1. Introduction
In recent years, it has been recognized that cellular kinetics of mesenchymal stem cells (MSCs) differs depending on their origin [
1]. Umbilical cord-derived mesenchymal stem cells (UC-MSCs), one type of MSCs, are reported to have features intermediate between fetal and adult cells and have a flexible differentiation potential [
2]. We have already verified the differentiation of UC-MSCs into adipocytes that were obtained according to the same culture method as that described in the present study [
3]. Although our previous studies confirmed that UC-MSCs have the potential to differentiate into osteoblasts, we failed to generate mature bone tissues by culturing UC-MSCs under the same conditions as the culture of bone marrow-derived mesenchymal stem cells (BM-MSCs) [
3,
4]. This suggested that the differentiation potential of UC-MSCs might be affected by factors other than the differentiation potential of MSCs derived from tissues such as bone marrow and adipose tissue. We deduced that this result was attributed to the immaturity of UC-MSCs, based on the fact that UC-MSCs are primitive and resemble fetal cells that are likely to be affected by the surrounding environment [
5]. Coculture allows humoral factors to be shared continuously [
6]. We hypothesized that UC-MSCs, which are likely to be affected by the surrounding environment, could be more easily induced to differentiate through sharing of humoral factors with cocultured cells.
For the purpose of clinical application, it is meaningful to explore methods that are simple to operate, are less invasive to cells, and mimic the physiological process of tissue formation, in addition to considering ethics and safety [
7]. Coculture meets these conditions [
6]. However, we did not find any reports on the differentiation potential of UC-MSCs that was evaluated using coculture techniques that were as simple as the technique performed in the present study. Coculture appears to be not only simple but also less stressful for cells because it seems to be more physiological and able to mimic the
in vivo environment. Thus, UC-MSCs were cocultured with chondrocytes and Schwann cells and the differentiation potential of UC-MSCs was evaluated. The present study is the first preliminary study investigating the potential of UC-MSC coculture. The examination of our hypothesis, which focuses on cell-cell interactions mediated by humoral factors, is expected to contribute to the realization of regenerative medicine using autologous tissues.
2. Materials and Methods
The present study was conducted under the approval of the Ethics Committee of Kitasato University (B-07-13). We obtained written informed consent from pregnant women and used the tissues that were normally discarded after delivery. The materials were collected under conditions ensuring the safety of neonates. The specimens provided were coded to prevent identification of individual donors.
2.1. Preparation of Cells
UC-MSCs were obtained by the out-growth method in accordance with our previous report [
3]. Our previous study confirmed that UC-MSCs had multiple differentiation potential [
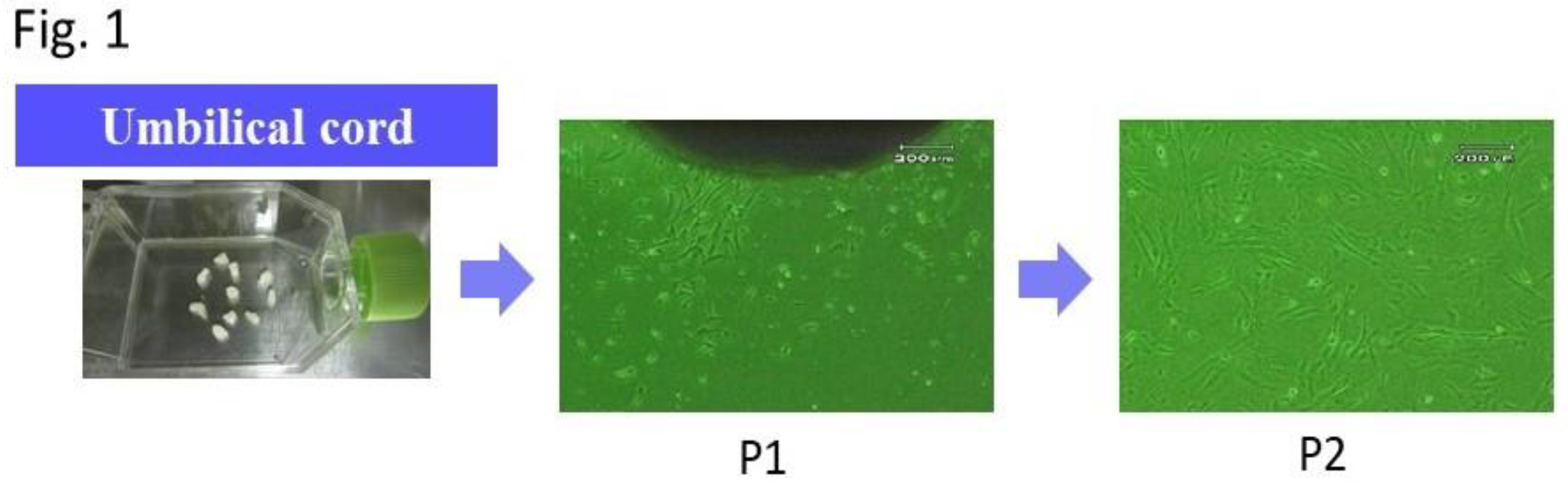
3]. The specific procedure is described here. The umbilical cord (UC) was collected after delivery of the placenta. Immediately after collection, the UC was placed in phosphate-buffered saline (PBS) and transported to the culture room at 20-25℃. The UC was washed of attached blood with PBS, cut into approximately 5-mm sections, and used for culture. These sections were incubated using Dulbecco’s modified Eagle’s medium (DMEM) in a 25-cm
2 flask (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) under the conditions of 5% CO
2 and 37℃. Media were replaced for the first time 1 week after the onset of the culture and every 3 days thereafter. Following the emergence of adhesive spindle-shaped cells, the UC sections were removed at approximately 2 weeks after the onset of the culture. The culture was continued until the moment when cells became subconfluent (
Figure 1). The cells that became 80% to 90% subconfluent were subcultured, and the second-passage cells were used in the experiments. Commercially-available chondrocytes (T0020-C, Cosmo Bio, Tokyo, Japan) were used. A mouse Schwann cell line (IMS32, Cosmo Bio, Tokyo, Japan) was used. They were cultured according to the respective, recommended methods. Commercially available chondrocytes (T0020-C, Cosmo Bio, Tokyo, Japan) and a mouse Schwann cell line (IMS32, Cosmo Bio, Tokyo, Japan) were cultured according to the respective manufacturer’s methods. The Schwann cells were used without subculturing.
2.2. Seeding on Scaffolds and Distribution of Specimens
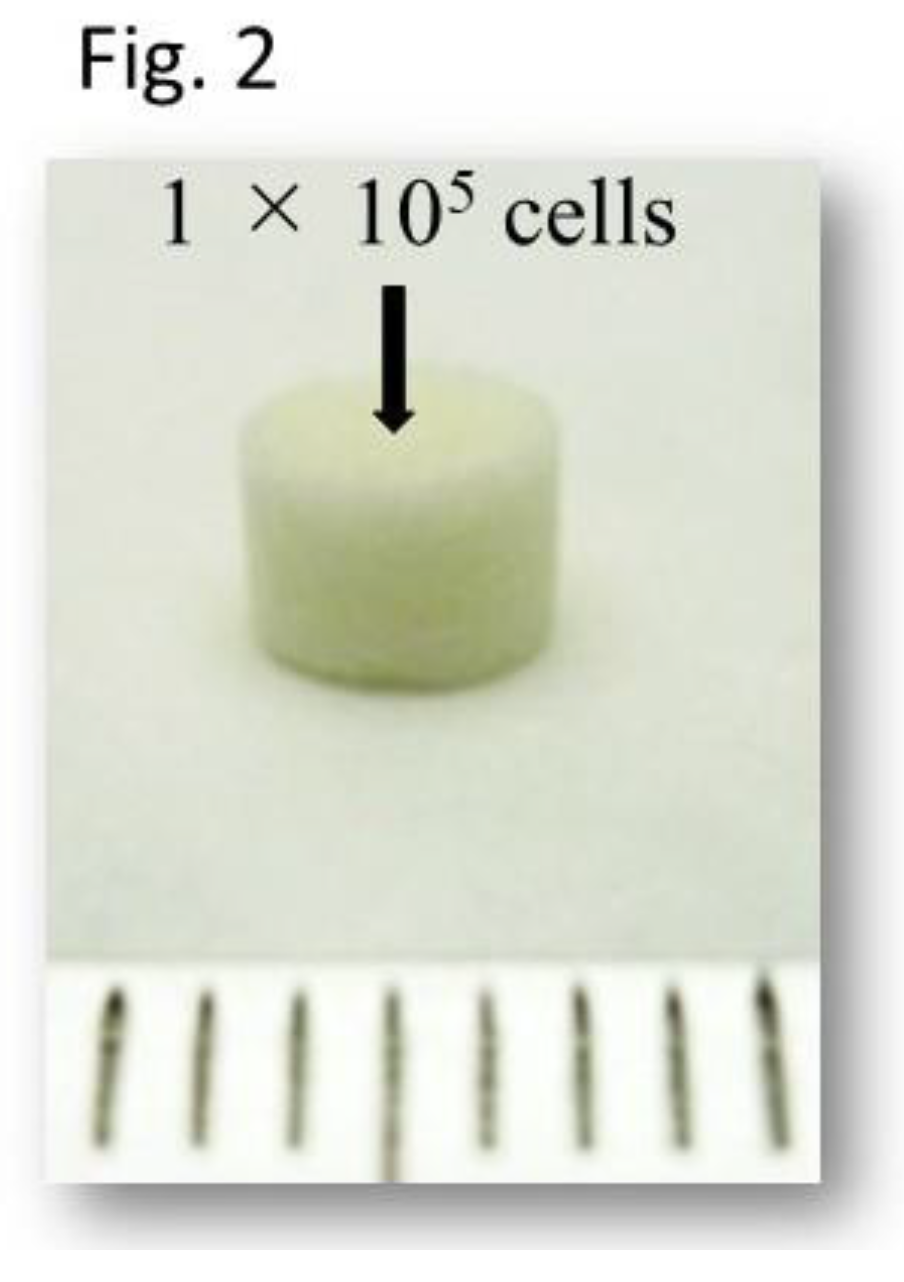
Atelocollagen sponge (Atelo Cell CSM-50, KOKEN Co., Ltd., Tokyo, Japan) was used as the scaffold (
Figure 2). Each scaffold was seeded with 1x10
5 UC-MSCs, which were regarded as one specimen. Seeding was performed according to the procedure described in the previous reports [
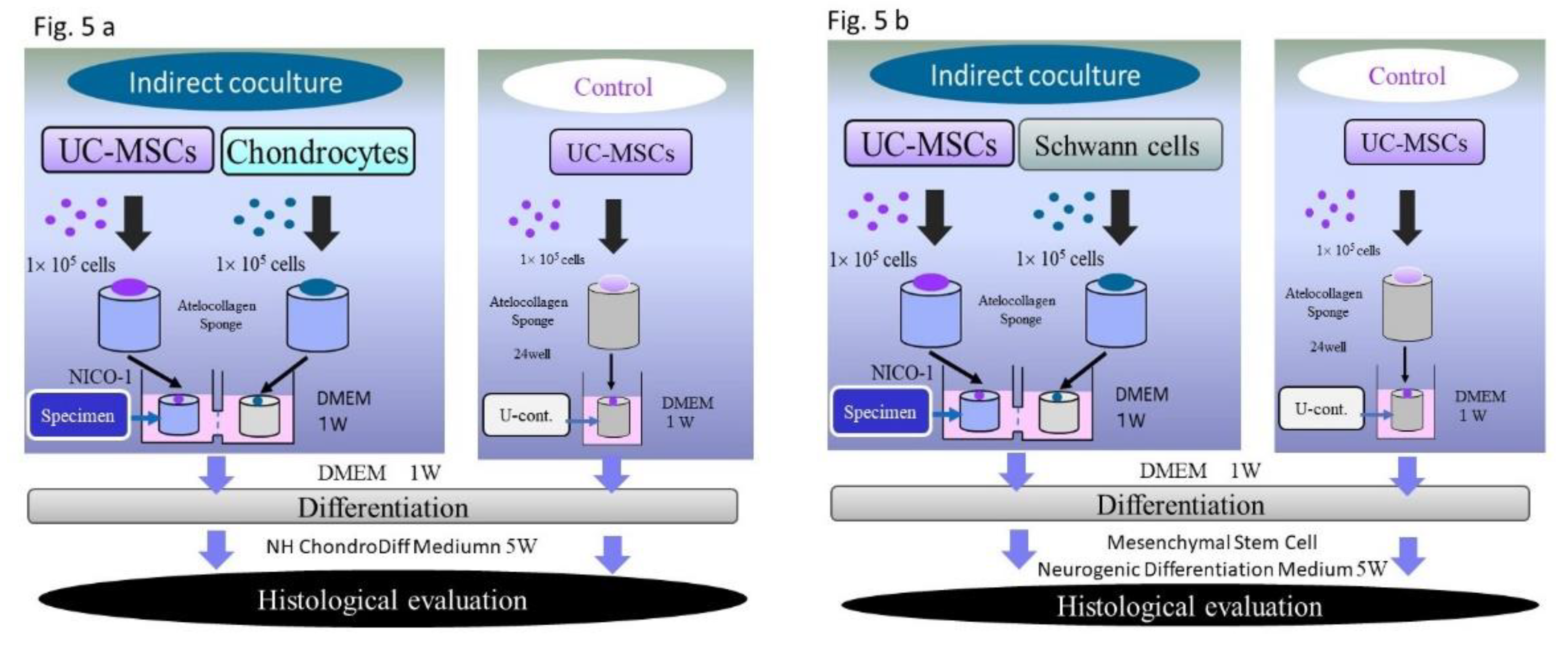
3]. Subsequently, specimens were placed in each vessel of coculture plate. The specimens were cultured in DMEM for one week to allow cell infiltration into the atelocollagen sponge. Six specimens were prepared. Three of them were used for induction of differentiation into chondrocytes and the other three were used for induction of differentiation into neural cells.
2.3. Coculture and Induction of Differentiation
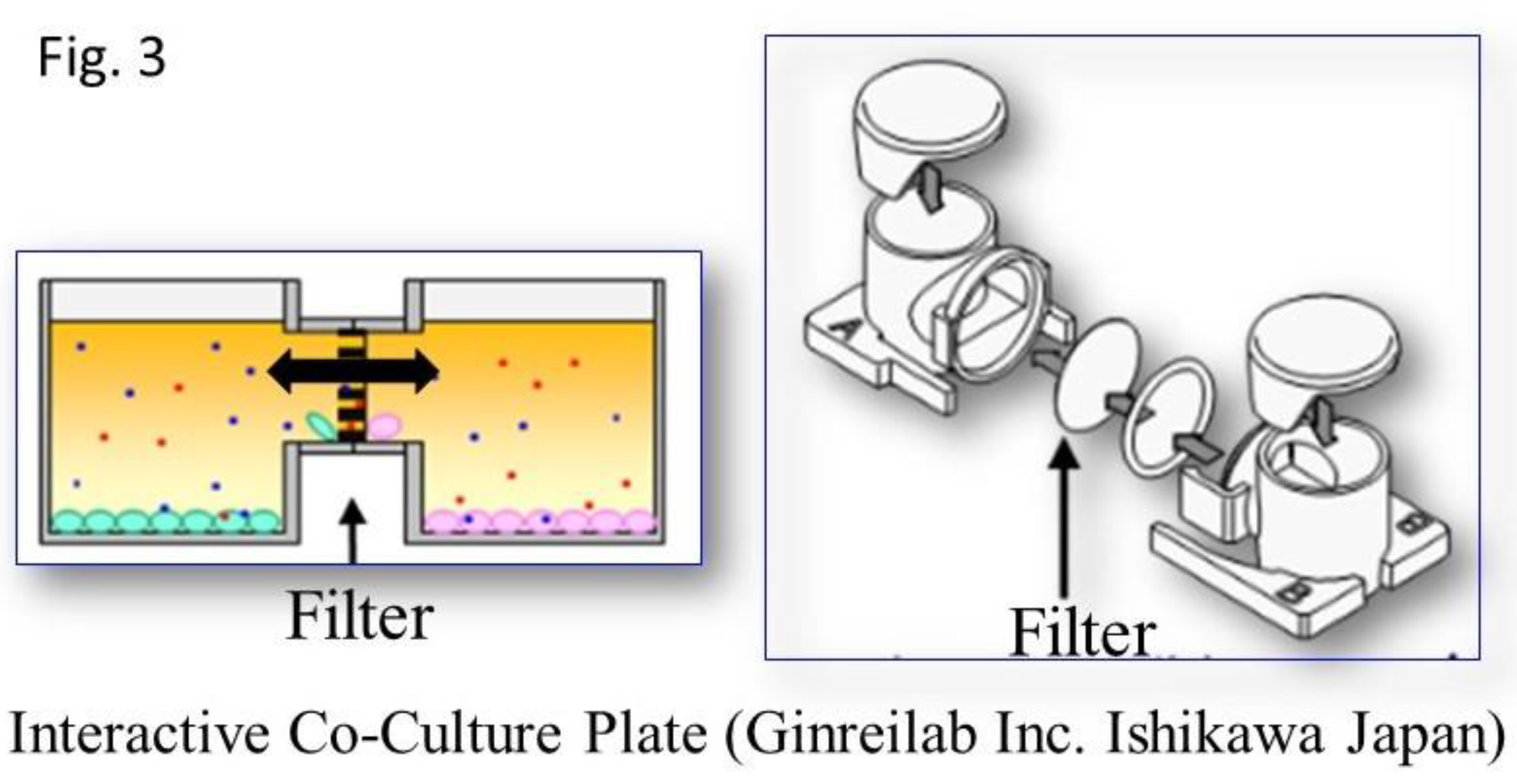
UC-MSCs that were seeded on scaffolds and cultured alone were used as the controls (control C: used for induction of differentiation into chondrocytes; control N, used for induction of differentiation into neural cells). For coculture, a horizontal interactive coculture plate (NOCO-1, Ginrei Lab, Ishikawa, Japan) was used (
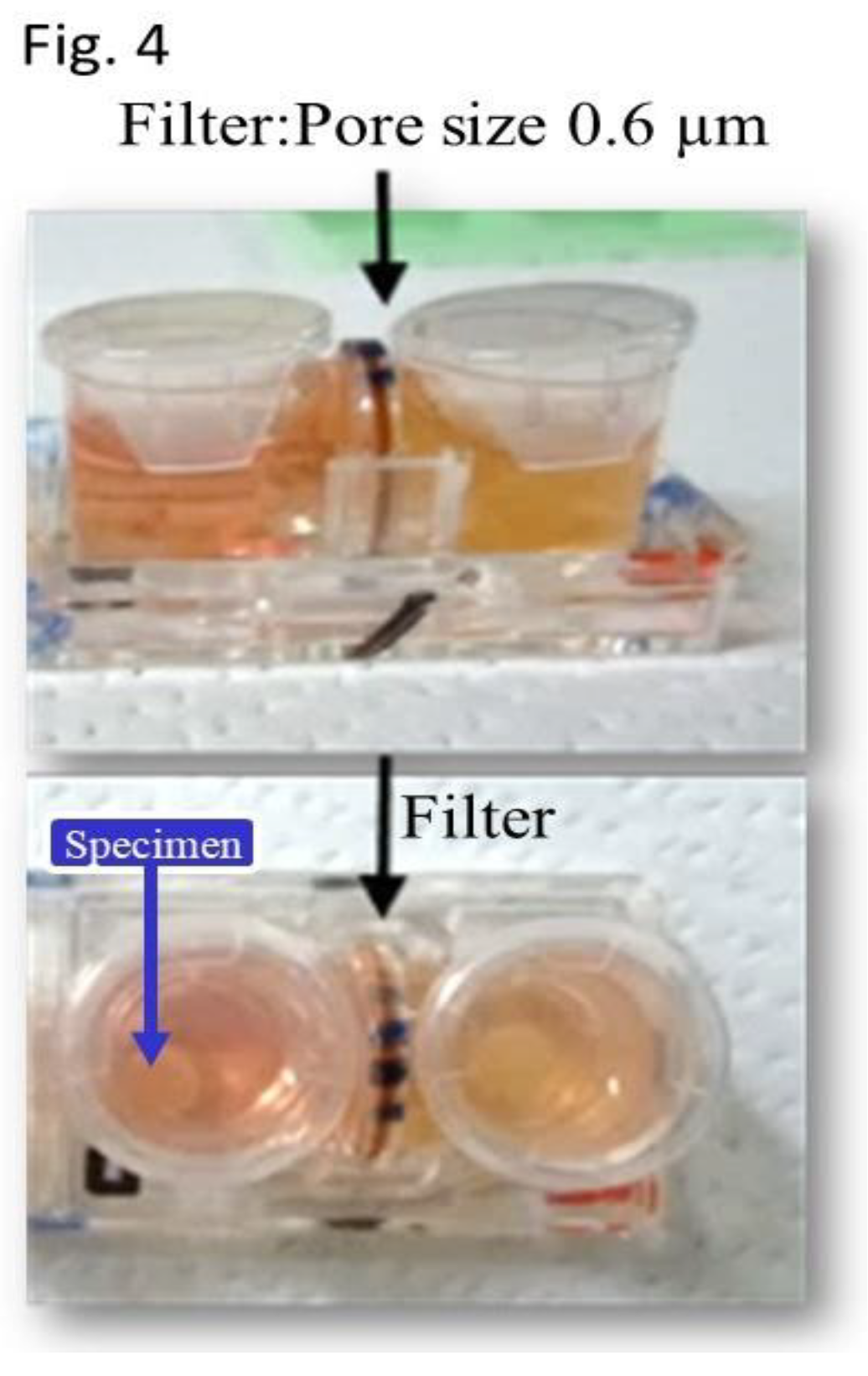
Figure 3). The specimens and controls were placed in the right and left vessels of the plate and a necessary and sufficient amount of culture medium was added. A filter with a pore size of 0.6 μm was placed between these vessels. This facilitated smooth and continuous passage of proteins and cytokines, including exosomes, from vessel to vessel. And The filter was precluding the cross-contamination of the two distinct cell cultures.
This provided an environment where the culture medium could be shared without mixing the cells (
Figure 4). The cells were also cultured at 37℃ in 5% CO
2 during coculture and induction of differentiation. For coculture of Control specimens (C) with chondrocytes, the culture medium was changed to ChondroDiff Media (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), and the specimens were cocultured for 5 weeks to be induced to differentiate into chondrocytes. In these cocultures, no additional components—such as growth factors—were added to the differentiation induction medium.
For coculture of Control specimens N (with Schwann cells), the culture medium was changed to Mesenchymal Stem Cell Neurogenic Differentiation Medium® (PromoCell GmbH, Heidelberg Germany), and the specimens were cocultured for 5 weeks to be induced to differentiate into Schwann cells. No substances other than the commercially-available differentiation induction media were added for induction of differentiation of either specimen. The flow of the experiments is shown in
Figure 5.
2.4. Histological Evaluation
Specimens removed from NOCO-1 were fixed with 10% formalin. After washing, the samples were embedded in paraffin, cut to approximately 3-mm sections, stained with hematoxylin and eosin (H&E), and observed with an optical microscope. Specimens C and controls C were stained with toluidine blue O (Toluidine Blue O: CI 52040. Kanto Chemical CO., INK Tokyo Japan). Specimens N and controls C were stained with S100 protein (Polyclonal Rabbit Anti-S100: DAKO Agilent. CA USA). Specimens were stained according to the respective manufacturer’s methods, and observed with an optical microscope.
3. Results
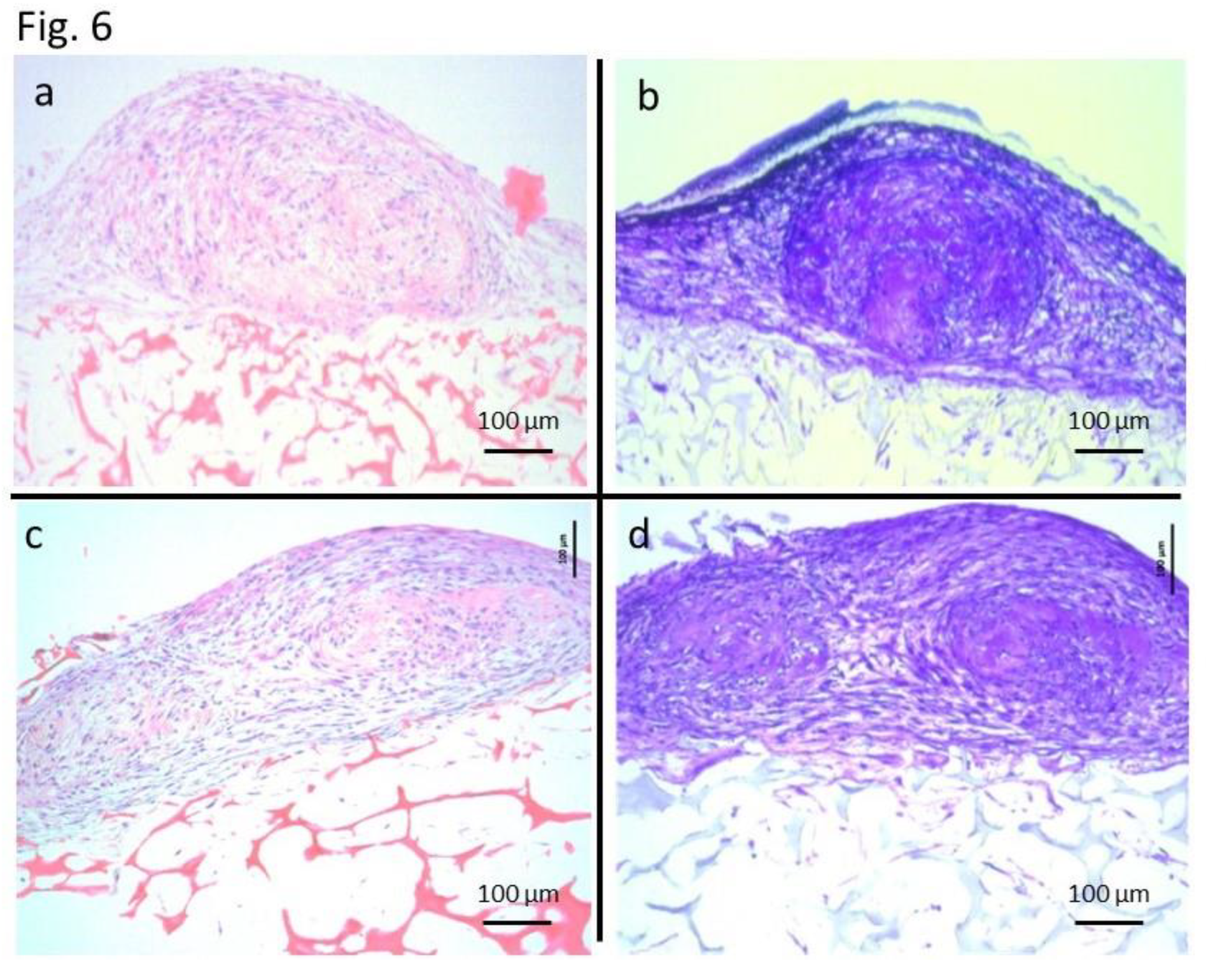
In specimens C and controls C, small elastic hard masses were formed outside the scaffolds. These small masses were cartilage matrices that were metachromatically-stained with toluidine blue. H&E staining also showed findings consistent with those of cartilage tissue (
Figure 6). In H&E staining of the controls, cartilage-like tissues detected in the controls were smaller than those in the specimens. And, in toluidine blue staining of the controls, metachromatically stained cartilage matrices were also detected in a smaller area in the controls than in the specimens. Controls N showed no cells stained with S100 protein, whereas specimens N showed cells positive for S100 protein. H&E staining revealed that the morphology of S100 protein-positive cells in specimens N was consistent with that of Schwann cells (
Figure 7). In H&E staining of the controls N, no cells showed morphology specific to Schwann cells.
4. Discussion
The availability of autologous tissues as biomedical materials for regenerative medicine suggests that they may be safe for use in patients. The number of pregnant women attending our department for prenatal consultations about their fetuses diagnosed with an alveolar cleft is not rare. As a consequence, we have focused on the umbilical cord (UC) as a source of autologous regenerative medicine. Collecting these autologous tissues at birth poses no difficulty. Recent studies have revealed the presence of mesenchymal stem cells (MSCs) in many tissues, including the UC. The presence of UC-MSCs has become well known in recent years, and attention is paid to their usefulness. In this study, we focused on the UC, which has the following advantages: (1) it is autologous tissue; (2) it can be noninvasively collected; and (3) it provides an abundant volume of tissue. The purpose of this study was to obtain autologous tissues for tissue regeneration in the form of UC-MSCs.
Currently, investigators are conducting studies to determine the process of tissue regeneration. However, not all elements of the process, such as timing, substances, concentrations, and combinations and ratios of substances, have been elucidated. When tissues are formed in the body, cells continuously release various factors, such as exosomes, and interact to regenerate tissues. In the case of
in vitro tissue formation, essential operations that have been reported are performed. However, the
in vitro operations may not necessarily mimic the physiological tissue formation in the body. For example, adding bone morphogenic protein (BMP) to BM-MSCs induces BM-MSCs to differentiate into osteoblasts so that bone tissues can be obtained. Nevertheless, this is achieved in an environment different from the complex
in vivo environment [
8]. As a means to solve this issue, we focused on coculture. The advantage of coculture is that the factors released from cocultured cells can continuously be used at physiological timing and concentrations during coculture [
6,
9]. This outcome is achievable regardless of whether the details have been known. Because UC-MSCs are more primitive and more likely to be affected by the surrounding environment than adult tissue-derived MSCs [
10,
11], we hypothesized that coculture would benefit tissue regeneration with UC-MSCs and facilitate tissue formation compared to tissue formation by culturing UC-MSCs alone.
The coculture techniques used in recent years are classified mainly into two types [
9]. These are direct coculture, by which cells are not separated but mixed together to contact each other in one well during culture; and indirect coculture, by which multiple different types of cells are cultured simultaneously in a system with separate vessels that keep the cells from being mixed and contacting each other [
6,
9]. In indirect coculture, humoral factors mediate cell-tocell interactions [
12,
13]. There are two types of indirect coculture containers: vertical and horizontal. Horizontal coculture vessels were employed in our study, as they are believed to facilitate more efficient and continuous exchange of humoral factors between cell types. This configuration optimizes the interaction between cells by promoting a stable and consistent environment for factor diffusion, which may enhance the overall coculture system's performance and reliability [
12].
Figure 6 shows a difference in the color of the medium between the left and right vessels. This discrepancy is likely due to the presence of a filter. Nevertheless, considering the results of the differentiation assays, indirect horizontal coculture appears to have been beneficial for the continuous exchange of humoral factors in observed this study.
Mesenchymal stem cells are well established as essential for regenerative medicine and are found in many tissues, e.g., bone marrow and adipose tissue. Bone marrow is the most common supply source of mesenchymal stem cells for osteogenesis. Recent studies revealed the presence of mesenchymal stem cells many other tissues, including the UC [
1,
2]. UC, whose abundant tissue volume is available noninvasively from the neonate at birth, has good clinical potential for the patient him/herself if it can be used as autologous tissue. This causes less ethical, medical, and safety issues [
3]. Our purpose is the clinical application of regenerative medicine using autologous tissue-derived materials to reduce surgical invasiveness in the future. Direct coculture seems more physiological and more likely to cause interactions through contact of cells than indirect coculture [
14]. However, if cells derived from other people or species are mixed, implantation will be difficult even after successful formation of targeted tissues. In recent years, investigators have studied the importance of the actions of humoral factors, such as exosomes, on cells [
15]. In the present study, US-MSCs in the control N, which were induced to differentiate only in the commercially-available differentiation induction medium, did not differentiate into Schwann cells. UC-MSCs in Control N differentiated into Schwann cells, which suggests that humoral factors shared through the filter in coculture might have contributed to the induction of differentiation into neural cells. As for the induction of differentiation into cartilage, the histological evaluation revealed cartilage-like tissues in both control C and specimen C. However, specimen C showed more mature and larger cartilage-like tissues that were macroscopically detectable. On the basis of a previous report [
16] and our previous experiences, we consider that the induction of UC-MSCs to differentiate into neural cells is not as easy as induction to differentiate into chondrocytes. In the present study, however, we were able to induce differentiation into both chondrocytes and Schwann cells with similar culture techniques using commercially-available differentiation induction media without any special manipulation of cells or inventive additives to the culture media. The results of the present study suggest that coculture is useful for the induction of differentiation of UC-MSCs.
Limitation: The sample size was limited because clinically obtained specimens were used.
5. Conclusions
Coculture with chondrocytes and Schwann cells was effective in inducing differentiation of UC-MSCs. Our results using indirect horizontal coculture vessels demonstrated that humoral factors released by chondrocytes and Schwann cells significantly induced differentiation of UC-MSCs. This suggests that coculture is not only a simple and less stressful technique but also a physiologically relevant method for enhancing the differentiation potential of UC-MSCs, making it a useful tool for regenerative medicine applications utilizing UC-MSCs as autologous tissue.
Author Contributions
Conceptualization, K.B.; methodology, K.B.; validation, Y.S., K.M., K.K and T.S.; investigation, Y.S. and Y.K.; resources, K.B.; data curation, K.B.; writing—original draft preparation, K.B.; writing—review and editing, A.T.; supervision, Y.Y.; project administration, A.T.; funding acquisition, K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant-in-aid for scientific research (C)20K09871 from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Kitasato University (B-07-13).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding author/s.
Acknowledgments
Support was provided by a grant-in-aid for scientific research (C)20K09871 from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We would like to thank Honyaku Center Inc. for English language editing.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collec-tion, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sarugaser, R.; Lickorish, D.; Baksh, D.; Hosseini, M.M.; Davies, J.E. Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells 2005, 23, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Yamazaki, Y.; Ikemoto, S.; Aoyagi, K.; Takeda, A.; Uchinuma, E. Osteogenic potential of human umbilical cord-derived mesenchymal stromal cells cultured with umbilical cord blood-derived autoserum. J. Cranio-Maxillofac. Surg. 2012, 40, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Yamazaki, Y.; Ishiguro, M.; Kumazawa, K.; Aoyagi, K.; Ikemoto, S.; Takeda, A.; Uchinuma, E. Osteogenic potential of human umbilical cord-derived mesenchymal stromal cells cultured with umbilical cord blood-derived fibrin: A preliminary study. J. Cranio-Maxillofac. Surg. 2013, 41, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties. Stem Cells 2020, 38, 1250–1263. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, T.; Yamamoto, S.; Omura, R.; Ito, K.; Nishide, Y.; Yamada, H.; Ohtomo, K.; Ishisaka, T.; Okano, K.; Ogawa, T.; Tsuji, H.; Matsuo, Y.; Minamoto, T.; Tomosugi, N.; Ferain, E.; Ochiya, T. Novel platform for regulation of extracellular vesicles and metabolites secretion from cells using a multi-linkable horizontal co-culture plate. Micromachines 2021, 12, 1431. [Google Scholar] [CrossRef] [PubMed]
- Ohgushi, H.; Kitamura, S.; Kotobuki, N.; Hirose, M.; Machida, H.; Muraki, K.; et al. Clinical application of marrow mesenchymal stem cells for hard tissue repair. Yonsei Med. J. 2004, 45, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Yamazaki, Y.; Sons, Y.; Sugimoto, Y.; Moriyama, K. ; Sugimoto, T; Kumazawa, K. , Shimakura Y., Takeda, A. An in vitro long-term study of cryopreserved umbilical cord blood derived platelet-rich plasma containing growth factors-PDGF-BB, TGF-b, and VEGF. J. Cranio-Maxillofac. Surg. 2019, 47, 668–675. [Google Scholar] [CrossRef]
- Shimasaki, T.; Yamamoto, S.; Arisawa, T. Exosome research and co-culture study. Biol. Pharm. Bull. 2018, 41, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Pivorinūas, A.; Bernotiene, E.; Unguryte, A.; Valiuniene, S.; Drasutiene, G.; Venalis, A. Isolation and differentiation of mesenchymal stem-like cells from human umbilical cord vein endothelium and subendothelium. Biologija 2006, 2, 99–103. [Google Scholar] [CrossRef]
- Bieback, K.; Brinkmann, I. Mesenchymal stromal cells from human perinatal tissues: From biology to cell therapy. World J. Stem Cells 2010, 2, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Sumi, S.; Kawagoe, M.; Abe, R.; Yanai, G.; Yang, K.C.; Shirouzu, Y. A multiple-funnels cell culture insert for the scale-up production of uniform cell spheroids. Regen. Ther. 2017, 7, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, F.S.; Vrtacnik, D.; Neuzil, P.; Iliescu, C. Microfluidic technology for clinical applications of exosomes. Micromachines 2019, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, J.; Magal, P.; Boulange-Lecomte, C.; Webb, G.; Le Foll, F. Consequences of cell-to-cell P-glycoprotein transfer on acquired multidrug resistance in breast cancer: A cell population dynamics model. Biol. Direct 2011, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Royo, F.; Thery, C.; Falcon-Perez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for separation and characterization of extracellular vesicles: Results of a worldwide survey performed by the ISEV rigor and standardization subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, R.B.; Ullah, I.; Kim, E.J.; Jang, S.J.; Lee, W.J.; Jeon, R.H.; Kang, D.; Lee, S.L.; Park, B.W.; Rho, G.J. Characterization and evaluation of neuronal trans-differentiation with electrophysiological properties of mesenchymal stem cells isolated from porcine endometrium. Int. J. Mol. Sci. 2015, 16, 10934–10951. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).