Submitted:
30 July 2024
Posted:
01 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reagents
2.3. Moisture Content and Color of Pumpkin Flesh
2.4. Extraction of Carotenoids and Determination of Total Carotenoid Content of Pumpkin Pulp
2.5. Cell Culture Conditions
2.5.1. Cell Count and Viability: Acridine Orange/DAPI Double Staining
2.6. In Vitro Antioxidant Activities
2.6.1. Free Radical-Scavenging Activity Using ABTS (ABTS Assay)
2.6.2. Oxygen Radical Absorbance Capacity Assay (ORAC Assay)
2.7. HPLC-DAD Analysis of Carotenoids
2.8. LC-HRMS Analysis for Carotenoids Structural Confirmation
2.9. Statistical Analysis
3. Results
3.1. General Characteristics of Pumpkin Cultivars
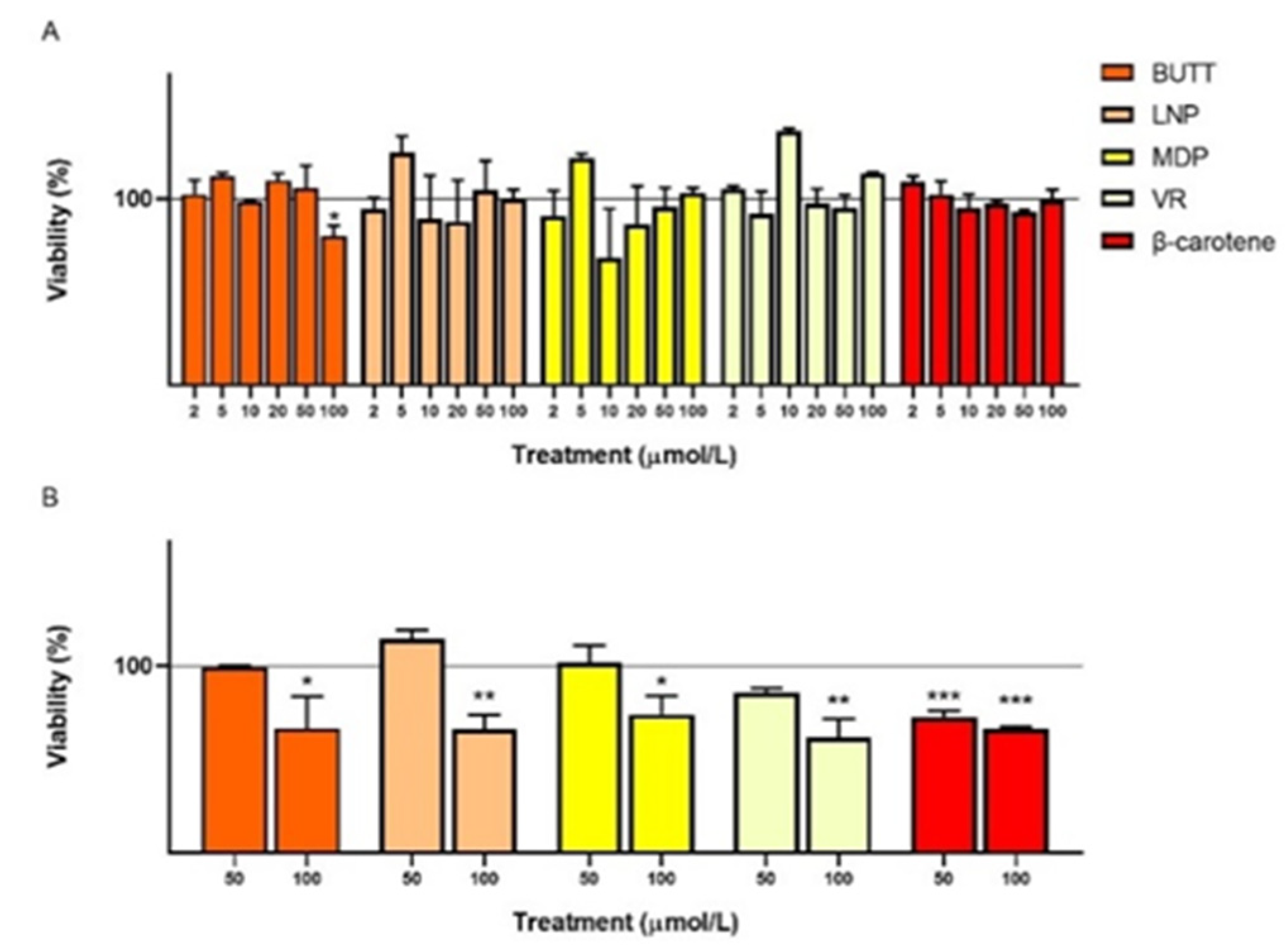
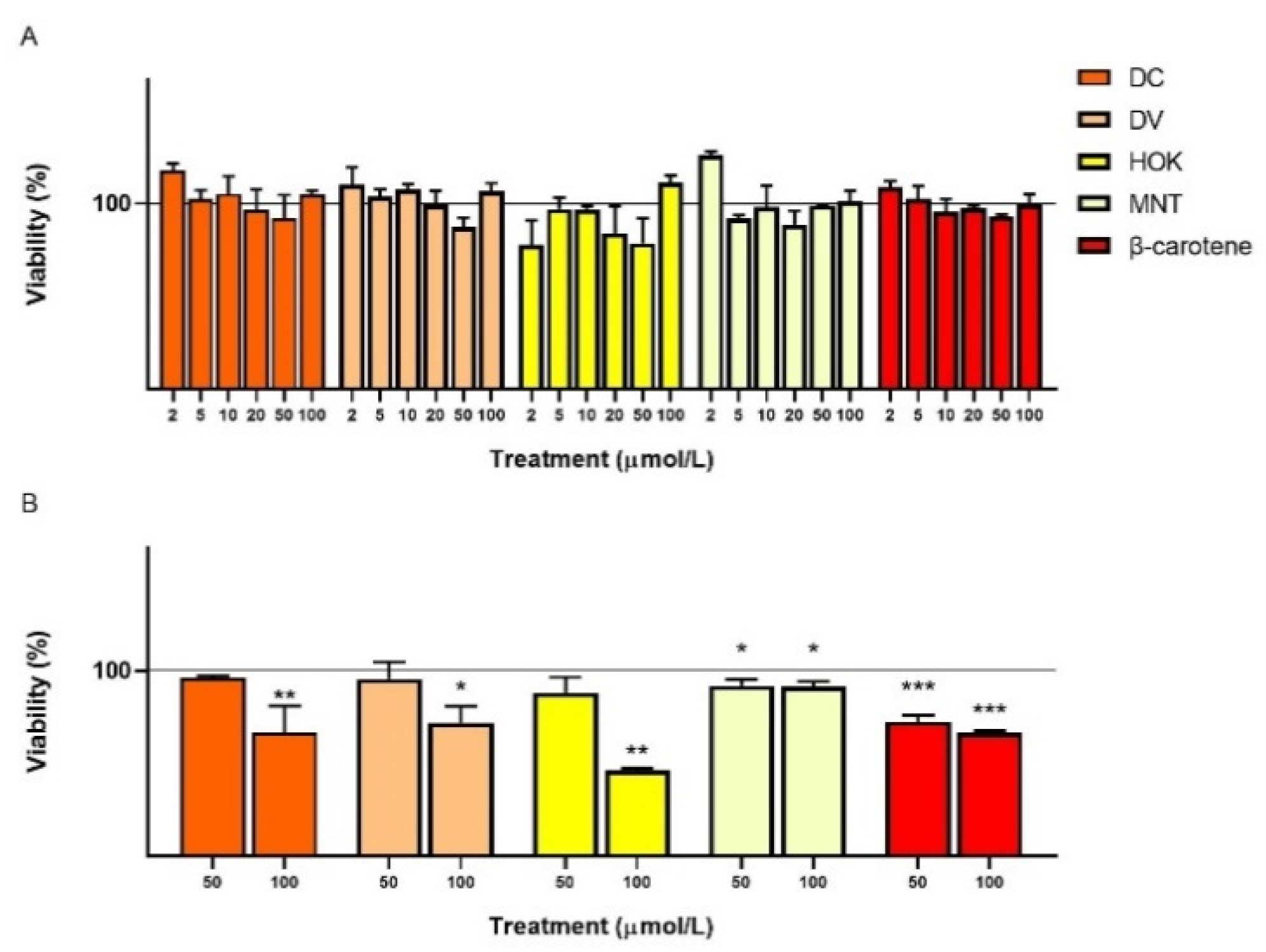
3.2. Cell Count and Viability: Acridine Orange/DAPI Double Staining
3.3. TCC and Antioxidant Activity of Pumpkin Pulp
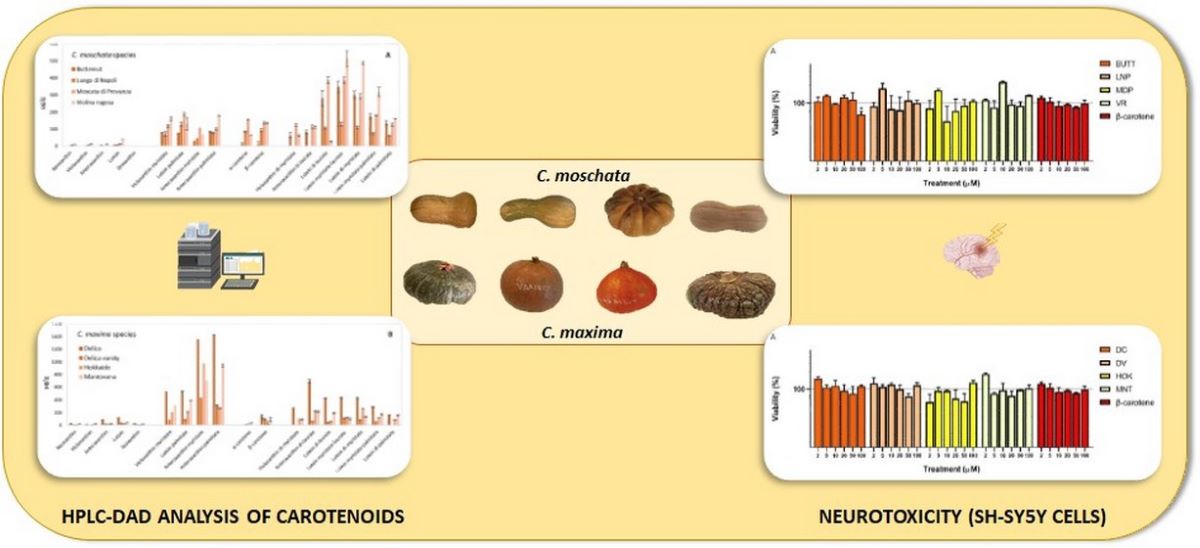
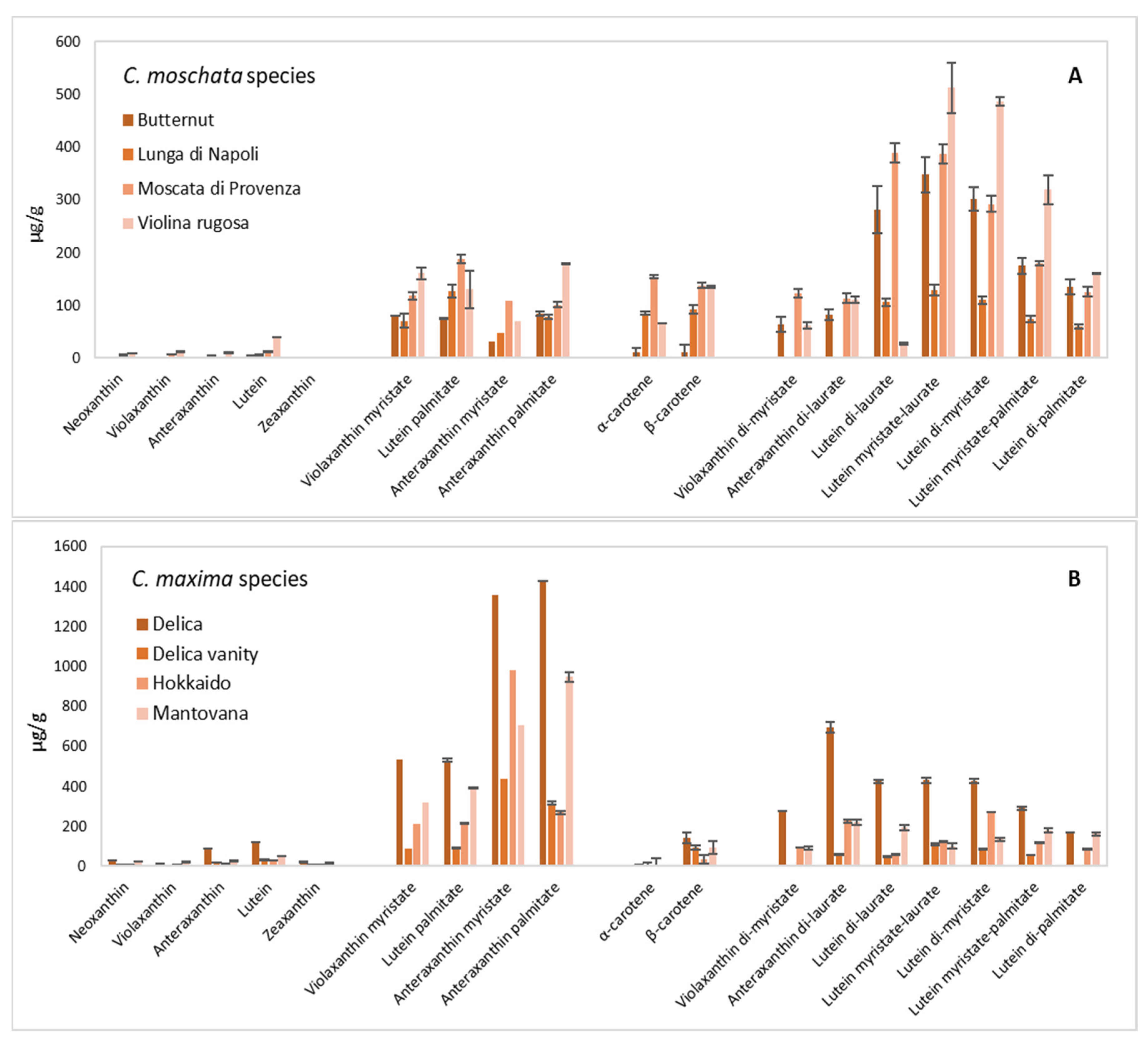
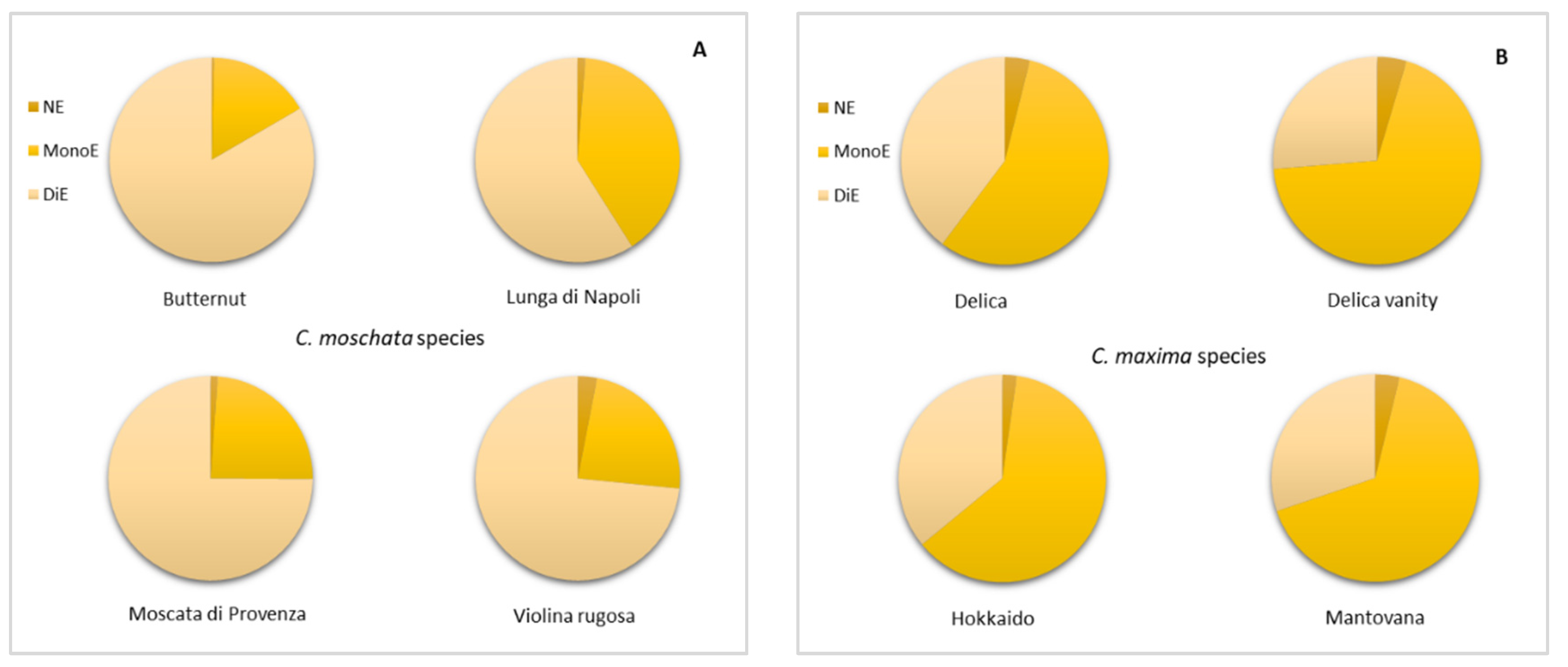
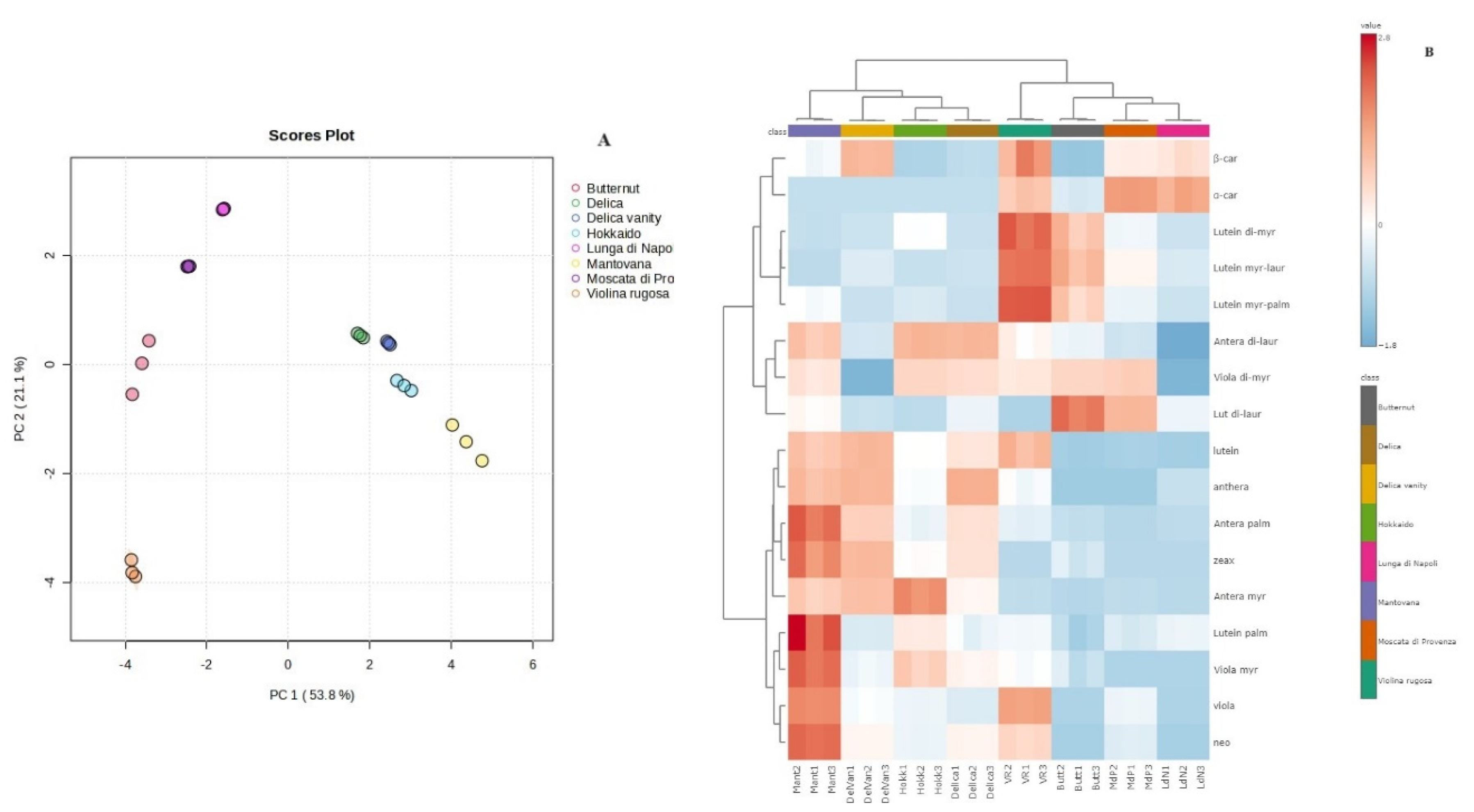
3.4. Carotenoid Composition of Pulp and Multivariate Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization 2013. WHO. Health 2020. A European policy framework and strategy for the 21st century. Available on line: https://pns.dgs.pt/files/2022/02/Health2020-Long.pdf. (accessed on 29 July 2024).
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; Corte-Real, J.; van Helden, Y.; Loizzo, M.R.; Poljšak, B.; Porrini, M.; Roob, J.; Trebše, P.; Tundis, R.; Wawrzyniak, A.; Rühl, R.; Dulińska-Litewka, J. Mechanistic aspects of carotenoid health benefits–where are we now? Nutr Res Rev 2021, 34, 276–302. [Google Scholar] [CrossRef] [PubMed]
- Batool, M.; Ranjha, M.M.A.N.; Roobab, U.; Manzoor, M.F.; Farooq, U.; Nadeem, H.R.; Nadeem, M.; Kanwal, R.; AbdElgawad, H.; Al Jaouni, S.K.; Selim, S.; Ibrahim, S.A. Nutritional value, phytochemical potential, and therapeutic benefits of pumpkin (Cucurbita sp.). Plants 2022, 11, 1394. [CrossRef]
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; Aadil, R.M. Sonication, a potential technique for extraction of phytoconstituents: a systematic review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Sharma, M.; Bhat, R. Extraction of carotenoids from pumpkin peel and pulp: comparison between innovative green extraction technologies (ultrasonic and microwave-assisted extractions using corn oil). Foods 2021, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.G.; Eller, F.J.; Wang, L.; Jane, J.L.; Wang, T.; Inglett, G.E. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J Agric Food Chem 2007, 55, 4005–4013. [Google Scholar] [CrossRef] [PubMed]
- Nawirska-Olszańska, A.; Kita, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars. Food Chem 2013, 139, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Gramza-Michałowska, A. The profile of secondary metabolites and other bioactive compounds in Cucurbita pepo L. and Cucurbita moschata pumpkin cultivars. Molecules 2019, 24, 2945. [Google Scholar] [CrossRef]
- Kostecka-Gugała, A.; Kruczek, M.; Ledwożyw-Smoleń, I.; Kaszycki, P. Antioxidants and health-beneficial nutrients in fruits of eighteen Cucurbita cultivars: analysis of diversity and dietary implications. Molecules 2020, 25, 1792. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Antioxidant potential of phytochemicals in pumpkin varieties belonging to Cucurbita moschata and Cucurbita pepo species, CyTA – J Food 2020, 18, 472-484. [CrossRef]
- Armesto, J.; Rocchetti, G.; Biancamaria, S.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Lucini, L.; Lorenzo, J.M. Nutritional characterization of Butternut squash (Cucurbita moschata D.): Effect of variety (Ariel vs. Pluto) and farming type (conventional vs. organic). Food Res Int 2020, 132, 109052. [CrossRef]
- Qi, X.; Jha, S.K.; Jha, N.K.; Dewanjee, S.; Dey, A.; Deka, R.; Pritam, P.; Ramgopal, K.; Liu, W.; Houet, K. Antioxidants in brain tumors: current therapeutic significance and future prospects. Mol Cancer 2022, 21, 204. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Büsselberg, D.; Giordano, F.A.; Golubnitschaja, O.; Kubatka, P. Carotenoids in cancer apoptosis-the road from bench to bedside and back. Cancers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Moccia, S.; Russo, M.; Durante, M.; Lenucci, M.S.; Mita, G.; Russo, G.L. A carotenoid-enriched extract from pumpkin delays cell proliferation in a human chronic lymphocytic leukemia cell line through the modulation of autophagic flux. Curr Res Biotech 2020, 2, 74–82. [Google Scholar] [CrossRef]
- Murakoshi, M.; Takayasu, J.; Kimura, O.; Kohmura, E.; Nishino, H.; Iwashima, A.; Okuzumi, J.; Sakai, T.; Sugimoto, T.; Imanishi, J.; Iwasakiet, R. Inhibitory effects of alpha-carotene on proliferation of the human neuroblastoma cell line GOTO. J Natl Cancer Inst 1989, 81, 1649-1652. [CrossRef]
- Kim, Y.S.; Lee, H.A.; Lim, J.K.; Kim, Y.; Jung, C.H.; Yoo, S.H.; Kim, Y. β-Carotene inhibits neuroblastoma cell invasion and metastasis in vitro and in vivo by decreasing level of hypoxia-inducible factor-1α. J Nutr Biochem 2014, 25, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Pinna, N.; Ianni, F.; Blasi, F.; Stefani, A.; Codini, M.; Sabatini, S.; Schoubben, A.; Cossignani, L. Unconventional extraction of total non-polar carotenoids from pumpkin pulp and their nanoencapsulation. Molecules 2022, 27, 8240. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- EasyRGB (2024). Convert color data into different standards and color spaces. Available on line: https://www.easyrgb.com/en/convert.php. (accessed on 29 July 2024).
- Villarini, M.; Acito, M.; Di Vito, R.; Vannini, S.; Dominici, L.; Fatigoni, C.; Pagiotti, R.; Moretti, M. Pro-apoptotic activity of artichoke leaf extracts in human HT-29 and RKO colon cancer cells. Int J Environ Res Public Health 2021, 18, 4166. [Google Scholar] [CrossRef] [PubMed]
- Pollini, L.; Rocchi, R.; Cossignani, L.; Mañes, J.; Compagnone, D.; Blasi, F. Phenol profiling and nutraceutical potential of Lycium spp. leaf extracts obtained with ultrasound and microwave assisted techniques. Antioxidants 2019, 8, 260. [Google Scholar] [CrossRef]
- Persichetti, L.E.; De Michele, A.; Codini, M.; Traina, G. Antioxidative capacity of Lactobacillus fermentum LF31 evaluated in vitro by oxygen radical absorbance capacity assay. Nutrition 2014, 30, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Montesano, D.; Rocchetti, G.; Cossignani, L.; Senizza, B.; Pollini, L.; Lucini, L.; Blasi, F. Untargeted metabolomics to evaluate the stability of extra-virgin olive oil with added Lycium barbarum carotenoids during storage. Foods 2019, 8, 179. [Google Scholar] [CrossRef]
- Pinna, N.; Ianni, F.; Selvaggini, R.; Urbani, S.; Codini, M.; Grispoldi, L.; Cenci-Goga, B.T.; Cossignani, L.; Blasi, F. Valorization of pumpkin byproducts: antioxidant activity and carotenoid characterization of extracts from peel and filaments. Foods 2023, 12, 4035. [Google Scholar] [CrossRef]
- Kurz, C.; Carle, R.; Schieber, A. HPLC-DAD-MSn characterisation of carotenoids from apricots and pumpkins for the evaluation of fruit product authenticity. Food Chem 2008, 110, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Petry, F.C.; Mercadante, A.Z. Composition by LC-MS/MS of new carotenoid esters in mango and citrus. J Agric Food Chem 2016, 64, 8207–8224. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; Xia, J. MetaboAnalyst 6.0: towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res 2024, 52, W398–W406. [CrossRef]
- Hosen, M.; Rafii, M.Y.; Mazlan, N.; Jusoh, M.; Oladosu, Y.; Chowdhury, M.F.N.; Muhammad, I.; Khan, M.M.H. Pumpkin (Cucurbita spp.): a crop to mitigate food and nutritional challenges. Hortic 2021, 7, 352. [CrossRef]
- Zhou, C.L.; Mi, L.; Hu, X.Y.; Zhu, B.H. Evaluation of three pumpkin species: correlation with physicochemical, antioxidant properties and classification using SPME-GC–MS and E-nose methods. J Food Sci Technol 2017, 54, 3118–3131. [Google Scholar] [CrossRef]
- Paciulli, M.; Rinaldi, M.; Rodolfi, M.; Ganino, T.; Morbarigazzi, M.; Chiavaro, E. Effects of high hydrostatic pressure on physico-chemical and structural properties of two pumpkin species. J Food Chem 2019, 274, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Norfezah, M.N.; Hardacre, A.; Brennan, C.S. Comparison of waste pumpkin material and its potential use in extruded snack foods. Food Sci Technol Int 2011, 17, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Itle, R.A.; Kabelk, E.A. Correlation between L*a*b* color space values and carotenoid content in pumpkins and squash (Cucurbita spp.). Hortsci 2009, 44, 633-637.
- Karanja, J.K.; Mugendi, B.J. , Khamis, F.M.; Muchugi, A.N. Nutritional Evaluation of Some Kenyan Pumpkins (Cucurbita spp.), Int J Agric For 2014, 4, 195-200. [CrossRef]
- Rodriguez-Amaya, D.B. A guide to carotenoid analysis in foods; ILSI Press: Washington, DC, USA, 2001; ISBN 978-1-57881-072-7. [Google Scholar]
- Colon, N.C.; Chung, D.H. Neuroblastoma. Adv Pediatr 2011, 58, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Darendelioğlu, E. Studies of anticancer activity of beta-carotene, alpha-tocopherol and ascorbic acid in SH-SY5Y neuroblastoma cells. J Inst Sci Technol 2019, 9, 1657–1665. [Google Scholar] [CrossRef]
- de Carvalho, L.M.J.; Gomes, P.B.; de Oliveira Godoy, R.L.; Pacheco, S.; do Monte, P.H.F.; de Carvalho, J.L.V. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): A preliminary study. Food Res Int 2012, 47, 337–340. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Sehar, S.; Sarwar, A.; Ashraf, A.H.; Jamil, M.A.; Noreen, S.; Rafique, A.; Iftikhar, K.; Quddoos, M.Y.; Aslam, J.; Majeed Hussain, M.A. A Comprehensive review of functional ingredients, especially bioactive compounds present in pumpkin peel, flesh and seeds, and their health benefits. Food Chem Adv 2022, 1, 100067. [Google Scholar] [CrossRef]
- Biesiada, A.; Nawirska, A.; Kucharska, A.; Sokół-Łętowska, A. Chemical composition of pumpkin fruit depending on cultivar and storage. Ecol Chem Eng A 2011, 18, 9–18. [Google Scholar]
- Azizah, A.H.; Wee, K.C.; Azizah, O.; Azizah, M. Effect of boiling and stir frying on total phenolics, carotenoids and radical scavenging activity of pumpkin (Cucurbita moschata). Int Food Res 2009, 16, 45–51. [Google Scholar]
- Grassino, A.N.; Brnčić, S.R.; Sabolović, M.B.; Žlabur, J.Š.; Marović, R.; Brnčić, M. Carotenoid content and profiles of pumpkin products and by-products. Molecules 2023, 28, 858. [Google Scholar] [CrossRef]
- Kreck, M.; Kurbel, P.; Ludwig, M.; Paschold, P.J.; Dietrich, H. Identification and quantification of carotenoids in pumpkin cultivars (Cucurbita maxima L.) and their juices by liquid chromatography with ultraviolet-diode array detection. J Appl Bot Food Qual 2006, 80, 93-99.
- Murkovic, M.; Mülleder, U.; Neunteufl, H. Carotenoid content in different varieties of pumpkins. J Food Comp Anal 2002, 15, 633–638. [Google Scholar] [CrossRef]
- Dhenge, R.; Rinaldi, M.; Ganino, T.; Santi, S.; Ferrarese, I.; Dall’Acqua, S. Variations of polyphenols, sugars, carotenoids, and volatile constituents in pumpkin (Cucurbita moschata) during high pressure processing: A kinetic study. Inn Food Sci Emerg Technol 2022, 78, 103005. [Google Scholar] [CrossRef]
- Khachik, F.; Beecher, G.R. Separation and identification of carotenoids and carotenol fatty acid esters in some squash products by liquid chromatography. 1. Quantification of carotenoids and related esters by HPLC. J Agric Food Chem 1988, 36, 929–937. [Google Scholar] [CrossRef]
- Ouyang, M.; Huang, Y.; Wang, Y.; Luo, F.; Liao, L. Stability of carotenoids and carotenoid esters in pumpkin (Cucurbita maxima) slices during hot air drying. Food Chem 2022, 367, 130710. [Google Scholar] [CrossRef] [PubMed]
- Mariutti, L.R.B.; Mercadante, A.Z. Carotenoid esters analysis and occurrence: What do we know so far? Arch Biochem Biophys 2018, 648, 36–43. [Google Scholar] [CrossRef]
- Lewis, E.; Nogueira, R.M.; Enfissi, E.M.A.; Fraser, P.D. The esterification of xanthophylls in Solanum lycopersicum (tomato) chromoplasts; the role of a non-specific acyltransferase. Phytochem 2021, 191, 112912. [Google Scholar] [CrossRef]
- Mitra, S.; Rauf, A.; Tareq, A.M.; Jahan, S.; Bin Emran, T.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rebezov, M. ; Uddin, Md S.; Jeandet, P., Shah, Z.A., Shariati, M.A., Eds.; Rengasamy, K.R. Potential health benefits of carotenoid lutein: An updated review. Food Chem Toxicol 2021, 154, 112328. [Google Scholar] [CrossRef]
- Ebadi, M.; Mohammadi, M.; Pezeshki, A.; Jafari, S. M. Health benefits of beta-carotene. In: Jafari, S.M., Rashidinejad, A., Simal-Gandara, J. (eds) Handbook of Food Bioactive Ingredients. Springer, Cham. [CrossRef]
| Fruit appearance |  |
 |
 |
 |
| C. moschata species | Butternut | Lunga di Napoli | Moscata di Provenza | Violina rugosa |
| Skin colour | Orange | Green | Orange with green spot | Orange |
| Fruit shape | Pear-like shape | Pear-like shape | Round-shape | Pear-like shape |
| Fruit weight, kg | 3,318 | 2,537 | 5,433 | 3,120 |
| Flesh colour | Orange | Orange | Orange | Orange |
| Usage | For food use | For food use | For food use | For food use |
| Rind | Smooth and regular | Smooth and regular | Smooth and regular | Smooth and regular |
| Harvest time | October | October | October | October |
| Fruit appearance |  |
 |
||
| C. maxima species | Delica | Delica vanity | Hokkaido | Mantovana |
| Skin colour | Green | Orange | Orange/Red | Brown with green spot |
| Fruit shape | Round-shape | Round-shape | Round-shape | Round-shape |
| Fruit weight, kg | 1,350 | 1,011 | 0,539 | 4,490 |
| Flesh colour | Orange | Orange | Orange | Orange |
| Usage | For food use | For food use | For food use | For food use |
| Rind | Rough and irregular | Rough and irregular | Smooth and regular | Rough and irregular |
| Harvest time | October | October | October | October |
| Cultivar | L* | a* | b* | C* | H* |
| C. moschata species | |||||
| Butternut | 51.10 ± 0.89a,d | 7.28 ± 0.61a,d | 44.73 ± 0.58a,e | 45.32 ± 0.71a,e | 80.76 ± 0.78a |
| Lunga di Napoli | 55.58 ± 0.93a,e | 3.97 ± 0.12b | 40.15 ± 1.09a | 40.35 ± 0.87b | 84.35 ± 0.20a,c,d |
| Moscata di Provenza | 55.17 ± 1.20a,e | 4.87 ± 0.93a,b | 40.98 ± 0.29a | 41.27 ± 0.75a,b | 83.22 ± 1.19a,c,d |
| Violina rugosa | 34.52 ± 0.85b | 14.98 ± 0.31c | 33.98 ± 0.41b | 37.13 ± 0.33b | 66.21 ± 1.15b |
| C. maxima species | |||||
| Delica | 52.71 ± 1.08a,e | 4.87 ± 0.21a,b | 49.28 ± 0.89c,e | 49.52 ± 0.97c,e | 84.36 ± 1.51c,d |
| Delica vanity | 26.98 ± 1.08c | 10.47 ± 0.42d | 25.19 ± 1.13d | 27.28 ± 1.22d | 67.43 ± 1.94b |
| Hokkaido | 45.98 ± 1.95d | 5.18 ± 0.18a,b | 41.58 ± 2.43a | 41.90 ± 1.97a,b | 82.90 ± 1.08c |
| Mantovana | 56.87 ± 2.47e | 2.58 ± 0.05b | 48.62 ± 1.77e | 48.69 ± 1.15e | 86.96 ± 2.03d |
| Data are reported as mean value ± SD of three independent measurements (n = 3) and are expressed on dry weight. L*, color lightness; a*, color in the range from green (negative) to red (positive); b*, color from blue (negative) to yellow (positive); C*, Chroma; H*, Hue angle. Different letters in each column indicate significant differences with p < 0.01 | |||||
| Cultivar | Yield (%) | TCC (μg β-CE/g) | ABTS (μg TE/g) | ORAC (μg TE/g) |
| C. moschata species | ||||
| Butternut | 1.63 ± 0.20a,b | 161.08 ± 7.80a | 280.91 ± 27.45a | 1352.34 ± 10.34a |
| Lunga di Napoli | 1.29 ± 0.06a,d | 303.27 ± 2.08b | 343.13 ± 18.82a,b | 1802.54 ± 76.54a,b |
| Moscata di Provenza | 2.00 ± 0.25b,e | 365.73 ± 9.49c | 417.62 ± 53.94b,c,e | 1500.30 ± 53.04a,d |
| Violina rugosa | 1.42 ± 0.15a,d | 443.89 ± 7.58d | 525.39 ± 32.24c,f | 2560.11 ± 324.24b |
| C. maxima species | ||||
| Delica | 4.15 ± 0.00c | 379.36 ± 44.08c | 1192.11 ± 48.44d | 3996.18 ± 72.58c |
| Delica vanity | 1.03 ± 0.01d | 241.32 ± 21.55e | 313.23 ± 16.22a,e | 1267.86 ± 166.22a |
| Hokkaido | 1.58 ± 0.06a,b | 310.77 ± 23.82b | 548.41 ± 68.45f | 2341.24 ± 68.45b,d |
| Mantovana | 2.20 ± 0.28e | 247.05 ± 13.62e | 500.90 ± 11.69c,f | 2615.96 ± 52.10b |
| Data are reported as mean value ± SD of three independent measurements (n = 3) and are expressed on dry weight. TCC, total carotenoid content; ABTS, 2,20-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt; ORAC, oxygen radical absorbance capacity; TE, Trolox equivalents; β-CE, β-carotene equivalents. Different letters in each column indicate significant differences with P < 0.01. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





