1. Introduction
Cytokines are small proteins secreted by cells of both the innate and adaptive immune systems that regulate a diverse array of biological processes within and outside the immunological system. IL-10 is an immune-related cytokine capable of inhibiting co-stimulatory signals via CD28 in naïve T cells, reducing differentiation of Th1 cells and the development of dendritic cells, decreasing survival of Th2 cells, suppressing the activity of Th17 cells, and preserving Foxp3 expression in Tregs [
1,
2,
3,
4,
5,
6,
7]. In addition, IL-10 has also been described as having inhibitory and stimulatory effects on human CD8+ T cells. While inhibition of CD8+ T cells is an indirect effect resulting from down-modulation of costimulatory molecules and MHC-I molecules by antigen presenting cells, stimulation is associated with expression of the IL-2 receptor α chain [
8,
9,
10]. TGF-β is a known regulator of a variety of processes, including cell activation, proliferation, and differentiation. Thus, TGF-β has been shown to inhibit T cell differentiation, promote formation and expansion of Treg cells, impair maturation of dendritic cells, inhibit Th17 cell differentiation, promote
trans-differentiation between Th17 and Tregs, and vice-versa, and inhibit cytolytic activity of cytotoxic T lymphocytes [
11,
12,
13,
14,
15,
16,
17,
18]. In contrast to IL-10 and TGF-β, the cytokines IFN-g and IL-17A are immune-related cytokines capable to cause cell activation and inflammation. IFN-g is primarily produced by NK, NKT, Th1, and cytotoxic CD8+ T cells [
19]. In addition to its well-known role in augmenting MHC expression on a variety of immune and non-immune cells, IFN-g has been long known as a cytokine with a marked direct effect on the activation of human CD8+ T cells [
20,
21]. Although IL-17A shares some biological effects with IFN-g, the scientific data of its effects on T cells are scarce or lacking. Thus, in addition to its involvement in the activation of a variety of non-immune cells, IL-17 alone or in combination with IFN-g recruits and activates innate cells at sites of inflammation, therefore contributing to augment the inflammatory process [
22,
23].
Despite this body of knowledge, the effect that these cytokines have on the antigen-independent activation of CD3+ T cells, namely by cytokines such as IL-15, is presently unknown. We have previously shown that IL-15 functions as a homeostatic cytokine that regulates the activation and expansion of naïve CD8+ T cells in vitro [
24,
25,
26]. We, and others, have also shown that T cell activation leads to changes in the physiological equilibrium between closed, i.e., β2m-associated, W6/32-reactive, and open, i.e., β2m-free, HC-10-reactive, HLA class I [HLA-I] molecules/conformers [
27,
28]. The existence of open HLA-I conformers is physiologically and functionally relevant in normal and clinical settings, such as during normal T cell activation [
27], transplantation [
29], autoimmunity [
30] and malignancy [
31,
32]. This is due to the special molecular features of these molecules, which allow them to interact in
cis and
trans with NK receptors and growth factor receptors [
33]. However, whether IL-15 itself or in combination with any of the aforementioned immune-related cytokines has an effect on the expression levels of open HLA-I conformers on the cell surface of activated T cells, is not known. Since IL-15 and the aforementioned cytokines are likely to share the same microenvironments at different phases of an inflammatory process, we were interested in examining the effects of IL-10, TGF-β, IFN-g and IL-17A on in vitro IL-15-activated human T cells, namely on their proliferative capacity and on the physiological equilibrium between closed and open HLA-I conformers.
2. Results
To elucidate the impact of immune-related cytokines on the IL-15-activation and proliferation of ex vivo human T cells, fresh peripheral blood lymphocytes (PBL) preparations were cultured in vitro for 12 days in the presence of IL-15 alone (10ng/mL) or in combination with IL-10, TGF-β, IL-17A, and IFN-g at two different concentrations (1ng/mL and 10ng/mL). While IL-15 was added at the start of the culture and six days later, the other cytokines were all only added at the start of the culture.
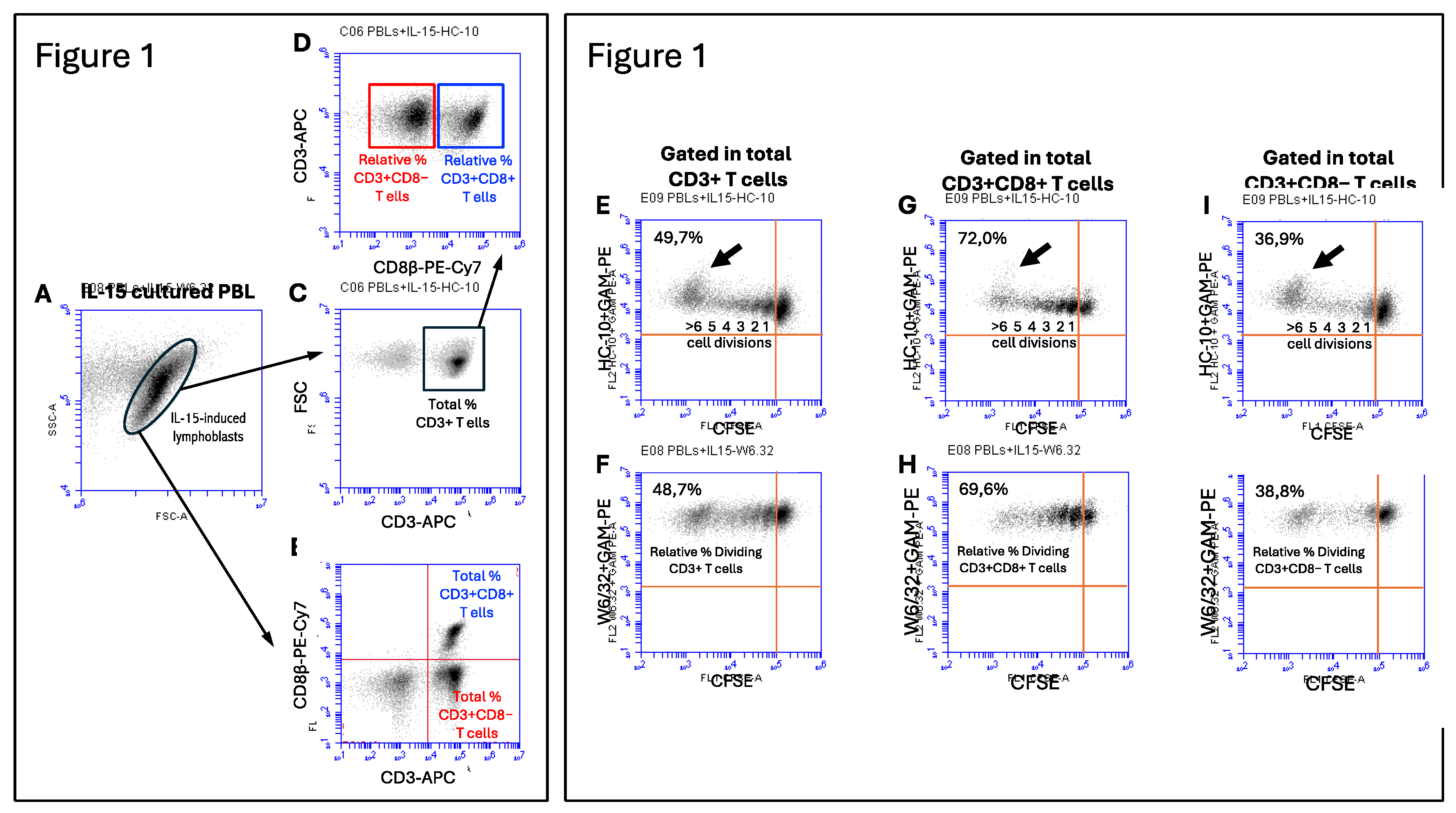
Figure 1 (left panel) shows the gating strategy to determine the total and relative percentages of CD3+, CD3+CD8+ and CD3+CD8− T cells in cultures PBL in the presence of IL-15 for 12 days. IL-15-activation of PBL induced the formation of a population of lymphoblasts (
Figure 1A), which contained a mixture of CD3+CD8+ T cells, CD3+CD8−T cells and lymphocytes negative for both T cell markers, most likely NK cells (
Figure 1B), which allowed us to calculate the percentage of the two T cell populations. Cells within the IL-15-induced lymphoblasts were predominantly CD3+ T cells (
Figure 1C), and after gating in the total CD3+ T cells (black square), we could determine the relative percentage of CD3+CD8+ T cells (blue square) and CD3+CD8−T cells (red square) at the end of the cultures (see
Figure 1D). As previously shown by us [
25,
26], the percentage of CD3+CD8+ T cells that divided in response to IL-15 outnumbered the percentage of CD3+CD8− T cells (most likely CD3+CD4+ T cells) in a proportion of approximately 2:1 (75.7% vs. 40.8%, p=0.0001; n=4) (see also
Figure 1, right panel). The preferential expansion of CD3+CD8+ T cells was seen with all combinations of cytokines (Suppl.
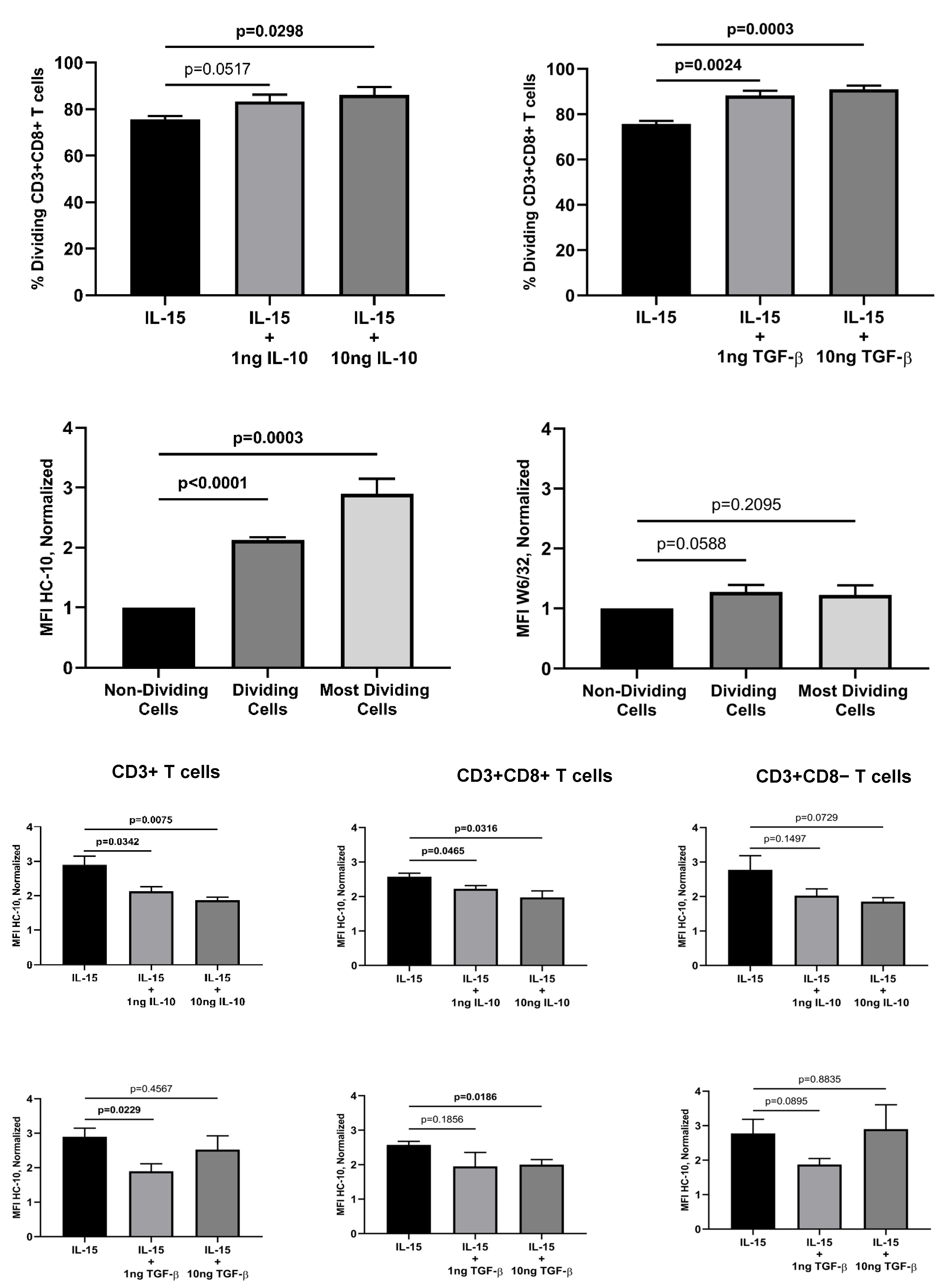
Figure 1). The same type of analysis was performed in cultures with combinations of IL-15 and the other cytokines. Interestingly, addition of IL-10 and TGF-β, both used at 10ng/mL to the IL-15-stimulated PBL cultures significantly increased the relative percentage of CD3+CD8+ T cells, but not of CD3+CD8− T cells, at the end of the culture (
Figure 2A). In marked contrast, IL-17A and IFN-g had no effect on the IL-15-induced CD3+CD8+ T cell proliferation (data not shown).
Next, we were interested in examining the expression of HLA class I (HLA-I) molecules at the cell surface of the activated and dividing CD3+ T cells, and the subsets within. Thus, previous studies have shown that mature b2m-free HLA-I molecules, also known as open HLA-I conformers, are expressed at the cell surface by antigen-activated normal T cells and transformed T and B cell lines, and are involved in regulating key plasma membrane-associated events, including intracellular parthways mediated by signaling receptors and receptor endocytosis [
27,
34,
35,
36,
37]. Thus, we wanted to ascertain whether IL-15, or combinations of IL-15 with the other immune-related cytokines, influenced expression of HLA-I molecules, either as β2m-associated/W6/32-reactive (i.e., closed HLA-I conformers) or as β2m-free/HC-10-reactive (i.e., open HLA-I conformers).
Figure 1 (right panels) shows the gating strategy to determine the mean fluorescence intensity (MFI) expression values of HC-10-reactive and W6/32-reactive HLA-I forms in CD3+ T cells (Figures 1E and 1F), CD3+CD8+ T cells (Figures 1G and 1H), and CD3+CD8− T cells (Figures 1I and 1J) in the dividing (CFSE halving) cells. Analysis of the expression of closed and open HLA-I conformers at the cell surface of the dividing CD3+ T cells revealed interesting results. While expression of closed HLA-I conformers remained steady during the cell divisions (
Figure 1F), expression of open conformers consistently increased with each cycle of cell division, being maximal in CD3+ T cells that underwent ≥6 cell division cycles (
Figure 1E, see black arrow). These results were observed both in CD3+CD8+ and CD3+CD8− T cells (Figures 1G, 1H, 1I and 1J). Given the differences in MFI values obtained between experiments, we normalized these values to the MFI values obtained in non-dividing cells (i.e., arbitrary units of fluorescence, a.u.f.). While the W6/32 MFI values in non-dividing cells ranged between 279.225 and 582.286 a.u.f., the HC-10 MFI values in non-dividing cells ranged between 2.434 and 12.406 a.u.f.. On average, the level of expression of closed HLA-I conformers was about 35-fold higher that the levels of expression of open HLA-I conformers, with the background fluorescence levels being below 1.000 a.u.f (data not shown).
As shown in
Figure 2B, the normalized HC-10 (open HLA-I conformers) MFI values in IL-15-activated CD3+ T cells were statistically significantly higher (approx. 2-fold increase; p<0001, n=4) when the total fraction of dividing CD3+ T cells was compared to the non-dividing cells. This increase was even more striking (approx. 3-fold increase; p=0.003, n=4) when only the most dividing CD3+ T cells (indicated by the black arrow in
Figure 1E) were considered. The increase in HC-10-reactive HLA-I molecules was observed both in CD3+CD8+ T cells (
Figure 1G, p<0.0001)) and CD3+CD8− T cells (
Figure 1I, p<0.005). In marked contrast, while no significant changes were observed in the expression of closed HLA-I conformers in CD3+ T cells (p=0.209) and CD3+ CD8+ T cells (p=0.541), a small, but signifiant increase, was observed in CD3+CD8− T cells (p=0.037). Noteworthy, when the same analysis was performed for the combination of IL-15 with the other cytokines, IL-10 and TGF-β significantly reverted the increase in open HLA-I conformers induced by IL-15 in the most dividing cells (i.e., ≥6 cycles of cell division). As shown in
Figure 2C, this reversion was seen in CD3+ T cells (IL-10 at 1ng and 10ng; TGF-β at 1ng) and CD3+CD8+ T cells (IL-10 at 1ng and 10ng; TGF-β at 10ng) but not in CD3+CD8− T cells. In contrast, IL-17A and IFN-g did not have any significant effect on the expression of HC-10-reactive HLA-I molecules (data not shown). Regarding the expression of closed HLA-I conformers, any of the cytokines studied altered the levels of expression, except for of 10ng of TGF-β that significantly increased W6/32-reactive HLA-I forms in CD3+CD8− T cells (p=0.013).
3. Discussion
This study aimed to elucidate the impact of several immune-related cytokines, namely IL-10, TGF-β, IL-17A and IFN-g, on the IL-15-induced activation of human CD3+ T cells. Some of these cytokines play important roles in the biology of T cells both in vitro and
in vivo. Moreover, some of them are presently being used as part of immunotherapies aimed at expanding/inhibiting cytotoxic/auto-reactive T cells to treat cancer, autoimmune disorders, and downplaying inflammation in chronic disorders [
38,
39,
40]. Thus, ascertaining whether these cytokines have an enhancing or inhibitory effect on the IL-15-mediated activation of CD3+ T cells, and subsets within, may provide important insights in our understanding of the biology of these cytokines. IL-15 is known to be involved in the activation and proliferation of human CD8+ T cells, with some of them differentiating into effector-memory CD8+ T cells expressing variable levels of CD45RA (i.e., CD8+ T
emra) akin to a variety of NK receptors, and expressing CD8⍺⍺ homodimers [
25,
26,
41]. However, this is the first time that the IL-15-mediated activation of CD3+ T cells also induces the expression of open HLA-I conformers at the plasma membrane, adding to the body of knowledge indicating that these HLA-I conformers are expressed as a result of cell activation, proliferation and differentiation regardless of the nature of the stimuli [
28,
33]. Of note, the potentiating effect that the presence of IL-10 and TGF-β had on the proliferation of the IL-15-activated CD3+CD8+ T cells was associated with a significant reduction on the expression of the HLA-I conformers not associated with β2m. These results are concordant with the lack of effect observed with IL-17A and IFN-g both in potentiating IL-15-mediated T cell proliferation and in increasing HC-10-reactive open HLA-I conformers.
Since IL-10 and TGF-β are cytokines associated with immunosuppressive processes, their effect in the proliferation of CD3+CD8+ T cells and in the increase of the percentage of CD3+CD8+ T cells at the end of the culture appear conflicting. TGF-β is best known for influencing T cell differentiation in combination with other cytokines [
42,
43], but not necessarily their proliferation. IL-10, on the other hand, is known for inhibiting or stimulating T cell proliferation depending on the cell status at the time of exposure and whether an APC is present or not [
8,
9]. Perhaps, IL-15 may also induce an increase in the expression of IL-10 and TGF-β receptors at the plasma membrane of CD3+CD8+ T cells, allowing for a more pronounced effect of these cytokines. Alternatively, these cytokines may enhance the generation of CD8+ T
emra cells and CD3+CD8⍺⍺ T cells, which contain subsets of suppressor/regulatory T cells [
41,
44,
45]. In either case, IL-10 and TGF-β emerge as modulators of the physiological equilibrium between closed (i.e., β2m-associated, W6/32-reactive) and open (i.e., β2m-free, HC-10-reactive) HLA class I conformers at the cell surface of activated CD3+ T cells. Further studies on the effect of IL-10 and TGF-β on the IL-15-induced activation and proliferation of human CD3+ T are warranted to disclose the exact mechanisms behind this interplay.
4. Materials and Methods
Ethics Statement
Human peripheral blood mononuclear cells (PBMC) were obtained from buffy coats of anonymous healthy regular blood donors kindly provided by the Centro do Sangue e da Transplantação de Coimbra (CST-C, Portugal) under a protocol approved by the Portuguese Institute of Blood and Transplantation (IPST, IP, Lisbon), the University of Beira Interior (UBI), and the Faculty of Health Sciences (FCS-UBI). The study protocol was approved by the Ethics Committee of the University in accordance with the Declaration of Helsinki (Ref. Number CE-UBI-Pj-2017-012).
Cells Isolation, CFSE Labeling and Culture Conditions
PBMC were isolated as previously described by us, from buffy coats after centrifugation over Lymphoprep (STEMCELL Technologies, France). Contaminating red blood cells were lysed in lysis solution (10 mM Tris, 155 mM NH4Cl, pH 7.4) for 10 minutes at 37°C. Enriched peripheral blood lymphocytes (PBL) were obtained after incubation of PBMC in Petri dishes for 1 hour at 37°C and 5% CO2. Freshly isolated PBL were immediately labelled with CellTraceTM CFSE Cell Proliferation kit (Thermo-Fisher Scientific, USA) at a final concentration of 5μM for 5 minutes at room temperature (RT) in phosphate-buffered saline (PBS) with occasional mixing, followed by three washes with RPMI-1640 medium (Thermo-Fisher Scientific) containing 10% heat-inactivated fetal bovine serum (FBS). Then, CFSE-labeled PBL (1.0x106/mL) were cultured in 24-well plates (Greiner Bio-One, Austria) in RPMI-1640 GlutaMAX medium (Thermo-Fisher Scientific) supplemented with 5% human serum (Sigma-Aldrich, USA) and 1% antibiotic-antimycotic solution (Sigma-Aldrich) at 37°C, 5% CO2, and 95% humidity for 12 days. PBL were cultured in the presence of IL-15 alone (10ng/mL) added at day 0 and 6, or in combination with 1ng and 10ng of IL-10, TGF-β, IFN-g and L-17A (R&D Systems, USA), added at day 0.
Flow Cytometry Studies
For cell surface staining, approximately 0.5x106 cells were incubated in 96-well round-bottom plates at 4°C in the dark for 30 minutes with combinations of different unconjugated and fluorochrome-conjugated antibodies diluted in staining solution (PBS, 0.2% BSA, 0.1% NaN3). Briefly, cells were first separately incubated with the unconjugated W6/32 antibodies (Thermo-Fisher Scientific), which recognize a monomorphic epitope on all classical HLA class I heavy chains, dependent on the presence of β2m (28, and references herein), or HC-10 antibodies (Thermo-Fisher Scientific), which recognize HLA class I heavy chains not associated with β2m and peptide and having the peptide sequence PxxWDR in the α1 domain (28, and references herein), followed by incubation with PE-conjugated goat anti-mouse antibodies (GAM-PE, BioLegend, USA). After washing, W6/32 and HC-10 labeled cells were incubated with APC-conjugated mouse anti-CD3 (CD3-APC, Thermo-Fisher Scientific) and PE-Cy7-conjugated mouse anti-CD8β (CD8β-PE-Cy7; BioLegend) antibodies. Irrelevant IgG2a-PE-Cy7 mouse antibodies were used as control for background fluorescence (Thermo-Fisher Scientific). After washing, a minimum of 20,000 events were acquired in a BD Accuri C6 (BD Biosciences, USA). Labeled cells were analyzed using BD Accuri C6 software (BD Biosciences).
Quantification of Cell Proliferation
T cell divisions were determined by sequential halving of CFSE fluorescence intensity after the period of culture. In all the experiments performed, CFSE halving allowed to distinguish the different cycles of cell division. To quantitate changes in the percentages of CD3+, CD3+CD8+ and CD3+CD8− T cells and in the expression of W6/32+ and HC-10+ HLA-I conformers, electronic regions were created around CD3+, CD3+CD8+ and CD3+CD8− T cells and the mean fluorescence intensity (MFI) values of the two HLA-I forms determined. MFI values of cells that did not divide were used to normalize the MFI values of the dividing cells as follows: (MFI dividing cells / MFI non-dividing cells) x 100.
Statistical Analysis
For flow cytometry data, statistical analysis was performed using the software Graph Pad Prism 8 [GraphPad software Inc., USA]. Continuous variables were expressed as the Mean ± Standard Error of the Mean (SEM). Differences between means of two continuous variables were analyzed using the Student’s T-test. All data were checked for normality. Statistical significance was defined as p<0.05. Normalization of HC-10 and W6/32 MFI values were performed comparing the values obtained in total dividing and most dividing T cells with non-dividing T cells.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, FAA; methodology, LD, HP, AE, EC, FA; formal analysis, LH, HP, AE, EC, FA; investigation, LD, HP, AE, EC; writing—original draft preparation, FA; writing—review and editing, LD, HP, AE, EC; supervision, FA; project administration, FA; funding acquisition, LD, HP, FA. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Beira Interior [Ref. Number CEUBI-Pj-2017-012].
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors like to thank the personnel of the Blood and Transfusion Center of Coimbra (CST-C, Portugal) for kindly providing buffy coats from regular healthy blood donors used in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Akdis CA, Joss A, Akdis M, Faith A, Blaser K. A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding. FASEB J. 2000;14(12):1666-8. [CrossRef]
- de Waal Malefyt R, Haanen J, Spits H et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174(4):915-24. [CrossRef]
- Tian Y, Mollo SB, Harrington LE, Zajac AJ. IL-10 Regulates Memory T Cell Development and the Balance between Th1 and Follicular Th Cell Responses during an Acute Viral Infection. J Immunol. 2016;197(4):1308-21. [CrossRef]
- Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28(1):359-69. [CrossRef]
- Coomes SM, Kannan Y, Pelly VS, Entwistle LJ, Guidi R, Perez-Lloret J, Nikolov N, Müller W, Wilson MS. CD4+ Th2 cells are directly regulated by IL-10 during allergic airway inflammation. Mucosal Immunol. 2017;10:150-161. [CrossRef]
- Huber S, Gagliani N, Esplugues E, O’Connor W Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, Roncarolo MG, Battaglia M, Flavell RA. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34(4):554-65. [CrossRef]
- Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10(11):1178-84. [CrossRef]
- Cohen SBA, Katsikis PD, Feldmann M, Londei M. IL-10 enhances expression of the IL-2 receptor α chain on T cells. Immunology 1994;83:329–32. PMID: 7835955.
- Groux H, Bigler M, de Vries JE, Roncarolo M-G. Inhibitory and Stimulatory Effects of IL-10 on Human CD8 + T Cells. J Immunol. 1998;160:3188–93. PMID: 9531274.
- Rowbottom AW, Lepper MA, Garland RJ, Cox CV, Corley EG. Interleukin-10-induced CD8 cell proliferation. Immunology. 1999;98(1):80-9. [CrossRef]
- Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505.
- Chen CH, Seguin-Devaux C, Burke NA et al. Transforming growth factor beta blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J Exp Med. 2003;197(12):1689-99. [CrossRef]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875-86. [CrossRef]
- Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, Amigorena S, Hermine O, Durandy A. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567–4575. PMID: 10201996.
- Denniston AK, Kottoor SH, Khan I, Oswal K, Williams GP, Abbott J, Wallace GR, Salmon M, Rauz S, Murray PI, Curnow SJ. Endogenous cortisol and TGF-beta in human aqueous humor contribute to ocular immune privilege by regulating dendritic cell function. J Immunol. 2011;186(1):305-11. [CrossRef]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits Th17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236-40. [CrossRef]
- Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS, Ching T, Weaver C, Zi X, Pan X, Fan R, Garmire LX, Cotton MJ, Drier Y, Bernstein B, Geginat J, Stockinger B, Esplugues E, Huber S, Flavell RA. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523(7559):221-5. [CrossRef]
- Thomas DA, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8(5):369-80. [CrossRef]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-g: An overview of signals, mechanisms and functions. J. Leukoc Biol. 2004;75:163–189. [CrossRef]
- Schoenborn JR, Wilson CB. Regulation of Interferon-g During Innate and Adaptive Immune Responses. Adv Immunol. 2007;96:41–101. [CrossRef]
- Siegel JP. Effects of interferon-gamma on the activation of human T lymphocytes. Cell Immunol. 1988;111(2):461-72. [CrossRef]
- Berry SPD, Dossou C, Kashif A, Sharifinejad N, Azizi G, Hamedifar H, Sabzvari A, Zian Z. The role of IL-17 and anti-IL-17 agents in the immunopathogenesis and management of autoimmune and inflammatory diseases. Int Immunopharmacol. 2022;102:108402. [CrossRef]
- McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019;50:892–906. [CrossRef]
- Alves NL, Hooibrink B, Arosa FA, van Lier RA. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood. 2003;102(7):2541-6. [CrossRef]
- Correia MP, Cardoso EM, Pereira CF, Neves R, Uhrberg M, Arosa FA. Hepatocytes and IL-15: A favorable microenvironment for T cell survival and CD8+ T cell differentiation. J Immunol. 2009;182(10):6149-59. [CrossRef]
- Correia MP, Costa AV, Uhrberg M, Cardoso EM, Arosa FA. IL-15 induces CD8+ T cells to acquire functional NK receptors capable of modulating cytotoxicity and cytokine secretion. Immunobiology. 2011;216(5):604-12. [CrossRef]
- Santos SG, Powis SJ, Arosa FA. Misfolding of major histocompatibility complex class I molecules in activated T cells allows cis-interactions with receptors and signaling molecules and is associated with tyrosine phosphorylation. J Biol Chem. 2004;279:53062–70. [CrossRef]
- Arosa FA, Santos SG, Powis SJ. Open conformers: The hidden face of MHC-I molecules. Trends Immunol. 2007;28(3):115-23. [CrossRef]
- Ravindranath MH, Filippone EJ, Amato-Menker CJ, Arosa FA, Das B, Ou Y, Norin AJ. Antibodies to cryptic epitopes on HLA class I and class II heavy chains bound to single antigen beads: Clinically relevant? Transpl Immunol. 2021;69:101482. [CrossRef]
- Hudson LE, Allen R. Leukocyte Ig-Like Receptors—A Model for MHC Class I Disease Associations. Front. Immunol. 2016;7:281. [CrossRef]
- Smith C, Santi M, Rajan B, Rushing EJ, Choi MR, Rood BR, Cornelison R, MacDonald TJ, Vukmanovic S. A novel role of HLA class I in the pathology of medulloblastoma. J Transl Med. 2009;7:59. [CrossRef]
- Cardoso EM, Esgalhado AJ, Patrão L, Santos M, Neves VP, Martinez J, Patto MAV, Silva H, Arosa FA. Distinctive CD8+ T cell and MHC class I signatures in polycythemia vera patients. Ann Hematol. 2018;97(9):1563-1575. [CrossRef]
- Arosa FA, Esgalhado AJ, Reste-Ferreira D, Cardoso EM. Open MHC Class I Conformers: A Look through the Looking Glass. Int J Mol Sci. 2021;22(18):9738. [CrossRef]
- Ramalingam TS, Chakrabarti A, Edidin M. Interaction of class I human leukocyte antigen (HLA-I) molecules with insulin receptors and its effect on the insulin-signaling cascade. Mol Biol Cell. 1997;8(12):2463-74. [CrossRef]
- Santos SG, Antoniou AN, Sampaio P, Powis SJ, Arosa FA. Lack of tyrosine 320 impairs spontaneous endocytosis and enhances release of HLA-B27 molecules. J Immunol. 2006 Mar 1;176(5):2942-9. [CrossRef]
- Smith C, Santi M, Rajan B, Rushing EJ, Choi MR, Rood BR, Cornelison R, MacDonald TJ, Vukmanovic S. J Transl Med. 2009;7:59. [CrossRef]
- Lin K, Bieri G, Gontier G, Müller S, Smith LK, Snethlage CE, White CW 3rd, Maybury-Lewis SY, Villeda SA. MHC class I H2-Kb negatively regulates neural progenitor cell proliferation by inhibiting FGFR signaling. PLOS Biology. 2021;19(6): e3001311. [CrossRef]
- Alspach E, Lussier EDM, Schreiber RD. Interferon g and its important roles in promoting and inhibiting spontaneous and therapeutic cancer immunity. Cold Spring Harb Perspect Biol. 2019;11(3):1-20. [CrossRef]
- Czaja AJ. Immune Inhibitory Properties and Therapeutic Prospects of Transforming Growth Factor-Beta and Interleukin 10 in Autoimmune Hepatitis. Dig Dis Sci. 2022;67(4):1163-1186. [CrossRef]
- Zannikou M, Fish EN, Platanias LC. Signaling by Type I Interferons in Immune Cells: Disease Consequences. Cancers (Basel). 2024;16(8):1600. [CrossRef]
- Esgalhado AJ, Reste-Ferreira D, Weinhold S, Uhrberg M, Cardoso EM, Arosa FA. In vitro IL-15-activated human naïve CD8+ T cells down-modulate the CD8β chain and become CD8αα T cells. Front Immunol. 2024;15:1252439. [CrossRef]
- Lee GR. The Balance of Th17 versus Treg Cells in Autoimmunity. Int J Mol Sci. 2018;19(3):730. [CrossRef]
- Itatani Y, Kawada K, Sakai Y. Transforming Growth Factor-β Signaling Pathway in Colorectal Cancer and Its Tumor Microenvironment. Int J Mol Sci. 2019;20:1–16. [CrossRef]
- Arosa FA, Esgalhado AJ, Padrão CA, Cardoso EM. Divide, Conquer, and Sense: CD8+CD28- T Cells in Perspective. Front Immunol. 2017;7:665. [CrossRef]
- Sheng H, Marrero I, Maricic I, Fanchiang SS, Zhang S, Sant’Angelo DB, Kumar K. Distinct PLZF+CD8aa+ Unconventional T cells enriched in liver use a cytotoxic mechanism to limit autoimmunity. J Immunol. 2019;203:2150–62. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).