Submitted:
30 July 2024
Posted:
31 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Spectroscopic Characterization of Zein Protein (ZP)
2.1.1. UV-Vis Absorption
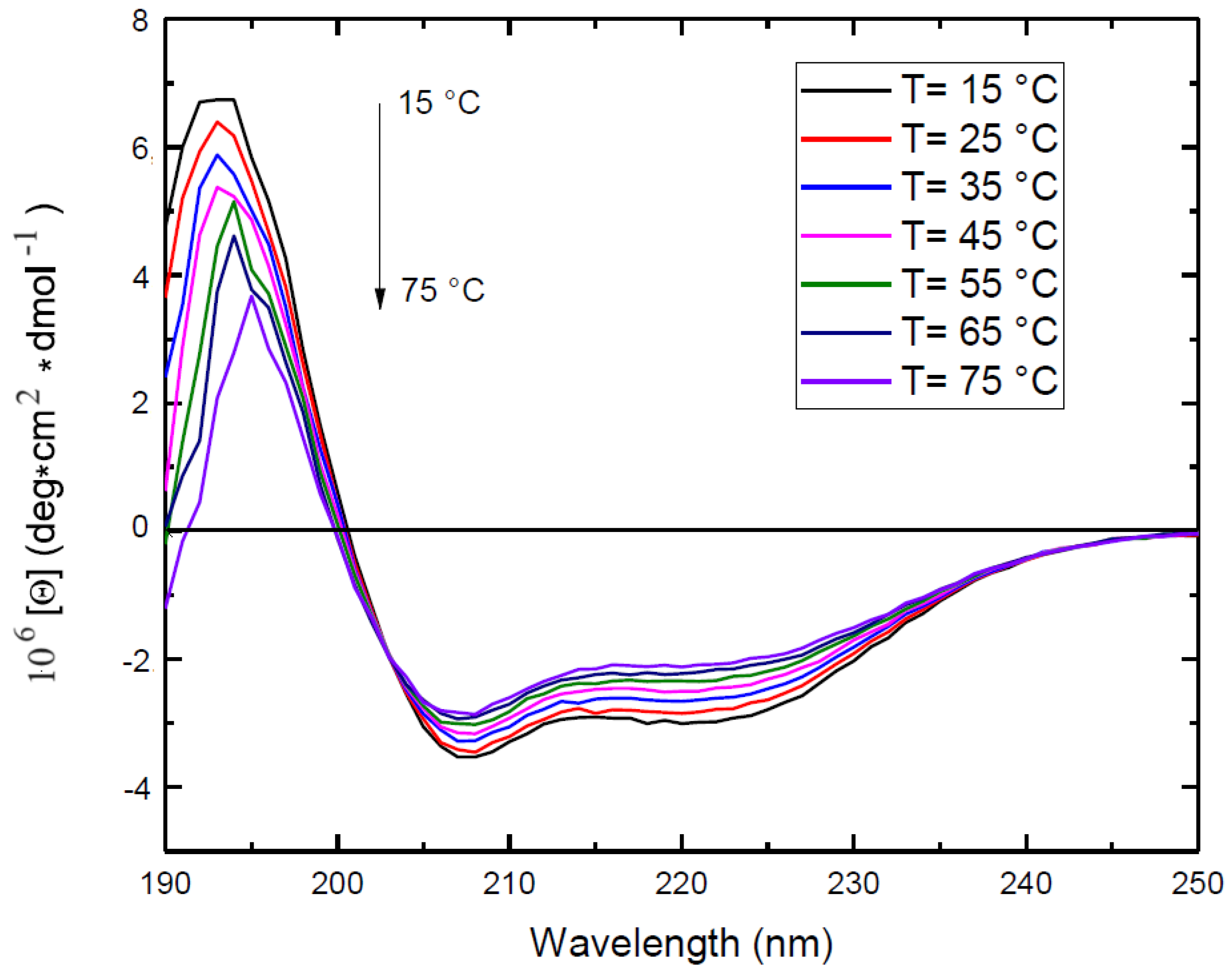
2.1.2. Electronic Circular Dichroism
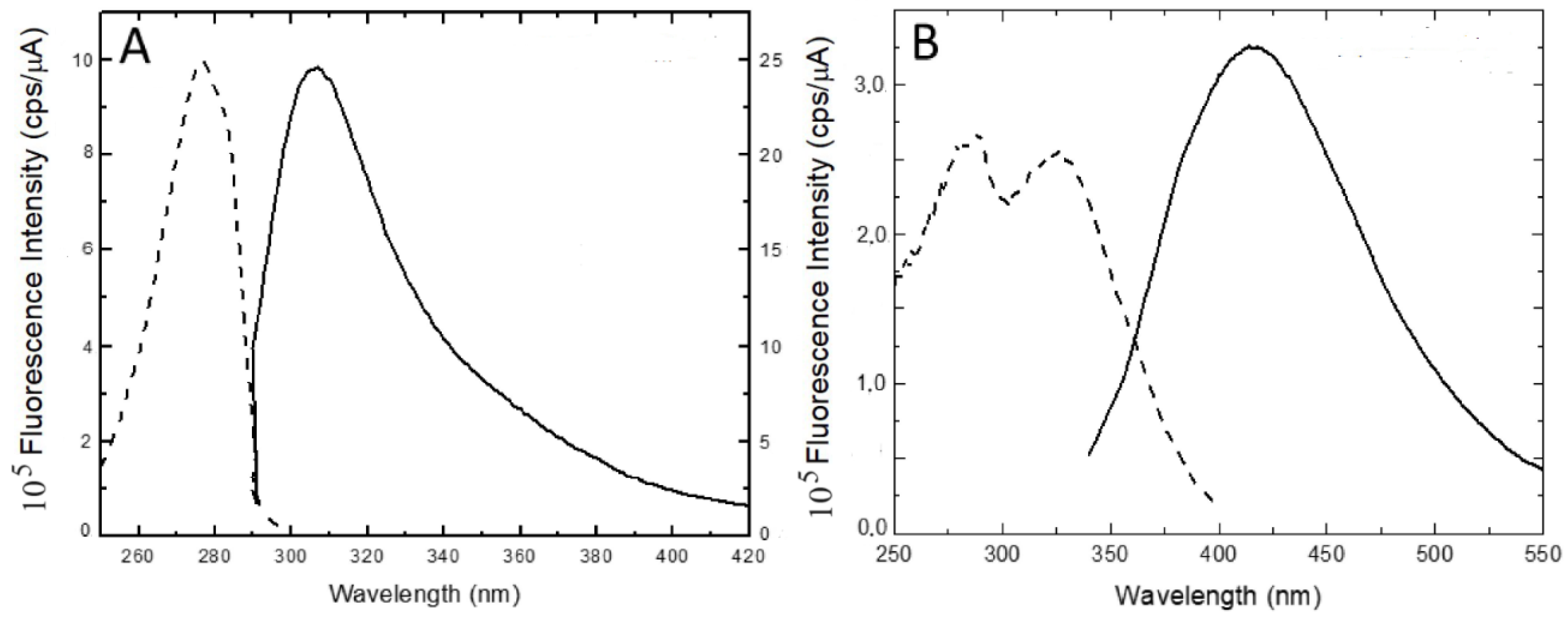
2.1.3. Steady-State Fluorescence
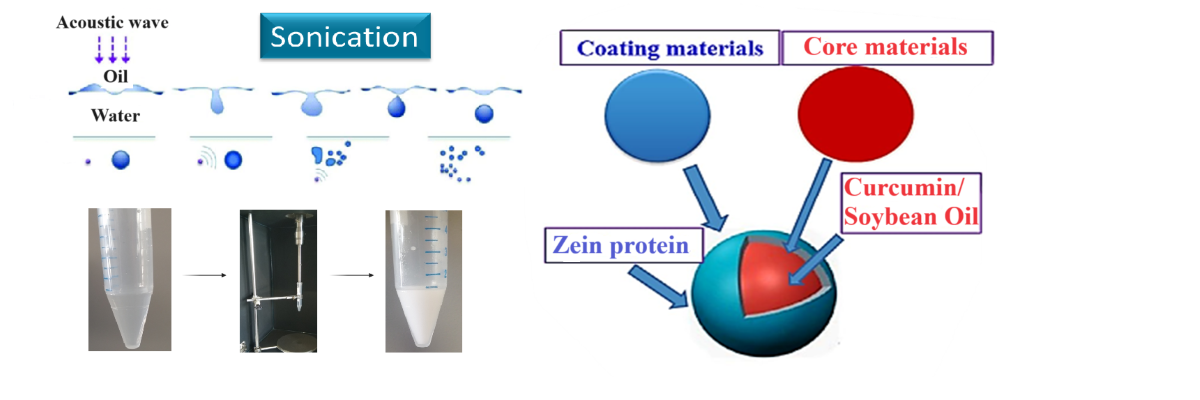
2.2. Zein Protein Microcapsules
2.2.1. Zein Microcapsules Synthesis: Procedure and Optimization of the Experimental Conditions
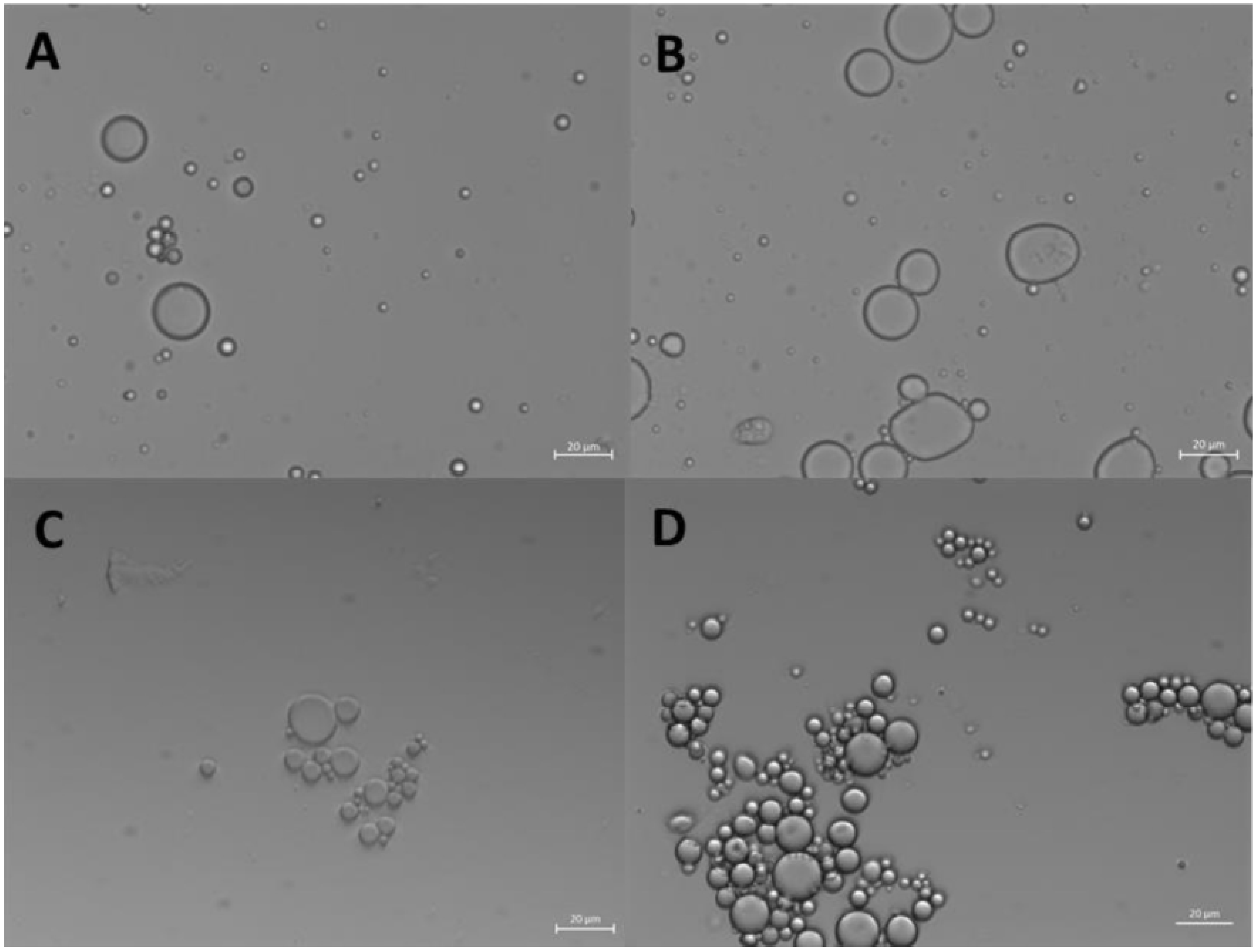
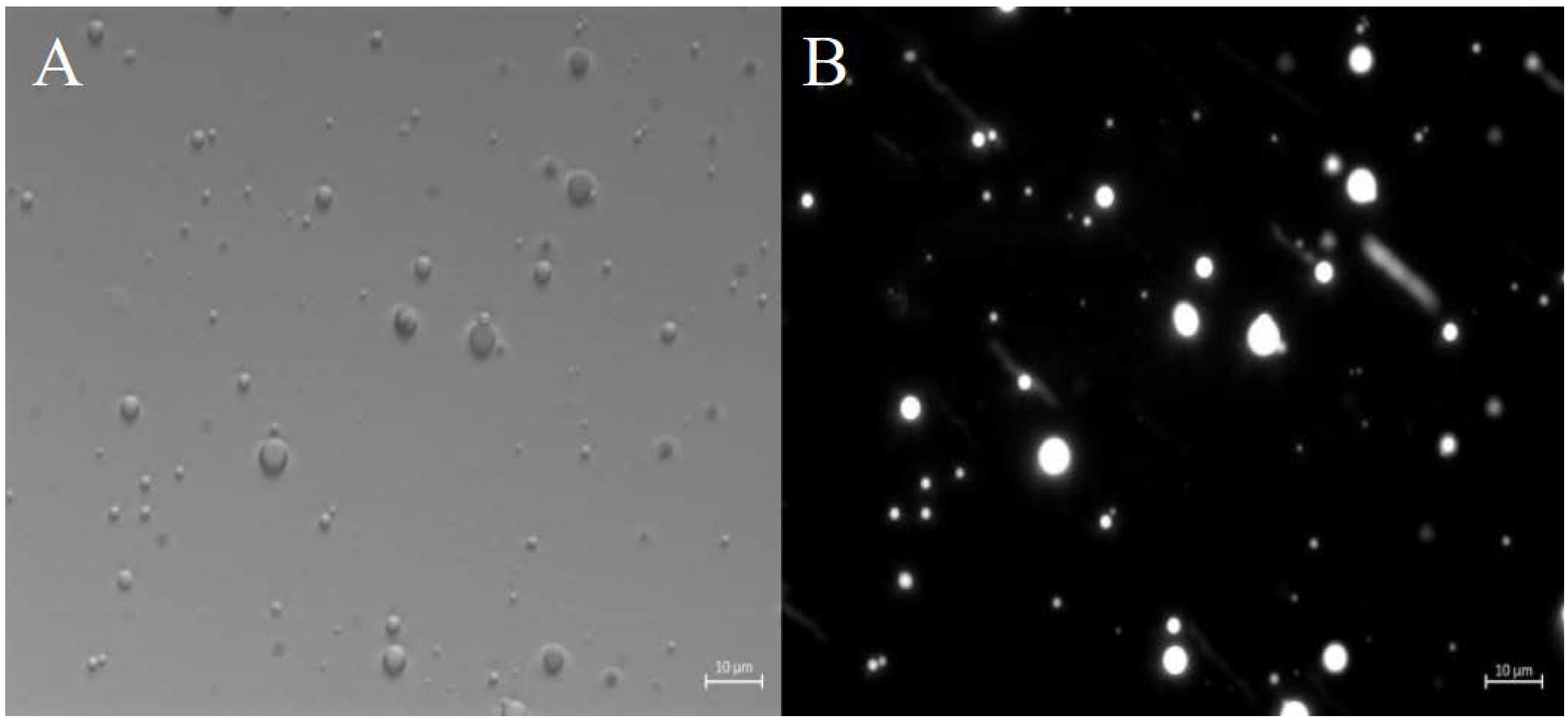
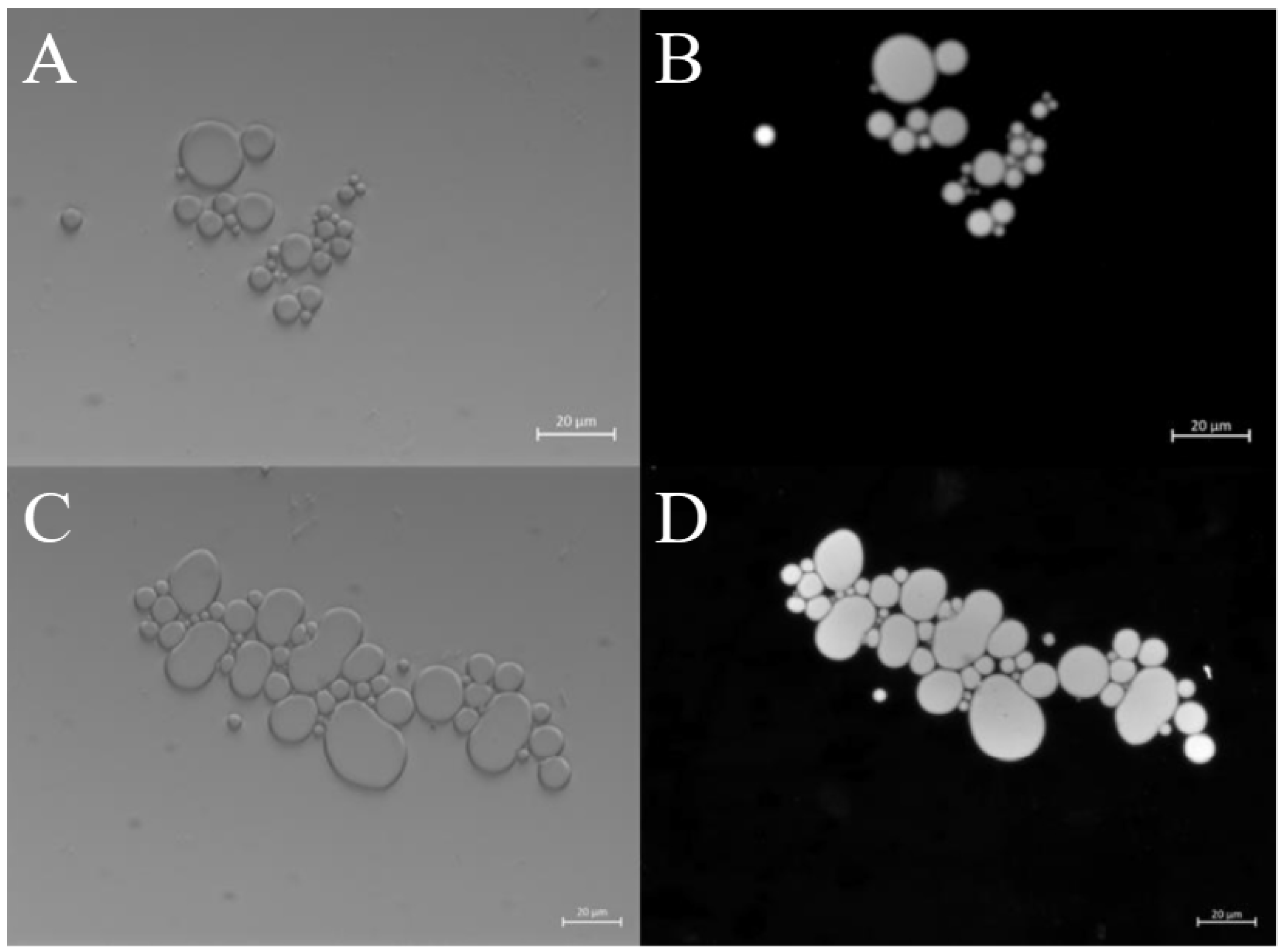
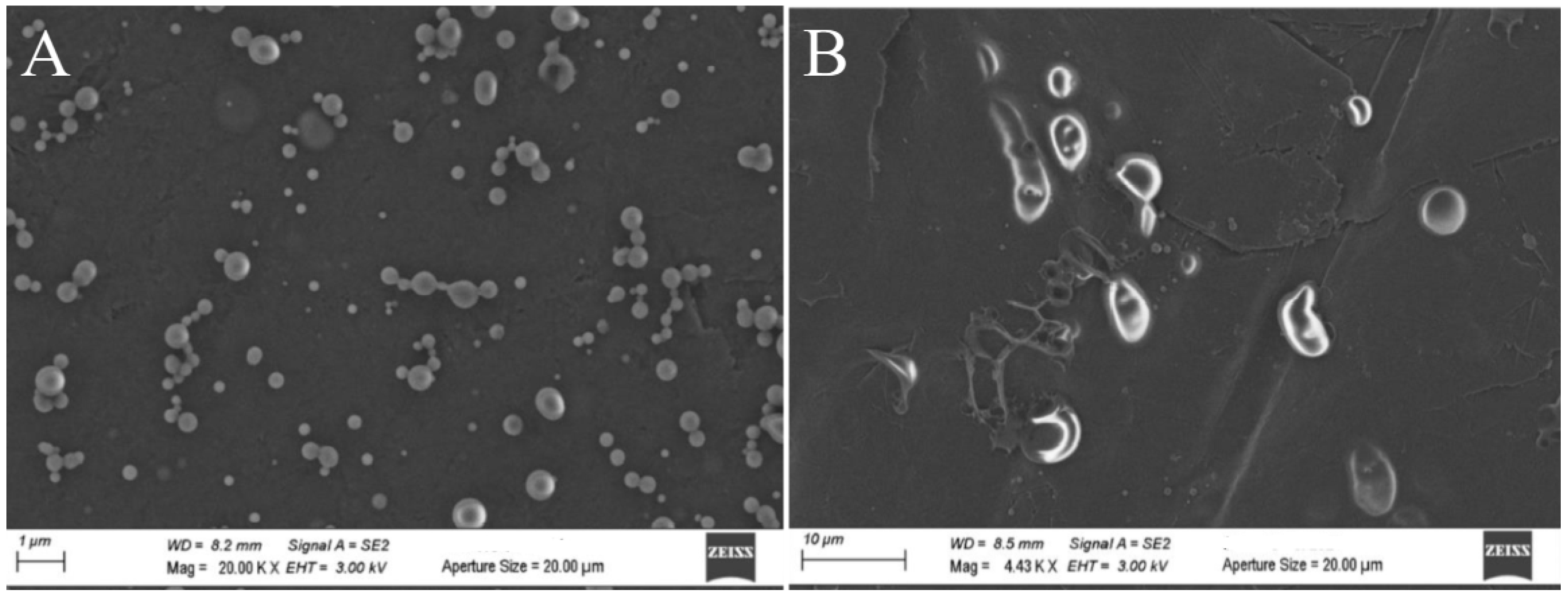
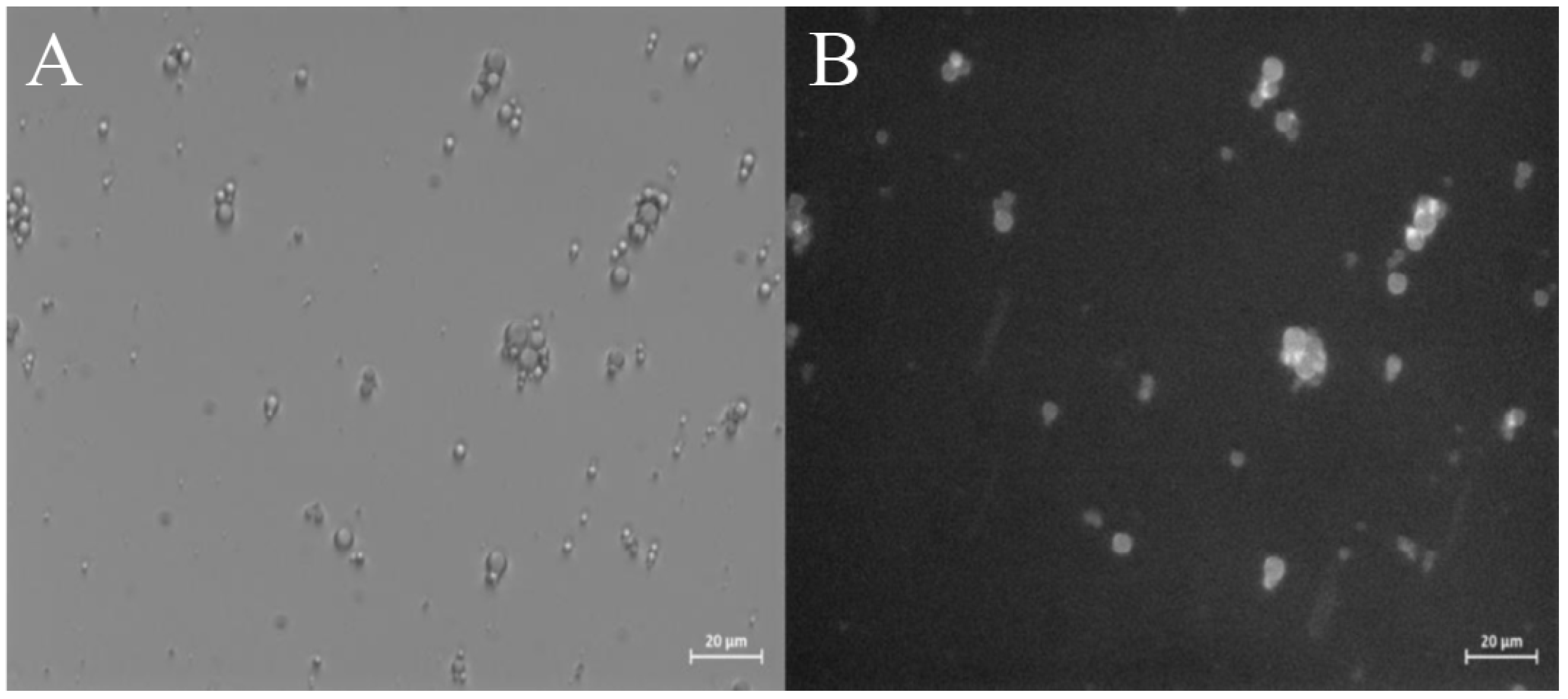
2.2.2. Morphological Characterization of ZP Microcapsules
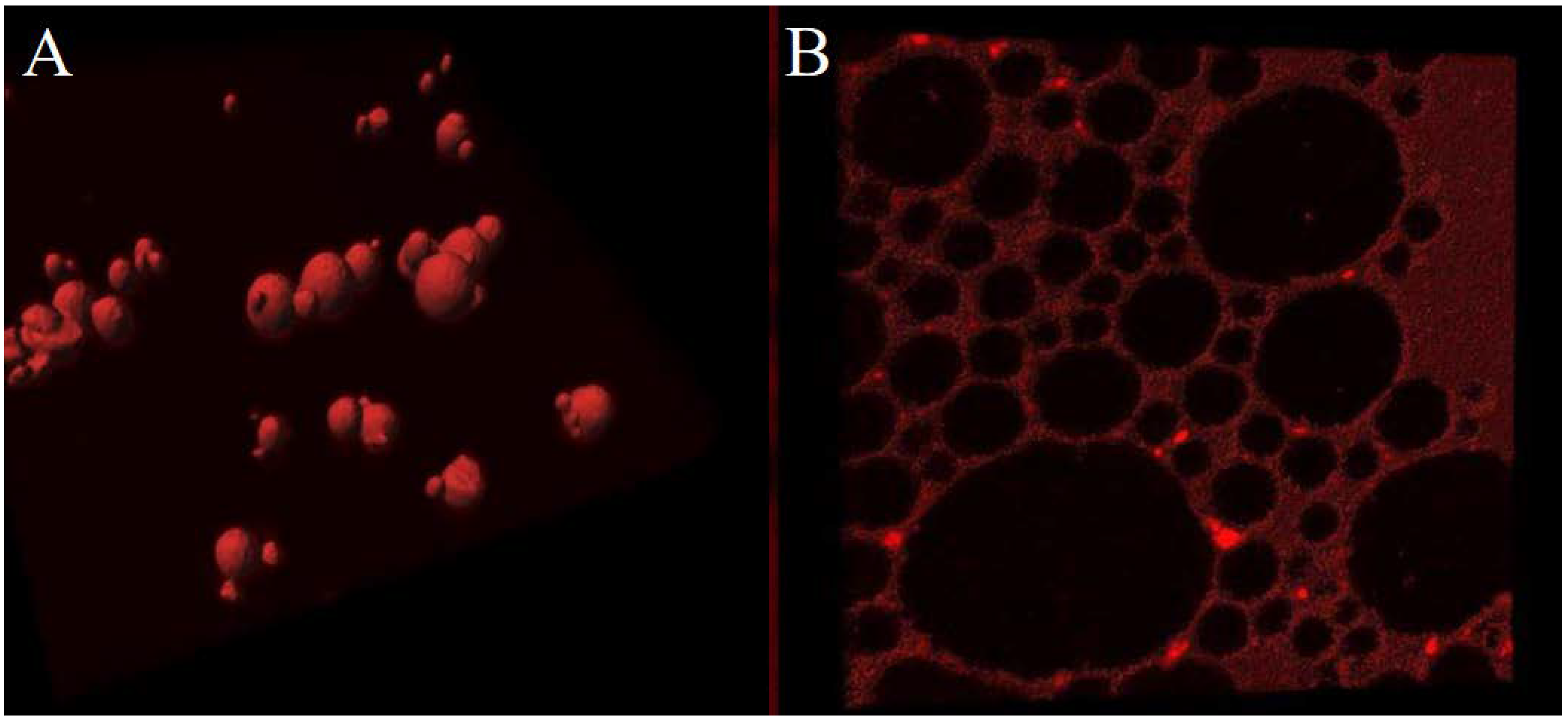
2.2.3. Encapsulation of Curcumin into ZP microcaspules
3. Materials and Methods
3.1. Materials
3.2. Methods and Instrumentation
3.2.1. Protein Solubilization
3.2.2. UV–Vis Absorption Spectrophotometry
3.2.3. Circular Dichroism
3.2.4. Steady-State Fluorescence
3.2.5. Optical Microscopy
3.2.6. Field Emission - Environmental Scanning Electron Microscope (FE-ESEM)
3.2.7. Light Scattering
3.2.8. Synthesis of Zein Microcapsules
3.2.9. Inclusion of Dyes and Active Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bah, M.G.; Bilal, H.M.; Wang, J. Fabrication and application of complex microcapsules: A review. Soft Matter 2020, 16, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Emam, A.; Ismaiel, A.; Ashokkumar, M.; Xu, X.; Pan, S.; Hu, H. Ultrasonic emulsification: An overview on the preparation of different emulsifiers-stabilized emulsions. Trends Food Sci. Technol. 2020, 105, 363–377. [Google Scholar] [CrossRef]
- Leong, T.S.H.; Martin,G. J.O.; Ashokkumar M. Ultrasonic encapsulation – A review. Ultrason. Sonochem. 2017, 35, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Tortora, M.; Cavalieri, F.; Mosesso, P.; Ciaffardini, F.; Melone, F.; Crestini, C. Ultrasound driven assembly of lignin into microcapsules for storage and delivery of hydrophobic molecules. Biomacromolecules 2014, 15, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Bernard, J.; Ganachaud, F.; Miserez, A. Protein-based encapsulation strategies: Toward micro- and nanoscale carriers with increased functionality. Small Sci. 2022, 2, 2100095. [Google Scholar] [CrossRef]
- Lawton, J.W. Zein: A history of processing and use. Cereal Chem. 2002, 79, 1–18. [Google Scholar] [CrossRef]
- Kim, S.; Xu, J. Aggregate formation of Zein and its structural inversion in aqueous ethanol. J. Cereal Sci. 2008, 47, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.; Padua, G.W. Nanoscale characterization of Zein self-assembly. Langmuir 2012, 28, 2429–2435. [Google Scholar] [CrossRef]
- Wang, Q.; Xian, W.; Li, S.; Liu, C.; Padua, G.W. Topography and biocompatibility of patterned hydrophobic/hydrophilic Zein layers. Acta Biomater. 2008, 4, 844–851. [Google Scholar] [CrossRef]
- Turasan, H.; Kokini, J.L. Advances in understanding the molecular structures and functionalities of biodegradable Zein-based materials using spectroscopic techniques: A review. Biomacromolecules 2017, 18, 331–354. [Google Scholar] [CrossRef]
- Wang, Y.; Su, C.-P.; Schulmerich, M.; Padua, G. W. Characterization of core–shell structures formed by Zein. Food Hydrocoll. 2013, 30, 487–494. [Google Scholar] [CrossRef]
- 1rache, J.M.; González-Navarro, C.J. Zein nanoparticles as vehicles for oral delivery purposes. Nanomed. 2017, 12, 1209–1211. [Google Scholar] [CrossRef]
- Girija Aswathy, R.; Sivakumar, B.; Brahatheeswarani, D.; Fukuda, T.; Yoshida, Y.; Maekawa, T.; Sakthi Kumar, D. Biocompatible fluorescent Zein nanoparticles for simultaneous bioimaging and drug delivery application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 025006. [Google Scholar] [CrossRef]
- Weissmueller, N.T.; Lu, H.D.; Hurley, A.; Prud’homme, R.K. Nanocarriers from GRAS Zein proteins to encapsulate hydrophobic actives. Biomacromolecules 2016, 17, 3828–3837. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-López, P.; Murdan, S. Zein microspheres as drug/antigen carriers: A study of their degradation and erosion in the presence and absence of enzymes. J. Microencapsul. 2006, 23, 303–314. [Google Scholar] [CrossRef]
- Sharif, N.; Fabra, M.J.; López-Rubio, A. Nanostructures of Zein for encapsulation of food ingredients, in Biopolymer Nanostructures for Food Encapsulation Purposes, Elsevier, 2019, 217–245. [CrossRef]
- Beck, M.I.; Tomka, I.; Waysek, E. Physico-chemical characterization of Zein as a film coating polymer: A direct comparison with ethyl cellulose. Int. J. Pharm. 1996, 141, 137–150. [Google Scholar] [CrossRef]
- Song, R.; Llaca, V.; Linton, E.; Messing, J. Sequence, regulation, and evolution of the maize 22-kD α-Zein gene family. Genome Res. 2001, 11, 1817–1825. [Google Scholar] [CrossRef]
- “zein AND reviewed:yes in UniProtKB.” https://www.uniprot.org/uniprot/?query=zein&fil=reviewed%3Ayes&sort=score (accessed , 2024). 17 July.
- “alpha zein AND reviewed:yes in UniProtKB.” https://www.uniprot.org/uniprot/?query=alpha%20zein&fil=reviewed%3Ayes&sort =score (accessed , 2024). 17 July.
- Cabra, V.; Arreguin, R.; Galvez, A.; Quirasco, M.; Vazquez-Duhalt, R.; Farres, A. Characterization of a 19 kDa α-Zein of high purity. J. Agric. Food Chem., 2005, 53, 725–729. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, C.; Hu, Y.; Gao, M.; Luan, G. Zein as a structural protein in Gluten-free systems: An overview. Food Sci. Hum. Wellness, 2021, 10, 270–277. [Google Scholar] [CrossRef]
- Wang, Y.; Padua, G.W. Formation of Zein microphases in ethanol−water. Langmuir 2010, 26, 12897–12901. [Google Scholar] [CrossRef]
- Mannheim, A.; Cheryan, M. Water-soluble Zein by enzymatic modification in organic solvents. Cereal Chem. 1993, 70, 115–121. [Google Scholar]
- Forato, L.A.; Bicudo, T.D.C.; Colnago, L.A. Conformation of alpha Zeins in solid state by Fourier Transform IR. Biopolymers 2003, 72, 421–426. [Google Scholar] [CrossRef]
- Tatham, A. S.; Field, J.M; Morris, V.J.; l’Anson, K.J.; Cardle, L.; Dufton, M.J.; Shewry, P.R. Solution conformational analysis of the alpha-Zein proteins of maize. J. Biol. Chem. 1993, 268, 26253–26259. [Google Scholar] [CrossRef] [PubMed]
- Forato, L.A.; Doriguetto, A.C.; Fischer, H.; Mascarenhas, Y.P.; Craievich, A.F.; Colnago,L. A. Conformation of the Z19 Prolamin by FTIR, NMR, and SAXS. J. Agric. Food Chem. 2004, 52, 2382–2385. [Google Scholar] [CrossRef] [PubMed]
- Argos, P.; Pedersen, K.; Marks, M.D.; Larkins, B.A. A Structural model for maize Zein proteins. J. Biol. Chem. 1982, 257, 9984–9990. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, N.; Danno, G.; Takezawa, H.; Izumi, Y. Three-dimensional structure of maize alpha-Zein proteins studied by small-sngle X-ray scattering. Biochim. Biophys. Acta. 1997, 1339, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Momany, F.A.; Sessa, D.J.; Lawton, J.W.; Selling, G.W.; Hamaker, S.A.H. Willett, J.L. Structural characterization of alpha-Zein. J. Agric. Food Chem. 2006, 54, 543–547. [Google Scholar] [CrossRef]
- Padua, G.W.; Guardiola, L.V. Microcapsules produced from Zein, in Microencapsulation and Microspheres for Food Applications, Elsevier, 2015, pp. 3– 20. [CrossRef]
- Oh, Y.K.; Flanagan, D.R. Swelling and permeability characteristics of Zein membranes. PDA J. Pharm. Sci. Technol. 2003, 57, 208–217. [Google Scholar]
- Wu, Y.; Luo, Y.; Wang, Q. Antioxidant and antimicrobial properties of essential oils encapsulated in Zein nanoparticles prepared by liquid–liquid dispersion method. LWT - Food Sci. Technol. 2012, 48, 283–290. [Google Scholar] [CrossRef]
- Beaven, G.H.; Holiday, E.R. Ultraviolet absorption spectra of proteins and amino acids. Advances in Protein Chemistry. [CrossRef]
- Malencik, D.A.; Anderson, S.R. Dityrosine as a product of oxidative stress and fluorescent probe. Amino Acids 2003, 25, 233–247. [Google Scholar] [CrossRef]
- Heinecke, J.W.; Li, W.; Daehnke, H.L.; Goldstein, J. A. Dityrosine, a specific marker of oxidation, is synthesized by the Myeloperoxidase-Hydrogen Peroxide system of human neutrophils and macrophages. J. Biol. Chem. 1993, 268, 4069–4077. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Johnson, S.K.; Clarke, M.W. Identification and quantification of dityrosine in grain proteins by isotope dilution liquid chromatography-tandem mass spectrometry. Food Anal. Methods, 2017, 10, 3321–3328. [Google Scholar] [CrossRef]
- BeStSel: a web server for accurate protein secondary structure prediction and fold recognition from the circular dichroism spectra. Nucleic Acids Research, Oxford Academic. https://academic.oup.com/nar/article/46/W1/W315/5035652?login=false (accessed , 2024). 23 July.
- Cabra, V.; Arreguin, R.; Vazquez-Duhalt, R.; Farres, A. Effect of temperature and pH on the secondary structure and processes of oligomerization of 19 kDa alpha-Zein. Biochim. Biophys. Acta BBA - Proteins Proteomics 2006, 1764, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Taylor, J.; Wellner, N.; Byaruhanga, Y.B.; Parker, M.L.; Mills, E.N.C.; Belton, P.S. Effect of preparation conditions on protein secondary structure and bofilm formation of Kafirin. J. Agric. Food Chem. [CrossRef]
- Richardson, J.S.; Richardson, D.C. Natural β-sheet proteins use negative design to avoid edge-to-edge aggregation. Proc. Natl. Acad. Sci. 2002, 99, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Batterman-Azcona, S.J.; Hamaker, B.R. Changes occurring in protein body structure and α-Zein during cornflake processing. Cereal Chem. J. 1998, 75, 217–221. [Google Scholar] [CrossRef]
- Klymchenko, A.S. Fluorescent probes for lipid membranes: From the cell surface to organelles. Acc. Chem. Res. 2023, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, W.; Mercadé-Prieto, R.; Chen X., D. Dye-protein interactions between Rhodamine B and Whey proteins that affect the photoproperties of the dye. J. Photochem. Photobiol. Chem. 2021, 408, 113092. [Google Scholar] [CrossRef]
- Stokes, D.J.; Thiel, B.L.; Donald, A.M. Direct observation of water−oil emulsion systems in the liquid state by Environmental Scanning Electron Microscopy. Langmuir 1998, 14, 4402–4408. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its’ effects on human health, Foods 2017, 10, 92. 10. [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and chemical stability of Curcumin in aqueous solutions and emulsions: Impact of pH, temperature, and molecular environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Chignell, C.F.; Bilski, P.; Reszka, K.J.; Motten, A. G.; Sik, R. H.; Dahl, T. A. Spectral and photochemical properties of Curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef]
- Hu, K.; Huang, X.; Gao, Y.; Huang, X.; Xiao, H.; McClements, D.J. Core-shell biopolymer nanoparticle delivery systems: Synthesis and characterization of Curcumin fortified Zein-Pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Sun, C.; Li, R.; Mao, L.; Liu, F.; Gao, Y. Structural characterization, formation mechanism and stability of Curcumin, in Zein-Lecithin composite nanoparticles fabricated by antisolvent co-precipitation. Food Chem. 2017, 237, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jing, Y.; Han, C.; Zhang, H.; Tian, Y. Encapsulation of Curcumin in Zein/Caseinate/sodium Alginate nanoparticles with improved physicochemical and controlled release properties. Food Hydrocoll. 2019, 93, 432–442. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.H.; Huang, J.Y.; Dai, L.; Du, J. , McClements, D. J.; Mao, L.; Liu, J.; Gao, Y.X. Fabrication and characterization of layer-by-layer composite nanoparticles based on Zein and Hyaluronic acid for co-delivery of Curcumin and Queercetagetin, ACS Appl. Mater. Interf. 2019, 11, 16922–16933. [Google Scholar] [CrossRef]
- Feng, S.; Sun, Y.; Wang, D.; Sun, P.; Shao, P. Effect of adjusting pH and Chondroitin sulfate in the formation of Curcumin Zein nanoparticles: Synthesis, characterization and morphology. Carbohydr. Polym. 2020, 250, 116970. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.H.; Dai, L.; Mao, L.; Gao, Y.X. Co-delivery of Curcumin and Piperine in Zein-Carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhang, H.; Liu, S.; Yang, M.; Cui, M.; Zhang, T.; Yu, Y.; Xiao, H.; Du, Z. Fabrication, characterization and functional attributes of Zein-Egg White derived peptides (EWDP)-chitosan tertiary nanoparticles for encapsulation of Curcumin: Role of EWDP. Food Chem. 2022, 372, 131266. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, X.; Gu, P.; Cheng, W.; Zhang, R.; Hu, K. Curcumin-loaded Zein/Pectin nanoparticles: Caco-2 cellular uptake and the effects on cell cycle arrest and apoptosis of human hepatoma cells (HepG2). J. Drug Deliv. Sci. Technol. 2022, 74, 103497. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, M.; Zhu, Q.; Qu, Y.; Jia, X.; Yin, L. Curcumin loaded Zein-Alginate nanogels with “core-shell” structure: Formation, characterization and simulated digestion. Int. J. Biol. Macromol. 2023, 251, 126201. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, X.; Li, Y.; Li, S.; Zhang, L. Huang, X.; Yang, M.; Du, X.; Liu, J.; Zhang, T. Enhancing the stability and biological activity of Curcumin through incorporating Zein-sodium Alginate-egg white peptides hybrid assemblies. Food Bioscience 2024, 59, 103868. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Moulik, S. P. Stability of Curcumin in different solvent and solution media: UV–Visible and steady-state fluorescence spectral study. J. Photochem. Photobiol. B 2016, 158, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed. New York: Springer, 2006.
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int., 2004, 11, 36–42. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K. W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods. [CrossRef]
- Kirk, S.E.; Skepper, J.N.; Donald, A.M. Application of Environmental Scanning Electron Microscopy to determine biological surface structure. J. Microsc. 2009, 233, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Selling, G.W.; Hamaker, S.A.H.; Sessa, D.J. Effect of solvent and temperature on secondary and tertiary structure of Zein by Circular Dichroism. Cereal Chem. J. 2007, 84, 265–270. [Google Scholar] [CrossRef]
- Brotons-Canto, A.; González-Navarro, C.J.; Gil, A.G.; Asin-Prieto, E.; Saiz, M.J.; Llabrés, J.M. Zein nanoparticles improve the oral bioavailability of Curcumin in Wistar rats. Pharmaceutics 2021, 13, 361. [Google Scholar] [CrossRef]
- Pan, Y.; Tikekar, R.V.; Wang, M.S.; Avena-Bustillos, R.J.; Nitin, N. Effect of barrier properties of Zein colloidal particles and oil-in-water emulsions on oxidative stability of encapsulated bioactive compounds. Food Hydrocoll. 2015, 43, 82–90. [Google Scholar] [CrossRef]
| Secondary 刘structure | ZP (fresh)刘(%) | ZP (1 day)刘(%) | ZP (10 days)刘(%) | ZP*刘(%) |
|---|---|---|---|---|
| α-helix | 48.6 | 45.0 | 45.8 | 56.7 |
| antiparallel β-sheet | 6.1 | 0.8 | 1.2 | 7.1 |
| turns | 13.9 | 13.3 | 13.4 | 8.2 |
| random coil | 31.3 | 40.9 | 39.7 | 28.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





