Submitted:
30 July 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Insect Culture
2.3. Host Plants
2.4. Analyses of Nutritional and Mineral Profiles of Host Plants
2.5. Experimentation
2.6. Feeding Indices
2.7. Fitness Indices
2.8. Statistical Data Analyses
3. Results
3.1. Proximate Composition of Different Host Plants Used to Feed S. frugiperda Larvae
3.2. Mineral Composition Analysis of Different Host Plants Used to Feed S. frugiperda Larvae
3.3. Feeding Indices Parameters for S. frugiperda Larvae Feeding on Different Host Plants
3.4. Growth Parameters for S. frugiperda Larvae Feeding on Different Host plants
3.4.1. Larval Length and Weight
3.4.2. Pupal Length and Weight
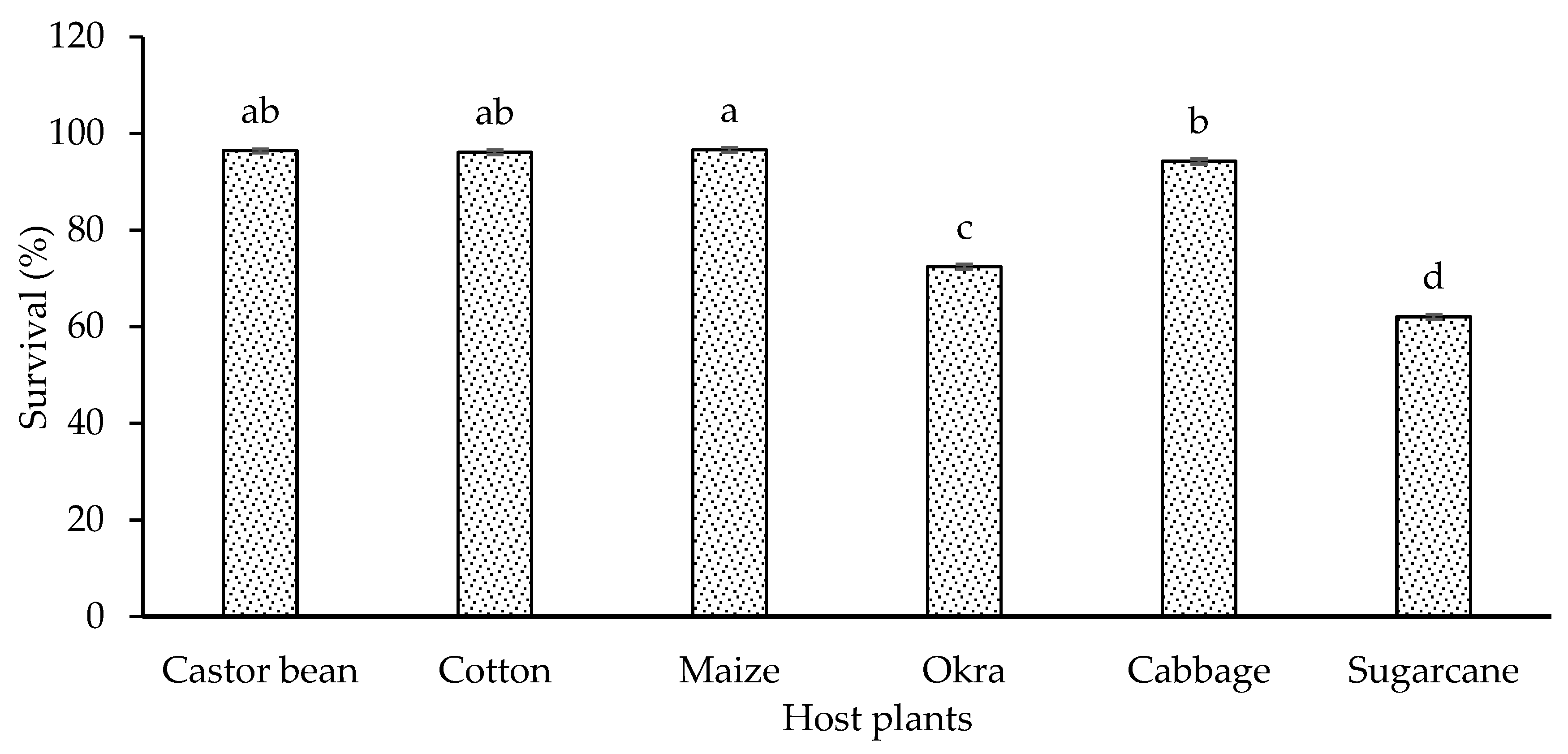
3.4.3. Total Larval Survival Rate
3.5. Growth Fitness Indices Parameters for S. frugiperda Larvae Feeding on Different Host Plants
3.6. Matrix of Pearson’s Correlation Coefficient among Proximate Analysis with Growth and Feeding Indices of S. frugiperda
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarmento, R.A.; Lemos, F.; Bleeker, P.M.; Schuurink, R.C.; Pallini, A.; Oliveira, M.G.A.; Lima, E.R.; Kant, M.; Sabelis, M.W.; Janssen, A. A herbivore that manipulates plant defence. Ecol. Lett. 2011, 14, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.J.; Rausher, M.D. Evolution of resistance to a multiple-herbivore community: genetic correlations, diffuse coevolution, and constraints on the plant's response to selection. Evolution 2013, 67, 1767–1779. [Google Scholar] [CrossRef] [PubMed]
- Kant, M.; Jonckheere, W.; Knegt, B.; Lemos, F.; Liu, J.; Schimmel, B.; Villarroel, C.; Ataide, L.; Dermauw, W.; Glas, J. Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Ann. Bot. 2015, 115, 1015–1051. [Google Scholar] [CrossRef] [PubMed]
- Kant, M.R.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Intraspecific variation in a generalist herbivore accounts for differential induction and impact of host plant defences. Proc. R. Soc. Ser. B Biol. Sci. 2008, 275, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Pentzold, S.; Zagrobelny, M.; Roelsgaard, P.S.; Møller, B.L.; Bak, S. The multiple strategies of an insect herbivore to overcome plant cyanogenic glucoside defence. PloS One 2014, 9, e91337. [Google Scholar] [CrossRef] [PubMed]
- Halon, E.; Eakteiman, G.; Moshitzky, P.; Elbaz, M.; Alon, M.; Pavlidi, N.; Vontas, J.; Morin, S. Only a minority of broad-range detoxification genes respond to a variety of phytotoxins in generalist Bemisia tabaci species. Sci. Rep. 2015, 5, 17975. [Google Scholar] [CrossRef] [PubMed]
- Katsanis, A.; Rasmann, S.; Mooney, K.A. Herbivore diet breadth and host plant defense mediate the tri-trophic effects of plant toxins on multiple coccinellid predators. PLoS One 2016, 11, e0155716. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J.; Ray, S.; Davidson-Lowe, E.; Ali, J.; Luthe, D.S.; Felton, G. Plant nutrition influences resistant maize defense responses to the fall armyworm (Spodoptera frugiperda). Front. Ecol. Evol. 2022, 10, 844274. [Google Scholar] [CrossRef]
- Pentzold, S.; Zagrobelny, M.; Rook, F.; Bak, S. How insects overcome two-component plant chemical defence: plant β-glucosidases as the main target for herbivore adaptation. Biol. Rev. 2014, 89, 531–551. [Google Scholar] [CrossRef]
- Beran, F.; Petschenka, G. Sequestration of plant defense compounds by insects: from mechanisms to insect–plant coevolution. Annu. Rev. Entomol. 2022, 67, 163–180. [Google Scholar] [CrossRef]
- Bala, K.; Sood, A.; Pathania, V.S.; Thakur, S. Effect of plant nutrition in insect pest management: A review. J. Pharmacognosy Phytochem. 2018, 7, 2737–2742. [Google Scholar]
- Castagneyrol, B.; Jactel, H.; Vacher, C.; Brockerhoff, E.G.; Koricheva, J. Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. J. Appl. Ecol. 2014, 51, 134–141. [Google Scholar] [CrossRef]
- Bernays, E.A.; Chapman, R.F. Host-plant selection by phytophagous insects; Springer Science & Business Media: 2007.
- Wang, D.; Zhou, L.; Wang, Q.; Ding, J. Plant chemistry determines host preference and performance of an invasive insect. Front. Plant Sci. 2020, 11, 594663. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Simpson, C. The mechanisms of nutritional compensation by phytophagous insects. Insect-Plant Interactions 2017, 111–160. [Google Scholar]
- Singh, V.; Sood, A. Plant Nutrition: A tool for the management of hemipteran insect-pests-A review. Agri. Rev. 2017, 38, 260–270. [Google Scholar] [CrossRef]
- Queiroz, R.B.; Lopes, M.C.; Costa, T.L.; da Silva, R.S.; Galdino, T.V.; Gontijo, P.d.C.; Martinez, H.E.P.; Picanço, M.C. Influence of tomato plants nutritional status on the fitness and damage of Tuta absoluta (Lepidoptera: Gelechiidae). Agric. For. Entomol. 2022, 24, 260–266. [Google Scholar] [CrossRef]
- Cease, A.J.; Harrison, J.F.; Hao, S.; Niren, D.C.; Zhang, G.; Kang, L.; Elser, J.J. Nutritional imbalance suppresses migratory phenotypes of the Mongolian locust (Oedaleus asiaticus). R. Soc. Open Sci. 2017, 4, 161039. [Google Scholar] [CrossRef]
- Sarwar, M.; Ahmad, N.; Toufiq, M. Identification of susceptible and tolerant gram (Cicer arietinum L.) genotypes against gram pod borer (Helicoverpa armigera)(Hubner). Pak. J. Bot.
- Kassi, A.K.; Javed, H.; Mukhtar, T. Relationship of physico-morphic characters of okra cultivars with their resistance to Helicoverpa armigera. Pak. J. Zool. 2019, 51, 835. [Google Scholar] [CrossRef]
- Snyder, L.D.; Gómez, M.I.; Power, A.G. Crop varietal mixtures as a strategy to support insect pest control, yield, economic, and nutritional services. Front. Sustainable Food Syst. 2020, 4, 60. [Google Scholar] [CrossRef]
- Jaworski, C.C.; Thomine, E.; Rusch, A.; Lavoir, A.V.; Wang, S.; Desneux, N. Crop diversification to promote arthropod pest management: A review. Agri. Comm. 2023, 1, 100004. [Google Scholar] [CrossRef]
- Ratnadass, A.; Fernandes, P.; Avelino, J.; Habib, R. Plant species diversity for sustainable management of crop pests and diseases in agroecosystems: a review. Agron. Sustainable Dev. 2012, 32, 273–303. [Google Scholar] [CrossRef]
- Khan, A.G.; Imran, M.; Khan, A.-u.-H.; Fares, A.; Šimůnek, J.; Ul-Haq, T.; Alsahli, A.A.; Alyemeni, M.N.; Ali, S. Performance of spring and summer-sown maize under different irrigation strategies in Pakistan. Sustainability 2021, 13, 2757. [Google Scholar] [CrossRef]
- Yousaf, S.; Rehman, A.; Masood, M.; Ali, K.; Suleman, N. Occurrence and molecular identification of an invasive rice strain of fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) from Sindh, Pakistan, using mitochondrial cytochrome c oxidase I gene sequences. J. Plant Dis. Prot. 2022, 129, 71–78. [Google Scholar] [CrossRef]
- Montezano, D.G.; Sosa-Gómez, D.; Specht, A.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.d.; Peterson, J.A.; Hunt, T. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Malo, M.; Hore, J. The emerging menace of fall armyworm (Spodoptera frugiperda JE Smith) in maize: A call for attention and action. J. Entomol. Zool. Stud. 2020, 8, 455–465. [Google Scholar]
- Yang, X.; Sun, X.; Zhao, S.; Li, J.; Chi, X.; Jiang, Y.; Wu, K. Population occurrence, spatial distribution and sampling technique of fall armyworm Spodoptera frugiperda in wheat fields. Plant Prot. 2020, 46, 23. [Google Scholar]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J. Fall armyworm: impacts and implications for Africa. Outlooks Pest Manage. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Gilal, A.A.; Bashir, L.; Faheem, M.; Rajput, A.; Soomro, J.A.; Kunbhar, S.; Mirwani, A.S.; Mastoi, G.S.; Sahito, J.G.M. First record of invasive fall armyworm (Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae)) in corn fields of Sindh, Pakistan. Pak. J. Agri. Res. 2020, 33(2), 47. [Google Scholar] [CrossRef]
- FAO. Integrated management of the fall armyworm on maize: a guide for farmer field schools in Africa. 2018.
- Gergs, A.; Baden, C.U. A dynamic energy budget approach for the prediction of development times and variability in Spodoptera frugiperda rearing. Insects 2021, 12, 300. [Google Scholar] [CrossRef]
- Waldbauer, G. The consumption and utilization of food by insects. In Adv. Insect Physiol., Elsevier, 1968, 5, 229–288. [Google Scholar]
- Ali, S.; Ullah, M.I.; Saeed, M.F.; Khalid, S.; Saqib, M.; Arshad, M.; Afzal, M.; Damalas, C.A. Heavy metal exposure through artificial diet reduces growth and survival of Spodoptera litura (Lepidoptera: Noctuidae). Environ. Sci. Poll. Res. 2019, 26, 14426–14434. [Google Scholar] [CrossRef]
- Cabezas, M.; Nava, D.; Geissler, L.; Melo, M.; Garcia, M.; Krüger, R. Development and leaf consumption by Spodoptera cosmioides (Walker) (Lepidoptera: Noctuidae) reared on leaves of agroenergy crops. Neotrop. Entomol. 2013, 42, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Barros, E.M.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol. Exp. Appl. 2010, 137, 237–245. [Google Scholar] [CrossRef]
- Neelima, C.; Nameirakam, L.; Devi, Y.K.; Scholar, M. The invasive fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) in Maize, status and infestation taken control under sustainable management: A Review. J. Emerg. Technol. Innov. Res 2020, 7, 1459–1471. [Google Scholar]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Haldhar, S.M.; Bhargava, R.; Choudhary, B.; Pal, G.; Kumar, S. Allelochemical resistance traits of muskmelon (Cucumis melo) against the fruit fly (Bactrocera cucurbitae) in a hot arid region of India. Phytoparasitica 2013, 41, 473–481. [Google Scholar] [CrossRef]
- Shah, J.; Walling, L. Advances in plant-hemipteran interactions. Front. Plant Sci. 2017, 8, 1652. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.K.; Prakash, A.; Johri, B. Induced systemic resistance (ISR) in plants: mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef]
- De Sousa Ramalho, F.; Azeredo, T.L.; de Nascimento, A.R.B.; Fernandes, F.S.; Nascimento Júnior, J.L.; Malaquias, J.B.; da Silva, C.A.D.; Zanuncio, J.C. Feeding of fall armyworm, Spodoptera frugiperda, on Bt transgenic cotton and its isoline. Entomol. Exp. Appl. 2011, 139, 207–214. [Google Scholar] [CrossRef]
- Hutasoit, R.T.; Kalqutny, S.H.; Widiarta, I.N. Spatial distribution pattern, bionomic, and demographic parameters of a new invasive species of armyworm Spodoptera frugiperda (Lepidoptera; Noctuidae) in maize of South Sumatra, Indonesia. Biodivers. J. Biol. Divers. 2020, 21. [Google Scholar] [CrossRef]
- Maharani, Y.; Puspitaningrum, D.; Istifadah, N.; Hidayat, S.; Ismail, A. Biology and life table of fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) on maize and rice. Serangga 2021, 26, 161–174. [Google Scholar]
- Cock, M.J.; Beseh, P.K.; Buddie, A.G.; Cafá, G.; Crozier, J. Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Sci. Rep. 2017, 7, 4103. [Google Scholar] [CrossRef] [PubMed]
- Ganiger, P.; Yeshwanth, H.; Muralimohan, K.; Vinay, N.; Kumar, A.; Chandrashekara, K. Occurrence of the new invasive pest, fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), in the maize fields of Karnataka, India. Curr. Sci. 2018, 115, 621–623. [Google Scholar] [CrossRef]
- Sharanabasappa, S.; Kalleshwaraswamy, C.; Maruthi, M.; Pavithra, H. Biology of invasive fall army worm Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) on maize. Indian J. Entomol. 2018, 80, 540–543. [Google Scholar] [CrossRef]
- Rashid, M.M.; Jahan, M.; Islam, K.S. Impact of nitrogen, phosphorus and potassium on brown planthopper and tolerance of its host rice plants. Rice Sci. 2016, 23, 119–131. [Google Scholar] [CrossRef]
- Hosseininejad, A.; Naseri, B.; Razmjou, J. Comparative feeding performance and digestive physiology of Helicoverpa armigera (Lepidoptera: Noctuidae) larvae-fed 11 corn hybrids. J. Insect Sci. 2015, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Altaf, N.; Idrees, A.; Ullah, M.I.; Arshad, M.; Afzal, A.; Afzal, M.; Rizwan, M.; Li, J. Biotic potential induced by different host plants in the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Cowie, B.W.; Strathie, L.W.; Goodall, J.M.; Venter, N.; Witkowski, E.T.; Byrne, M.J. Does host plant quality constrain the performance of the Parthenium beetle Zygogramma bicolorata? Biol. Cont. 2019, 139, 104078. [Google Scholar] [CrossRef]
- Wang, W.; He, P.; Zhang, Y.; Liu, T.; Jing, X.; Zhang, S. The population growth of Spodoptera frugiperda on six cash crop species and implications for its occurrence and damage potential in China. Insects 2020, 11, 639. [Google Scholar] [CrossRef]
- Wouters, F.C.; Blanchette, B.; Gershenzon, J.; Vassão, D.G. Plant defense and herbivore counter-defense: benzoxazinoids and insect herbivores. Phytochem. Rev. 2016, 15, 1127–1151. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.; Gadratagi, B.G.; Paramesh, V.; Kumar, P.; Madivalar, Y.; Narayanappa, N.; Ullah, F. Sustainable management of invasive fall armyworm, Spodoptera frugiperda. Agronomy 2022, 12, 2150. [Google Scholar] [CrossRef]
- Xie, W.; Zhi, J.; Ye, J.; Zhou, Y.; Li, C.; Liang, Y.; Yue, W.; Li, D.; Zeng, G.; Hu, C. Age-stage, two-sex life table analysis of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) reared on maize and kidney bean. Chem. Biol. Technol. Agric. 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.; Wang, Q.; Liu, X.; Fu, Y.; Zhang, Y.; Chen, J. Push–pull plants in wheat intercropping system to manage Spodoptera frugiperda. J. Pest Sci. 2023, 96, 1579–1593. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.-Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Khedr, M.A.; Al-Shannaf, H.M.; Mead, H.M.; Shaker, S.A. Comparative study to determine food consumption of cotton leafworm, Spodoptera littoralis, on some cotton genotypes. J. Plant Prot. Res. 2015, 55. [Google Scholar] [CrossRef]
- Chen, W.H.; Itza, B.; Kafle, L.; Chang, T.Y. Life table study of fall armyworm (Spodoptera frugiperda) (Lepidoptera: Noctuidae) on three host plants under laboratory conditions. Insects 2023, 14, 329. [Google Scholar] [CrossRef]
- He, L.M.; Wang, T.L.; Chen, Y.C.; Ge, S.S.; Wyckhuys, K.A.; Wu, K.M. Larval diet affects development and reproduction of East Asian strain of the fall armyworm, Spodoptera frugiperda. J. Integr. Agric. 20, 736–744. [CrossRef]
- He, L.M.; Wu, Q.L.; Gao, X.W.; Wu, K.M. Population life tables for the invasive fall armyworm, Spodoptera frugiperda fed on major oil crops planted in China. J. Integr. Agric. 20, 745–754. [CrossRef]
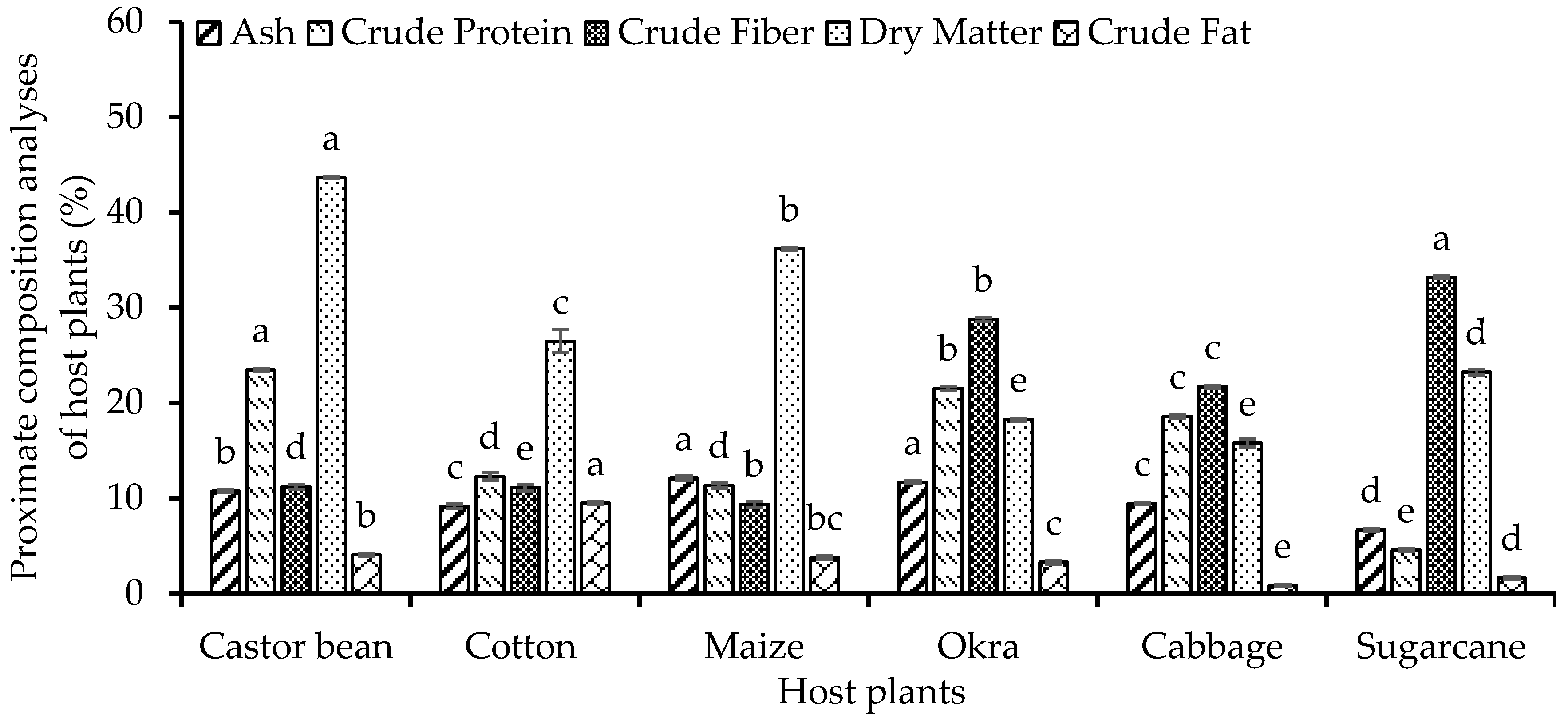

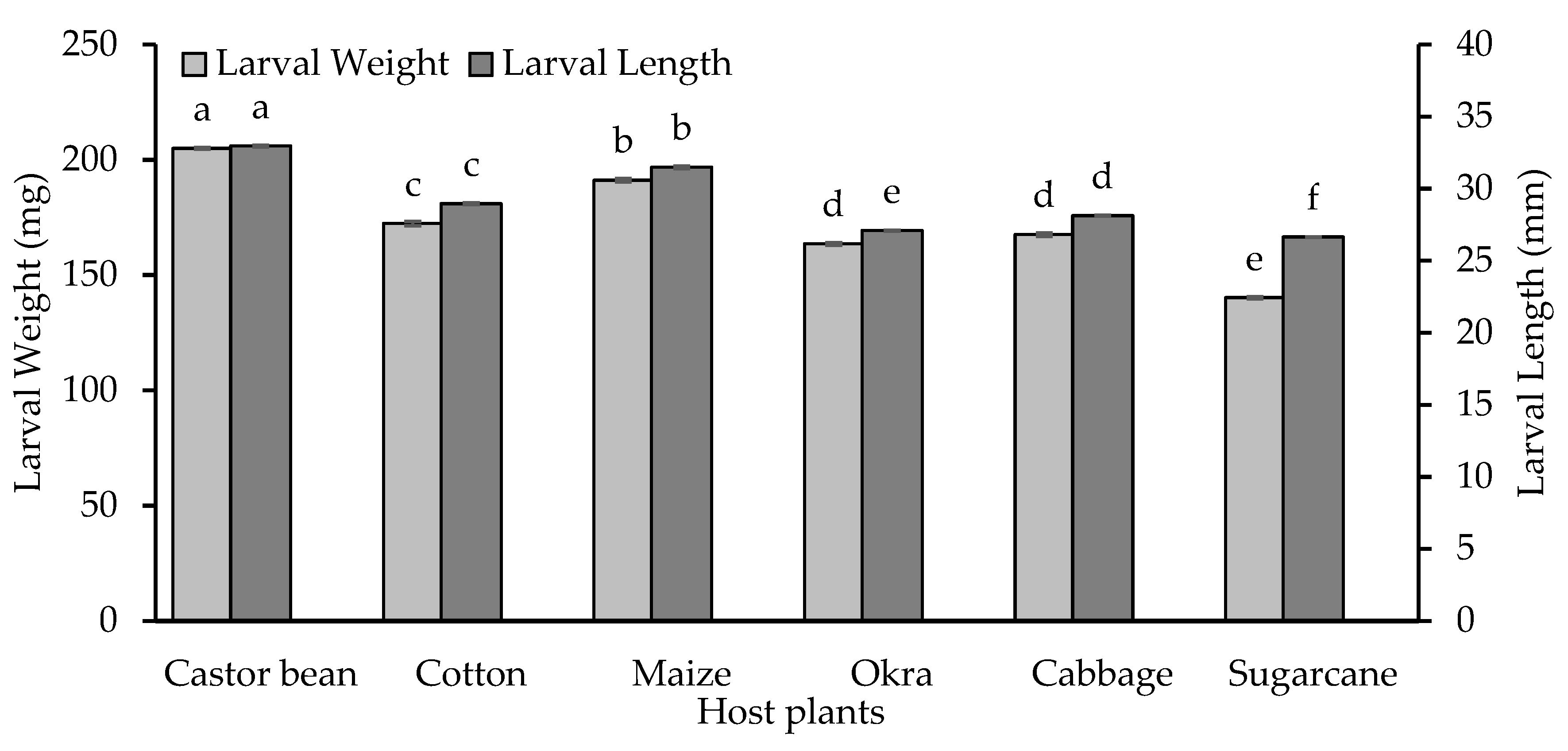
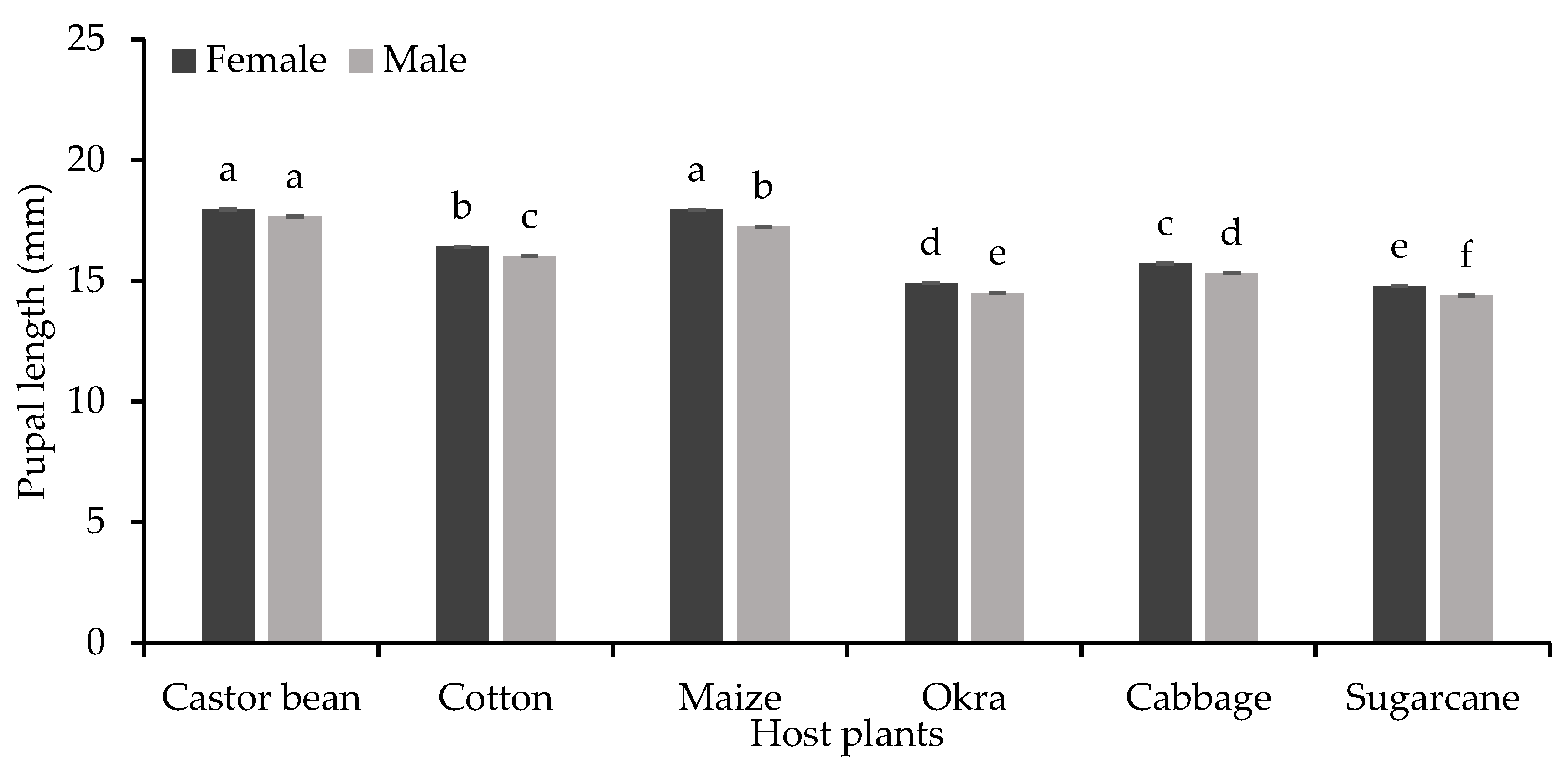
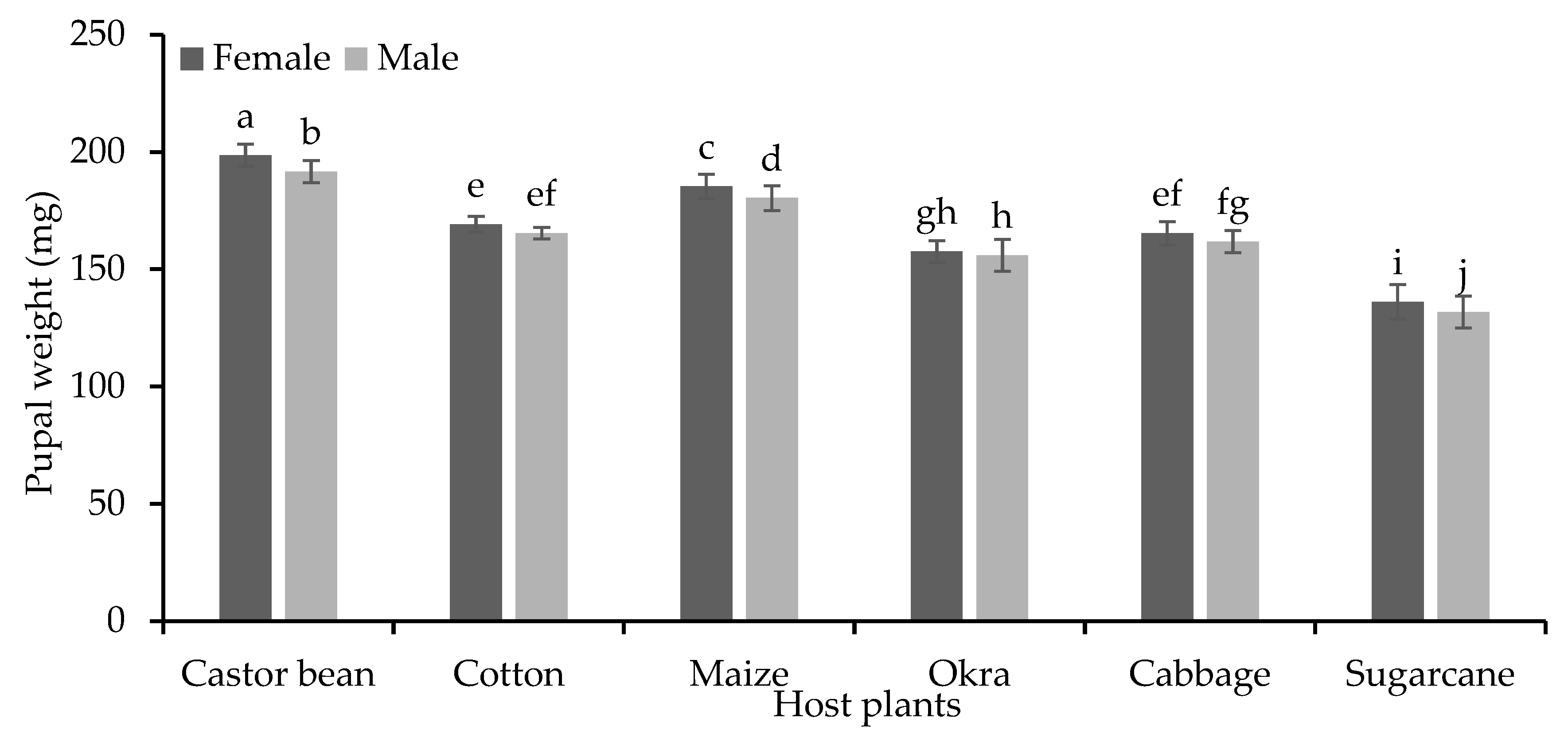
| Host plants | RGR | RCR | ECI | CI |
|---|---|---|---|---|
| Castor bean | 0.97±0.03b | 47.13±0.45b | 71.80±1.25a | 67.68ab |
| Cotton | 0.85±0.01c | 33.49±0.47c | 71.11±1.32a | 67.84ab |
| Maize | 1.13±0.03a | 50.47±0.61a | 70.77±1.45a | 68.39a |
| Okra | 0.71±0.03d | 19.66±0.46d | 69.22±2.03a | 67.08ab |
| Cabbage | 0.77±0.01cd | 33.22±0.50c | 69.87±1.40a | 67.47ab |
| Sugarcane | 0.59±0.01e | 19.2±0.40d | 70.02±1.69a | 65.69b |
| df | 5 | 5 | 5 | 5 |
| F-value | 63.4 | 588 | 3.45 | 3.14 |
| p-value | <0.001 | <0.001 | 0.08 | 0.03 |
| Host plants | Larval growth Index | Pupal Growth Index | Immature Growth Index | Standard Growth Index | Fitness Index |
|---|---|---|---|---|---|
| Castor bean | 10.21±0.85b | 20.69±0.52a | 7.55±0.15a | 21.53±0.17b | 408.21±41.04b |
| Cotton | 5.48±0.34d | 15.47±1.50b | 6.05±0.82ab | 15.62±0.51d | 177.81±10.52c |
| Maize | 13.13±0.66a | 23.38±0.41a | 5.33±0.36a | 26.59±1.22a | 606.79±27.95a |
| Okra | 8.57±0.71bc | 12.9±0.58b | 4.96±0.95b | 17.87±0.36c | 233.08±19.96c |
| Cabbage | 7.18±0.56cd | 15.95±0.82b | 4.82±0.88b | 16.52±0.46cd | 192.15.75c |
| Sugarcane | 0.72±0.10e | 0±0.00c | -- | 8.56±1.88e | -- |
| df | 5 | 5 | 4 | 5 | 4 |
| F-value | 51.4 | 108 | 2.5 | 74.7 | 52 |
| p-value | <0.001 | <0.001 | 0.04 | <0.001 | <0.001 |
| DM | CP | CF | EE | Ash | LGI | PGI | IGI | SGI | |
|---|---|---|---|---|---|---|---|---|---|
| CP | 0.43* | ||||||||
| CF | -0.30 | -0.73** | |||||||
| EE | 0.13 | -0.04 | -0.59** | ||||||
| Ash | 0.26 | 0.62** | -0.38* | 0.10 | |||||
| LGI | -0.15 | 0.19 | -0.45* | 0.64** | 0.47** | ||||
| PGI | 0.14 | 0.39* | -0.60** | 0.68** | 0.69** | 0.87** | |||
| IGI | 0.06 | 0.40* | -0.58** | 0.57** | 0.58** | 0.82** | 0.88** | ||
| SGI | -0.05 | 0.21 | -0.50** | 0.70** | 0.48** | 0.93** | 0.90** | 0.80** | |
| FI | -0.09 | 0.00 | -0.41* | 0.80** | 0.34 | 0.95** | 0.85** | 0.76** | 0.92** |
| RGR | RCR | DM | CP | CF | EE | Ash | |
|---|---|---|---|---|---|---|---|
| RCR | 0.91** | ||||||
| DM | 0.50** | 0.64** | |||||
| CP | 0.21 | 0.23 | 0.43* | ||||
| CF | -0.10 | -0.05 | -0.30 | -0.73** | |||
| EE | 0.29 | 0.21 | 0.13 | -0.04 | -0.59 | ||
| ASH | 0.69** | 0.53** | 0.26 | 0.62** | -0.38 | 0.10 | |
| ECI | -0.30 | 0.43 | 0.01 | 0.13 | 0.64 | 0.52 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





