality), independently of age (P=0.37, P=0.41 and P=0.88 for the three subsets respectively), with no significant interaction between age group and modality (P=0,9, P=0.88 and P=0.9, respectively). Proportions of CD4+CD28-CD57+ T cells remained non-significant for dialysis modality and age group (
Figure 2). In contrast, CD8+ subsets were affected by age in ANCOVA (P=0.003, P=0.006 and P=0.01 for CD28+CD57-, CD28- and CD28-CD57- respectively), while neither dialysis modality nor age were significant in ANCOVA for CD8+CD28-CD57- T cells proportion (P=0.06, P=0.61 and P=0.358 for modality, age and interaction, respectively)(
Figure 2).
Moreover, there was a significant difference in percentage of exhausted CD4+PD1+ cells [14.1(8.9-19.4) vs 8.5(6.8-11.7)% for HD and HDF respectively, P=0.005], whereas the percentage of CD8+PD1+ cells did not differ significantly between the two groups [33.8(12.7-52.2) vs 17.4(8.5-41)% for HD and HDF respectively, P=0.06]. The impact of age in ANCOVA was not significant neither in the CD4+PD1+ (P=0.42) nor in CD8+PD1+ T cells percentages (P=0.82). The significance of dialysis modality was retained after adjusting for age only for CD4+PD1+ T cell percentage (
Figure 2).
Counts and percentages of naïve and memory T and B cells did not differ between the two groups. Moreover, levels of urea, creatinine, and phosphorous levels did not correlate with any of the studied T and B cells subsets (data not shown).
2.3. Effect of HFV
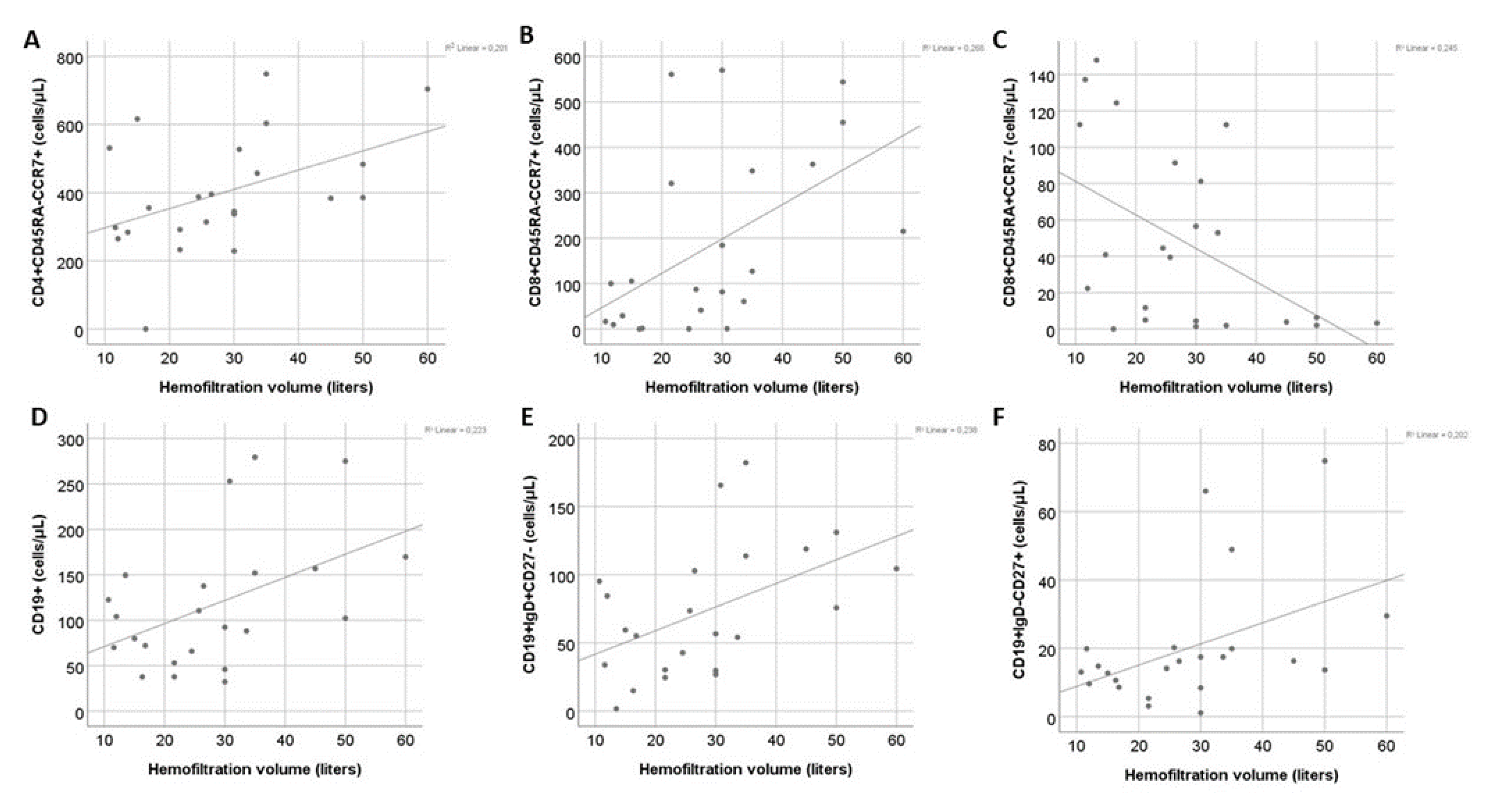
Correlation of T cells subsets with hemofiltration volume in patients on HDF revealed a significant positive association between HFV and central memory CD4+ (r=0.46, P=0.03) and CD8+ cell counts (r=0.51, P=0.01) and a significant negative correlation with CD8+ EMRA T cells count (r=-0.46, P=0.03). Moreover, a positive correlation between HFV with total B cell count was observed (r=0.46, P=0.03). Out of the B cell subpopulations HFV mainly affected naïve (r=0.53, P=0.008) and switched memory B cells (r=0.5, P=0.02), whereas its association with IgD-CD27- B cells (r=0.41, P=0.05) was marginal. (
Figure 3). In univariate regression model the effect of HFV remained significant for all the above subsets except for IgD-CD27- B cells (
Table 2).
Multivariate analysis performed for each lymphocyte population, including age and HFV, showed that HFV was an independent factor for CD8+ central memory and EMRA T cell population, whereas age was the main factor affecting CM CD4+ , total B and naïve B cell count (
Table 2).
2.4. Effect of Vintage
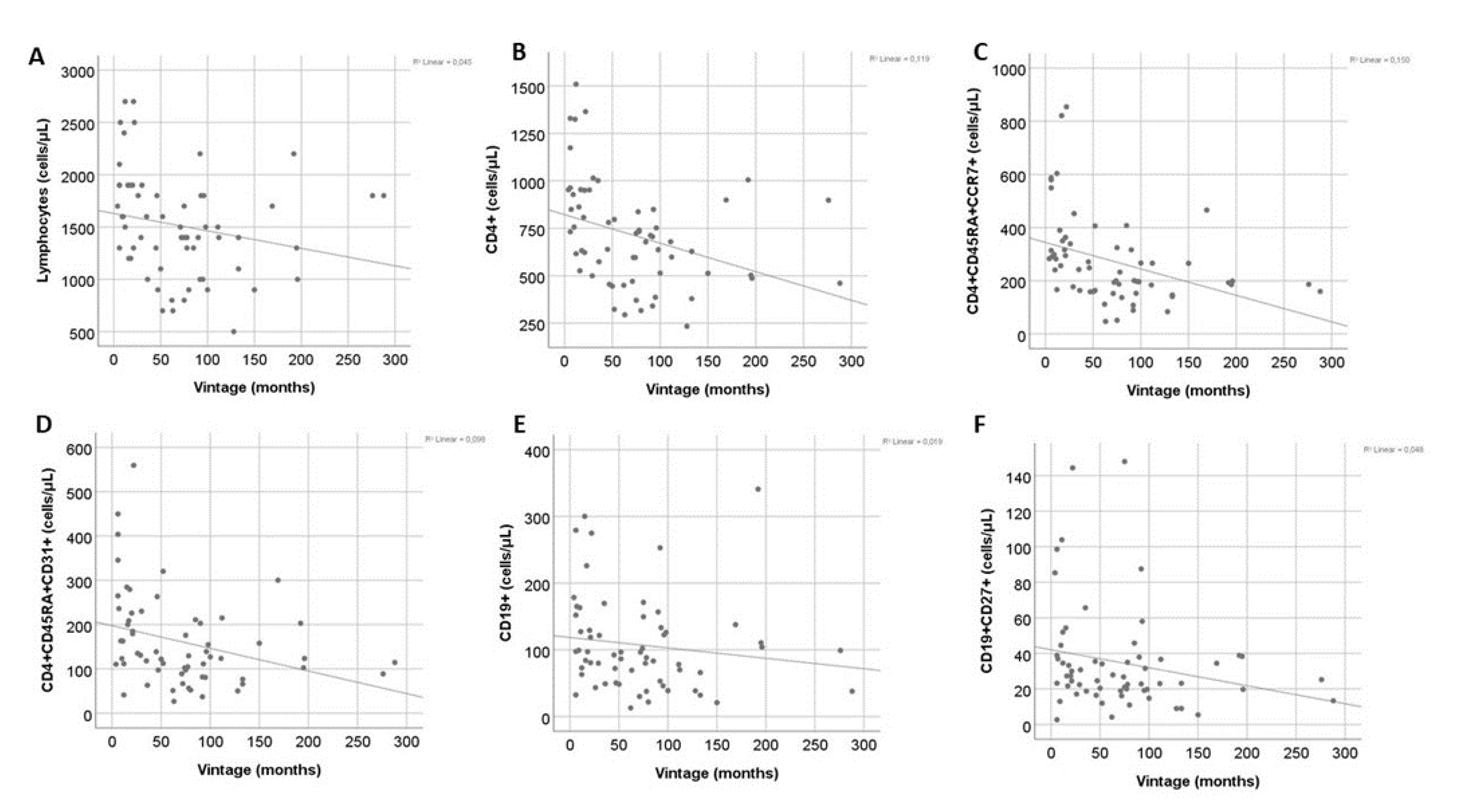
Dialysis Vintage was negatively correlated with the total count of T cells and CD4+ T cells (r=-0.35, P=0.005 and r=-0.46, P<0.001, respectively), while total CD8+ T cells were not affected. The effect of vintage was evident only in low differentiation CD4+ T cell subsets (r=-0.51, P<0.001, r=-0.38, P=0.002 and r=-0.31, P=0.01 for naïve, RTEs and CM CD4+ T cells respectively). Moreover, there was a significant negative correlation with CM CD8+ T cells (r=-0.29, P=0.02). Finally, total B cell and memory B cell count were also negatively correlated with dialysis vintage (r=-0.26, P=0.04 and r=-0.25, P=0.04) (
Figure 4). However, in multivariate analysis for vintage and age the effect of vintage remained significant only for total CD4+ and naïve CD4+ count, whereas age was the main predictive factor for CD4+ RTEs and B cell subsets (
Table 3). Total lymphocytes and CM cells, in both CD4+ and CD8+ compartments, were not affected neither by vintage nor age in the multivariate model.
2.5. Immune Phenotype May Be Related to Increased Mortality
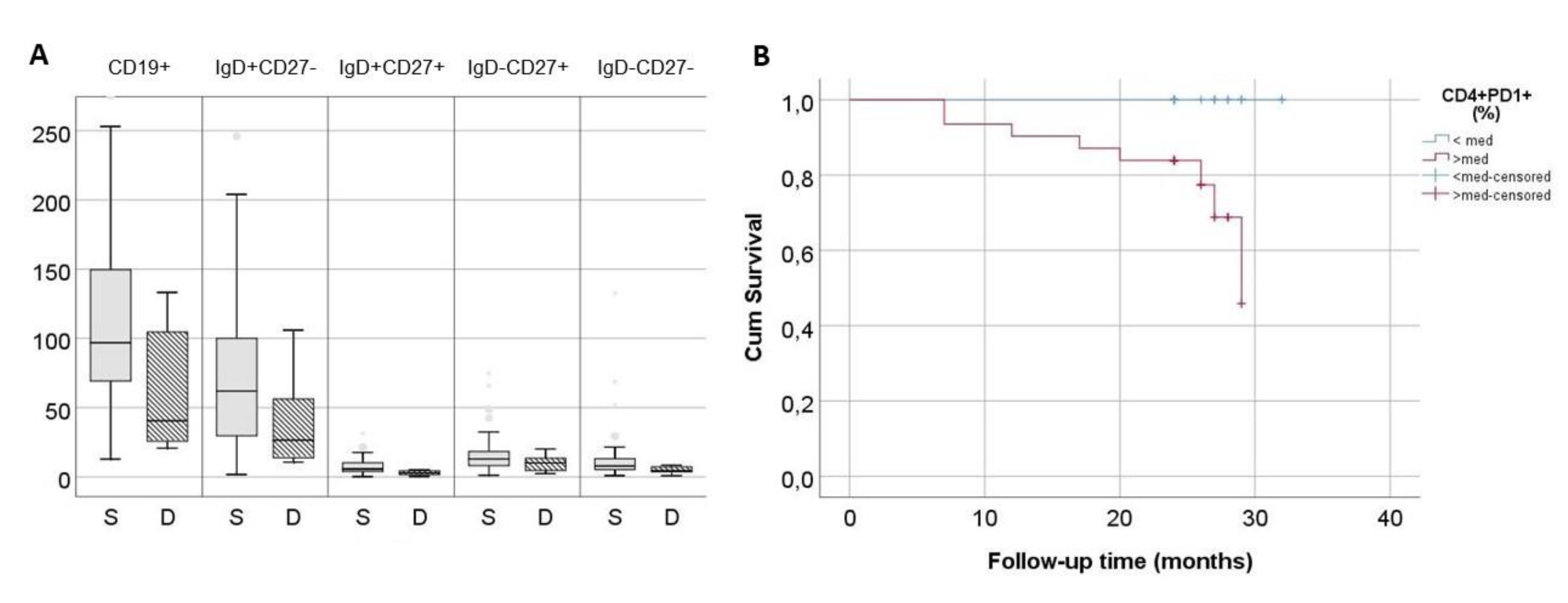
All patients were followed up prospectively after phenotypic evaluation for at least 24 months and deaths were recorded. A 13% (8/62) of patients died during the follow-up period. Deceased patients had significantly higher proportion of exhausted CD4+PD1+ T cells in comparison to survivors [18.8(14-25) vs 11.5(7-16.9)% for respectively, P=0.006] and decreased count of total B cells [40(24-115) vs 97(70-150)cells/μL, respectively, P=0.02]. This difference affected mainly naïve and IgM memory B cells (
Figure 5A). Kaplan-Meier survival analysis for patients with proportion of CD4+PD1+ above and below median was significant (P=0.002) (
Figure 5B). Moreover, significant effect of exhausted CD4+PD1+ T cells proportion remained in Cox Regression either when examined alone as a continuous variable (P=0.003, 95%CI: 1.0, 1.2), or in combination with age (CD4+PD1+ proportion: P=0.03, 95% CI: 1.01, 1.23, age: P=0.02, 95% CI: 1.01, 1.44).
3. Discussion
Alterations of acquired immunity have been studied to a certain extend in ESKD. However, the effect of dialysis modality and prescription has not been studied to date. Scope of the present study was to examine differences of senescence-related phenotype of T and B lymphocytes between patients on conventional HD or online HDF, determine the effect of hemofiltration volume and dialysis vintage and investigate the impact of T and B cells subsets to mortality.
Dialysis modality appeared to have significant effect on T cells phenotype, in terms of the expression of CD28 and PD1 molecules on CD4+ and CD8+ T lymphocytes. Patients on HDF had an improved phenotype with higher percentage of low differentiation CD28+ and lower percentage of senescent CD28-CD57- T cells. CD28, a costimulatory molecule is downregulated after multiple activations of a T cell clone [
18], or as a result of cytokine action in absence of antigenic stimulation [
19,
20,
21], and has been documented in many chronic inflammatory conditions, included ESKD [8, 22–23]. High percentages of CD4+CD28- T cells have been associated to instable angina and to increased risk for relapse of acute coronary event or stroke [
24,
25,
26,
27,
28]. Moreover, these cells have been found within atheromatous plaque in animals and have been associated to vascular smooth muscle cell apoptosis and increased risk of plaque rupture [
29]. CD8+CD28- T cells were also found to be increased in dialysis patients with cardiovascular disease [
23].
Significant correlations between T cell phenotype with the hemofiltration volume were found in HDF patients. Our analysis revealed a clear positive effect of increased hemofiltration volume on central memory T cells, both of CD4+ and CD8+ compartment, while hemofiltration volume was negatively correlated with CD8+ EMRA T cells, a terminally differentiated subset with strong senescent characteristics [
30]. Interestingly, CD8+ EMRA cell population has been positively associated with a protein bound uremic toxin, p-cresyl sulfate [
31]. Hence, increased clearance of protein-bound uremic toxins, potentially achieved with HDF may contribute to the improvement of the immune phenotype of dialysis patients. Finally, a potential positive effect was also found on B cell compartment, however, in multivariate analysis, age was the main determinant of B cell subsets.
Dialysis vintage was also associated with several immunophenotype alterations. Vintage was the main determinant of total CD4+ and naïve CD4+ T cells, whereas age of CD4+ RTE and B cells in the multivariate model, despite vintage being significant in the linear correlation analysis. In 2011, Borges et al. reported a positive association of CD4+ apoptotic cells with dialysis vintage, a finding consistent to the results of the present study [
32]. Central memory T cell count had a significant negative correlation to vintage, which, however, was not retained in multivariate analysis. Results from previous studies are controversial, as a recent large multicenter study supported the effect dialysis vintage on the percentage of central memory CD4+ cells [
31], while other investigators claimed no significant correlation [
22]. In consistency with our results, Stefanidis et al, have recently reported a gradual reduction in T lymphocyte telomere length during dialysis vintage [
33].
Finally, according to the findings of the present study, immune phenotype might predict all-cause mortality in dialysis patients. Literature on the role of immune phenotype on dialysis patients’ survival is, to date, poor. One study, published in 2020, associated decreased levels of low-differentiation T cell subsets to increased all-cause mortality in dialysis patients [
34]. These findings were not confirmed by the present study, potentially due to lower number of patients and shorter follow-up time. B cells count < 100/μL were also associated to increased cardiovascular and all-cause mortality in dialysis patients, according to a study of Molina et al [
35]. The present study adds one more potential predictive factor, associated with mortality in this patients’ group, the percentage of exhausted CD4+ T cells.
Limitations of the study include the relatively low number of patients enrolled and the limited follow-up interval. Moreover, patients were not initiated in a randomized way in HD of HDF before initiation of the study. Thus, more studies are needed to confirm our results.
In conclusion, HDF is potentially beneficial to patients as it was associated with improved immune phenotype in terms of CD28 and PD1 expression on T cells. An additional benefit was observed in high hemofiltration volumes, which were associated to decreased count of terminally differentiated CD8+ EMRA T cells. Moreover, dialysis vintage affected total and naïve CD4+ T cell counts, the latter, however, being an unmodifiable factor. Finally, exhausted CD4+ T cells might be a predictive marker for all-cause mortality in dialysis patients. .
4. Materials and Methods
4.1. Patients
In this prospective, observational study, a total of 62 adult patients on chronic maintenance HD were included and followed up for at least two years. All the patients received a thrice-weekly dialysis program with HD or online HDF for at least one year, with Kt/V>1.2. Patients with active malignancy, hematological or autoimmune diseases, recent infection, or vaccination (< 3 months), or patients who had received immunosuppressive treatment one year before enrollment were excluded from the study. As diabetes mellitus has been shown to provoke alterations to cellular immunity, diabetic patients were deemed not eligible for the study [36-37]. Moreover, patients who changed from HD to HDF of vice versa, for any reason, were also excluded. As many lymphocyte subpopulations are affected by ageing, though not in a linear way, we decided to stratify patients in three age groups, 20-40, 41-60 and 61-80 years. Patients were followed up for two years and cardiovascular events, de novo cancer diagnosis and deaths were recorded. The study was approved by the Institutional Review Board of the Medical School of the Aristotle University of Thessaloniki (ref No 2273/15-12-2020) and was conducted according to the principles of the Declaration of Helsinki. All study participants signed an informed consent prior to enrollment.
4.2. Flow Cytometry
Flow cytometry was performed in fresh total blood, collected immediately before the initiation of a mid-week dialysis session, processed no more than 12 hours after collection. Specimens were kept in the refrigerator at 4oC until examination. Proportions of CD4+, CD8+, and B lymphocytes subsets were determined using a cell counter (Navios Flow Cytometer, Beckman Coulter), according to the manufacturer’s recommendations, as described previously [
38]. For each sample four different panels of markers were prepared. Briefly, the lymphocytes were stained with conjugated antibodies for CD45, CD3, CD4, CD8, CD45RA, CCR7, CD28, CD31, CD57, PD1, CD19, CD27 and IgD, in four different tubes, as described in Supplemental
Table 2, and lymphocyte subsets were defined as shown in Supplemental
Table 3. Gating strategy is shown on Supplemental
Figure 1.
4.3. Statistical Analysis
Statistical analysis was performed with SPSS.25 for Windows (IBM, Armonk, NY, USA). Continuous variables are reported as median(25th–75th percentile). Differences between groups were evaluated using Chi-square test for categorical variables. Comparisons of continuous variables were evaluated with Mann–Whitney U test for comparison between two groups or Kruskal–Wallis test for comparisons among three groups. Analysis of Covariance (ANCOVA) was used to evaluate the effect of age in comparisons among subgroups. Spearman Rank Correlation Coefficient was used to estimate correlation between continuous variables. Multivariate linear regression was used for the significance of multiple continuous variables. For clinical outcomes of patients, Kaplan-Meier and Cox regression model analysis were performed. A p-value < 0.05 was considered statistically significant.
Author Contributions
M.S., M.F and G.L conception and study design, M.F and A.X. laboratory processing, T.T, M.C, E.M, S.S and G.L. data collection and interpretation, G.L, M.S., Manuscript drafting, A.P and M.S study supervision.
Funding
Dr Lioulios received a grand from the Hellenic Society of Nephrology to conduct the study (Grant No: 48-26/5/2021).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Institutional Review Board of the Medical School of the Aristotle University of Thessaloniki (ref No 2273/15-12-2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will be available from the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cohen, G. Immune Dysfunction in Uremia 2020. Toxins (Basel). 2020 Jul;12(7).
- Kramer A, Pippias M, Noordzij M, Stel VS, Andrusev AM, Aparicio-Madre MI, et al. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: a summary. Clin Kidney J. 2019 Oct;12(5):702–20.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C yuan. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. New England Journal of Medicine. 2004;351(13):1296–305. [CrossRef]
- Vogelzang JL, van Stralen KJ, Noordzij M, Diez JA, Carrero JJ, Couchoud C, et al. Mortality from infections and malignancies in patients treated with renal replacement therapy: data from the ERA-EDTA registry. Nephrol Dial Transplant. 2015 Jun;30(6):1028–37. [CrossRef]
- Lioulios G, Fylaktou A, Asouchidou D, Xochelli A, Nikolaidou V, Stai S, et al. Effect of Lymphocyte Phenotypic Alterations on the Humoral Response to Vaccination Against SARS-COV-2 in Dialysis Patients. Ann Lab Med. 2023 Sep 1;43(5):451–60. [CrossRef]
- Van Laecke S, Van Damme K, Dendooven A. Immunosenescence: an unexplored role in glomerulonephritis. Vol. 11, Clinical and Translational Immunology. John Wiley and Sons Inc; 2022. [CrossRef]
- Lioulios G, Fylaktou A, Papagianni A, Stangou M. T cell markers recount the course of immunosenescence in healthy individuals and chronic kidney disease. Clin Immunol. 2021 Apr;225:108685. [CrossRef]
- Sampani E, Stangou M, Daikidou DV, Nikolaidou V, Asouchidou D, Dimitriadis C, et al. Influence of end stage renal disease on CD28 expression and T-cell immunity. Nephrology. 2021 Feb 1;26(2):185–96. [CrossRef]
- Litjens NHR, van Druningen CJ, Betjes MGH. Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin Immunol. 2006 Jan;118(1):83–91. [CrossRef]
- Yadav AK, Lal A, Jha V. Cytotoxic CD4+CD28null T lymphocytes, systemic inflammation and atherosclerotic risk in patients with chronic kidney disease. Nephron Clin Pract. 2012 Oct;120(4). [CrossRef]
- Ducloux D, Legendre M, Bamoulid J, Saas P, Courivaud C, Crepin T. End-Stage Renal Disease-Related Accelerated Immune Senescence: Is Rejuvenation of the Immune System a Therapeutic Goal? Vol. 8, Frontiers in Medicine. Frontiers Media S.A.; 2021.
- Losappio V, Franzin R, Infante B, Godeas G, Gesualdo L, Fersini A, et al. Molecular mechanisms of premature aging in hemodialysis: The complex interplay between innate and adaptive immune dysfunction. Vol. 21, International Journal of Molecular Sciences. MDPI AG; 2020. [CrossRef]
- Glorieux G, Gryp T, Perna A. Gut-derived metabolites and their role in immune dysfunction in chronic kidney disease. Vol. 12, Toxins. MDPI AG; 2020. [CrossRef]
- Blankestijn PJ, Fischer KI, Barth C, Cromm K, Canaud B, Davenport A, et al. Benefits and harms of high-dose haemodiafiltration versus high-flux haemodialysis: The comparison of high-dose haemodiafiltration with high-flux haemodialysis (CONVINCE) trial protocol. BMJ Open. 2020 Feb 5;10(2). [CrossRef]
- Tattersall JE, Ward RA; EUDIAL group. Online haemodiafiltration: definition, dose quantification and safety revisited. Nephrol Dial Transplant. 2013 Mar;28(3):542-50. [CrossRef] [PubMed]
- Lang T, Zawada AM, Theis L, Braun J, Ottillinger B, Kopperschmidt P, et al. Hemodiafiltration: Technical and Medical Insights. Bioengineering. 2023 Jan 21;10(2):145. [CrossRef]
- Susantitaphong P, Siribamrungwong M, Jaber BL. Convective therapies versus low-flux hemodialysis for chronic kidney failure: a meta-analysis of randomized controlled trials. Nephrol Dial Transplant. 2013 Nov;28(11):2859–74. [CrossRef]
- Vallejo AN, Bryl E, Klarskov K, Naylor S, Weyand CM, Goronzy JJ. Molecular basis for the loss of CD28 expression in senescent T cells. J Biol Chem. 2002 Dec;277(49):46940–9. [CrossRef]
- Chiu WK, Fann M, Weng N ping. Generation and growth of CD28nullCD8+ memory T cells mediated by IL-15 and its induced cytokines. J Immunol. 2006 Dec;177(11):7802–10. [CrossRef]
- Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000 Jul;12(7):1005–13.
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008 Dec;29(6):848–62. [CrossRef]
- Meijers RW, Litjens NH, de Wit EA, Langerak AW, van der Spek A, Baan CC, et al. Uremia causes premature ageing of the T cell compartment in end-stage renal disease patients. Immun Ageing. 2012 Sep;9(1):19. [CrossRef]
- Crépin T, Legendre M, Carron C, Vachey C, Courivaud C, Rebibou JM, et al. Uraemia-induced immune senescence and clinical outcomes in chronic kidney disease patients. Nephrol Dial Transplant. 2020 Apr;35(4):624–32. [CrossRef]
- Zal B, Kaski JC, Arno G, Akiyu JP, Xu Q, Cole D, et al. Heat-Shock Protein 60-Reactive CD4+CD28null T Cells in Patients with Acute Coronary Syndromes. Circulation. 2004 Mar 16;109(10):1230–5.
- Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000 Jun;101(25):2883–8. [CrossRef]
- Liuzzo G, Kopecky SL, Frye RL, O’Fallon WM, Maseri A, Goronzy JJ, et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999 Nov;100(21):2135–9. [CrossRef]
- Liuzzo G, Biasucci LM, Trotta G, Brugaletta S, Pinnelli M, Digianuario G, et al. Unusual CD4+CD28null T Lymphocytes and Recurrence of Acute Coronary Events. J Am Coll Cardiol. 2007 Oct 9;50(15):1450–8. [CrossRef]
- Nowik M, Nowacki P, Grabarek J, Drechsler H, Białecka M, Widecka K, et al. Can we talk about CD4+CD28- Lymphocytes as a risk factor for ischemic stroke? Eur Neurol. 2007 Jul;58(1):26–33.
- Sato K, Niessner A, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. Journal of Experimental Medicine. 2006 Jan 23;203(1):239–50. [CrossRef]
- Callender LA, Carroll EC, Beal RWJ, Chambers ES, Nourshargh S, Akbar AN, et al. Human CD8(+) EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell. 2018 Feb;17(1).
- Chiu YL, Shu KH, Yang FJ, Chou TY, Chen PM, Lay FY, et al. A comprehensive characterization of aggravated aging-related changes in T lymphocytes and monocytes in end-stage renal disease: The iESRD study. Immunity and Ageing. 2018 Nov 8;15(1). [CrossRef]
- Borges A, Borges M, Fernandes J, Nascimento H, Sameiro-Faria M, Miranda V, et al. Apoptosis of peripheral CD4++ T-lymphocytes in end-stage renal disease patients under hemodialysis and rhEPO therapies. Ren Fail. 2011;33(2):138–43. [CrossRef]
- Stefanidis I, Voliotis G, Papanikolaou V, Chronopoulou I, Eleftheriadis T, Kowald A, et al. Telomere Length in Peripheral Blood Mononuclear Cells of Patients on Chronic Hemodialysis Is Related With Telomerase Activity and Treatment Duration. Artif Organs. 2015 Sep 1;39(9):756–64. [CrossRef]
- Xiang F, Chen R, Cao X, Shen B, Chen X, Ding X, et al. Premature aging of circulating T cells predicts all-cause mortality in hemodialysis patients. BMC Nephrol. 2020 Jul 13;21(1). [CrossRef]
- Molina M, Allende LM, Ramos LE, Gutiérrez E, Pleguezuelo DE, Hernández ER, et al. CD19+ B-cells, a new biomarker of mortality in hemodialysis patients. Front Immunol. 2018 Jun 15;9(JUN). [CrossRef]
- Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front Immunol. 2020 Jul 22;11:1582. [CrossRef] [PubMed] [PubMed Central]
- Sampani E, Daikidou DV, Lioulios G, Xochelli A, Mitsoglou Z, Nikolaidou V. CD28null and Regulatory T Cells Are Substantially Disrupted in Patients with End-Stage Renal Disease Due to Diabetes Mellitus. Int J Mol Sci. 2021 Mar 15;22(6):2975. [CrossRef] [PubMed] [PubMed Central]
- Lioulios G, Fylaktou A, Xochelli A, Sampani E, Tsouchnikas I, Giamalis P, et al. Clustering of End Stage Renal Disease Patients by Dimensionality Reduction Algorithms According to Lymphocyte Senescence Markers [Internet]. Vol. 13, Frontiers in Immunology . 2022. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2022.841031.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).