Submitted:
30 July 2024
Posted:
31 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
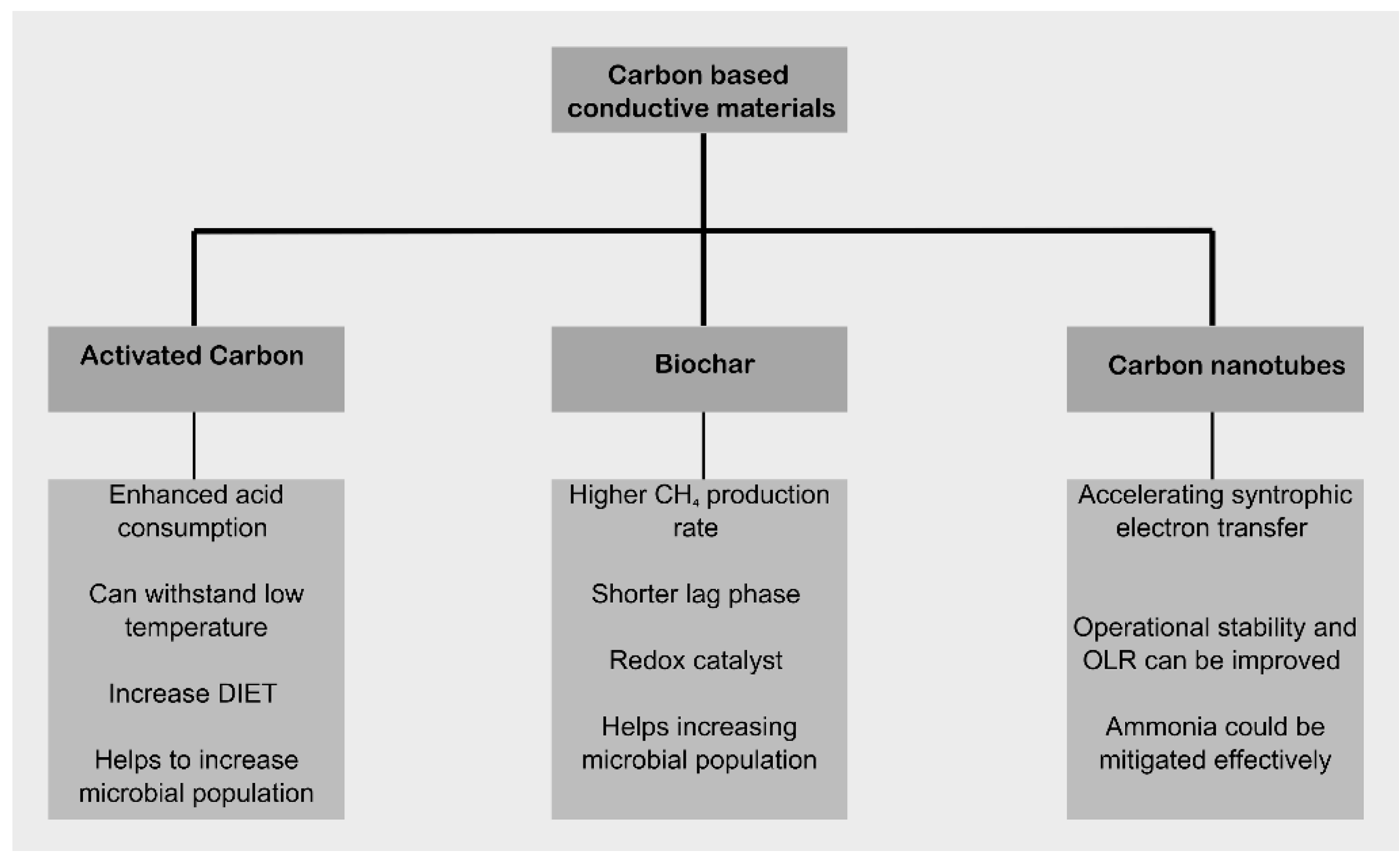
2. Carbon–Based Conductive Materials
2.1. GAC and PAC
2.2. Biochar
2.3. SWNTs and MWNTs
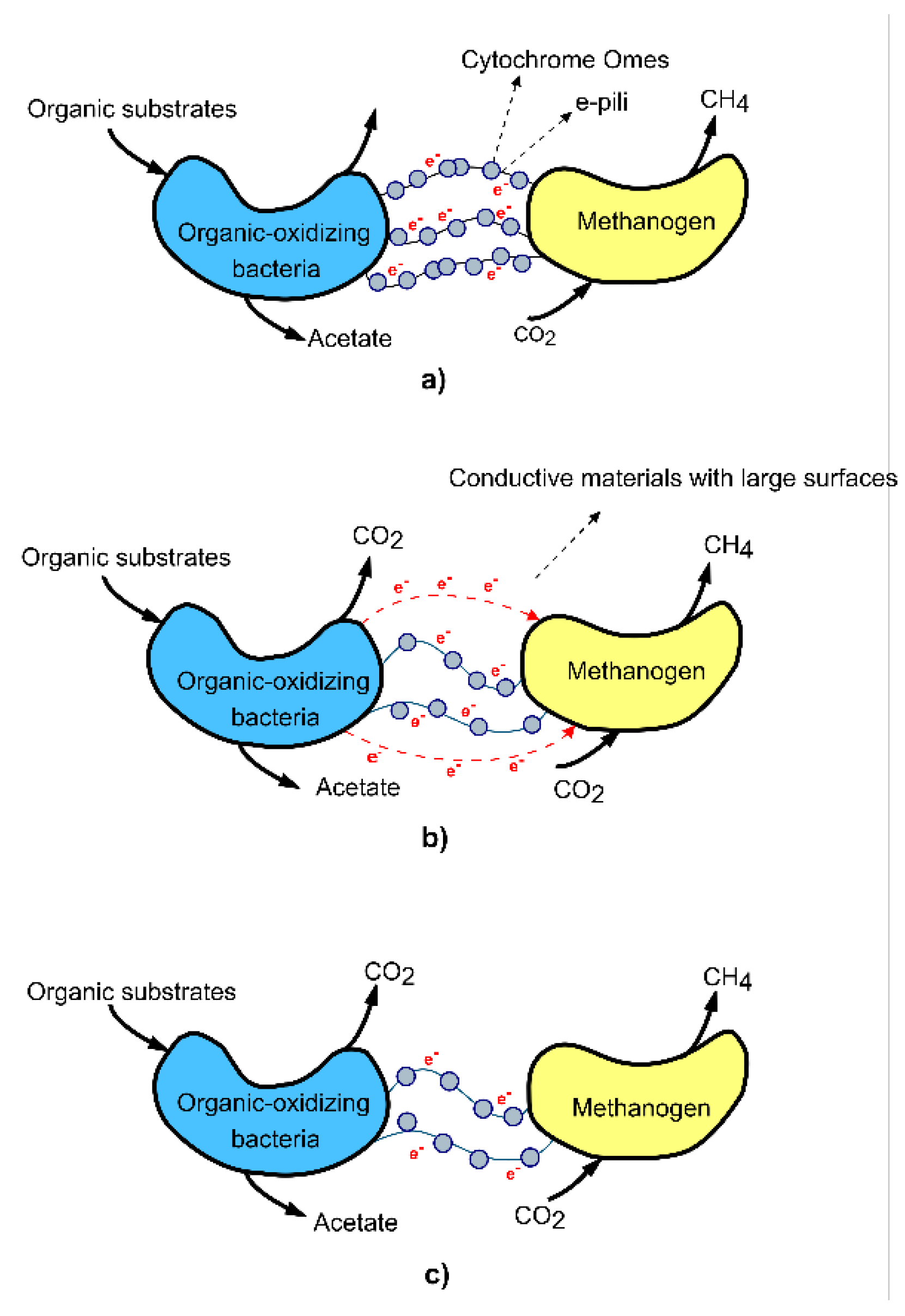
2.4. DIET Mechanism
3. Metal Oxide Nanomaterial
| Type of additive | Type of substrate | Yield of biogas/biomethane | References |
|---|---|---|---|
| Al2O3 | animal fat | increase in biogas production by 285% | [64] |
| Al2O3 | sewage sludge | increase in biogas production by 23.4% | [66] |
| Al2O3 | waste-activated sludge | increase in methane production by 14.8% | [69] |
| Fe2O3 | animal fat | increase in biogas production by 45.87% | [64] |
| Fe2O3 | granular sludge | increase in methane production by 38% | [7] |
| Fe2O3 | waste activated sludge | increase in methane production by 117% | [58] |
| Fe3O4 | corn straw and sewage sludge | increase in methane production by 60.47% | [71] |
| Fe3O4 | waste sludge | increase in methane yield by 58.7% | [75] |
| Fe3O4 | wastewater sludge | increase in biogas production by 96%increase in methane production by 144% | [68] |
| Fe3O4 | wastewater sludge | increase in biogas production by 107%increase in methane production by 153% | [68] |
| Fe3O4 | municipal solid waste | increase in methane yield by 72.09% | [76] |
| TiO2 | fresh dairy cattle manure | increase in methane yield by 121% | [67] |
| TiO2 | anaerobic sludge | increase in methane yield by 14.9% | [69] |
| CeO2 | waste-activated sludge | increase in methane production by 9.2% | [77] |
| MnO2 | seed sludge | decrease in methane production by 93% | [72] |
| MgO | waste activated sludge | decrease in methane production by 99% | [58] |
| CoO | sewage sludge | decrease in biogas production by 60% | [69] |
| CeO2 | sludge | decrease in biogas production by 35% | [58] |
| CeO2 | cellulose | decrease in biogas production by 100% | [58] |
| CuO | cattle manure | decrease in biogas production by 96% | [58] |
| CuO | sewage sludge | decrease in biogas production by 17.3% | [66] |
| ZnO | animal fat | decrease in biogas production by 17% | [64] |
| ZnO | municipal solid waste | decrease in biogas production by 15% | [78] |
| ZnO | waste-activated sludge | decrease in methane production by 50% | [73] |
| ZnO | sewage sludge | decrease in biogas production by 90.2% | [66] |
4. Trace Elements
- Iron (Fe) plays many roles in anaerobic processes, mainly due to its exceptionally high reduction potential. Because of its properties, it plays a special role in energy metabolism. Iron is crucial for the activity of various enzymes, including hydrogenases and ferredoxins, which are involved in electron transfer and hydrogen metabolism [82,89]. In summary, this metal is utilised in the transport of methanogenic bacteria for the conversion of CO2 to CH4 and serves as both an electron acceptor and donor [5]. Adequate iron levels can enhance biogas production and stabilise the AD process.
- Nickel (Ni) is essential for the function of several enzymes, such as methyl-coenzyme M reductase, which is key in the final step of methanogenesis. Anaerobic bacteria are heavily dependent on nickel when carbon dioxide and hydrogen are the sole sources of energy. The nickel tetrapyrrole, coenzyme F430, is known to bind to methyl-S-CoM reductase, which catalyses methane formation from methyl-S-CoM in both acetoclastic and hydrogenotrophic methanogens [90]. This coenzyme is part of the methyl-coenzyme M reductase enzyme, which reduces methyl-coenzyme M to methane [84,91]. Nickel supplementation can improve methane production, especially in nickel-deficient substrates.
- Cobalt (Co) is a critical component of vitamin B12, which activates carboxypeptidase and is required for the metabolism of certain methanogens. Corrinoids, such as vitamin B12, containing a cobalt ion, bind to methyl-coenzyme M (CoM) reductase, which catalyses methane formation in both acetoclastic methanogens and hydrogenotrophic bacteria [84]. The enzyme carbon monoxide dehydrogenase (CODH) also utilises cobalt [92]. Therefore, cobalt can increase methane yield and improve the metabolic activities of acetoclastic methanogens.
- Molybdenum (Mo) is a cofactor for enzymes like formate dehydrogenase, which participates in the conversion of formate to carbon dioxide. The Mo enzyme is synthesised only when Mo is present in the growth medium [82]. This metal can inhibit sulfate-reducing bacteria, limiting the formation of sulphides. Molybdenum can also stimulate methane production from corn silage and municipal waste substrates [84,93]. In summary, molybdenum enhances formate decomposition, thereby supporting the entire AD process.
- Selenium and Tungsten (Se and W) are parts of several selenoproteins that play roles in protecting cells from oxidative damage and participating in redox reactions [82]. Selenium, like tungsten, is a component of the enzyme formate dehydrogenase (FDH), which catalyses formate production by propionate oxidizers. Certain methanogenic bacteria contain W and Mo enzymes for the same purpose [94]. Few studies have been conducted on the effects of Se and W on methanogenesis. One study conducted on a laboratory scale, with food industry waste, showed evidence of increased methane production under the influence of Se and W, and additionally in combination with Co [95].
- Zinc (Zn) is involved in enzyme function, stabilising protein structures, and regulating gene expression. Zinc is a part of enzymes such as formate dehydrogenase (FDH), superoxide dismutase (SODM), and hydrogenase [82,83]. Zn has been found in remarkably high concentrations (50–630 ppm) in 10 methanogenic bacteria [96]. This metal is necessary for maintaining microbial activity and diversity in AD.
- Copper (Cu) functions in redox reactions and electron transport. In general, the role of copper in methanogenesis is contradictorily perceived. It has rarely been studied, making it difficult to fully understand the role of Cu in biogas production. However, it has not been found to have a noticeable stimulating effect on biogas production [82]. It is important to note that while Cu is essential in small amounts, its excess can be toxic to microorganisms [86].
5. Biological Additives
5.1. Enzyme Supplementation
5.2. Bioaugmentation
| Type of additive | Type of substrate | Yield of biogas/biomethane | References |
|---|---|---|---|
| cellulases, xylanases, β-glucosidases |
ensiled forage ley | increase in methane production by 19% | [111] |
| lipase from Aspergillus | animal fat | increase in methane production by 80.8% | [110] |
| arachis oil | increase in methane production by 26.9% | [110] | |
| floatable grease | increase in methane production by 37% | [110] | |
| lipase from Candida | animal fat | increase in methane production by 157.7% | [110] |
| arachis oil | increase in methane production by 53.8% | [110] | |
| floatable grease | increase in methane production by 40.7% | [110] | |
| bio-additive Digest P3 (carbohydrases, pectinase, xylanase) |
poultry litter | increase in biogas production by 59.7% increase in methane production by 91.4% |
[136] |
| bio-additive APD (Aerobacter, Pseudomonas, Alcaligenes, cellulase, lipase) |
igniscum silage | increase in biogas production by 6% decrease in methane production by 7% |
[18] |
| maize silage | increase in biogas production by 53% increase in methane production by 74% |
[18] | |
| bio-additive PPT (Pseudomonas, Flavobacterium, Lactobacillus, cellulase, lipase) |
igniscum silage | increase in biogas production by 16% increase in methane production by 26% |
[18] |
| maize silage | increase in biogas production by 62% increase in methane production by 79% |
[18] | |
| bio-additive HAP (Clostridium, Micrococcus, cellulase, lipase) |
igniscum silage | increase in biogas production by 12% increase in methane production by 30% |
[18] |
| maize silage | increase in biogas production by 32% increase in methane production by 46% |
[18] | |
| bio-additive JENOR (Pichia, Trichoderma, cellulase, lipase) |
igniscum silage | increase in biogas production by 13% increase in methane production by 16% |
[18] |
| maize silage | increase in biogas production by 17% increase in methane production by 26% |
[18] | |
| Orpinomyces sp. | barley, triticale, rye, wheat, cow manure |
increase in methane production by 33% | [117] |
| Ochrobactrum sp. | sewage sludge | increase in biogas production by 22.06% | [137] |
| Caldicellulosiruptor bescii | birch wood chips | increase in methane production by 44% | [120] |
| Clostridium thermocellum | wheat straw, cow manure |
increase in methane production by 39% | [116] |
|
Clostridium cellulolyticumClostridium cellulovorans Clostridium aceticum Mesotoga infera Methanosarcina barkeri Methanosaeta concilii |
Axonopus compressus | increase in methane production by 20.7% | [126] |
|
Neocallimastix sp. Orpinomyces sp. fermentative bacteria |
wheat straw | increase in methane production of 290% | [127] |
| mushroom spent straw | increase in methane production by 330% | [127] | |
|
Aspergillus sp. Trichoderma viride |
maize straw | increase in methane production by 31.7% | [128] |
| Trichoderma atroviride | water hyacinth | increase in biogas production by 65% increase in methane production by 117% |
[118] |
| Trichoderma reesei | rice straw and soybean straw |
increase in biogas production by 318% increase in methane production by 807% |
[138] |
|
Orpinomyces sp. Piromyces sp. Anaeromyces sp. Neocallimastix frontalis |
algal biomass | increase in methane production by 40.6% | [123] |
| cow manure | increase in methane production by 60% | [124] |
6. AD Additives Research – Summary
7. Feasibility and Perspectives
8. Conclusions
References
- Pilarska, A.A.; Wolna-Maruwka, A.; Pilarski, K.; Janczak, D.; Przybył, K.; Gawrysiak-Witulska, M. The Use of Lignin as a Microbial Carrier in the Co-Digestion of Cheese and Wafer Waste. Polymers 2019, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- Amani, T.; Nosrati, M.; Sreekrishnan, T.R. Anaerobic digestion from the viewpoint of microbiological, chemical, and operational aspects - A review. Environ. Rev. 2010, 18, 255–278. [Google Scholar] [CrossRef]
- Gadirli, G.; Pilarska, A.A.; Dach, J.; Pilarski, K.; Kolasa-Więcek, A.; Borowiak, K. Fundamentals, Operation and Global Prospects for the Development of Biogas Plants - A Review. Energies 2024, 17, 568. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Zain, S.M.; Pramanik, B.K. The anaerobic digestion process of biogas production from food waste: Prospects and constraints. Bioresour. Technol. Rep. 2019, 8, 100310. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Leca, E.; Zennaro, B.; Hamelin, J.; Carrère, H.; Sambusiti, C. Use of additives to improve collective biogas plant performances: A comprehensive review. Biotechnol. Adv. 2023, 65, 108129. [Google Scholar] [CrossRef] [PubMed]
- Ambuchi, J.J.; Zhang, Z.; Feng, Y. Biogas Enhancement Using Iron Oxide Nanoparticles and Multi-Wall Carbon Nanotubes. Int. J. Chem. Biomol. Eng. 2016, 10, 1305–1311. [Google Scholar]
- Barua, S.; Zakaria, B.S.; Lin, L.; Dhar, B.R. Magnetite Doped Granular Activated Carbon as an Additive for High-Performance Anaerobic Digestion. Mater. Sci. Energy Technol. 2019, 2, 377–384. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.H.; Lee, S.H.; Jung, S.P.; Kim, S.H. Enhancing anaerobic digestion for rural wastewater treatment with granular activated carbon (GAC) supplementation. Bioresour. Technol. 2020, 315, 123890. [Google Scholar] [CrossRef]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi) conductive iron-oxide minerals. Environ. Microbiol. 2012, 14, 1646–1654. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Q.; Sun, D.; Wang, Y.; Dong, H.; Dang, Y.; Holmes, D.E. Applying potentials to conductive materials impairs High-loading anaerobic digestion performance by affecting direct interspecies electron transfer. Bioresour. Technol. 2020, 297, 122422. [Google Scholar] [CrossRef]
- Chen, L.; Fang, W.; Chang, J.; Liang, J.; Zhang, P.; Zhang, G. Improvement of Direct Interspecies Electron Transfer via Adding Conductive Materials in Anaerobic Digestion: Mechanisms, Performances, and Challenges. Front. Microbiol. 2022, 13, 860749. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, H.J.; Park, K.H.; Park, H.D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, A.A.; Wolna-Maruwka, A.; Niewiadomska, A.; Grządziel, J.; Gałązka, A.; Paluch, E.; Borowiak, K.; Pilarski, K. Quantitative and Qualitative Changes in the Genetic Diversity of Bacterial Communities in Anaerobic Bioreactors with the Diatomaceous Earth/Peat Cell Carrier. Cells 2022, 11, 2571. [Google Scholar] [CrossRef] [PubMed]
- Guskos, N.; Papadopoulos, G.J.; Likodimos, V.; Patapis, S.; Yarmis, D.; Przepiera, A.; Przepiera, K.; Majszczyk, J.; Typek, J.; Wabia, M.; Aidinis, K.; Drazek, Z. Photoacoustic, EPR and electrical conductivity investigations of three synthetic mineral pigments: Hematite, goethite and magnetite. Mater. Res. Bull. 2002, 37, 1051–1061. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, T.; Chen, S.; Hu, Q.; Cheng, X.; Sun, D.; Vupputuri, S.; Qiu, B.; Liu, H.; Guo, Z. Conductive Polyaniline Hydrogel Enhanced Methane Production from Anaerobic Wastewater Treatment. J. Colloid Interface Sci. 2021, 581, 314–322. [Google Scholar] [CrossRef]
- Mutschlechner, M.; Illmer, P.; Wagner, A.O. Biological pre-treatment: Enhancing biogas production using the highly cellulolytic fungus Trichoderma viride. Waste Manag. 2015, 43, 98–107. [Google Scholar] [CrossRef]
- Fugol, M.; Prask, H.; Szlachta, J.; Dyjakon, A.; Pasławska, M.; Szufa, S. Improving the Energetic Efficiency of Biogas Plants Using Enzymatic Additives to Anaerobic Digestion. Energies 2023, 16, 1845. [Google Scholar] [CrossRef]
- Hou, D.; Li, K.; Ma, R.; Liu, Q. Influence of order degree of coaly graphite on its structure change during preparation of graphene oxide. Journal of Materiomics, 2020, 6, 628–641. [Google Scholar] [CrossRef]
- Muratçobanoğlu, H.; Gökçek, Ö.B.; Mert, R.A.; Zan, R.; Demirel, S. Simultaneous Synergistic Effects of Graphite Addition and Co-Digestion of Food Waste and Cow Manure: Biogas Production and Microbial Community. Bioresour. Technol. 2020, 309, 123365. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, Y.; Lin, P.; Li, J.; Dong, H.; Yu, H.; Qi, L.; Ren, L. Effects of graphite, graphene, and graphene oxide on the anaerobic co-digestion of sewage sludge and food waste: Attention to methane production and the fate of antibiotic resistance genes. Bioresour. Technol. 2021, 339, 125585. [Google Scholar] [CrossRef] [PubMed]
- Burachevskaya, M.; Mandzhieva, S.; Bauer, T.; Minkina, T.; Rajput, V.; Chaplygin, V.; Fedorenko, A.; Chernikova, N.; Zamulina, I.; Kolesnikov, S.; Sushkova, S.; Perelomov, L. The Effect of Granular Activated Carbon and Biochar on the Availability of Cu and Zn to Hordeum sativum Distichum in Contaminated Soil. Plants 2021, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Guo, B.; Zhou, Y.; Gao, M.; Sharaf, A.; Liu, Y. Granular activated carbon stimulated microbial physiological changes for enhanced anaerobic digestion of municipal sewage. Chem. Eng. J. 2020, 400, 125838. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, H.; Zhang, Y.; Cui, M.H.; Fu, B.; Liu, H.B. Insight into sludge anaerobic digestion with granular activated carbon addition: Methanogenic acceleration and methane reduction relief. Bioresour. Technol. 2021, 319, 124131. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; He, C.; Luo, L.; Lü, F.; He, P.; Cui, L. Comparing activated carbon of different particle sizes on enhancing methane generation in upflow anaerobic digester. Bioresour. Technol. 2015, 196, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wei, H.; Su, Y.; Gu, W.; Wang, B.; Xie, B. Powdered activated carbon facilitates methane productivity of anaerobic co-digestion via acidification alleviating: Microbial and metabolic insights. Bioresour. Technol. 2020, 313, 123706. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.Y.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, J.H.; Seong, H.; Sul, W.J.; Jin, K.H.; Park, H.D. Metagenomic insight into methanogenic reactors promoting direct interspecies electron transfer via granular activated carbon. Bioresour. Technol. 2018, 259, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, R.; Wang, H.; Yang, K. Direct interspecies electron transfer stimulated by granular activated carbon enhances anaerobic methanation efficiency from typical kitchen waste lipid-rapeseed oil. Sci. Total Environ. 2020, 704, 135282. [Google Scholar] [CrossRef]
- Patrícya Florentino, A.; Sharaf, A.; Zhang, L.; Liu, Y. Overcoming Ammonia Inhibition in Anaerobic Blackwater Treatment with Granular Activated Carbon: The Role of Electroactive Microorganisms. Environ. Sci. Water Res. Technol. 2019, 5, 383–396. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, L.; Wijaya, S.M.; Zhou, Y. Unveiling the role of activated carbon on hydrolysis process in anaerobic digestion. Bioresour. Technol. 2020, 296, 122366. [Google Scholar] [CrossRef] [PubMed]
- Cuetos, M.J.; Martinez, E.J.; Moreno, R.; Gonzalez, R.; Otero, M.; Gomez, X. Enhancing anaerobic digestion of poultry blood using activated carbon. J. Adv. Res. 2017, 8, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Dastyar, W.; Mirsoleimani Azizi, S.M.; Meshref, M.N.A.; Dhar, B.R. Powdered activated carbon amendment in percolate tank enhances high-solids anaerobic digestion of organic fraction of municipal solid waste. Process. Saf. Environ. Prot. 2021, 151, 63–70. [Google Scholar] [CrossRef]
- Poluszyńska, J.; Ślęzak, E.; Wieczorek, P.P. Biowęgiel jako środek polepszający właściwości gleby. Przemysł Chemiczny, 2019, 1, 100–107. [Google Scholar] [CrossRef]
- Hassan, S.; Ngo, T.; Khudur, L.S.; Krohn, C.; Dike, C.C.; Hakeem, I.G.; Shah, K.; Surapaneni, A.; Ball, A.S. Biosolids-Derived Biochar Improves Biomethane Production in the Anaerobic Digestion of Chicken Manure. Resources 2023, 12, 123. [Google Scholar] [CrossRef]
- Lü, F.; Liu, Y.; Shao, L.; He, P. Powdered biochar doubled microbial growth in anaerobic digestion of oil. Appl. Energy 2019, 247, 605–614. [Google Scholar] [CrossRef]
- Baral, K.R.; McIlroy, J.; Lyons, G.; Johnston, C. The Effect of Biochar and Acid Activated Biochar on Ammonia Emissions during Manure Storage. Environ. Pollut. 2023, 317, 120815. [Google Scholar] [CrossRef]
- Mumme, J.; Srocke, F.; Heeg, K.; Werner, M. Use of biochars in anaerobic digestion. Bioresour. Technol. 2014, 164, 189–197. [Google Scholar] [CrossRef]
- Giwa, A.S.; Xu, H.; Chang, F.; Wu, J.; Li, Y.; Ali, N.; Ding, S.; Wang, K. Effect of biochar on reactor performance and methane generation during the anaerobic digestion of food waste treatment at long-run operations. J. Environ. Chem. Eng. 2019, 7, 103067. [Google Scholar] [CrossRef]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef]
- Lim, E.Y.; Tian, H.; Chen, Y.; Ni, K.; Zhang, J.; Tong, Y.W. Methanogenic pathway and microbial succession during start-up and stabilization of thermophilic food waste anaerobic digestion with biochar. Bioresour. Technol. 2020, 314, 123751. [Google Scholar] [CrossRef] [PubMed]
- Gómez, X.; Meredith, W.; Fernández, C.; Sánchez-García, M.; Díez-Antolínez, R.; Garzón-Santos, J.; Snape, C.E. Evaluating the effect of biochar addition on the anaerobic digestion of swine manure: Application of Py-GC/MS. Environ. Sci. Pollut. Res. 2018, 25, 25600–25611. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Q.; Yuwen, C.; Gong, K.; Sheng, L.; Li, Y.; Xing, Y.; Chen, R. Biochar Triggers Methanogenesis Recovery of a Severely Acidified Anaerobic Digestion System via Hydrogen-Based Syntrophic Pathway Inhibition. Int. J. Hydrogen Energy 2021, 46, 9666–9677. [Google Scholar] [CrossRef]
- Salvador, A.F.; Martins, G.; Melle-Franco, M.; Serpa, R.; Stams, A.J.M.; Cavaleiro, A.J.; Pereira, M.A.; Alves, M.M. Carbon nanotubes accelerate methane production in pure cultures of methanogens and in a syntrophic coculture. Environ. Microbiol. 2017, 19, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Rahimian Koloor, S.S.; Alshehri, A.H.; Arockiarajan, A. Carbon Nanotube Characteristics and Enhancement Effects on the Mechanical Features of Polymer-Based Materials and Structures - A Review. J. Mater. Res. Technol. 2023, 24, 6495–6521. [Google Scholar] [CrossRef]
- Jeyakumar, R.B.; Vincent, G.S. Recent Advances and Perspectives of Nanotechnology in Anaerobic Digestion: A New Paradigm towards Sludge Biodegradability. Sustainability 2022, 14, 7191. [Google Scholar] [CrossRef]
- Li, L.L.; Tong, Z.H.; Fang, C.Y.; Chu, J.; Yu, H.Q. Response of anaerobic granular sludge to single-wall carbon nanotube exposure. Water Res. 2015, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Liang, Z.; Chen, Y.; Song, H.; Wan, J. Enhancement of syntrophic acetate oxidation pathway via single walled carbon nanotubes addition under high acetate concentration and thermophilic condition. Bioresour. Technol. 2020, 306, 123182. [Google Scholar] [CrossRef] [PubMed]
- Cavaleiro, A.J.; Salvador, A.F.; Martins, G.; Oliveira, C.C.; Liu, Y.; Martins, V.R.; Castro, A.R.; Soares, O.S.G.P.; Pereira, M.F.R.; Pereira, L.; Langenhoff, A.A.M.; Pereira, M.A.; Alves, M.M. Multi-Walled Carbon Nanotubes Enhance Methanogenesis from Diverse Organic Compounds in Anaerobic Sludge and River Sediments. Appl. Sci. 2020, 10, 8184. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Anaerobic Digestion of Chicken Manure Assisted by Carbon Nanotubes: Promotion of Volatile Fatty Acids Consumption and Methane Production. Fermentation 2022, 8, 641. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Dai, X.; Dai, L. Principles and advancements in improving anaerobic digestion of organic waste via direct interspecies electron transfer. Renew. Sustain. Energy Rev 2021, 148, 111367. [Google Scholar] [CrossRef]
- Madondo, N.I.; Rathilal, S.; Bakare, B.F.; Tetteh, E.K. Application of Bioelectrochemical Systems and Anaerobic Additives in Wastewater Treatment: A Conceptual Review. Int. J. Mol. Sci. 2023, 24, 4753. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Zhang, W. Enhanced Anaerobic Digestion Using Conductive Materials through Mediation of Direct Microbial Interspecies Electron Transfer: A Review. Fermentation 2023, 9, 884. [Google Scholar] [CrossRef]
- Chen, S.; Rotaru, A.E.; Liu, F.; Philips, J.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresour. Technol. 2014, 173, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, S.; Cai, J.; He, P.; Lü, F. Ability of biochar to facilitate anaerobic digestion is restricted to stressed surroundings. J. Clean. Prod. 2019, 238, 117959. [Google Scholar] [CrossRef]
- Barua, S.; Zakaria, B.S.; Dhar, B.R. Enhanced methanogenic co-degradation of propionate and butyrate by anaerobic microbiome enriched on conductive carbon fibers. Bioresour. Technol. 2018, 266, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Storck, T.; Virdis, B.; Batstone, D.J. Modelling extracellular limitations for mediated versus direct interspecies electron transfer. The ISME Journal. 2016, 10, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Baniamerian, H.; Isfahani, P.G.; Tsapekos, P.; Alvarado-Morales, M.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Application of Nano-Structured Materials in Anaerobic Digestion: Current Status and Perspectives. Chemosphere 2019, 229, 188–199. [Google Scholar] [CrossRef]
- Mu, H.; Chen, Y.; Xiao, N. Effects of metal oxide nanoparticles (TiO2, Al2O3, SiO2 and ZnO) on waste activated sludge anaerobic digestion. Bioresour. Technol. 2011, 102, 10305–10311. [Google Scholar] [CrossRef]
- Kökdemir Ünşar, E.; Perendeci, N.A. What Kind of Effects Do Fe2O3 and Al2O3 Nanoparticles Have on Anaerobic Digestion, Inhibition or Enhancement? Chemosphere 2018, 211, 726–735. [Google Scholar] [CrossRef]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and Multi Metal Oxide Nanoparticles: Synthesis, Antibacterial and Cytotoxic Properties. J. Nanobiotechnology 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.E.; Mohammad, A.; Ali, W.; Sharwani, A.A. Recent Developments in Properties and Applications of Metal Oxides. In Inorganic Anticorrosive Materials; Verma, E., Aslam, J., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 95–111. [Google Scholar]
- Ren, S.; Usman, M.; Tsang, D.C.W.; O-Thong, S.; Angelidaki, I.; Zhu, X.; Zhang, S.; Luo, G. Hydrochar-facilitated anaerobic digestion: evidence for direct interspecies electron transfer mediated through surface oxygen-containing functional groups. Environ. Sci. Technol. 2020, 54, 5755–5766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Pu, H.; Yang, Z. Study on the effect of different additives on the anaerobic digestion of hybrid Pennisetum: Comparison of nano-ZnO, nano-Fe2O3 and nano-Al2O3. Heliyon 2023, 9, e16313. [Google Scholar] [CrossRef] [PubMed]
- Arya, I.; Poona, A.; Dikshit, P.K.; Pandit, S.; Kumar, J.; Singh, H.N.; Jha, N.K.; Rudayni, H.A.; Chaudhary, A.A.; Kumar, S. Current Trends and Future Prospects of Nanotechnology in Biofuel Production. Catalysts 2021, 11, 1308. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Zhang, Y.; Xiang, Y.; Xu, R.; Jia, M.; Cao, J.; Xiong, W. Effects of different conductive nanomaterials on anaerobic digestion process and microbial community of sludge. Bioresour. Technol. 2020, 304, 123016. [Google Scholar] [CrossRef]
- Farghali, M.; Andriamanohiarisoamanana, F.J.; Ahmed, M.M.; Kotb, S.; Yamashiro, T.; Iwasaki, M.; Umetsu, K. Impacts of iron oxide and titanium dioxide nanoparticles on biogas production: Hydrogen sulfide mitigation, process stability, and prospective challenges. J. Environ. Manag. 2019, 240, 160–167. [Google Scholar] [CrossRef]
- Heikal, G.; Shakroum, M.; Vranayova, Z.; Abdo, A. Impact of Nanoparticles on Biogas Production from Anaerobic Digestion of Sewage Sludge. J. Ecol. Eng. 2022, 23, 222–240. [Google Scholar] [CrossRef]
- Ajay, C.M.; Mohan, S.; Dinesha, P.; Rosen, M.A. Review of impact of nanoparticle additives on anaerobic digestion and methane generation. Fuel 2020, 277, 118234. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Dong, B.; Dai, X. Magnetite triggering enhanced direct interspecies electron transfer: a scavenger for the blockage of electron transfer in anaerobic digestion of high-solids sewage sludge. Environ. Sci. Technol. 2018, 52, 7160–7169. [Google Scholar] [CrossRef]
- Li, P.; Wang, Q.; He, X.; Yu, R.; He, C.; Shen, D.; Jiao, Y. Investigation on the effect of different additives on anaerobic co-digestion of corn straw and sewage sludge: Comparison of biochar, Fe3O4, and magnetic biochar. Bioresour. Technol. 2022, 345, 126532. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, S.; Yu, C.; Zhou, J. Effects of nano-sized MnO2 on methanogenic propionate and butyrate degradation in anaerobic digestion. J. Hazard. Mater. 2019, 364, 11–18. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.; Zhang, Z.; Cang, D.; Nwe, K.A.; Zheng, L.; Li, Z.; Cheng, S. Evaluating the Influences of ZnO Engineering Nanomaterials on VFA Accumulation in Sludge Anaerobic Digestion. Biochem. Eng. J. 2017, 125, 206–211. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Tian, L.; Zhang, L.; Cheng, S.; Li, Z.; Cang, D. Mechanistic investigation of toxicological change in ZnO and TiO2 multi-nanomaterial systems during anaerobic digestion and the microorganism response. Biochem. Eng. J. 2019, 147, 62–71. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Wang, Y.; Zhao, Y.; She, Z.; Gao, M.; Guo, Y. Application of iron oxide (Fe3O4) nanoparticles during the two-stage anaerobic digestion with waste sludge: Impact on the biogas production and the substrate metabolism. Renewable Energy 2020, 146, 2724–2735. [Google Scholar] [CrossRef]
- Ali, A.; Mahar, R.B.; Soomro, R.A.; Sherazi, S.T.H. Fe3O4 nanoparticles facilitated anaerobic digestion of organic fraction of municipal solid waste for enhancement of methane production. Energy Source Part A 2017, 39, 1815–1822. [Google Scholar] [CrossRef]
- Ünşar, E.K.; Çığgın, A.S.; Erdem, A.; Perendeci, N.A. Long- and Short-Term Impacts of CuO, Ag and CeO2 Nanoparticles on Anaerobic Digestion of Municipal Waste Activated Sludge. Environ. Sci.: Processes Impacts 2016, 18, 277–288. [Google Scholar]
- Temizel, I.; Emadian, S.M.; Di Addario, M.; Onay, T.T.; Demirel, B.; Copty, N.K.; Karanfil, T. Effect of nano-ZnO on biogas generation from simulated landfills. Waste Manage 2017, 63, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, B.; Li, A.; Zhang, L.; Li, R.; Yang, T.; Xing, W. Mechanism of process imbalance of long-term anaerobic digestion of food waste and role of trace elements in maintaining anaerobic process stability. Bioresour. Technol. 2019, 275, 172–182. [Google Scholar] [CrossRef]
- Molaey, R.; Bayrakdar, A.; Sürmeli, R.Ö.; Çalli, B. Influence of trace element supplementation on anaerobic digestion of chicken manure: Linking process stability to methanogenic population dynamics. J. Clean. Prod. 2018, 181, 794–800. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Li, A. Enhanced anaerobic digestion of food waste by trace metal elements supplementation and reduced metals dosage by green chelating agent [S, S]-EDDS via improving metals bioavailability. Water Res. 2015, 2015 84, 266–277. [Google Scholar] [CrossRef]
- Nakhate, S.P.; Gulhane, M.; Singh, A.K.; Purohit, H.J.; Shah, M.P.; Khardenavis, A.A. Trace metals as key controlling switches regulating the efficiencies of aerobic and anaerobic bioprocesses. Biochem. Eng. J. 2023, 198, 108999. [Google Scholar] [CrossRef]
- Linville, J.L.; Shen, Y.; Schoene, R.P.; Nguyen, M.; Urgun-Demirtas, M.; Snyder, S.W. Impact of trace element additives on anaerobic digestion of sewage sludge with in-situ carbon dioxide sequestration. Process Biochem. 2016, 51, 1283–1289. [Google Scholar] [CrossRef]
- Pobeheim, H.; Munk, B.; Lindorfer, H.; Guebitz, G.M. Impact of nickel and cobalt on biogas production and process stability during semi-continuous anaerobic fermentation of a model substrate for maize silage. Water Res. 2011, 45, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Ketheesan, B.; Thanh, P.M.; Stuckey, D.C. Iron deficiency and bioavailability in anaerobic batch and submerged membrane bioreactors (SAMBR) during organic shock loads. Bioresour. Technol. 2016, 211, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Myszograj, S.; Stadnik, A.; Płuciennik-Koropczuk, E. The influence of trace elements on anaerobic digestion process. Civ. Environ. Eng. Rep. 2018, 28, 105–115. [Google Scholar] [CrossRef]
- Gustavsson, J.; Shakeri Yekta, S.; Sundberg, C.; Karlsson, A.; Ejlertsson, J.; Skyllberg, U.; Svensson, B.H. Bioavailability of cobalt and nickel during anaerobic digestion of sulfur-rich stillage for biogas formation. Appl. Energy 2013, 112, 473–477. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Loh, K.C. Enhanced food waste anaerobic digestion: An encapsulated metal additive for shear stress-based controlled release. J. Clean. Prod. 2019, 235, 85–95. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Boxriker, M.; Lemmer, A.; Oechsner, H.; Jungbluth, T.; Mathies, E.; Ramhold, D. Effect of ethylenediaminetetraacetic acid (EDTA) on the bioavailability of trace elements during anaerobic digestion. Chem. Eng. J. 2013, 223, 436–441. [Google Scholar] [CrossRef]
- Friedmann, H.C.; Klein, A.; Thauer, R.K. Structure and function of the nickel porphinoid, coenzyme F430, and of its enzyme, methyl coenzyme M reductase. FEMS Microbiol. Lett. 1990, 87, 339–348. [Google Scholar] [CrossRef]
- Gustavsson, J.; Yekta, S.S.; Karlsson, A.; Skyllberg, U.; Bo, H.S. Potential bioavailability and chemical forms of Co and Ni in the biogas process - An evaluation based on sequential and acid volatile sulfide extractions. Eng. Life Sci. 2013, 13, 572–579. [Google Scholar] [CrossRef]
- Murakami, E.; Ragsdale, S.W. Evidence for Intersubunit Communication during Acetyl-Coa Cleavage by the Multienzyme Co Dehydrogenase/Acetyl-Coa Synthase Complex from Methanosarcina thermophila Evidence That the Beta Subunit Catalyzes C-C and C-S Bond Cleavage. J. Biol. Chem. 2000, 275, 4699–4707. [Google Scholar] [CrossRef]
- Uemura, S. Mineral requirements for mesophilic and thermophilic anaerobic digestion of organic solid waste. Int. J. Environ. Res. 2010, 4, 33–40. [Google Scholar]
- Yu, B.; Zhang, D.; Shan, A.; Lou, Z.; Yuan, H.; Huang, X.; Yuan, W.; Dai, X.; Zhu, N. Methane-rich biogas production from waste-activated sludge with the addition of ferric chloride under a thermophilic anaerobic digestion system. RSC Adv. 2015, 5, 38538–38546. [Google Scholar] [CrossRef]
- Feng, X.M.; Karlsson, A.; Svensson, B.H.; Bertilsson, S. Impact of trace element addition on biogas production from food industrial waste ^ linking process to microbial communities. FEMS Microbiol. Ecol. 2010, 74, 226–240. [Google Scholar] [CrossRef]
- Scherer, P.; Lippert, H.; Wolff, G. Composition of the Major Elements and Trace Elements of 10 Methanogenic Bacteria Determined by Inductively Coupled Plasma Emission Spectrometry. Biol. Trace Elem. Res. 1983, 5, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.; Shimada, K.; Speece, R.E. Minimum Requirements for Trace Metals (Iron, Nickel, Cobalt, and Zinc) in Thermophilic and Mesophilic Methane Fermentation from Glucose. Water Environ. Res. 2011, 83, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Paritosh, K.; Yadav, M.; Chawade, A.; Sahoo, D.; Kesharwani, N.; Pareek, N.; Vivekanand, V. Additives as a Support Structure for Specific Biochemical Activity Boosts in Anaerobic Digestion: A Review. Front. Energy Res. 2020, 8, 88. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; He, Y.; Wang, Y.; Wang, S.; Zheng, Z.; Wang, S.; Xu, J.; Cai, Y.; Ying, H. A Comprehensive Review of the Strategies to Improve Anaerobic Digestion: Their Mechanism and Digestion Performance. Methane 2024, 3, 227–256. [Google Scholar] [CrossRef]
- Ferdeș, M.; Dincă, M.N.; Moiceanu, G.; Zăbavă, B.Ș.; Paraschiv, G. Microorganisms and Enzymes Used in the Biological Pretreatment of the Substrate to Enhance Biogas Production: A Review. Sustainability 2020, 12, 7205. [Google Scholar] [CrossRef]
- Kubiak, A.; Pilarska, A.A.; Wolna-Maruwka, A.; Niewiadomska, A.; Panasiewicz, K. The Use of Fungi of the Trichoderma Genus in Anaerobic Digestion: A Review. Int. J. Mol. Sci. 2023, 24, 17576. [Google Scholar] [CrossRef]
- Kim, M.; Li, D.; Choi, O.; Sang, B.I.; Chiang, P.C.; Kim, H. Effects of supplement additives on anaerobic biogas production. Korean J. Chem. Eng. 2017, 34, 2678–2685. [Google Scholar] [CrossRef]
- Ye, M.; Liu, J.; Ma, C.; Li, Y.Y.; Zou, L.; Qian, G.; Xu, Z.P. Improving the stability and efficiency of anaerobic digestion of food waste using additives: A critical review. J. Clean. Prod. 2018, 192, 316–326. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Leng, X. Improving Biogas Production Using Additives in Anaerobic Digestion: A Review. J. Clean. Prod. 2021, 297, 126666. [Google Scholar] [CrossRef]
- Rajendran, K.; Drielak, E.; Varma, V.S.; Muthusamy, S.; Kumar, G. Updates on the Pretreatment of Lignocellulosic Feedstocks for Bioenergy Production—A Review. Biomass Conv. Bioref. 2018, 8, 471–483. [Google Scholar] [CrossRef]
- Kainthola, J.; Podder, A.; Fechner, M.; Goel, R. An Overview of Fungal Pretreatment Processes for Anaerobic Digestion: Applications, Bottlenecks and Future Needs. Bioresour. Technol. 2021, 321, 124397. [Google Scholar] [CrossRef] [PubMed]
- Kovács, E.; Szűcs, C.; Farkas, A.; Szuhaj, M.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Pretreatment of Lignocellulosic Biogas Substrates by Filamentous Fungi. J. Biotechnol. 2022, 360, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.M.; Wright, M.M. Anaerobic digestion fundamentals, challenges, and technological advances. Phys. Sci. Rev. 2023, 8, 2819–2837. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Lues, R. Anaerobic Digestion of Lignocellulosic Biomass: Substrate Characteristics (Challenge) and Innovation. Fermentation 2023, 9, 755. [Google Scholar] [CrossRef]
- Meng, Y.; Luan, F.; Yuan, H.; Chen, X.; Li, X. Enhancing anaerobic digestion performance of crude lipid in food waste by enzymatic pretreatment. Bioresour. Technol. 2017, 224, 48–55. [Google Scholar] [CrossRef]
- Speda, J.; Johansson, M.A.; Odnell, A.; Karlsson, M. Enhanced Biomethane Production Rate and Yield from Lignocellulosic Ensiled Forage Ley by in Situ Anaerobic Digestion Treatment with Endogenous Cellulolytic Enzymes. Biotechnol. Biofuels 2017, 10, 129. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Wu, D.; Zhang, P.; Wang, H.; Tao, X.; Ye, J.; Nabi, M. Enzyme pretreatment enhancing biogas yield from corn stover: Feasibility, optimization, and mechanism analysis. J. Agric. Food Chem. 2018, 66, 10026–10032. [Google Scholar] [CrossRef] [PubMed]
- Herrero Garcia, N.; Benedetti, M.; Bolzonella, D. Effects of enzymes addition on biogas production from anaerobic digestion of agricultural biomasses. Waste Biomass Valor. 2019, 10, 3711–3722. [Google Scholar] [CrossRef]
- Weide, T.; Baquero, C.D.; Schomaker, M.; Brügging, E.; Wetter, C. Effects of enzyme addition on biogas and methane yields in the batch anaerobic digestion of agricultural waste (silage, straw, and animal manure). Biomass Bioenergy 2020, 132, 105442. [Google Scholar] [CrossRef]
- Bhatnagar, R.; Ryan, D.; Murphy, R.; Enright, A.M. Effect of Co-digestion Ratio and Enzyme Treatment on Biogas Production from Grass Silage and Chicken Litter. Waste Biomass Valor 2019, 10, 3271–3277. [Google Scholar] [CrossRef]
- Öner, B.E.; Akyol, Ç.; Bozan, M.; Ince, O.; Aydin, S.; Ince, B. Bioaugmentation with Clostridium thermocellum to enhance the anaerobic biodegradation of lignocellulosic agricultural residues. Bioresour. Technol. 2018, 249, 620–625. [Google Scholar] [CrossRef]
- Akyol, Ç.; Ince, O.; Bozana, M.; Ozbayramb, E.G.; Ince, B. Fungal bioaugmentation of anaerobic digesters fed with lignocellulosic biomass: What to expect from anaerobic fungus Orpinomyces sp. Bioresour. Technol. 2019, 277, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ilo, O.P.; Nkomo, S.L.; Mkhize, N.M.; Mutanga, O.; Simatele, M.D. The effects of Trichoderma atroviride pretreatment on the biogas production from anaerobic digestion of water hyacinth. Energy Environ. 2024, 35, 1339–1358. [Google Scholar] [CrossRef]
- Ali, S.S.; Mustafa, A.M.; Kornaros, M.; Manni, A.; Sun, J.; Khalil, M.A. Construction of novel microbial consortia CS-5 and BC-4 valued for the degradation of catalpa sawdust and chlorophenols simultaneously with enhancing methane production. Bioresour. Technol. 2020, 301, 122720. [Google Scholar] [CrossRef]
- Mulat, D.G.; Huerta, S.G.; Kalyani, D.; Horn, S.J. Enhancing methane production from lignocellulosic biomass by combined steam-explosion pretreatment and bioaugmentation with cellulolytic bacterium Caldicellulosiruptor bescii. Biotechnol. Biofuels 2018, 11, 19. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.; Li, L.; Sun, Y. Bioaugmentation for overloaded anaerobic digestion recovery with acid-tolerant methanogenic enrichment. Waste Manag. 2018, 79, 744–751. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Sun, Y.; Yuan, Z. Bioaugmentation Strategy for Enhancing Anaerobic Digestion of High C/N Ratio Feedstock with Methanogenic Enrichment Culture. Bioresour. Technol. 2018, 261, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Yildirim, E.; Ince, O.; Ince, B. Rumen anaerobic Fungi create new opportunities for enhanced methane production from microalgae biomass. Algal Res. 2017, 23, 150–160. [Google Scholar] [CrossRef]
- Yildirim, E.; Ince, O.; Aydin, S.; Ince, B. Improvement of biogas potential of anaerobic digesters using rumen fungi. Renew. Energy 2017, 109, 346–353. [Google Scholar] [CrossRef]
- Lebiocka, M.; Montusiewicz, A.; Cydzik-Kwiatkowska, A. Effect of Bioaugmentation on Biogas Yields and Kinetics in Anaerobic Digestion of Sewage Sludge. Int. J. Environ. Res. Public Health 2018, 15, 1717. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.E.; Wang, Q.; Lim, E.Y.; Liu, Z.; He, J.; Tong, Y.W. Optimization of Bioaugmentation of the Anaerobic Digestion of Axonopus Compressus Cowgrass for the Production of Biomethane. J. Clean. Prod. 2020, 258, 120932. [Google Scholar] [CrossRef]
- Ferraro, A.; Dottorini, G.; Massini, G.; Mazzurco Miritana, V.; Signorini, A.; Lembo, G.; Fabbricino, M. Combined bioaugmentation with anaerobic ruminal fungi and fermentative bacteria to enhance biogas production from wheat straw and mushroom spent straw. Bioresour. Technol. 2018, 260, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, Z.; Cai, Y.; Zhao, Y.; Zhang, Y.; Gao, Y.; Cui, Z.; Wang, X. Accelerated biomethane production from lignocellulosic biomass: Pretreated by mixed enzymes secreted by Trichoderma viride and Aspergillus sp. Bioresour. Technol. 2020, 309, 123378. [Google Scholar] [CrossRef]
- Vinzelj, J.; Joshi, A.; Insam, H.; Podmirseg, S.M. Employing anaerobic fungi in biogas production: Challenges & opportunities. Bioresour. Technol. 2020, 300, 122687. [Google Scholar]
- Wilken, S.E.; Saxena, M.; Petzold, L.R.; O’Malley, M.A. In Silico Identification of Microbial Partners to Form Consortia with Anaerobic Fungi. Processes 2018, 6, 7. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Jin, W.; Sharpton, T.J.; Mackie, R.I.; Cann, I.; Cheng, Y.; Zhu, W. Combined genomic, transcriptomic, proteomic, and physiological characterization of the growth of Pecoramyces sp. F1 in monoculture and co-culture with a syntrophic methanogen. Front. Microbiol. 2019, 10, 435. [Google Scholar] [CrossRef]
- Swift, C.L.; Brown, J.L.; Seppälä, S.; O’Malley, M.A. Co-cultivation of the anaerobic fungus Anaeromyces robustus with Methanobacterium bryantii enhances transcription of carbohydrate active enzymes. J. Ind. Microbiol. Biotechnol. 2019, 46, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yan, M.; Treu, L.; Angelidaki, I.; Fotidis, I.A. Hydrogenotrophic methanogens are the key for a successful bioaugmentation to alleviate ammonia inhibition in thermophilic anaerobic digesters. Bioresour. Technol. 2019, 293, 122070. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Liu, C.; Zhang, R.; Liu, G. Mitigation of ammonia inhibition through bioaugmentation with different microorganisms during anaerobic digestion: Selection of strains and reactor performance evaluation. Water Res. 2019, 155, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Undiandeye, J.; Gallegos, D.; Lenz, J.; Nelles, M.; Stinner, W. Effect of Novel Aspergillus and Neurospora Species-Based Additive on Ensiling Parameters and Biomethane Potential of Sugar Beet Leaves. Appl. Sci. 2022, 12, 2684. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A.M. Trace Element Supplementation and Enzyme Addition to Enhance Biogas Production by Anaerobic Digestion of Chicken Litter. Energies 2020, 13, 3477. [Google Scholar] [CrossRef]
- Poszytek, K.; Karczewska-Golec, J.; Ciok, A.; Decewicz, P.; Dziurzynski, M.; Gorecki, A.; Jakusz, G.; Krucon, T.; Lomza, P.; Romaniuk, K.; Styczynski, M.; Yang, Z.; Drewniak, L.; Dziewit, L. Genome-Guided Characterization of Ochrobactrum sp. POC9 Enhancing Sewage Sludge Utilization - Biotechnological Potential and Biosafety Considerations. Int. J. Environ. Res. Public Health 2018, 15, 1501. [Google Scholar] [CrossRef]
- Deng, Y.; Dai, B.; Xu, J.; Liu, X.; Xu, J. Anaerobic co-digestion of rice straw and soybean straw to increase biogas production by pretreatment with Trichoderma reesei RUT C30. Environ. Prog. Sustain. Energy 2018, 37, 1050–1057. [Google Scholar] [CrossRef]
- Fernández-García, M.; Rodriguez, J.A. Metal Oxide Nanoparticles. In Encyclopedia of Inorganic Chemistry; John Wiley & Sons.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Hamed, S.M.; Hassan, S.H.; Selim, S.; Kumar, A.; Khalaf, S.M.H.; Wadaan, M.A.M.; Hozzein, W.N.; AbdElgawad, H. Physiological and biochemical responses to aluminum-induced oxidative stress in two cyanobacterial species. Environ. Pollut. 2019, 251, 961–969. [Google Scholar] [CrossRef]
- Noyola, A.; Tinajero, A. Effect of biological additives and micronutrients on the anaerobic digestion of physicochemical sludge. Water Sci Technol, 2005, 52, 275–281. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Kulupa, T.; Kubiak, A.; Wolna-Maruwka, A.; Pilarski, K.; Niewiadomska, A. Anaerobic Digestion of Food Waste - A Short Review. Energies 2023, 16, 5742. [Google Scholar] [CrossRef]
- Pilarski, K.; Pilarska, A.A.; Kolasa-Więcek, A.; Suszanowicz, D. An Agricultural Biogas Plant as a Thermodynamic System: A Study of Efficiency in the Transformation from Primary to Secondary Energy. Energies 2023, 16, 7398. [Google Scholar] [CrossRef]
| Type of additive | Dosage (g/L) | Type of substrate | Effect | References |
|---|---|---|---|---|
| GAC | 50.0 | dog food | enables the process to proceed at high OLR values and improves process efficiency | [27] |
| GAC | 6.0 | acetic acid and ethanol |
increase in methane yield by 31% | [28] |
| GAC | – | rapeseed oil | increase in methane yield | [29] |
| GAC | 33.3 | black water (urine and feces) |
increase in methane yield by 57% | [30] |
| PAC | 5.0 10.0 |
food waste, vegetable waste |
higher yield provided by PAC than GAC, PAC degrades VFA more efficiently than GAC |
[26] |
| PAC | 0.125–1 | pre-treated activated sludge subjected to thermal hydrolysis |
stimulates hydrolysis activity, increases methanogenic activity, and speeds up VS removal |
[31] |
| PAC | 15.0 | poultry blood | enhances syntrophic metabolism | [32] |
| PAC | 15.0 | organic fraction of municipal solid waste |
reduction of inhibitor content (FAN, VFA), 17% higher methane yield |
[33] |
| Dose of biochar (g/L) |
Type of substrate | Effect | References |
|---|---|---|---|
| 10 | glucose | reduced downtime and faster fermentation start | [40] |
| 10 | food waste | increased average methane yield by 14% | [41] |
| – | waste water | improved methane yield and enhanced degradation of protein substances |
[42] |
| 20 | volatile fatty acids | reduced downtime and faster methane production start | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





