Submitted:
30 July 2024
Posted:
31 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Effect of Sucrose on Biological Parameters in Single- and Dual-Species Biofilms
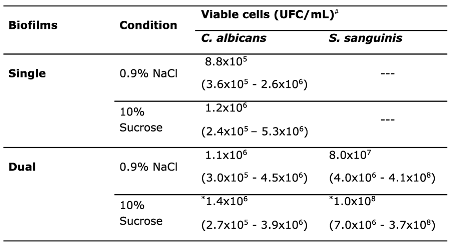
3.1.1. Cell Viability of C. albicans and S. sanguinis
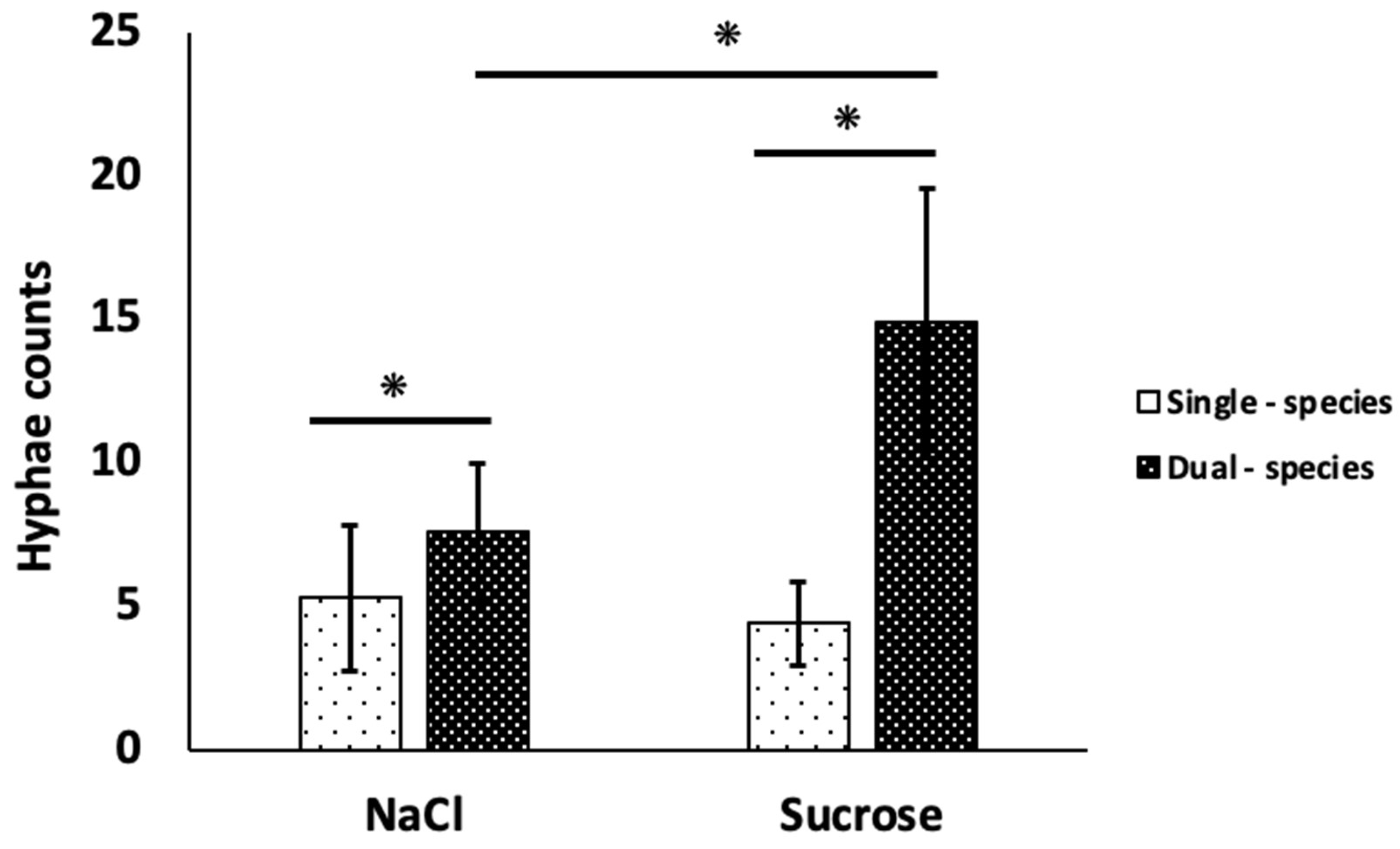
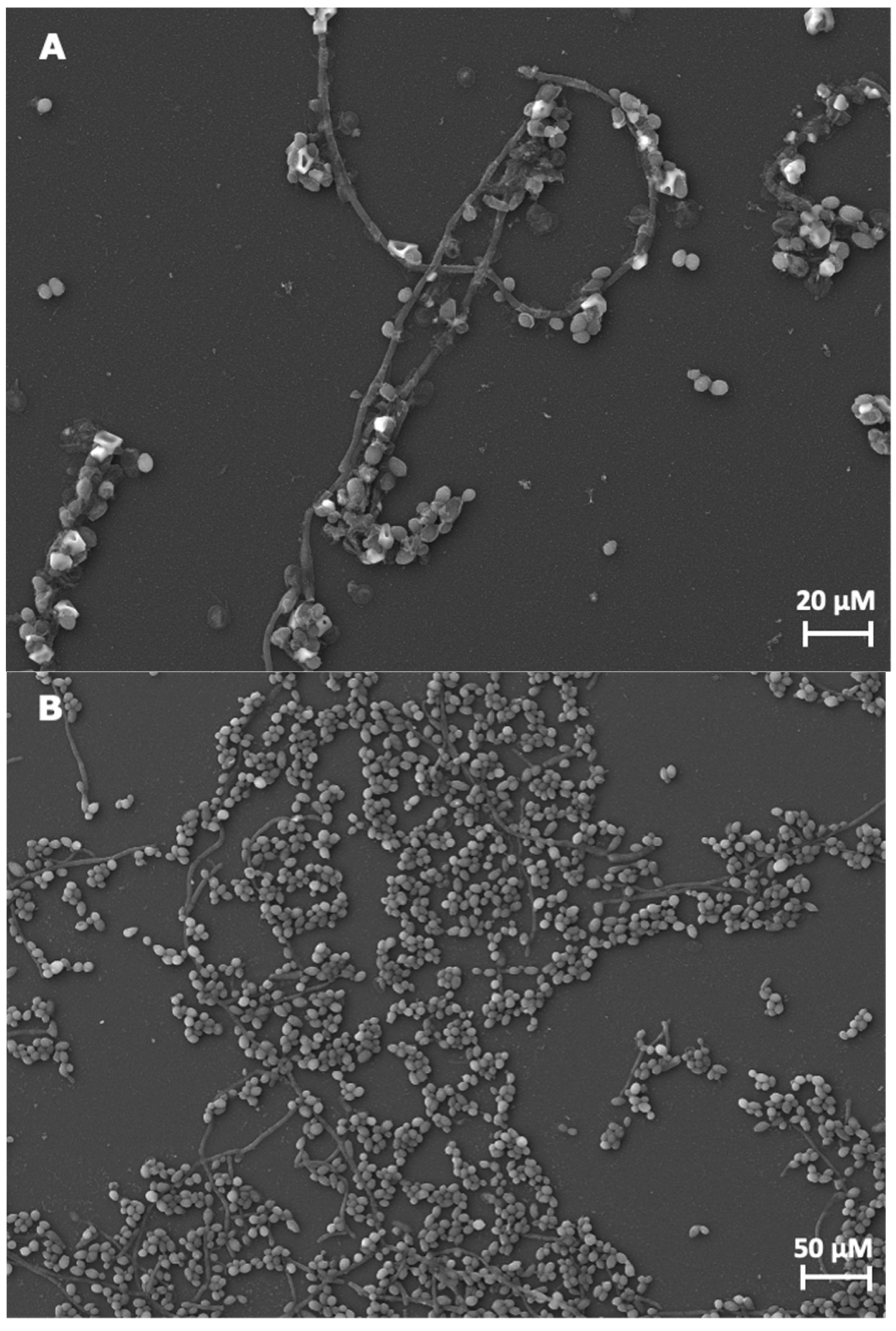
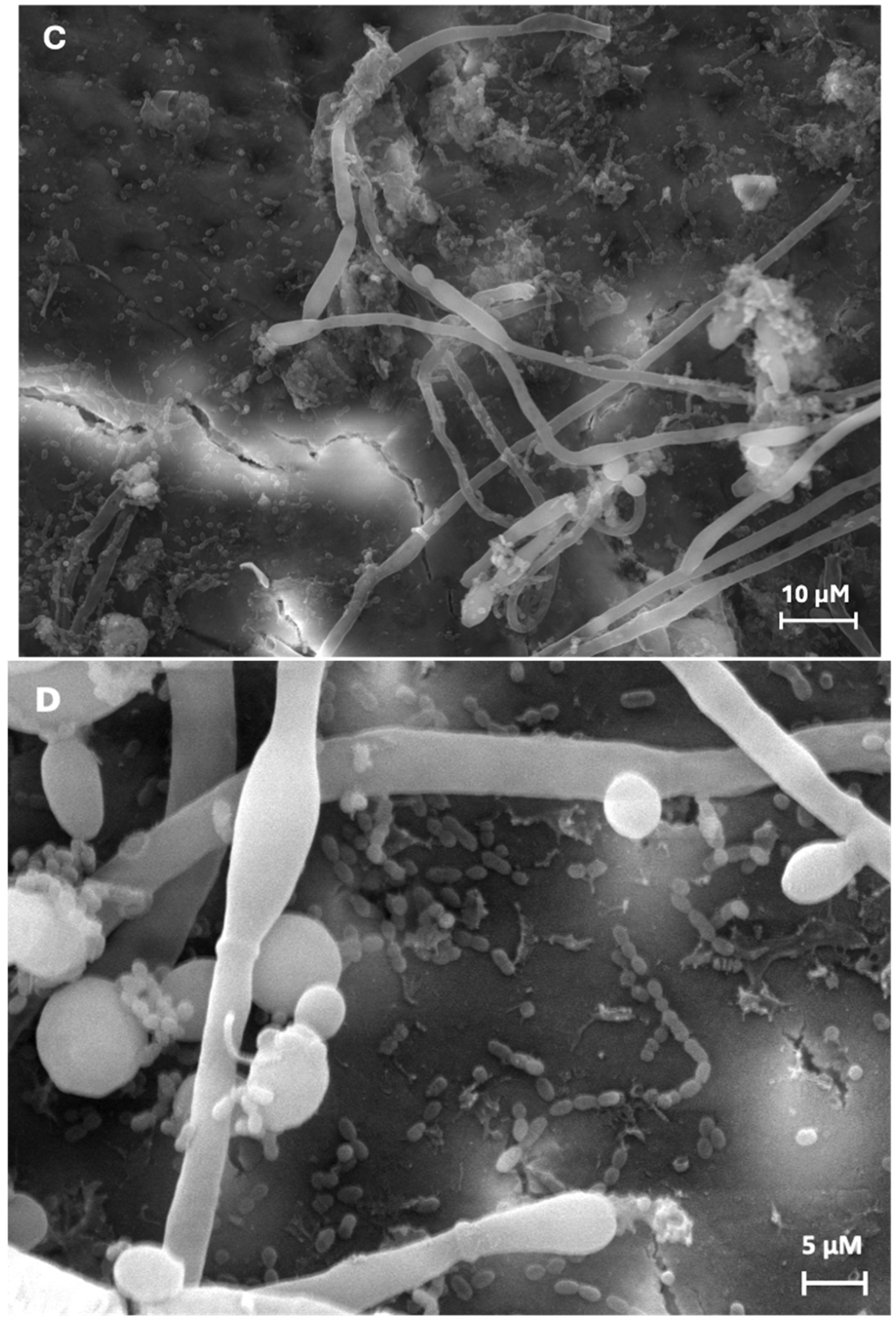
3.1.2. Filamentation of C. albicans
3.1.3. Biofilms Formation
3.2. Effect of Sucrose on Physico-Chemical Parameters in Single- and Dual-Species Biofilms
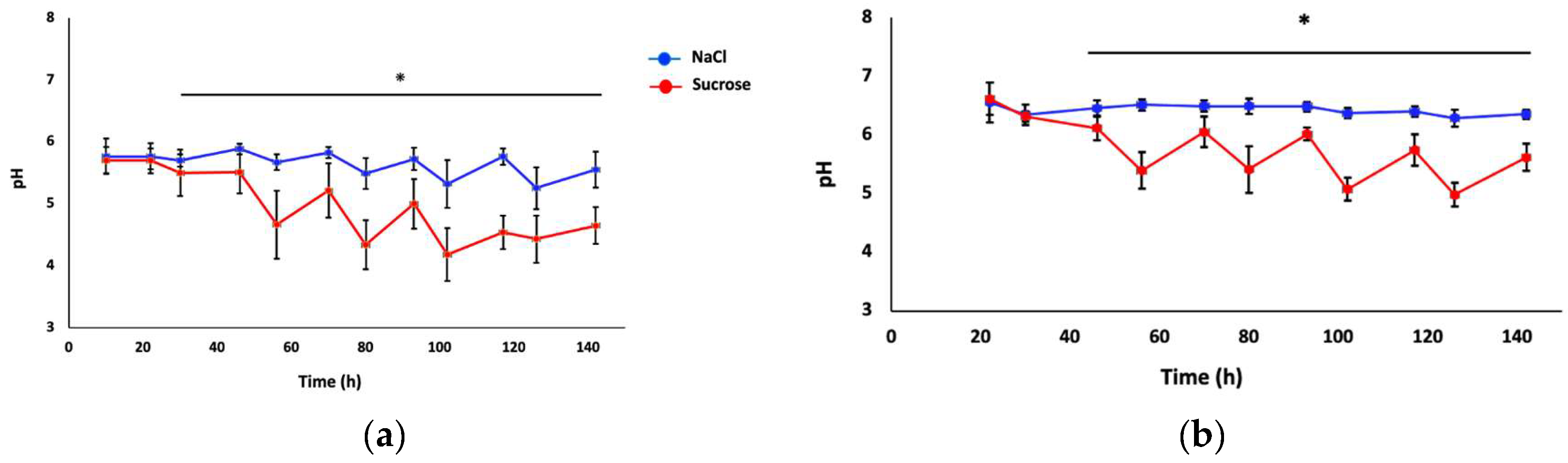
3.2.1. Acidogenicity Measurement in the Biofilms
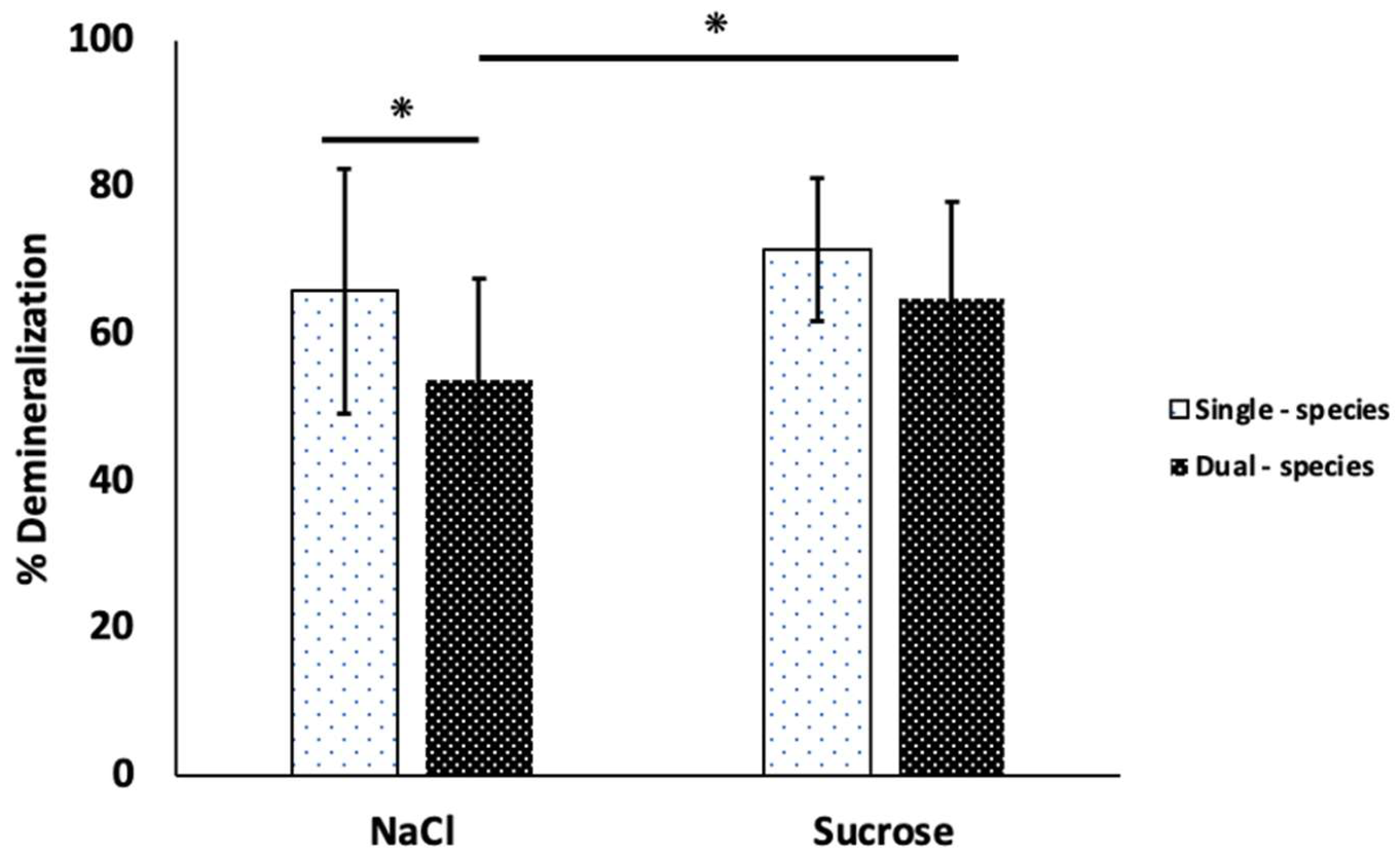
3.2.2. Surface Microhardness on Enamel Slabs
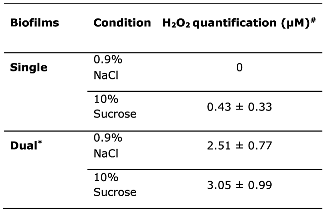
3.2.3. H2O2 Production in Biofilms
4. Discussion
Effect of Sucrose on Biological Parameters Evaluated in Single- and Dual-Species Biofilms
Cell Viability of C. albicans and S. sanguinis
Hyphal Formation in C. albicans
Effect of Sucrose on Physico-Chemical Parameters Evaluated in Single- and Dual-Species Biofilms
Acidogenicity of the Biofilms
Enamel Slabs Demineralization
H2O2 Production in Biofilms
Candida albicans Role in the Oral Biofilms Structure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- Simón-Soro, A.; Ren, Z.; Krom, B.P.; Hoogenkamp, M.A.; Cabello-Yeves, P.J.; Daniel, S.G.; Bittinger, K.; Tomas, I.; Koo, H.; Mira, A. Polymicrobial aggregates in human saliva build the oral biofilm. mBio. 2022, 13, e0013122. [Google Scholar] [CrossRef]
- Marsh, P.D.; Zaura, E. Dental biofilm: ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44, S12–S22. [Google Scholar] [CrossRef]
- Marsh, P.; Martin, M. Williams, D. In Oral Microbiology, 5th ed.; Lewis, MA., Ed.; Edinburgh London New York Oxford Philadelphia St Louis Sydney Toronto, Churchill Livingstone Elsevier, 2009.
- Kreth, J.; Giacaman, R.A.; Raghavan, R.; Merritt, J. The road less traveled - defining molecular commensalism with Streptococcus sanguinis. Mol. Oral Microbiol. 2017, 32, 181–196. [Google Scholar] [CrossRef]
- Zhu, L.; Kreth, J. The role of hydrogen peroxide in environmental adaptation of oral microbial communities. Oxid. Med. Cell. Longev. 2012, 2012, 717843. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Merrit, J.; Shi, W.; Qi, F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 2005, 187, 7193–7203. [Google Scholar] [CrossRef]
- Huffines, J.T.; Scoffield, J.A. Disruption of Streptococcus mutans and Candida albicans synergy by a commensal streptococcus. Sci. Rep. 2020, 10, 19661. [Google Scholar] [CrossRef] [PubMed]
- Redanz, S.; Cheng, X.; Giacaman, R.A.; Pfeifer, C.S.; Merritt, J.; Kreth, J. Live and let die: hydrogen peroxide production by the commensal flora and its role in maintaining a symbiotic microbiome. Mol. Oral Microbiol. 2018, 33, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Thein, Z.M.; Samaranayake, Y.H.; Samaranayake, L.P. Effect of oral bacteria on growth and survival of Candida albicans biofilms. Arch. Oral Biol. 2006, 51, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Romo, J.A.; Kumamoto, C.A. On commensalism of Candida. J Fungi 2020, 6, 16. [Google Scholar] [CrossRef]
- Simón-Soro, A.; Guillen-Navarro, M.; Mira, A. Metatranscriptomics reveals overall active bacterial composition in caries lesions. J. Oral Microbiol. 2014, 6, 25443. [Google Scholar] [CrossRef]
- Lozano, C.; Rodríguez, G.; Lefimil, C.; Morales-Bozo, I.; Urzúa-Orellana, B. Prevalence of Candida albicans and carriage of Candida non-albicans in the saliva of preschool children, according to their caries status. Acta Odontol. Scand. 2017, 75, 30–35. [Google Scholar] [CrossRef]
- Du, Q.; Ren, B.; He, J.; Peng, X.; Guo, Q.; Zheng, L.; Li, J.; Dai, H.; Chen, V.; Zhang, L. ; Zhou, X; Xu, X. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. ISME J. 2021, 15, 894–908. [Google Scholar] [CrossRef]
- Díaz, P.I.; Strausbaugh, L.D.; Dongari-Bagtzoglou, A. Fungal-bacterial interactions and their relevance to oral health: linking the clinic and the bench. Front. Cell. Infect. Microbiol. 2014, 4, 101–106. [Google Scholar] [CrossRef]
- Janus, M.M.; Willems, H.M.E.; Krom, B.P. Candida albicans in multispecies oral communities; A keystone commensal? Adv. Exp. Med. Biol. 2016, 931, 13–20. [Google Scholar] [CrossRef]
- Baraniya, D.; Chen, T.; Nahar, A.; Alakwaa, F.; Hill, J.; Tellez, M.; Ismail, A.; Puri, S.; Al-Hebshi, N.N. Supragingival mycobiome and inter-kingdom interactions in dental caries. J. Oral Microbiol. 2020, 12, 1729305–1729313. [Google Scholar] [CrossRef]
- Hong, B.Y.; Hoare, A.; Cardenas, A.; Dupuy, A.K.; Choquette, L.; Salner, A.L.; Schauer, P.K.; Hegde, U.; Petersen, D.E.; Dongari-Bagtzoglou, A.; Strausbauhg, L.D.; Díaz, P.I. The salivary mycobiome contains 2 ecologically distinct mycotypes. J. Dent. Res. 2020, 99, 730–738. [Google Scholar] [CrossRef]
- Li, W.; Yu, D.; Gao, S.; Lin, J.; Chen, Z.; Zhao, W. Role of Candida albicans-secreted aspartyl proteinases (Saps) in severe early childhood caries. Int. J. Mol. Sci. 2014, 15, 10766–10779. [Google Scholar] [CrossRef]
- de Abreu, T.C.; Barbosa, M.; Moraes, P.; Barbosa, G.F.; Cople, L.; Fonseca-Goncalves, A. Demineralizing potential of dental biofilm added with Candida albicans and Candida parapsilosis isolated from preschool children with and without caries. Microb. Pathog. 2016, 100, 51–55. [Google Scholar] [CrossRef]
- Lamont, R.J.; Jenkinson, H.F. Microbiology of Caries. In Oral Microbiology at a Glance, 1st ed.; Wiley-Blackwell publication, United Kingdom, 2010; Volume 1, pp. 34.
- Akküç, S.; Duruk, G.; Keles, A. Remineralization effect of three different agents on initial caries and erosive lesions: a micro-computed tomography and scanning electron microscopy analysis. BMC Oral Health 2023, 23, 106–117. [Google Scholar] [CrossRef]
- Soon-Hwan, O.; Martin-Yken, H.; Coleman, D.A.; Dague, E.; Hoyer, L.L. Development and use of a monoclonal antibody specific for the Candida albicans cell-surface protein Hwp1. Front. Cell. Infect. Microbiol. 2022, 12, 907453. [Google Scholar] [CrossRef]
- Ten Cate, J.M.; Klis, F.M.; Pereira-Cenci, T.; Crielaard, W.; de Groot, P.W.J. Molecular and cellular mechanisms that lead to Candida biofilm formation. J. Dent. Res. 2009, 88, 105–115. [Google Scholar] [CrossRef]
- do Rosário Palma, A.; Domingues, N.; de Barros, P.; Brito, G.; Cardoso, A. Influence of Streptococcus mitis and Streptococcus sanguinis on virulence of Candida albicans: in vitro and in vivo studies. Folia Microbiol. 2019, 64, 215–222. [Google Scholar] [CrossRef]
- Brown, G. Innate antifungal immunity: the key role of phagocytes. Ann. Rev. Immunol. 2011, 29, 1–21. [Google Scholar] [CrossRef]
- Nasution, O.; Srinivasa, K.; Kim, M.; Kim, Y.J.; Kim, W.; Jeong, W.; Choi, W. Hydrogen peroxide induces hyphal differentiation in Candida albicans. Eukaryot. Cell. 2008, 7, 2008–2011. [Google Scholar] [CrossRef]
- Koo, H.; Andes, D.R.; Krysan, D.J. Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018, 14, e1007342. [Google Scholar] [CrossRef]
- Komalapriya, C.; Kaloriti, D.; Tillmann, A.T.; Yin, Z.; Herrero-de-Dios, C.; Jacobsen, M.D.; Belmonte, R.C.; Cameron, G.; Haynes, K.; Grebogi, C.; de Moura, A.P.; Gow, N.A.; Thiel, M.; Quinn, J.; Brown, A.J.; Romano, M.C. Integrative Model of Oxidative Stress Adaptation in the Fungal Pathogen Candida albicans. PloS One 2015, 10, e0137750. [Google Scholar] [CrossRef]
- Díaz-Garrido, N.; Lozano, C.; Giacaman, R. Frequency of sucrose exposure on the cariogenicity of a biofilm-caries model. Eur. J. Dent. 2016, 10, 345–350. [Google Scholar] [CrossRef]
- Giacaman, R.A.; Umaña, R.; Nuñez, M.J.; Díaz-Garrido, N.; Echeverría, C.; García-Manríquez, N.; Mira, A.; Fernández, C.E.; Gambetta-Tessini, K.; Lozano, C.P. Saliva decreases sucrose-induced cariogenicity in an experimental biological caries model. Microorganisms 2023, 11, 1426. [Google Scholar] [CrossRef]
- Lozano, C.; Díaz-Garrido, N.; Kreth, J.; Giacaman, R. Streptococcus mutans and Streptococcus sanguinis Expression of Competition-Related Genes, Under Sucrose. Caries Res. 2019, 53, 194–203. [Google Scholar] [CrossRef]
- Bezerra, N.V.F.; Brito, A.C.M.; de Medeiros, M.M.D.; de França Leite, K.L.; Bezerra, I.M.; de Almeida, L.F.D.; Aires, C.P.; Cavalcanti, Y.W. Glucose supplementation effect on the acidogenicity, viability, and extracellular matrix of Candida single- and dual-species biofilms. J. Investig. Clin. Dent. 2019, 10, e12412. [Google Scholar] [CrossRef]
- Aitken-Saavedra, J.; Lund, R.G.; González, J.; Huenchunao, R.; Perez-Vallespir, I.; Morales-Bozo, I.; Urzúa, B.; Tarquinio, S.C.; Maturana-Ramírez, A.; Martos, J.; Fernandez-Ramires, R.; Molina-Berríos, A. Diversity, frequency and antifungal resistance of Candida species in patients with type 2 diabetes mellitus. Acta Odontol. Scand. 2018, 76, 580–586. [Google Scholar] [CrossRef]
- Domingues, P.C.A.; Oliveira, V.C.; Bim, F.L.; Aires, C.P.; Santos, A.P.D.; Castro, D.T.; Silva-Lovato, C.H.; Andrade, D.; Watanabe, E. Influence of glucose supplementation on biofilm formation of Candida albicans and Candida glabrata isolated from diabetic and non-diabetic individuals. Arch. Oral Biol. 2022, 134, 105339. [Google Scholar] [CrossRef]
- Lok, B.; Ahmad, M.A.; Mohd, L.Z.; Chukwudi, N.A.; Sandai, R.; Sandai, D. The assimilation of different carbon sources in Candida albicans: Fitness and pathogenicity. Med. Mycol. 2021, 59, 115–125. [Google Scholar] [CrossRef]
- Xiang, Z.; Wakade, R.S.; Ribeiro, A.A.; Hu, W.; Bittinger, K.; Simon-Soro, A.; Kim, D.; Li, J.; Krysan, D. J.; Liu, Y.; Koo, H. Human Tooth as a Fungal Niche: Candida albicans Traits in Dental Plaque Isolates. mBio. 2023, 14, e0276922. [Google Scholar] [CrossRef]
- Ev, L.D.; Damé-Teixeira, N.; DO, T.; Maltz, M.; Cavalcanti, C. The role of Candida albicans in root caries biofilms: an RNA-seq analysis. J. Appl. Oral Sci. 2020, 28, e20190578. [Google Scholar] [CrossRef]
- Ren, Z.; Jeckel, H.; Simon-Soro, A.; Xiang, Z.; Liu, Y.; Cavalcanti, I.M.; Xiao, J.; Tin, N.-N.; Hara, A.; Drescher, K.; Koo, H. Interkingdom assemblages in human saliva display group-level surface mobility and disease-promoting emergent functions. PNAS 2022, 119, e2209699119. [Google Scholar] [CrossRef]
- Díaz-Garrido, N.; Lozano, C.P.; Kreth, J.; Giacaman, R.A. Extended biofilm formation time by Streptococcus sanguinis modifies its non-cariogenic behavior, in vitro. Braz. Oral Res. 2022, 36, e107–10. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.; Ren, B.; Cheng, L. The regulation of hyphae growth in Candida albicans. Virulence 2020, 11, 337–348. [Google Scholar] [CrossRef]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef]
- Morse, D.J.; Wilson, M.J.; Wei, X.; Bradshaw, D.J.; Lewis, M.A.O.; Williams, D.W. Modulation of Candida albicans virulence in in vitro biofilms by oral bacteria. Lett. Appl. Microbiol. 2019, 68, 337–343. [Google Scholar] [CrossRef]
- Xu, H.; Sobue, T.; Bertolini, M.; Thompson, A.; Vickerman, M.; Nobile, C.J.; Dongari-Bagtzoglou, A.D. S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence 2017, 8, 1602–1617. [Google Scholar] [CrossRef]
- Basso, V.; d’Enfert, C.; Znaidi, S.; Bachellier-Bassi, S. From Genes to Networks: The Regulatory Circuitry Controlling Candida albicans Morphogenesis. Curr. Top. Microbiol. Immunol. 2019, 422, 61–99. [Google Scholar] [CrossRef]
- Merino Guzmán, G.; Cedillo Ramírez, L.; Silva Andrade, F.; Muñoz García, A.A.; Castañeda Roldán, E.I. Análisis morfológico de biopelículas de Candida albicans producidas en diferentes condiciones de pH y temperatura analizadas por microscopía óptica y de fuerza atómica. [In English: Morphological analysis of Candida albicans biofilms produced at different pH and temperature conditions analyzed by optic and atomic force microscopy]. Rev. Mex. Mic. 2011, 33, 1–8. [Google Scholar]
- Westwater, C.; Balish, E.; Schofield, D.A. Candida albicans-conditioned medium protects yeast cells from oxidative stress: a possible link between quorum sensing and oxidative stress resistance. Eukaryot. Cell. 2005, 4, 1654–1661. [Google Scholar] [CrossRef]
- Willems, H.M.; Kos, K.; Jabra-Rizk, M. A.; Krom, B.P. Candida albicans in oral biofilms could prevent caries. Pathog. Dis. 2016, 74, ftw039. [Google Scholar] [CrossRef]
- Eidt, G.; Gomes de Andrade, C.; de Cássia, T.; Arthur, R.A. Role of Candida albicans on enamel demineralization and on acidogenic potential of Streptococcus mutans in vitro biofilms. J. Appl. Oral Sci. 2019, 27, e20180593. [Google Scholar] [CrossRef]
- Huang, X.; Schulte, R.M.; Burne, R.A.; Nascimento, M.M. Characterization of the arginolytic microflora provides insights into pH homeostasis in human oral biofilms. Caries Res. 2015, 49, 165–176. [Google Scholar] [CrossRef]
- Díaz-Garrido, N.; Lozano, C.P.; Kreth, J.; Giacaman, R.A. Competition and caries on enamel of a dual-species biofilm model with Streptococcus mutans and Streptococcus sanguinis. Appl. Environ. Microbiol. 2020, 86, e01262–20. [Google Scholar] [CrossRef]
- Szabó, B.; Majoros, L.; Papp-Falusi, E.; Szabó, Z.; Szabó, J.; Márton, I.; Kelentey, B. Studies on the possible aetiological role of different Candida species in pathogenesis of dentine caries by monitoring the calcium release from tooth particles. Acta Microbiol. Immunol. Hung. 2014, 61, 11–17. [Google Scholar] [CrossRef]
- Charone, S.; Portela, M.B.; Martins, K.O.; Soares, R.M.; Castro, G.F. Role of Candida species from HIV infected children in enamel caries lesions: an in vitro study. J. Appl. Oral Sci. 2017, 25, 53–60. [Google Scholar] [CrossRef]
- Kreth, J.; Zhang, Y.; Herzberg, M. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J. Bacteriol. 2008, 190, 4632–4640. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, Z.; Itzek, A.; Herzberg, M.C.; Kreth, J. CcpA regulates biofilm formation and competence in Streptococcus gordonii. Mol. Oral Microbiol. 2012, 27, 83–94. [Google Scholar] [CrossRef]
- d’Enfert, C.; Kaune, AK.; Alaban, LR.; Chakraborty, S.; Cole, N.; Delavy, M.; et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef]
- Vila, T.; Kong, E.F.; Ibrahim, A.; Piepenbrink, K.; Shetty, A.C.; McCracken, C.; Bruno, V.; Jabra-Rizk, M.A. Candida albicans quorum-sensing molecule farnesol modulates staphyloxanthin production and activates the thiol-based oxidative-stress response in Staphylococcus aureus. Virulence 2019, 10, 625–642. [Google Scholar] [CrossRef]
- Sebaa, S.; Boucherit-Otmani, Z.; Courtois, P. Effects of tyrosol and farnesol on Candida albicans biofilm. Mol. Med. Rep. 2019, 19, 3201–3209. [Google Scholar] [CrossRef]
- Bowen, W.; Burne, R.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





