Submitted:
31 July 2024
Posted:
31 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Epidemiology
4. Risk Factors
4.1. Patient-Related Factors
4.2. Disease-Related Factors
5. Endoscopic Surveillance
6. IBD Therapy and Cancer
6.1. Management of IBD Therapy in Patients with a History of Previous Cancer
6.2. Management of IBD Therapy in Patients with Current Cancer
7. Management of Chemotherapy and Radiation Therapy in IBD
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Porter, R.J.; Arends, M.J.; Churchhouse, A.M.D. , e S. Din, «Inflammatory Bowel Disease-Associated Colorectal Cancer: Translational Risks from Mechanisms to Medicines», J. Crohns Colitis, vol. 15, fasc. 12, pp. 2131–2141, dic. 2021. [CrossRef]
- Shah, S.C. e S. H. Itzkowitz, «Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management», Gastroenterology, vol. 162, fasc. 3, pp. 715-730.e3, mar. 2022. [CrossRef]
- Sato, Y.; Tsujinaka, S.; Miura, T.; Kitamura, Y.; Suzuki, H. , e C. Shibata, «Inflammatory Bowel Disease and Colorectal Cancer: Epidemiology, Etiology, Surveillance, and Management», Cancers, vol. 15, fasc. 16, p. 4154, ago. 2023. [CrossRef]
- Westwood, M.; et al. , «Faecal immunochemical tests (FIT) can help to rule out colorectal cancer in patients presenting in primary care with lower abdominal symptoms: a systematic review conducted to inform new NICE DG30 diagnostic guidance», BMC Med., vol. 15, fasc. 1, p. 189, dic. 2017. [CrossRef]
- Yalchin, M.; Baker, A.-M.; Graham, T.A. , e A. Hart, «Predicting Colorectal Cancer Occurrence in IBD», Cancers, vol. 13, fasc. 12, p. 2908, giu. 2021. [CrossRef]
- Mattar, M.C.; Lough, D.; Pishvaian, M.J. , e A. Charabaty, «Current management of inflammatory bowel disease and colorectal cancer», Gastrointest. Cancer Res. GCR, vol. 4, fasc. 2, pp. 53–61, mar. 2011.
- Ananthakrishnan, A.N.; et al. , «Colonoscopy Is Associated With a Reduced Risk for Colon Cancer and Mortality in Patients With Inflammatory Bowel Diseases», Clin. Gastroenterol. Hepatol., vol. 13, fasc. 2, pp. 322-329.e1, feb. 2015. [CrossRef]
- Beaugerie, L. e S. H. Itzkowitz, «Cancers Complicating Inflammatory Bowel Disease», N. Engl. J. Med., vol. 372, fasc. 15, pp. 1441–1452, apr. 2015. [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A. , e F. Bray, «Global patterns and trends in colorectal cancer incidence and mortality», Gut, vol. 66, fasc. 4, pp. 683–691, apr. 2017. [CrossRef]
- Wheat, C.L.; Clark-Snustad, K.; Devine, B.; Grembowski, D.; Thornton, T.A. , e C. W. Ko, «Worldwide Incidence of Colorectal Cancer, Leukemia, and Lymphoma in Inflammatory Bowel Disease: An Updated Systematic Review and Meta-Analysis», Gastroenterol. Res. Pract., vol. 2016, pp. 1–18, 2016. [CrossRef]
- Jess, T.; Gamborg, M.; Matzen, P.; Munkholm, P. , e T. I. A. Sorensen, «Increased Risk of Intestinal Cancer in Crohn’s Disease: A Meta-Analysis of Population-Based Cohort Studies», Am. J. Gastroenterol., vol. 100, fasc. 12, pp. 2724–2729, dic. 2005. [CrossRef]
- Jess, T.; Rungoe, C. , e L. Peyrin–Biroulet, «Risk of Colorectal Cancer in Patients With Ulcerative Colitis: A Meta-analysis of Population-Based Cohort Studies», Clin. Gastroenterol. Hepatol., vol. 10, fasc. 6, pp. 639–645, giu. 2012. [CrossRef]
- Lutgens, M.W.M.D.; Van Oijen, M.G.H.; Van Der Heijden, G.J.M.G.; Vleggaar, F.P.; Siersema, P.D. , e B. Oldenburg, «Declining Risk of Colorectal Cancer in Inflammatory Bowel Disease: An Updated Meta-analysis of Population-based Cohort Studies», Inflamm. Bowel Dis., vol. 19, fasc. 4, pp. 789–799, mar. 2013. [CrossRef]
- Annese, V.; et al. , «European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies», J. Crohns Colitis, vol. 9, fasc. 11, pp. 945–965, nov. 2015. [CrossRef]
- Eaden, J.A. , «The risk of colorectal cancer in ulcerative colitis: a meta-analysis», Gut, vol. 48, fasc. 4, pp. 526–535, apr. 2001. [CrossRef]
- Selinger, C.P.; et al. , «Long-term Follow-up Reveals Low Incidence of Colorectal Cancer, but Frequent Need for Resection, Among Australian Patients With Inflammatory Bowel Disease», Clin. Gastroenterol. Hepatol., vol. 12, fasc. 4, pp. 644–650, apr. 2014. [CrossRef]
- Qiu, X.; Ma, J.; Wang, K. , e H. Zhang, «Chemopreventive effects of 5-aminosalicylic acid on inflammatory bowel disease-associated colorectal cancer and dysplasia: a systematic review with meta-analysis», Oncotarget, vol. 8, fasc. 1, pp. 1031–1045, gen. 2017. [CrossRef]
- Samadder, N.J.; et al. , «Family History Associates With Increased Risk of Colorectal Cancer in Patients With Inflammatory Bowel Diseases», Clin. Gastroenterol. Hepatol., vol. 17, fasc. 9, pp. 1807-1813.e1, ago. 2019. [CrossRef]
- Nuako, K.; Ahlquist, D.; Mahoney, D.; Schaid, D.; Siems, D. , e N. Lindor, «Familial predisposition for colorectal cancer in chronic ulcerative colitis: A case-control study», Gastroenterology, vol. 115, fasc. 5, pp. 1079–1083, nov. 1998. [CrossRef]
- Wijnands, A.M.; De Jong, M.E.; Lutgens, M.W.M.D.; Hoentjen, F.; Elias, S.G. , e B. Oldenburg, «Prognostic Factors for Advanced Colorectal Neoplasia in Inflammatory Bowel Disease: Systematic Review and Meta-analysis», Gastroenterology, vol. 160, fasc. 5, pp. 1584–1598, apr. 2021. [CrossRef]
- Söderlund, S.; et al. , «Inflammatory Bowel Disease Confers a Lower Risk of Colorectal Cancer to Females Than to Males», Gastroenterology, vol. 138, fasc. 5, pp. 1697-1703.e2, mag. 2010. [CrossRef]
- Soetikno, R.M.; Lin, O.S.; Heidenreich, P.A.; Young, H.S. , e M. O. Blackstone, «Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: A meta-analysis», Gastrointest. Endosc., vol. 56, fasc. 1, pp. 48–54, lug. 2002. [CrossRef]
- Magro, F.; et al. , «Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders», J. Crohns Colitis, vol. 11, fasc. 6, pp. 649–670, giu. 2017. [CrossRef]
- Ekbom, A.; Helmick, C.; Zack, M. , e H.-O. Adami, «Ulcerative Colitis and Colorectal Cancer: A Population-Based Study», N. Engl. J. Med., vol. 323, fasc. 18, pp. 1228–1233, nov. 1990. [CrossRef]
- Rutter, M.; et al. , «Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis», Gastroenterology, vol. 126, fasc. 2, pp. 451–459, feb. 2004. [CrossRef]
- Gupta, R.B.; et al. , «Histologic Inflammation Is a Risk Factor for Progression to Colorectal Neoplasia in Ulcerative Colitis: A Cohort Study», Gastroenterology, vol. 133, fasc. 4, pp. 1099–1105, ott. 2007. [CrossRef]
- Rubin, D.T.; et al. , «Inflammation Is an Independent Risk Factor for Colonic Neoplasia in Patients With Ulcerative Colitis: A Case–Control Study», Clin. Gastroenterol. Hepatol., vol. 11, fasc. 12, pp. 1601-1608.e4, dic. 2013. [CrossRef]
- Flores, B.M.; O’Connor, A. , e A. C. Moss, «Impact of mucosal inflammation on risk of colorectal neoplasia in patients with ulcerative colitis: a systematic review and meta-analysis», Gastrointest. Endosc., vol. 86, fasc. 6, pp. 1006-1011.e8, dic. 2017. [CrossRef]
- Kirchgesner, J.; et al. , «Nancy Index Scores of Chronic Inflammatory Bowel Disease Activity Associate With Development of Colorectal Neoplasia», Clin. Gastroenterol. Hepatol., vol. 18, fasc. 1, pp. 150-157.e1, gen. 2020. [CrossRef]
- Coelho-Prabhu, N. e J. D. Lewis, «Update on Endoscopic Dysplasia Surveillance in Inflammatory Bowel Disease», Am. J. Gastroenterol., vol. 118, fasc. 10, pp. 1748–1755, ott. 2023. [CrossRef]
- Huguet, J.M.; et al. , «Colorectal cancer screening and surveillance in patients with inflammatory bowel disease in 2021», World J. Gastroenterol., vol. 28, fasc. 5, pp. 502–516, feb. 2022. [CrossRef]
- Vento, P.; Lepistö, A.; Kärkkäinen, P.; Ristimäki, A.; Haglund, C. , e H. J. Järvinen, «Risk of cancer in patients with chronic pouchitis after restorative proctocolectomy for ulcerative colitis», Colorectal Dis., vol. 13, fasc. 1, pp. 58–66, gen. 2011. [CrossRef]
- Derikx, L. A. A. P. . L. H. C. Nissen, L. J. T. Smits, B. Shen, e F. Hoentjen, «Risk of Neoplasia After Colectomy in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis», Clin. Gastroenterol. Hepatol., vol. 14, fasc. 6, pp. 798-806.e20, giu. 2016. [CrossRef]
- Murthy, S.K.; Feuerstein, J.D.; Nguyen, G.C. , e F. S. Velayos, «AGA Clinical Practice Update on Endoscopic Surveillance and Management of Colorectal Dysplasia in Inflammatory Bowel Diseases: Expert Review», Gastroenterology, vol. 161, fasc. 3, pp. 1043-1051.e4, set. 2021. [CrossRef]
- Al Bakir, I.; Kabir, M.; Yalchin, M. , e A. Hart, «Optimising inflammatory bowel disease surveillance and dysplasia management—Where do we stand?», United Eur. Gastroenterol. J., vol. 10, fasc. 10, pp. 1054–1062, dic. 2022. [CrossRef]
- Laine, L.; et al. , «SCENIC International Consensus Statement on Surveillance and Management of Dysplasia in Inflammatory Bowel Disease», Gastroenterology, vol. 148, fasc. 3, pp. 639-651.e28, mar. 2015. [CrossRef]
- Rabinowitz, L.G.; Kumta, N.A. , e J. F. Marion, «Beyond the SCENIC route: updates in chromoendoscopy and dysplasia screening in patients with inflammatory bowel disease», Gastrointest. Endosc., vol. 95, fasc. 1, pp. 30–37, gen. 2022. [CrossRef]
- Lamb, C.A.; et al. , «British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults», Gut, vol. 68, fasc. Suppl 3, pp. s1–s106, dic. 2019. [CrossRef]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G. , e M. D. Long, «ACG Clinical Guideline: Ulcerative Colitis in Adults», Am. J. Gastroenterol., vol. 114, fasc. 3, pp. 384–413, mar. 2019. [CrossRef]
- Gordon, H.; et al. , «ECCO Guidelines on Inflammatory Bowel Disease and Malignancies», J. Crohns Colitis, vol. 17, fasc. 6, pp. 827–854, giu. 2023. [CrossRef]
- Jang, J.-Y. , «The Past, Present, and Future of Image-Enhanced Endoscopy», Clin. Endosc., vol. 48, fasc. 6, pp. 466–475, nov. 2015. [CrossRef]
- Marion, J.F.; et al. , «Chromoendoscopy-Targeted Biopsies Are Superior to Standard Colonoscopic Surveillance for Detecting Dysplasia in Inflammatory Bowel Disease Patients: A Prospective Endoscopic Trial», Am. J. Gastroenterol., vol. 103, fasc. 9, pp. 2342–2349, set. 2008. [CrossRef]
- Bisschops, R.; et al. , «Chromoendoscopy versus narrow band imaging in UC: a prospective randomised controlled trial», Gut, vol. 67, fasc. 6, pp. 1087–1094, giu. 2018. [CrossRef]
- Moussata, D.; et al. , «Are random biopsies still useful for the detection of neoplasia in patients with IBD undergoing surveillance colonoscopy with chromoendoscopy?», Gut, p. gutjnl-2016-311892, gen. 2017. [CrossRef]
- Kandiah, K.; et al. , «Multicentre randomised controlled trial on virtual chromoendoscopy in the detection of neoplasia during colitis surveillance high-definition colonoscopy (the VIRTUOSO trial)», Gut, vol. 70, fasc. 9, pp. 1684–1690, set. 2021. [CrossRef]
- Beaugerie, L.; et al. , «Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer», Gut, vol. 63, fasc. 9, pp. 1416–1423, set. 2014. [CrossRef]
- Acuna, S.A.; Huang, J.W.; Dossa, F.; Shah, P.S.; Kim, S.J. , e N. N. Baxter, «Cancer recurrence after solid organ transplantation: A systematic review and meta-analysis», Transplant. Rev., vol. 31, fasc. 4, pp. 240–248, ott. 2017. [CrossRef]
- Bernheim, O.; Colombel, J.-F.; Ullman, T.A.; Laharie, D.; Beaugerie, L. , e S. H. Itzkowitz, «The management of immunosuppression in patients with inflammatory bowel disease and cancer», Gut, vol. 62, fasc. 11, pp. 1523–1528, nov. 2013. [CrossRef]
- Shelton, E.; et al. , «Cancer Recurrence Following Immune-Suppressive Therapies in Patients With Immune-Mediated Diseases: A Systematic Review and Meta-analysis», Gastroenterology, vol. 151, fasc. 1, pp. 97-109.e4, lug. 2016. [CrossRef]
- Poullenot, F.; et al. , «Comparative Risk of Incident Cancer in Patients with Inflammatory Bowel Disease with Prior Non-digestive Malignancy According to Immunomodulator: a Multicentre Cohort Study», J. Crohns Colitis, vol. 16, fasc. 10, pp. 1523–1530, nov. 2022. [CrossRef]
- Poullenot, F. e D. Laharie, «Management of Inflammatory Bowel Disease in Patients with Current or Past Malignancy», Cancers, vol. 15, fasc. 4, p. 1083, feb. 2023. [CrossRef]
- Mañosa, M.; et al. , «Immunomodulatory Therapy Does Not Increase the Risk of Cancer in Persons With Inflammatory Bowel Disease and a History of Extracolonic Cancers», Am. J. Gastroenterol., vol. 114, fasc. 5, pp. 771–776, mag. 2019. [CrossRef]
- Laredo, V.; García-Mateo, S.; Martínez-Domínguez, S.J.; De La Cruz, J.L.; Gargallo-Puyuelo, C.J. , e F. Gomollón, «Risk of Cancer in Patients with Inflammatory Bowel Diseases and Keys for Patient Management», Cancers, vol. 15, fasc. 3, p. 871, gen. 2023. [CrossRef]
- Waljee, A.K.; et al. , «Anti-tumour necrosis factor-α therapy and recurrent or new primary cancers in patients with inflammatory bowel disease, rheumatoid arthritis, or psoriasis and previous cancer in Denmark: a nationwide, population-based cohort study», Lancet Gastroenterol. Hepatol., vol. 5, fasc. 3, pp. 276–284, mar. 2020. [CrossRef]
- Cohen, R.D.; Bhayat, F.; Blake, A. , e S. Travis, «The Safety Profile of Vedolizumab in Ulcerative Colitis and Crohn’s Disease: 4 Years of Global Post-marketing Data», J. Crohns Colitis, vol. 14, fasc. 2, pp. 192–204, feb. 2020. [CrossRef]
- Card, T.; Ungaro, R.; Bhayat, F.; Blake, A.; Hantsbarger, G. , e S. Travis, «Vedolizumab use is not associated with increased malignancy incidence: GEMINI LTS study results and post-marketing data», Aliment. Pharmacol. Ther., vol. 51, fasc. 1, pp. 149–157, gen. 2020. [CrossRef]
- Hong, S.J.; et al. , «Ustekinumab and Vedolizumab Are Not Associated With Subsequent Cancer in IBD Patients with Prior Malignancy», Inflamm. Bowel Dis., vol. 28, fasc. 12, pp. 1826–1832, dic. 2022. [CrossRef]
- Ghosh, S.; et al. , «Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis Through 5 Years in Crohn’s Disease and 4 Years in Ulcerative Colitis», J. Crohns Colitis, p. jjae013, feb. 2024. [CrossRef]
- Panés, J.; et al. , «Analysis of tofacitinib safety in ulcerative colitis from the completed global clinical developmental program up to 9.2 years of drug exposure», United Eur. Gastroenterol. J., p. ueg2.12584, mag. 2024. [CrossRef]
- Ferrante, M.; et al. , «Long-Term Safety and Efficacy of Risankizumab Treatment in Patients with Crohn’s Disease: Results from the Phase 2 Open-Label Extension Study», J. Crohns Colitis, vol. 15, fasc. 12, pp. 2001–2010, dic. 2021. [CrossRef]
- Blauvelt, A.; et al. , «Malignancy rates through 5 years of follow-up in patients with moderate-to-severe psoriasis treated with guselkumab: Pooled results from the VOYAGE 1 and VOYAGE 2 trials», J. Am. Acad. Dermatol., vol. 89, fasc. 2, pp. 274–282, ago. 2023. [CrossRef]
- Wetwittayakhlang, P.; Tselekouni, P.; Al-Jabri, R.; Bessissow, T. , e P. L. Lakatos, «The Optimal Management of Inflammatory Bowel Disease in Patients with Cancer», J. Clin. Med., vol. 12, fasc. 6, p. 2432, mar. 2023. [CrossRef]
- Sebastian, S. e S. Neilaj, «Practical guidance for the management of inflammatory bowel disease in patients with cancer. Which treatment?», Ther. Adv. Gastroenterol., vol. 12, p. 175628481881729, gen. 2019. [CrossRef]
- Khoury, W.; Lavery, I.C. , e R. P. Kiran, «Effects of Chronic Immunosuppression on Long-term Oncologic Outcomes for Colorectal Cancer Patients Undergoing Surgery», Ann. Surg., vol. 253, fasc. 2, pp. 323–327, feb. 2011. [CrossRef]
- Grimsdottir, S.; Attauabi, M.; Dahl, E.K.; Burisch, J. , e J. B. Seidelin, «Systematic Review with Meta-analysis: The Impact of Cancer Treatments on the Disease Activity of Inflammatory Bowel Diseases», J. Crohns Colitis, vol. 17, fasc. 7, pp. 1139–1153, lug. 2023. [CrossRef]
- Axelrad, J.E.; Fowler, S.A.; Friedman, S.; Ananthakrishnan, A.N. , e V. Yajnik, «Effects of Cancer Treatment on Inflammatory Bowel Disease Remission and Reactivation», Clin. Gastroenterol. Hepatol., vol. 10, fasc. 9, pp. 1021-1027.e1, set. 2012. [CrossRef]
- Axelrad, J.E.; et al. , «Hormone Therapy for Cancer Is a Risk Factor for Relapse of Inflammatory Bowel Diseases», Clin. Gastroenterol. Hepatol., vol. 18, fasc. 4, pp. 872-880.e1, apr. 2020. [CrossRef]
- Conceição, D.; Saraiva, M.R.; Rosa, I. , e I. Claro, «Inflammatory Bowel Disease Treatment in Cancer Patients—A Comprehensive Review», Cancers, vol. 15, fasc. 12, p. 3130, giu. 2023. [CrossRef]
- O’Reilly, M.; Mellotte, G.; Ryan, B. , e A. O’Connor, «Gastrointestinal side effects of cancer treatments», Ther. Adv. Chronic Dis., vol. 11, p. 204062232097035, gen. 2020. [CrossRef]
- Stein, A. W. Voigt, e K. Jordan, «Review: Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management», Ther. Adv. Med. Oncol., vol. 2, fasc. 1, pp. 51–63, gen. 2010. [CrossRef]
- Soularue, E.; et al. , «Enterocolitis due to immune checkpoint inhibitors: a systematic review», Gut, vol. 67, fasc. 11, pp. 2056–2067, nov. 2018. [CrossRef]
- Desmedt, V.; et al. , «Position statement on the management of the immune checkpoint inhibitor-induced colitis via multidisciplinary modified Delphi consensus», Eur. J. Cancer, vol. 187, pp. 36–57, lug. 2023. [CrossRef]
- Haanen, J.; et al. , «Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up», Ann. Oncol., vol. 33, fasc. 12, pp. 1217–1238, dic. 2022. [CrossRef]
- Marthey, L.; et al. , «Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease», J. Crohns Colitis, vol. 10, fasc. 4, pp. 395–401, apr. 2016. [CrossRef]
- Bergqvist, V.; et al. , «Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis», Cancer Immunol. Immunother., vol. 66, fasc. 5, pp. 581–592, mag. 2017. [CrossRef]
- Bishu, S.; Melia, J.; Sharfman, W.; Lao, C.D.; Fecher, L.A. , e P. D. R. Higgins, «Efficacy and Outcome of Tofacitinib in Immune checkpoint Inhibitor Colitis», Gastroenterology, vol. 160, fasc. 3, pp. 932-934.e3, feb. 2021. [CrossRef]
- Willett, C.G.; et al. , «Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms», Int. J. Radiat. Oncol., vol. 46, fasc. 4, pp. 995–998, mar. 2000. [CrossRef]
- Bodofsky, S.; et al. , «Inflammatory bowel disease-associated malignancies and considerations for radiation impacting bowel: a scoping review», J. Gastrointest. Oncol., vol. 13, fasc. 5, pp. 2565–2582, ott. 2022. [CrossRef]
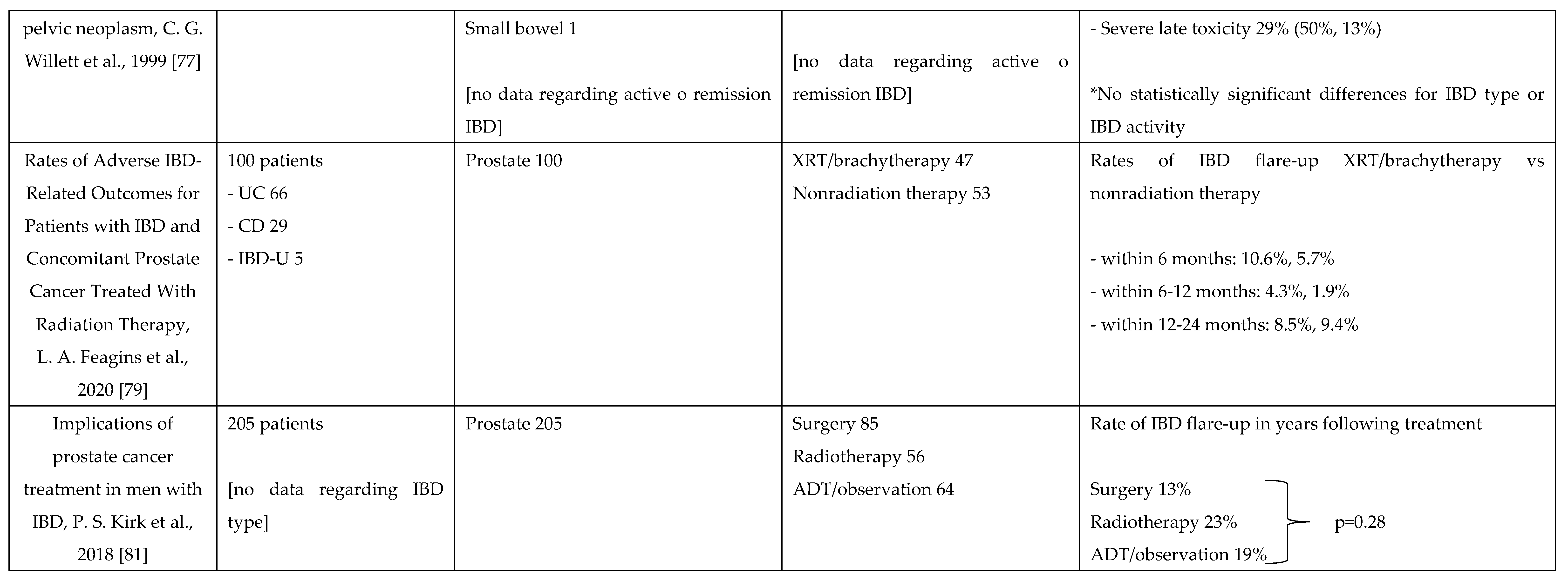
- Feagins, L.A.; et al. , «Rates of Adverse IBD-Related Outcomes for Patients With IBD and Concomitant Prostate Cancer Treated With Radiation Therapy», Inflamm. Bowel Dis., vol. 26, fasc. 5, pp. 728–733, apr. 2020. [CrossRef]
- Green, S.; Stock, R.G. , e A. J. Greenstein, «Rectal cancer and inflammatory bowel disease: natural history and implications for radiation therapy», Int. J. Radiat. Oncol., vol. 44, fasc. 4, pp. 835–840, lug. 1999. [CrossRef]
- Kirk, P.S.; et al. , «Implications of Prostate Cancer Treatment in Men With Inflammatory Bowel Disease», Urology, vol. 104, pp. 131–136, giu. 2017. [CrossRef]
- Brodersen, J.B.; Knudsen, T.; Kjeldsen, J.; Juel, M.A.; Rafaelsen, S.R. , e M. D. Jensen, «Diagnostic accuracy of pan-enteric capsule endoscopy and magnetic resonance enterocolonography in suspected Crohn’s disease», United Eur. Gastroenterol. J., vol. 10, fasc. 9, pp. 973–982, nov. 2022. [CrossRef]
- Solitano, V.; et al. , «Artificial Endoscopy and Inflammatory Bowel Disease: Welcome to the Future», J. Clin. Med., vol. 11, fasc. 3, p. 569, gen. 2022. [CrossRef]
- Da Rio, L.; et al. , «Artificial intelligence and inflammatory bowel disease: Where are we going?», World J. Gastroenterol., vol. 29, fasc. 3, pp. 508–520, gen. 2023. [CrossRef]
- Onuora, S. , «Cancer recurrence risk not increased by DMARDs», Nat. Rev. Rheumatol., vol. 17, fasc. 12, pp. 707–707, dic. 2021. [CrossRef]
- Sepriano, A.; et al. , «Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis», Ann. Rheum. Dis., vol. 79, fasc. 6, pp. 760–770, giu. 2020. [CrossRef]
- Lee, C.K. e G. Y. Melmed, «Multidisciplinary Team-Based Approaches to IBD Management: How Might “One-Stop Shopping” Work for Complex IBD Care?», Am. J. Gastroenterol., vol. 112, fasc. 6, pp. 825–827, giu. 2017. [CrossRef]
- Helwig, U.; et al. , «Transmural Response and Transmural Healing Defined by Intestinal Ultrasound: New Potential Therapeutic Targets?», J. Crohns Colitis, vol. 16, fasc. 1, pp. 57–67, gen. 2022. [CrossRef]
- D’Amico, F.; et al. , «International consensus on methodological issues in standardization of fecal calprotectin measurement in inflammatory bowel diseases», United Eur. Gastroenterol. J., vol. 9, fasc. 4, pp. 451–460, mag. 2021. [CrossRef]
- DʼHaens, G.; et al. , «Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease»:, Inflamm. Bowel Dis., vol. 18, fasc. 12, pp. 2218–2224, dic. 2012. [CrossRef]
- Theede, K.; Holck, S.; Ibsen, P.; Ladelund, S.; Nordgaard-Lassen, I. , e A. M. Nielsen, «Level of Fecal Calprotectin Correlates With Endoscopic and Histologic Inflammation and Identifies Patients With Mucosal Healing in Ulcerative Colitis», Clin. Gastroenterol. Hepatol., vol. 13, fasc. 11, pp. 1929-1936.e1, nov. 2015. [CrossRef]
- D’Amico, F.; Nancey, S.; Danese, S. , e L. Peyrin-Biroulet, «A Practical Guide for Faecal Calprotectin Measurement: Myths and Realities», J. Crohns Colitis, vol. 15, fasc. 1, pp. 152–161, gen. 2021. [CrossRef]
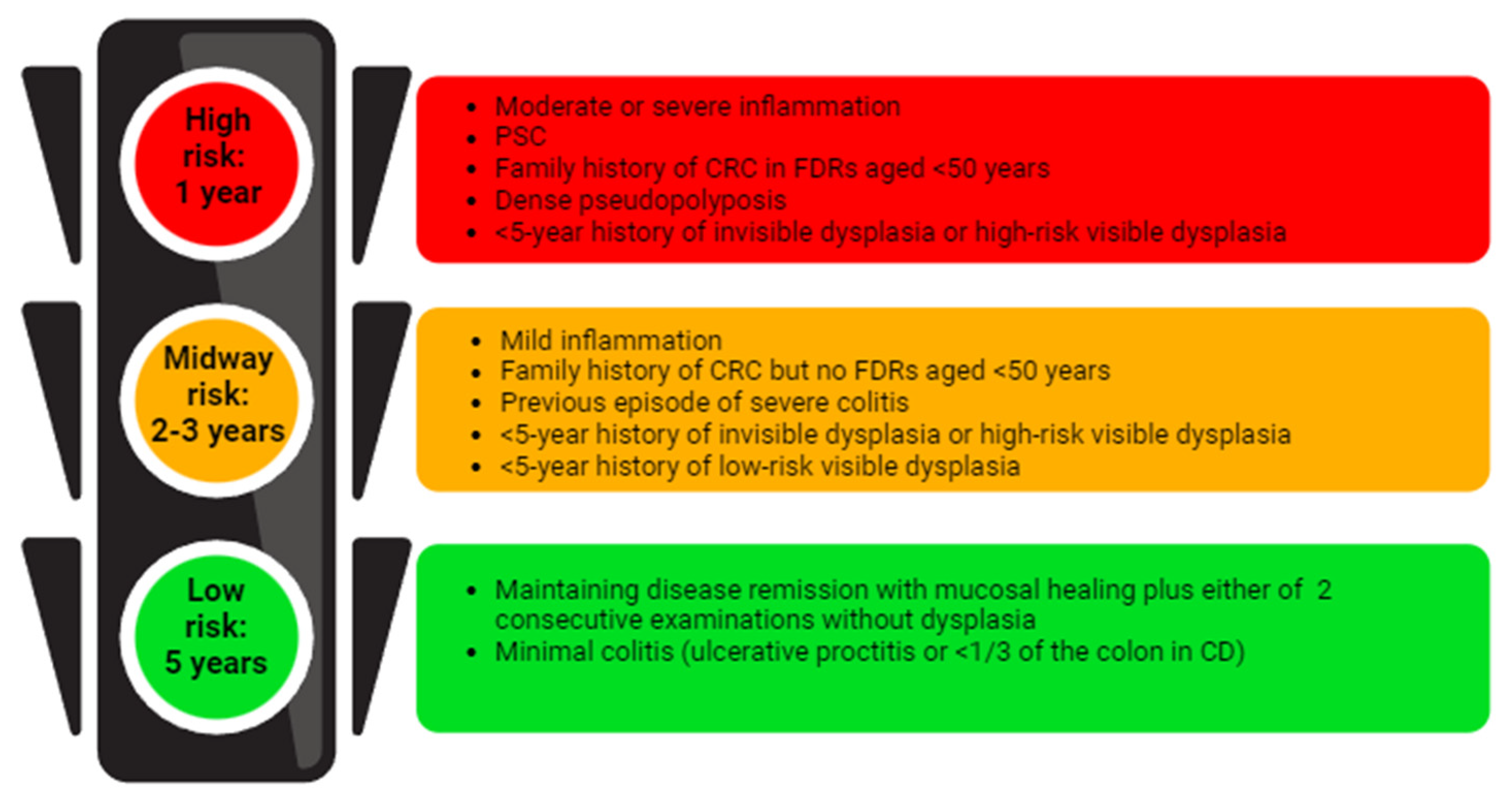
| Guideline (year of publication) | Type of endoscopic surveillance | Random or targeted biopsies |
| SCENIC Consensus (2015) [36] | HD recommended If SD, dye-CE recommended If HD, dye-CE suggested |
No consensus |
| SCENIC commentary (2022) [37] | HD-WLE, dye-CE, or VCE | Random limited to highest-risk groups only (PSC, prior dysplasia, atrophic scarred colon, ongoing active inflammation) |
| ECCO Guideline (2017) [23] | HD recommended | Random if WL Targeted only if dye-CE |
| ECCO Guideline (2023) [40] | HD-WLE, dye-CE, or VCE | Targeted biopsies Random in high-risk (PSC or history of dysplasia) |
| ACG Clinical Guideline (2019) [39] | If SD, dye-CE recommended If HD, dye-CE or VCE recommended |
No recommendation |
| AGA Clinical Practice update (2021) [34] |
HD recommended Dye-CE should be considered VCE acceptable alternative if HD |
Random if WL only and all patients with high risk (PSC or history of dysplasia) Targeted if dye-CE or VCE |
| BSG Guideline (2019) [38] | HD recommended If SD, dye-CE recommended If HD, dye-CE suggested NBI not suggested |
Targeted recommended |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





