1. Introduction
High-tibial osteotomy (HTO) has emerged as a valid and reliable proxy for the alleviation of pain, improvement of function, and delay of joint degeneration in patients with osteoarthritis (OA). Notably, it decreases the magnitude of external knee adduction moments during walking, thereby reducing the dynamic load on the medial tibiofemoral joint [
1,
2,
3].
At first, the lateral closed wedge technique was introduced. However, several disadvantages, such as fibula osteotomy, lateral muscle detachment, or dissection of the peroneal nerve were indicated. Recently, the numbers of medial open wedge high-tibial osteotomies (MOWHTOs) have increased with the use of the locking plate Tomofix®. However, several concerns exist owing to the documented losses of correction and delayed union or nonunion of the open wedge gap after osteotomy. Therefore, there is considerable interest in the use of bone substitutes for the improvement of bone repair.
Various void fillers can be used, including autograft, allograft, bone substitute fillers, and noncalcium–phosphate-based void fillers. Nicholas and Omer [
4,
5] reported in their systematic review that autografts exhibit superior union results compared with allografts. The use of autografts yielded more favorable outcomes than synthetic bone substitutes with regard to the rates of delayed union or nonunion, and mean time to union. Therefore, it has been indicated that there are no definitive advantages for OWHTO with any bone void filler in terms of union rates. Additionally, given the loss of correction and the use of synthetic bone substitutes, OWHTO cannot be recommended.
However, autografts were indicated as the treatment choice for salvage procedures in cases of nonunion following OWHTO owing to the harvest-site morbidity [
6,
7,
8]. The use of bone void filler for large osteotomy gaps was also recommended [
9]. Meanwhile, the addition of tri-calcium phosphate (TCP) into osteotomy gaps as a bone substitute filler does interfere with normal bone healing [
10]. Therefore, the use of bone substitute fillers in conjunction with advanced technologies has been proposed for the substitution of autografts.
Three-dimensional (3D) printing technologies have been used to fabricate the tissue engineered scaffolds because of the fabrication possibility of the 3D free-form structures with fully interconnected pores for adequate tissue regeneration [
11,
12]. Owing to the inherent brittleness of ceramic-based scaffolds in load-bearing scenarios [
13,
14], like HTO regions, in this study, we fabricated a customized 3D scaffold with the synthetic polymer polycaprolactone (PCL) and the ceramic beta-tricalcium phosphate (β-TCP) for HTO via 3D printing technology.
Generally, tissues in the body consist of tissue-specific extracellular matrix (ECM) that can modulate cellular activity. Therefore, for effective tissue regeneration, many researchers have been attempting to use decellularized extracellular matrix (dECM)-based materials [
15,
16,
17,
18]. To enhance the capability of bone regeneration, we produced dECM derived from bone tissue (bone dECM) and used it to cover the 3D scaffolds.
The present study aimed to evaluate the bone regeneration efficacy of customized 3D scaffolds covered with bone dECM for HTO. We conducted the analysis of scaffold characterization and in-vitro tests, such as proliferation and differentiation. To confirm the capability of bone tissue regeneration, we performed in vivo experiments with beagles. For the customization of 3D scaffold implantation, we fabricated a customized guide instrument with a correction angle for an osteotomy zig and exploited it for scaffold implantation. Accordingly, Microscopic computed tomography (micro-CT) analyses, quantitative data acquisition for bone regeneration, histological analyses, and radiologic evaluations, were conducted.
2. Materials and Methods
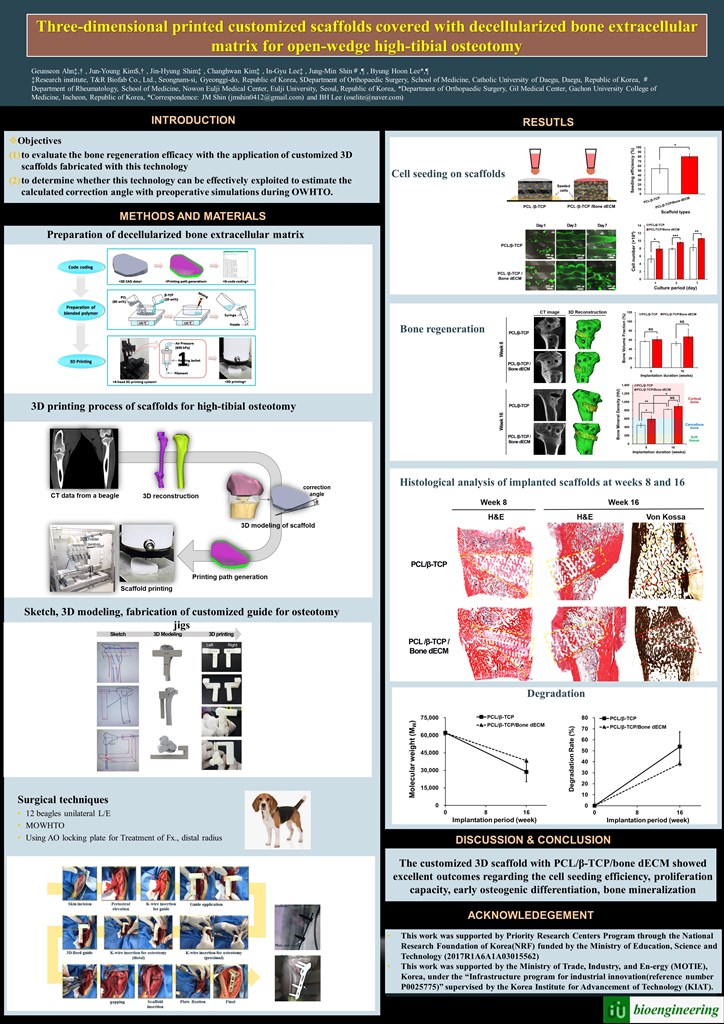
For the preparation of bone-dECM, normal bone was collected from 8-month-old beagles at a slaughterhouse, and soft tissue, periosteum, bone marrow, and epiphyseal cartilage were removed from the collected bone. The bone was freeze-dried and pulverized using a commercial pulverizer (DSMP-370, DukSan Co.). The size did not exceed 4 mm and was stored at -80°C. The freeze-dried and pulverized bone particles were decellularized to obtain the extracellular matrix. After that, the normal bone was demineralized in hydrochloric acid (HCl) solution for 12h and residual lipids were removed using a chloroform-methanol mixture (ratio 1:1). For cell removal, the demineralized bone powder was soaked in 0.05% trypsin and 0.02% ethylenediaminetetraacetic acid (EDTA) at 37°C for 2h. The samples were then rinsed with distilled water and 70% ethanol for more than 20h. The resulting product, dECM, was lyophilized until needed. The bone dECM was digested in a pepsin solution (3 mg mL−1 pepsin in 0.05 N HCl) with continuous stirring for more than 72h to obtain a final concentration of 20 mg of bone dECM per mL of pepsin solution. To fabricate a customized scaffold for the HTO, we reconstructed a 3D model from a beagle’s computed tomography (CT) image. Using this model, we designed and fabricated a customized scaffold for the HTO (
Figure 1).
After fabricating scaffolds using polycaprolactone (PCL, PURASORB® PC 12, Purac, Netherlands) and beta-tricalcium phosphate (β-TCP, 7758-87-4, Premier BioMaterials, Ireland) to form the bone, the PCL/β-TCP paste was loaded into a steel syringe and extruded through a steel nozzle using a 3D bioprinter (3DX printer, T&R Biofab Co., Ltd., South Korea) at a syringe temperature of 110 °C and a pneumatic pressure of 600 kPa. To prepare scaffolds covered with bone-dECM, the PCL/β-TCP scaffolds were immersed in the bone-dECM solution and freeze-dried for more than 48 h in an incubator at 37 °C. High-resolution scanning electron microscopy (SEM, Nova NanoSEM 450, Netherlands) analysis was performed to characterize the internal framework and chemical composition of the scaffold.
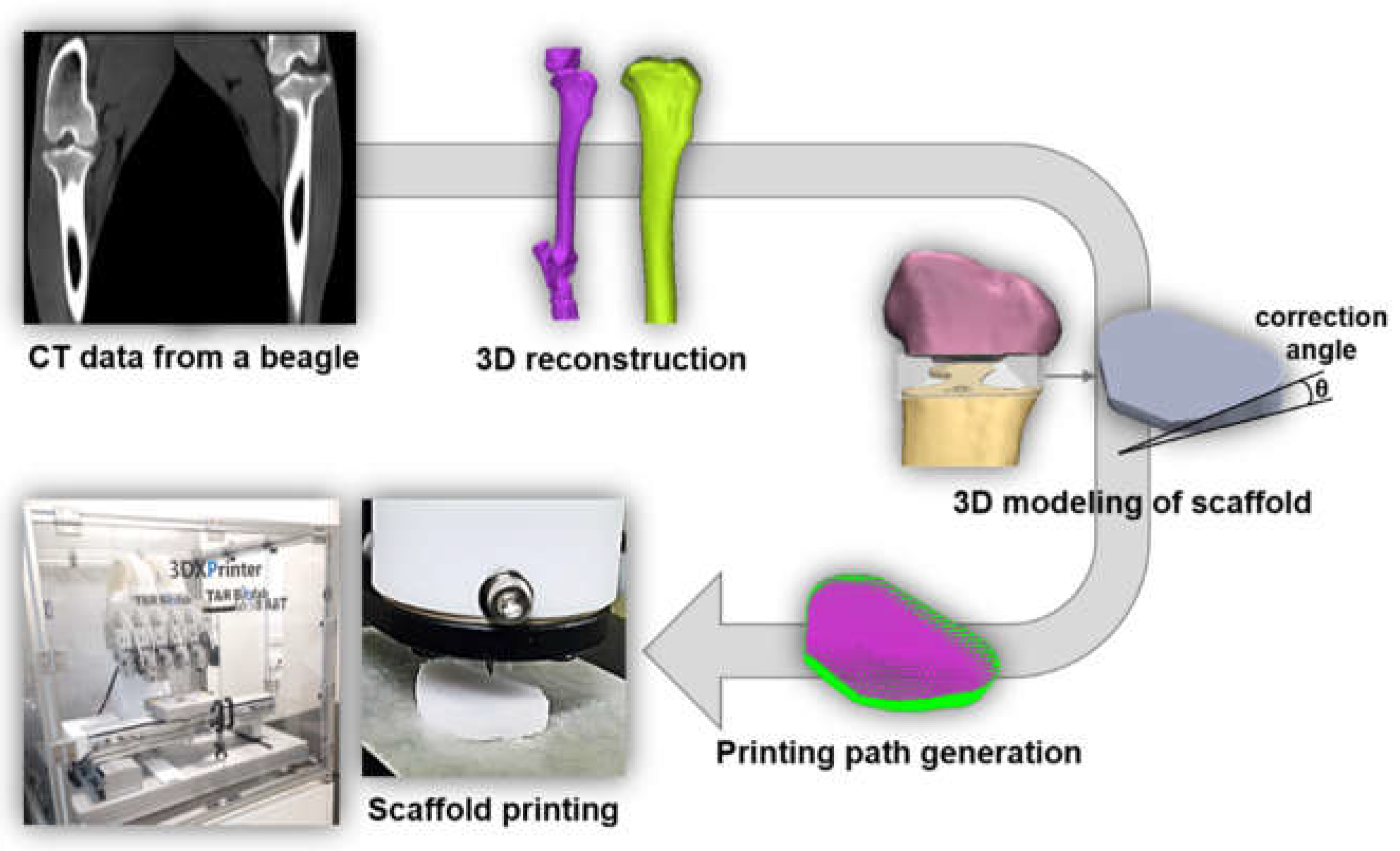
Then, the HTO fracture jig guide was designed and prepared in a “T” shape with a width of 50 mm, a length of 43 mm, and a height of 63 mm. Four holes were drilled into the guide for rigid fixation to the bone. The guide was fabricated using a commercial 3D printer (uPrint SE, Stratasys, USA). The osteotomy jig guide was designed for HTO with a lateral cortical hinge in the 3D plane [
19]. This can prevent secondary changes in posterior tilt and distal tibial rotation. 3D simulation techniques were applied to model the synthetic augmentation material for the osteotomy. The acquired micro-CT image data were exported to Mimics (Materialise, Leuven, Belgium) to create a 3D model of the proximal tibia. This created a true anteroposterior (AP) view. According to the simulation, there was no secondary change in the posterior tibial inclination angle in the sagittal plane or rotational change in the axial plane (
Figure 2). The aperture gap was set to about 10° in the coronal plane. After the HTO procedure, the angle between the upper and lower osteotomy plane X-ray images was measured for evaluation.
To validate the clinical efficacy of synthetic void fillers manufactured using 3D printing-based technology, 18 beagles were divided into groups according to whether or not they had lateral hinge fracture (LHF) during HTO [
20]. LHF can occur as a complication of medial open wedge HTO during the formation of an open gap. It can lead to nonunion at the fracture site and loss of correction [
21]. In the subsequent cell test, MC3T3-E1 (pre-osteoblast, #CRL-2593, ATCC, USA) cells were used. The cells were cultured in α-minimal essential medium (α-MEM, Gibco, USA) containing 10% fetal bovine serum (FBS, Gibco, USA) and 1% penicillin and streptomycin (Invitrogen, USA), and the medium was replaced every 2 days.
To determine the morphology of cell proliferation, the Cell Counting Kit-8 (CCK-8, Dojindo, Japan) assay and the live/deadTM Viability/Cytotoxicity Kit (Life Technologies, USA) were used. The ratio of CCK-8 solution to culture medium was 1:10 and incubated with all scaffolds for 4 h in an incubator (around 37°C, around 5% CO2). The cell number on the scaffolds was calculated using the OD value and standard value generated by MC3T3-E1 cells. The cell proliferation rate and cell morphology were checked at intervals of 1, 3, and 7 days. Live and dead cells were stained by mixing calcein AM, EthD-1, and phosphate buffered saline (PBS) at a ratio of 1:2:500 and incubated with the samples for 30 min in an incubator (37°C, 5% CO2). The samples were then washed three times with PBS. To determine osteogenic differentiation, cell-seeded scaffolds were cultured in osteogenic medium supplemented with α-MEM containing 20% FBS, 0.01 × 10-3 M dexamethasone, 0.2 × 10-3 M ascorbic acid, 10 × 10-3 M β-glycerol phosphate (Sigma–Aldrich, USA), and 1% penicillin/streptomycin. To assay alkaline phosphatase (ALP) activity, cells cultured on scaffolds were lysed in radioimmunoprecipitation lysis buffer (Millipore, USA) at 4-, 7-, and 14-day intervals. The lysates were then incubated in p-nitrophenyl phosphate (pNPP) liquid matrix system (Sigma–Aldrich, USA) at 37°C for 30 min and read at 405 nm with a microplate reader.
The extracted data were utilized in two groups (PCL/β-TCP and PCL/β-TCP/bone-dECM) experiments. Beagles were anesthetized with a combination of zoletil (5 mg/kg) and xylazine (2 mg/kg) and maintained with isofurane (1.5–2.0%). For the beagle surgery, a medial OWHTO with a lateral cortical hinge was performed using a self-designed jig and 3D printer. The osteotomy was planned to start along the diaphyseal flare proximal to the tibial tuberosity. After the tarsal tendon was separated, the superficial medial collateral ligament was transected, and a guide wire was inserted toward the tip of the fibular head (safe zone) along the planned osteotomy plane [
22]. The osteotomy was completed just anterior to the lateral cortex, and a scaffold was inserted into the osteotomy site. The correction angle was determined after scaffold insertion. The osteotomy was fixed with a fixed angle plate with interlocking screws (TomoFixTM104, Synthes, Switzerland), and the muscles and skin were sutured in a conventional manner. The Micro-CT analysis was used to investigate the degree of mineralization at 8 and 16 weeks after beagle transplantation. Bone volume and density were also measured for quantitative analysis of bone regeneration, and bone regeneration capacity and mineral density (BMD) were determined using the Hounsfield Unit (HU) scale. For hematoxylin and eosin (H&E) staining, sacrificed samples were fixed in 4% paraformaldehyde for 7 days, decalcified in 10% ethylenediaminetetraacetic acid (EDTA) for 2 weeks, embedded in paraffin, and sectioned using a microtome (Leika RM2145 microtome, Germany) to obtain sections of approximately 5 μm in thickness. For von Kossa staining, sacrificed samples were fixed in 4% formalin in PBS (pH = 7.4) for 7 days, dehydrated in alcohol, and embedded in methyl methacrylate. The surfaces of all sections were wet-polished with 35, 15, and 5 μm diamond pastes and ultrasonically cleaned to remove foreign matter. The sections were then ultraviolet-transmitted, treated with 5% silver nitrate solution for 20 min, and 5% sodium triphosphate was added for Von Kossa staining.
After the above procedure, gel permeation chromatography (GPC) analysis was performed to evaluate the degradation characteristics. The analysis was performed on an EcoSEC HLC-8320 GPC (Tosoh, Japan) equipped with a Shodex LF-804 column and Shodex KF-802.5 (7.8× 3.00 mm). The sample was dissolved in CHCl3 to adjust the concentration to 30 mg/mL and filtered using a 0.45 μm polytetrafluoroethylene filter. The molecular weight arrangement of the scaffold was confirmed using a refractive index detector at around 40°C. The EcoSEC program was used for data analysis. All data were expressed as the mean and standard deviation. Statistical significance was defined as *p < 0.05, **p < 0.01, ***p < 0.005, and ****p < 0.001.
3. Results
3.1. Characterization of 3D Printed Scaffolds
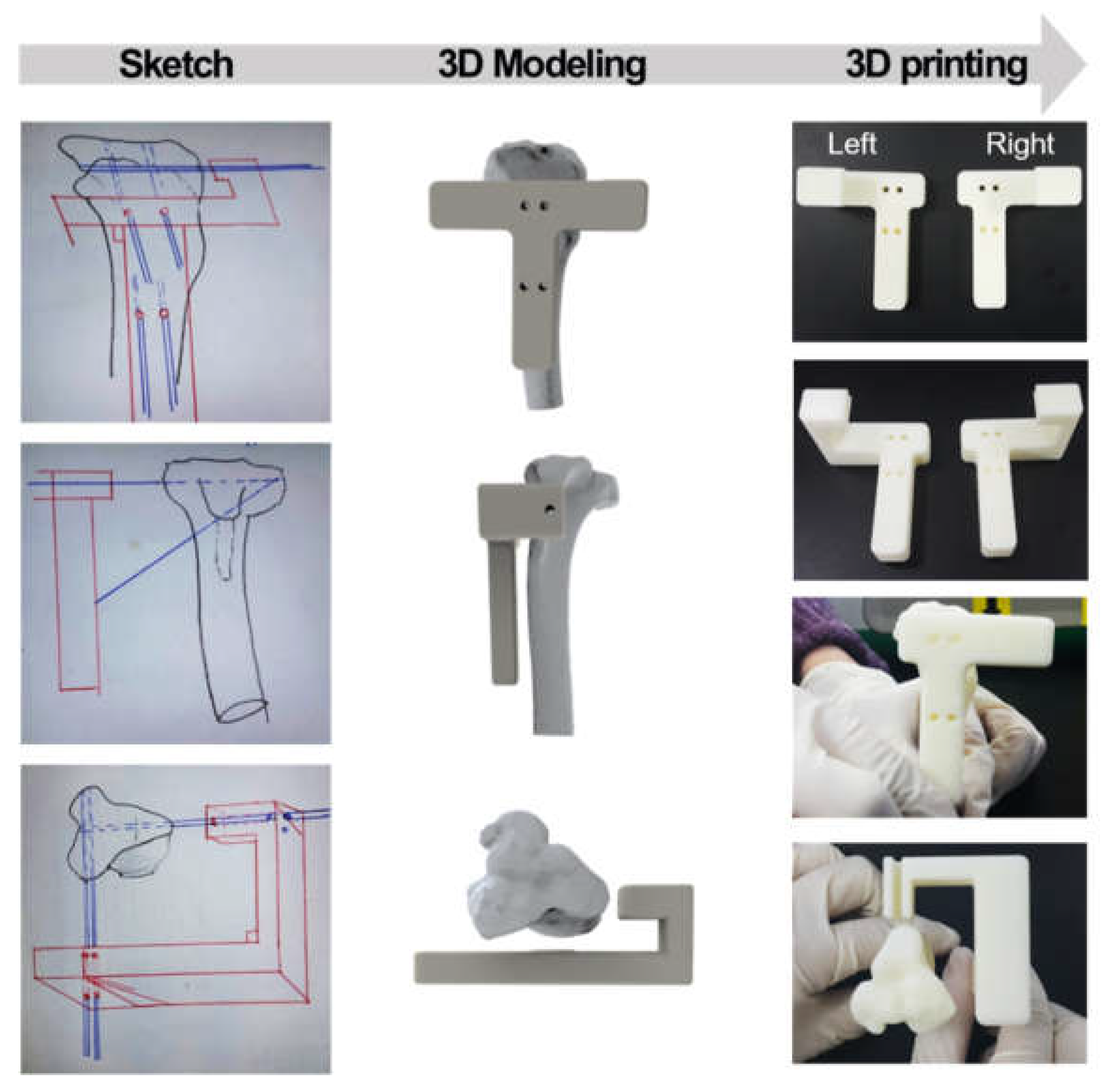
The line width and gap of the scaffolds with a 10° correction angle were 350 μm (
Figure 3(A)). In this study, two different scaffolds were prepared to compare the effect of bone regeneration. PCL/β-TCP and PCL/β-TCP/bone dECM scaffolds were fabricated via 3D printing technology and a simple coating method with bone dECM (
Figure 3(B)). On SEM imaging, the pore parts of the PCL/β-TCP scaffolds were cleaned, whereas the pore parts of the PCL/β-TCP/ bone dECM scaffolds were filled with bone dECM (
Figure 3(C)).
3.2. Characterization of Customized Guide Instrument
The osteotomy guide for HTO was a customized instrument for creating gaps with a certain size of tibia part. For this purpose, the angles of the pin holes were set in advance to guide the saw direction into the tibia part. As shown in
Figure 2, holes were designed to insert guide pins at a preset angle, and simulations were performed with a tibial model.
3.3. Cell Seeding Efficiency and Proliferation Results
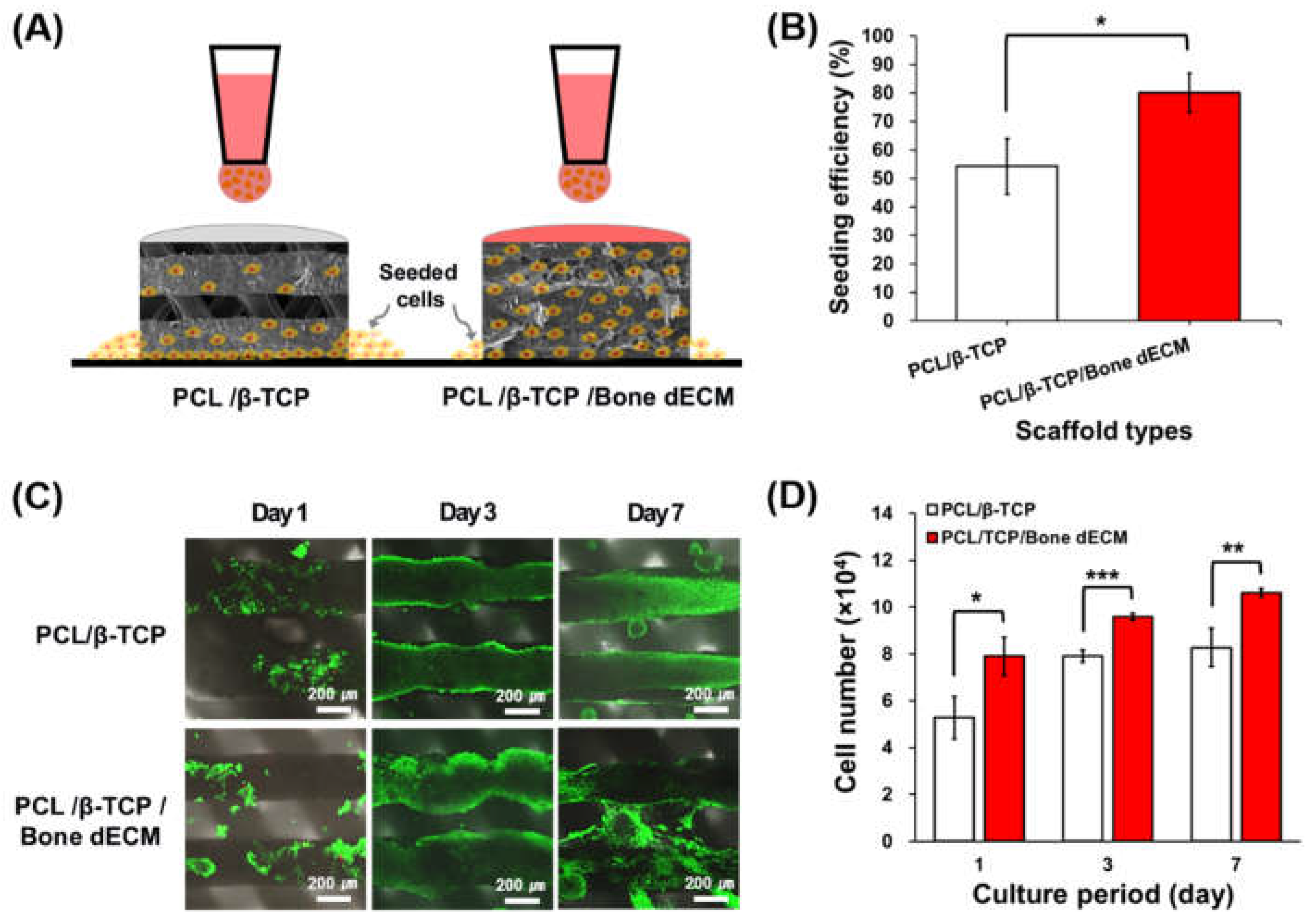
Pre-osteoblasts (MC3T3-E1) were seeded on the PCL/β-TCP and PCL/β-TCP/bone dECM scaffolds (
Figure 4(A)). Regarding cell the seeding efficiency, PCL/β-TCP/bone dECM scaffolds yielded significantly higher cell seeding levels than those in the PCL/β-TCP scaffolds (p < 0.05,
Figure 4(B)). At day 1, cells on the PCL/β-TCP and PCL/β-TCP/bone dECM scaffold yielded increased survival rates with > 90% viability (
Figure 4(C)). Live and dead staining and CCK-8 assays were performed at days 1, 3, and 7, for the analysis of the cell viability and proliferation on scaffolds. As shown in
Figure 4(C), the cells in PCL/β-TCP scaffolds were only attached on the scaffold lines, whereas the cells in the PCL/β-TCP/bone dECM scaffold were attached on the scaffold line and bony dECM parts which filled the pore part. Throughout the entire culture period, PCL/β-TCP/bone dECM scaffolds exhibited excellent proliferation capacity compared with PCL/β-TCP scaffolds based on the initial cell seeding efficiency (
Figure 4(D)). Based on the above results, the bone dECM is concluded to help the initial cell attachment and proliferation.
3.4. Cell Differentiation Results
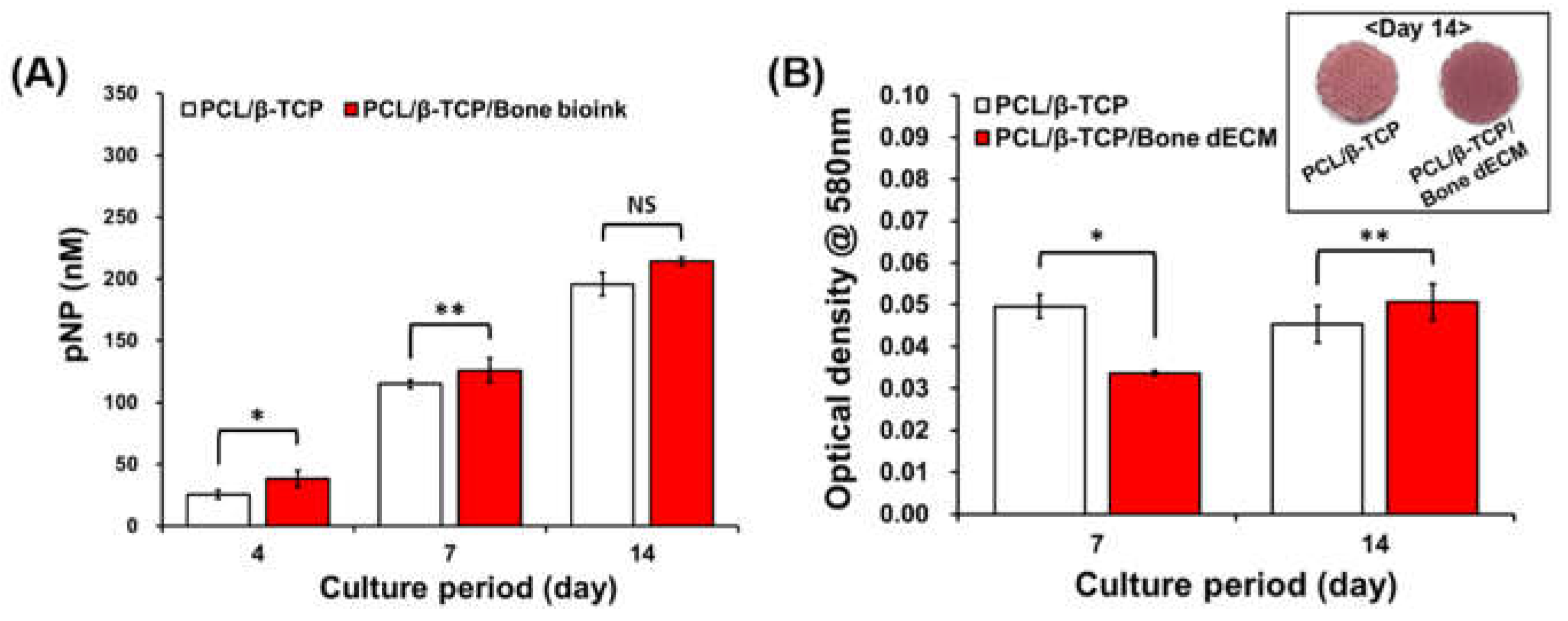
To confirm the effect of bone dECM on osteogenic differentiation, ALP activity and alizarin red S staining were performed after the inducement of osteogenic differentiation with osteogenic media. ALP expression levels constitute an early osteogenic differentiation marker that significantly increased in PCL/β-TCP/bone dECM scaffolds compared with those related to PCL/β-TCP scaffolds at early time points (days 4 and 7). At day 14, the ALP expression level in the PCL/β-TCP scaffolds reached the same level as that of the PCL/β-TCP/bone dECM scaffolds (
Figure 5(A)). Thus, bone dECM could promote early osteogenic differentiation. To confirm the mineralization, alizarin red S staining was performed at days 7 and 14. On day 7, PCL/β-TCP scaffolds that had direct contact between β-TCP and cells were more effective in osteogenic differentiation than PCL/β-TCP/bone dECM scaffolds. However, the PCL/β-TCP/bone dECM scaffold group yielded a higher level of mineralization at day 14 compared with that of the PCL/β-TCP scaffold group (
Figure 5(B)). Moreover, the stained images showed the same result at day 14. Thus, the bone dECM did not only help the initial cell attachment and proliferation but also the osteogenic differentiation. It is thus concluded that bone dECM could provide an effective environment for bone tissue engineering.
3.5. In Vivo Results
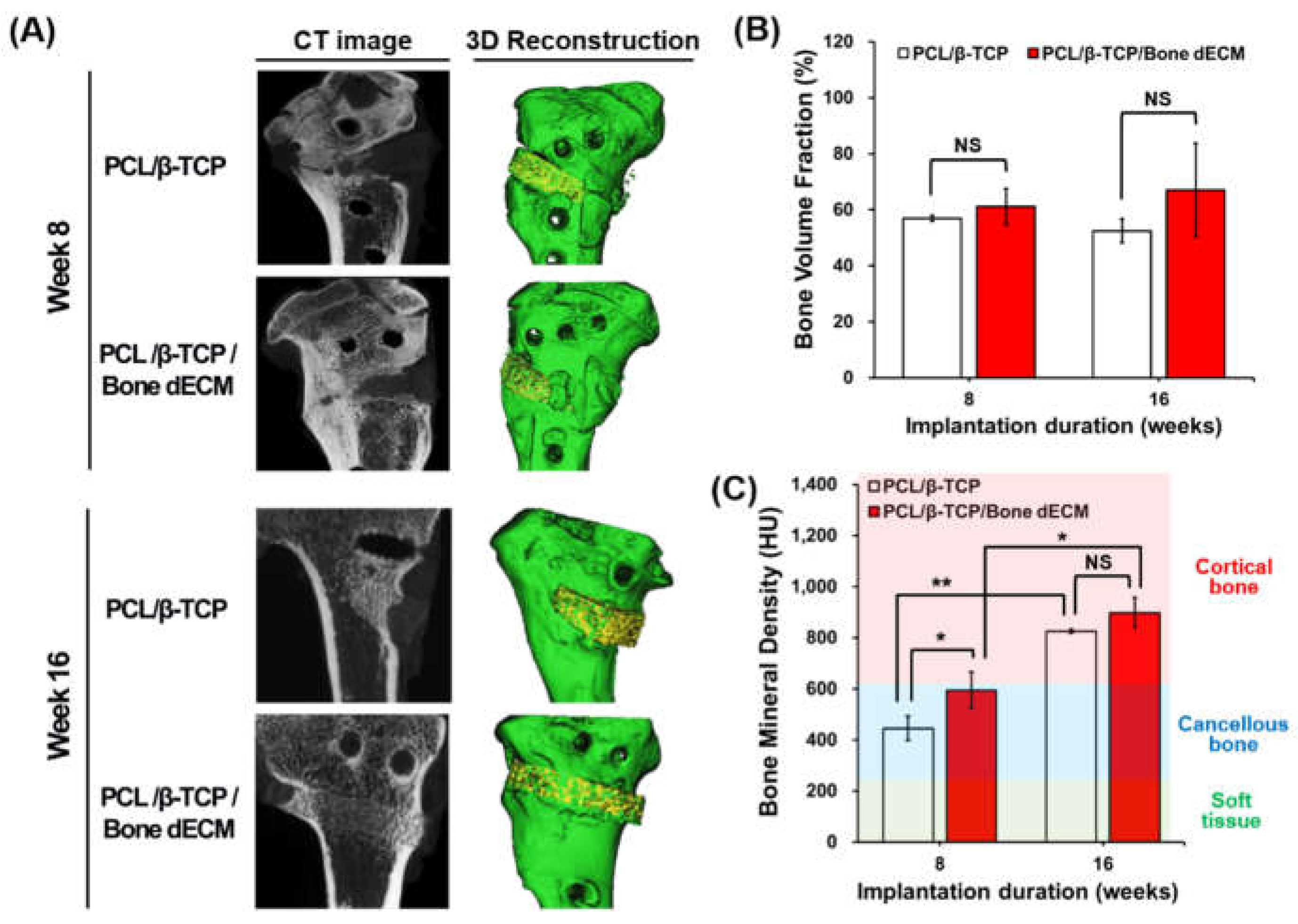
After harvesting the samples, micro-CT images were obtained to confirm the bone regeneration capacity of the scaffolds. 3D Micro-CT models were reconstructed with images at weeks 8 and 16. In the 3D reconstructions, newly regenerated bone and native bone (included scaffold parts) were respectively presented with yellow and green colors (
Figure 6(A)). For quantitative analyses of new bone formation at the defect site, the bone volume fraction (BV/TV, %) and bone mineral density (BMD) were calculated based on the HU values obtained from micro-CT data. Bone volume fractions increased as time progressed, even though these changes were not significantly different in the two scaffold groups throughout the entire period (
Figure 6(B)). In the analyses of bone mineral density, soft tissue (~225 HU), cancellous bone (226–661 HU), and cortical bone (662–1988 HU) were separated based on the HU values [
18]. At week 8, even though the HU values of PCL/β-TCP and PCL/β-TCP/bone dECM were comparable to those for cancellous bone, PCL/β-TCP/bone dECM scaffolds exhibited significantly higher HU values than PCL/β-TCP scaffolds (p < 0.05). At week 16, PCL/β-TCP and PCL/β-TCP/bone dECM had comparable densities to those of cortical bone. At week 16, PCL/β-TCP scaffolds had reached similar bone mineral densities compared with PCL/β-TCP/bone dECM scaffolds. This result suggested that bone dECM in scaffolds could help promote bone mineralization at early time points.
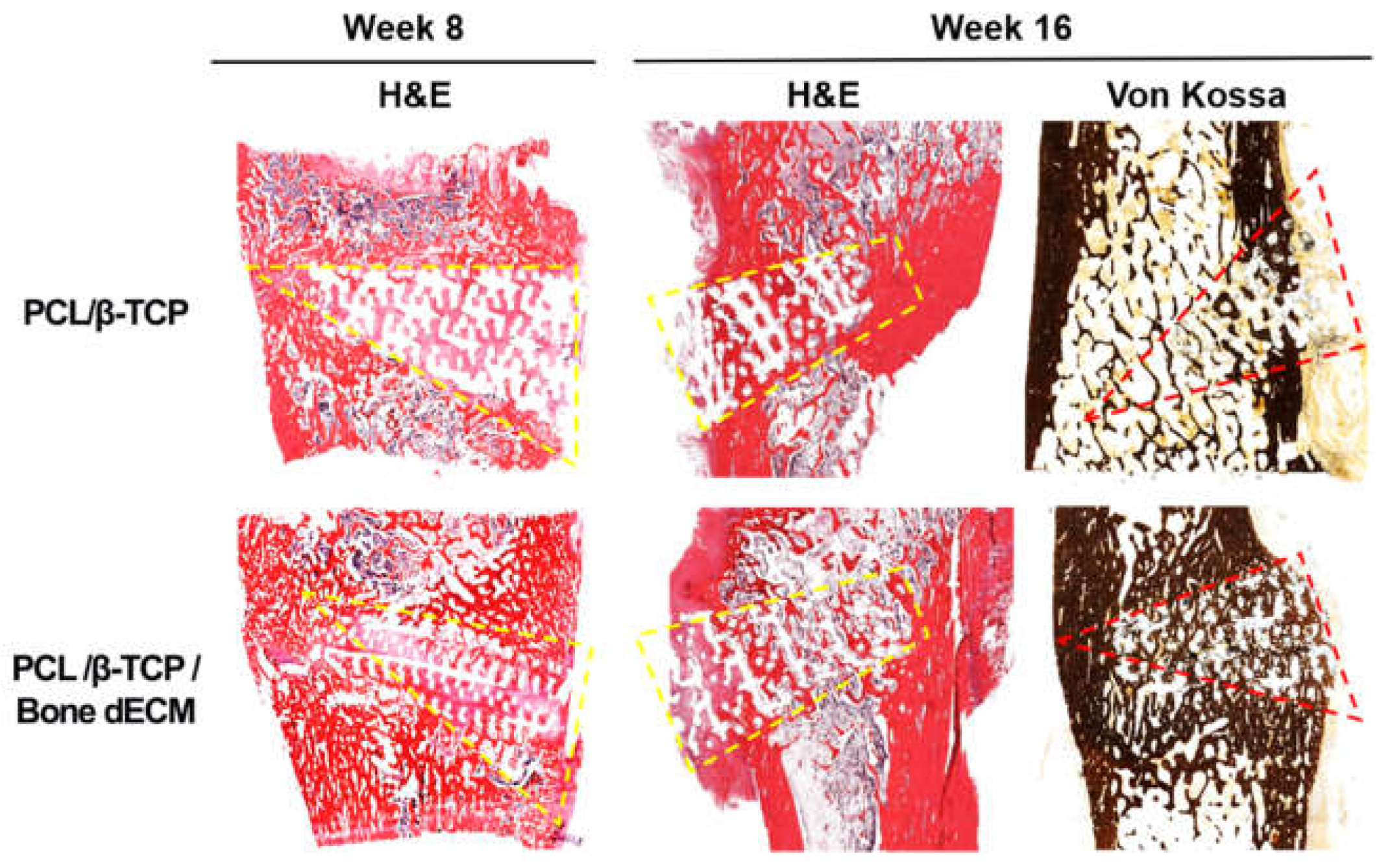
To confirm the level of new bone formation in this animal test, histological analyses were performed (
Figure 7). H&E and Von Kossa staining were performed to confirm the new bone formation and mineralization. At the eighth week after implantation, H&E staining showed tissue penetration in the pores of the scaffolds and partially new bone formation was documented in the rims of both implanted scaffolds. PCL/β-TCP/bone dECM scaffold group yielded additional new bone formation than that documented in the PCL/β-TCP scaffold group in the area between the native bone and scaffolds. After 16 weeks, the area occupied by the scaffolds decreased and new bone tissue was generated in the rest of the space. New bone tissue was confirmed and was mineralized by Von Kossa staining. Specifically, the outcomes from the implanted PCL/β-TCP/bone dECM scaffolds confirmed that new bone tissue and bone marrow were combined. These results suggested that bone dECM could enhance the initial, new bone formation, and mineralization.
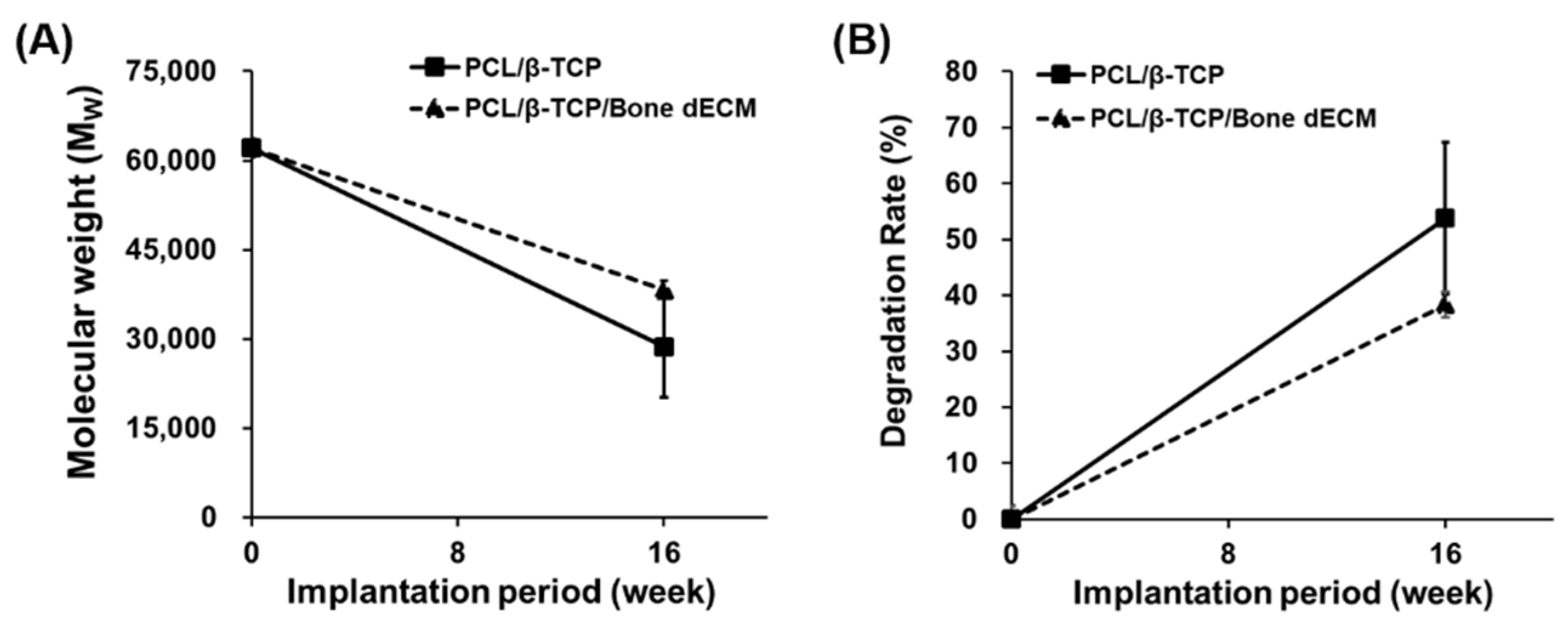
3.6. Degradation Results
PCL and β-TCP are biodegradable polymers and exhibit natural degradation properties within the human body. To confirm the degree of degradation of the scaffolds during implantation, GPC analysis was performed at week 16 after implantation (
Figure 8). PCL/β-TCP scaffolds were degraded by approximately 54% after 16 weeks. PCL/β-TCP/bone dECM scaffolds were degraded by approximately 38% after 16 weeks. Although there were differences in the extent of degradation, all the scaffolds showed that the in vivo degradation process was an ongoing process. Thus, scaffolds composed of PCL, β-TCP, and bone dECM had biodegradable properties and could be degraded in vivo and replaced with autologous tissues.
3.7. Radiologic Evaluations
The correction angle measured after osteotomy using synthetic void filler manufactured to attain opening gap of 10° in coronal planes exhibited high accuracy with low errors (10.3 ± 0.9°). Additionally, even with LHFs, the correction angle was not significantly different (
Table 1).
4. Discussion
Various studies have been published with scaffolds in the field of bone tissue engineering [
12,
13,
14,
15]. In particular, the bone regeneration effects of ceramic materials, such as HA and TCP are well known because of their similar properties to bone tissues [
13,
14,
18]. However, few studies show the synergistic effects of TCP and bone dECM incorporated in scaffolds. The present study was conducted to address two principal hypotheses. First, the construction of customized 3D scaffolds covered with bone dECM for HTO exhibited outstanding reparability for bone healing at the opening gap compared with the construct synthesized based on the isolation of the TCP from placed scaffolds at the knee joints of beagles. A closer analysis of the in vitro and in vivo test outcomes showed that scaffolds with bone dECM (PCL/β-TCP/bone dECM scaffolds) had superior structural capabilities and retained the surrounding cells at an early stage and promoted cell attachment and proliferation. In addition, it was confirmed that differentiation was initially promoted by the bone-like microenvironment of bone dECM that contained various bone tissue-derived biomolecules, such as collagen and ECM proteins. However, as time progressed, it was confirmed that the bone regeneration effects of PCL/β-TCP scaffolds became similar to those of the PCL/β-TCP/bone dECM scaffolds. This indicates that β-TCP plays a major role in bone regeneration. Bone dECM produces good results (attachment, proliferation, and bone formation) in the early stages, but they cannot be maintained in the long-term as it is degraded by enzymes in the body. In conclusion, as confirmed in this study, the synergistic effects of β-TCP and bone dECM were excellent. Specifically, in the case of scaffolds with relatively large volumes that are applicable to clinical trials, bone dECM contained in scaffolds could retain stem cells in the surrounding bone marrow. It is thought that they could promote bone healing.
For void fillers, autologous bone grafts are still the standard materials because they have osteogenic, osteoinductive, and osteoconductive effects [
23]. Conversely, they also have some disadvantages, such as prolonged surgical times and additional donor site morbidities. Allogenous bone grafts that have no harmful effects on the donor site, are a good alternative option to autologous bone grafts. However, they are associated with the potential risks of immune response or disease transmission [
24]. Therefore, synthetic bone grafts, such as hydroxyapatite (HA) and TCP are introduced for open wedge HTO. Previous studies have shown comparable results with allogenous bone grafts. Specifically, one of the advantages of synthetic grafts is that they can be initially grafted owing to their additional structural contribution if manufactured in wedge forms. However, several studies have demonstrated radiologically and histologically that synthetic grafts showed poor integration compared with other grafts [
23,
25]. The lack of graft absorption is still a concern after synthetic material grafting. From this point-of-view, scaffolds that use PCL have slow degradation properties, and can provide the structural component for supporting the implant site during bone tissue regeneration. As confirmed by the degradation and histological results, molecular weight reduction of ~50% occurred after 16 weeks, but no structural collapse was observed in the histological results. This is a particularly important feature for bone regeneration that requires structural support. In animal experiments, the PCL/β-TCP scaffold also yielded an excellent bone regeneration efficacy, but the PCL/β-TCP/bone dECM scaffold had a better initial bone regeneration efficacy. This means that bone regeneration was promoted based on a) the ability of the scaffold to retain the bone marrow-derived stem cells at an early stage and b) the bone tissue microenvironment of the bony dECM.
Second, this 3D printing-based platform technology can be effectively exploited to attain the desired correction angle with preoperative simulations. Synthetic void filler manufactured with 3D printing-based platform technology according to preoperative simulations was exploited for scaffold implantation with the use of a fabricated customized guide instrument with a correction angle for an osteotomy zig. Following postoperative radiologic evaluations in 18 beagles, the correction angles yielded high-accuracy and reliability regardless of the absence or presence of LHF at the HTO procedure. A LHF may occur as a complication of medial open-wedge HTO during the formation of the opening gap, which causes instability at the osteotomy site, and may lead to delayed union or nonunion. Correspondingly, it could thus result in correction losses after the medial open-wedge HTO [
21]. Therefore, our technologies showed the new clinical availability of synthetic void filler manufactured by 3D printing-based platform technology using preoperative simulation. The filling methods used for successful gap healing in the formed gap created during open-wedge HTO—which is a surgical option for medial compartmental osteoarthritis or osteonecrosis with varus deformity of the knee joint [
26,
27]—would lead to a wide clinical applicability in the orthopedic field, including nonunion, osteomyelitis, and to malunion correction.
Given that 3D printing technologies enable free-form fabrications, these technologies are extensively used in the fabrication of porous structures that are advantageous for inducing tissue regeneration [
12,
28,
29,
30]. Particularly, extrusion-based 3D printing systems have been applied in the fields of various tissue regeneration and regenerative medicine because of their ability to use a variety of biopolymers, such as PCL [
12,
29,
30]. For bone tissue regeneration, ceramics, such as hydroxyapatite (HA), and TCPs have been considered as potential candidates for effective bone reconstruction [
29,
30,
31,
32]. Furthermore, some studies have conducted bone-tissue-derived biomaterials that consist of ECM components of bone tissue for enhancing bone tissue regeneration [
15,
16,
17].
For these reasons, we fabricated the customized scaffolds using PCL, β-TCP, and bone dECM. These scaffolds were customized structures designed and printed with the desired correction angle for open-wedge HTO. The scaffolds were composed of a) PCL that constitutes a supporting part, and b) β-TCP and bone dECM that can promote bone tissue regeneration. The β-TCP in the scaffolds play important roles in bone regeneration owing to their composition, but also have roles in the promotion of the degradation rate of PCL by expanding its surface area given that its degradation rate (approximately 3–6 months) is faster than that (about over 12 months) of PCL [
30]. Additionally, bone dECM is consisted of bone tissue in the microenvironment and it is thus effective for bone regeneration. This is a key feature that can overcome the disadvantage that the cells are attached only on the surface of the porous scaffolds, and the fact that it is difficult to retain many cells within the scaffolds. Nevertheless, these cells possess tremendous potential when seeded in scaffolds for effective bone tissue regeneration.
5. Conclusions
The customized 3D scaffold with PCL/β-TCP/bone dECM showed excellent outcomes regarding the cell seeding efficiency, proliferation capacity, early osteogenic differentiation, and bone mineralization. Given that the scaffolds provide the proper environment that promotes bone tissue regeneration, their capacities to achieve their integrations with native tissue in vivo were excellent. It is expected to solve the disadvantages related to bone union in MOWHTO and to replace autografts in the future.
Author Contributions
Geunseon Ahn and Jun-Young Kim contributed equally to this study. G. Ahn: Conceptualization, Investigation, Methodology, Formal analysis, Data curation, Validation, Writing—original draft, review and editing. J.-H. Shim: Conceptualization, Investigation, Methodology, Formal analysis, Data curation, Validation, Writing—original draft, review and editing. C. Kim: Investigation, Formal analysis, Writing- original draft. I.-G. Lee: Investigation, Formal analysis, Writing- original draft. S. Ahn: Investigation, Formal analysis, Writing- original draft. J-M Shin: Investigation, Formal analysis, Writing- original draft. G. Ahn and J-M Shin: Methodology, Validation, Writing—review and editing. J.-Y. Kim: Conceptualization, Methodology, Validation, Supervision, Writing—original draft, review and editing. B.H. Lee: Conceptualization, Methodology, Validation, Supervision, Writing—original draft, review and editing.
Funding
This research received no external funding
Institutional Review Board Statement
This research was approved by the Institutional Animal Care and Use Committee (IACUC) of DGMIF-LAC (No. DGMIF-17012604-00).
Informed Consent Statement
Not applicable
Data Availability Statement
Not applicable
Acknowledgments
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education, Science and Technology (2017R1A6A1A03015562), and supported by the Ministry of Trade, Industry, and Energy (MOTIE), Korea, under the “Infrastructure program for industrial innovation(reference number P0025775)” supervised by the Korea Institute for Advancement of Technology (KIAT). This work was supported by the Gachon University research fund of 2023.(GCU-202309330001)".
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahn, G.; Lee, J.-S.; Yun, W.-S.; Shim, J.-H.; Lee, U.-L. Cleft Alveolus Reconstruction Using a Three-Dimensional Printed Bioresorbable Scaffold With Human Bone Marrow Cells. J. Craniofacial Surg. 2018, 29, 1880–1883. [CrossRef]
- Andriacchi, T.P.; Mündermann, A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr. Opin. Rheumatol. 2006, 18, 514–518. [CrossRef]
- Aryee, S.; Imhoff, A.B.; Rose, T.; Tischer, T. Do we need synthetic osteotomy augmentation materials for opening-wedge high tibial osteotomy. Biomaterials 2008, 29, 3497–3502. [CrossRef]
- Blaker, J.; Nazhat, S.; Maquet, V.; Boccaccini, A. Long-term in vitro degradation of PDLLA/Bioglass® bone scaffolds in acellular simulated body fluid. Acta Biomater. 2010, 7, 829–840. [CrossRef]
- Brouwer RW, Raaij van TM, Bierma-Zeinstra SM, et al. Osteotomy for treating knee osteoarthritis. The Cochrane database of systematic reviews. 2007(3):Cd004019. [CrossRef]
- Cai S, Xu GH, Yu XZ, et al. Fabrication and biological characteristics of beta-tricalcium phosphate porous ceramic scaffolds reinforced with calcium phosphate glass. Journal of materials science Materials in medicine. 2009;20(1):351-358. [CrossRef]
- Cheng, C.W.; Solorio, L.D.; Alsberg, E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol. Adv. 2014, 32, 462–484. [CrossRef]
- Fuentes, E.; de Viteri, V.S.; Igartua, A.; Martinetti, R.; Dolcini, L.; Barandika, G. Structural Characterization and Mechanical Performance of Calcium Phosphate Scaffolds and Natural Bones: A Comparative Study. J. Appl. Biomater. Biomech. 2010, 8, 159–165. [CrossRef]
- Gothard, D.; Smith, E.L.; Kanczler, J.M.; Black, C.R.; Wells, J.A.; Roberts, C.A.; White, L.J.; Qutachi, O.; Peto, H.; Rashidi, H.; et al. In Vivo Assessment of Bone Regeneration in Alginate/Bone ECM Hydrogels with Incorporated Skeletal Stem Cells and Single Growth Factors. PLOS ONE 2015, 10, e0145080. [CrossRef]
- Han, J.H.; Kim, H.J.; Song, J.G.; Yang, J.H.; Bhandare, N.N.; Fernandez, A.R.; Park, H.J.; Nha, K.W. Is Bone Grafting Necessary in Opening Wedge High Tibial Osteotomy? A Meta-Analysis of Radiological Outcomes. Knee Surg. Relat. Res. 2015, 27, 207–220. [CrossRef]
- Han SB, Lee DH, Shetty GM, et al. A “safe zone” in medial open-wedge high tibia osteotomy to prevent lateral cortex fracture. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2013;21(1):90-95. [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [CrossRef]
- Kim, J.-Y.; Ahn, G.; Kim, C.; Lee, J.-S.; Lee, I.-G.; An, S.-H.; Yun, W.-S.; Kim, S.-Y.; Shim, J.-H. Synergistic Effects of Beta Tri-Calcium Phosphate and Porcine-Derived Decellularized Bone Extracellular Matrix in 3D-Printed Polycaprolactone Scaffold on Bone Regeneration. Macromol. Biosci. 2018, 18, e1800025. [CrossRef]
- Lash NJ, Feller JA, Batty LM, Wasiak J, Richmond AK. Bone grafts and bone substitutes for opening-wedge osteotomies of the knee: a systematic review. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2015;31(4):720-730. [CrossRef]
- Lee, B.H.; Ha, C.W.; Moon, S.W.; Chang, M.; Kim, H.Y.; Park, S.H.; Wang, J.H. Three-dimensional relationships between secondary changes and selective osteotomy parameters for biplane medial open-wedge high tibial osteotomy. Knee 2017, 24, 362–371. [CrossRef]
- Lee, O.-S.; Lee, K.J.; Lee, Y.S. Comparison of bone healing and outcomes between allogenous bone chip and hydroxyapatite chip grafts in open wedge high tibial osteotomy. J. Mater. Sci. Mater. Med. 2017, 28, 189–189. [CrossRef]
- Lee, S.C.; Jung, K.A.; Nam, C.H.; Jung, S.H.; Hwang, S.H. The Short-term Follow-up Results of Open Wedge High Tibial Osteotomy with Using an Aescula Open Wedge Plate and an Allogenic Bone Graft: The Minimum 1-Year Follow-up Results. Clin. Orthop. Surg. 2010, 2, 47–54. [CrossRef]
- Miller, B.S.; Dorsey, W.O.P.; Bryant, C.R.; Austin, J.C. The Effect of Lateral Cortex Disruption and Repair on the Stability of the Medial Opening Wedge High Tibial Osteotomy. Am. J. Sports Med. 2005, 33, 1552–1557. [CrossRef]
- Miyazaki, T.; Wada, M.; Kawahara, H.; Sato, M.; Baba, H.; Shimada, S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann. Rheum. Dis. 2002, 61, 617–622. [CrossRef]
- Nha KW, Oh SM, Ha YW, et al. A Retrospective Comparison of Union Rates After Open Wedge High Tibial Osteotomies With and Without Synthetic Bone Grafts (Hydroxyapatite and beta-tricalciumphosphate) at 2 Years. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2018;34(9):2621-2630. [CrossRef]
- Rangavittal N, Landa-Canovas AR, Gonzalez-Calbet JM, Vallet-Regi M. Structural study and stability of hydroxyapatite and beta-tricalcium phosphate: two important bioceramics. Journal of biomedical materials research. 2000;51(4):660-668. [CrossRef]
- Ryu, S.M.; Park, J.W.; Na, H.D.; Shon, O.J. High Tibial Osteotomy versus Unicompartmental Knee Arthroplasty for Medial Compartment Arthrosis with Kissing Lesions in Relatively Young Patients. Knee Surg. Relat. Res. 2018, 30, 17–22. [CrossRef]
- Santic, V.; Tudor, A.; Sestan, B.; Legovic, D.; Sirola, L.; Rakovac, I. Bone allograft provides bone healing in the medial opening high tibial osteotomy. Int. Orthop. 2009, 34, 225–229. [CrossRef]
- Sawkins, M.; Bowen, W.; Dhadda, P.; Markides, H.; Sidney, L.; Taylor, A.; Rose, F.; Badylak, S.; Shakesheff, K.; White, L. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. 2013, 9, 7865–7873. [CrossRef]
- Schroter S, Ateschrang A, Ihle C, et al. [Lateral hinge fractures in open wedge high tibial osteotomy]. Der Orthopade. 2014;43(11):1000-1007. [CrossRef]
- Shim, J.-H.; Jeong, J.-H.; Won, J.-Y.; Bae, J.-H.; Ahn, G.; Jeon, H.; Yun, W.-S.; Bae, E.-B.; Choi, J.-W.; Lee, S.-H.; et al. Porosity effect of 3D-printed polycaprolactone membranes on calvarial defect model for guided bone regeneration. Biomed. Mater. 2017, 13, 015014. [CrossRef]
- Shim JH, Won JY, Park JH, et al. Effects of 3D-Printed Polycaprolactone/beta-Tricalcium Phosphate Membranes on Guided Bone Regeneration. International journal of molecular sciences. 2017;18(5). [CrossRef]
- Slevin, O.; Ayeni, O.R.; Hinterwimmer, S.; Tischer, T.; Feucht, M.J.; Hirschmann, M.T. The role of bone void fillers in medial opening wedge high tibial osteotomy: a systematic review. Knee Surgery, Sports Traumatol. Arthrosc. 2016, 24, 3584–3598. [CrossRef]
- Warden, S.J.; Morris, H.G.; Crossley, K.M.; Brukner, P.D.; Bennell, K.L. Delayed- and non-union following opening wedge high tibial osteotomy: surgeons? results from 182 completed cases. Knee Surgery, Sports Traumatol. Arthrosc. 2004, 13, 34–37. [CrossRef]
- Won JY, Park CY, Bae JH, et al. Evaluation of 3D printed PCL/PLGA/beta-TCP versus collagen membranes for guided bone regeneration in a beagle implant model. Biomedical materials (Bristol, England). 2016;11(5):055013. [CrossRef]
- Yacobucci, G.N.; Cocking, M.R. Union of Medial Opening-Wedge High Tibial Osteotomy Using a Corticocancellous Proximal Tibial Wedge Allograft. Am. J. Sports Med. 2008, 36, 713–719. [CrossRef]
- Yoo, M.-J.; Shin, Y.-E. Open Wedge High Tibial Osteotomy and Combined Arthroscopic Surgery in Severe Medial Osteoarthritis and Varus Malalignment: Minimum 5-Year Results. Knee Surg. Relat. Res. 2016, 28, 270–276. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).