Submitted:
31 July 2024
Posted:
31 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Lessons to Be Learnt from LiBs to Develop Thermally Resilient SEI Layer in SiBs
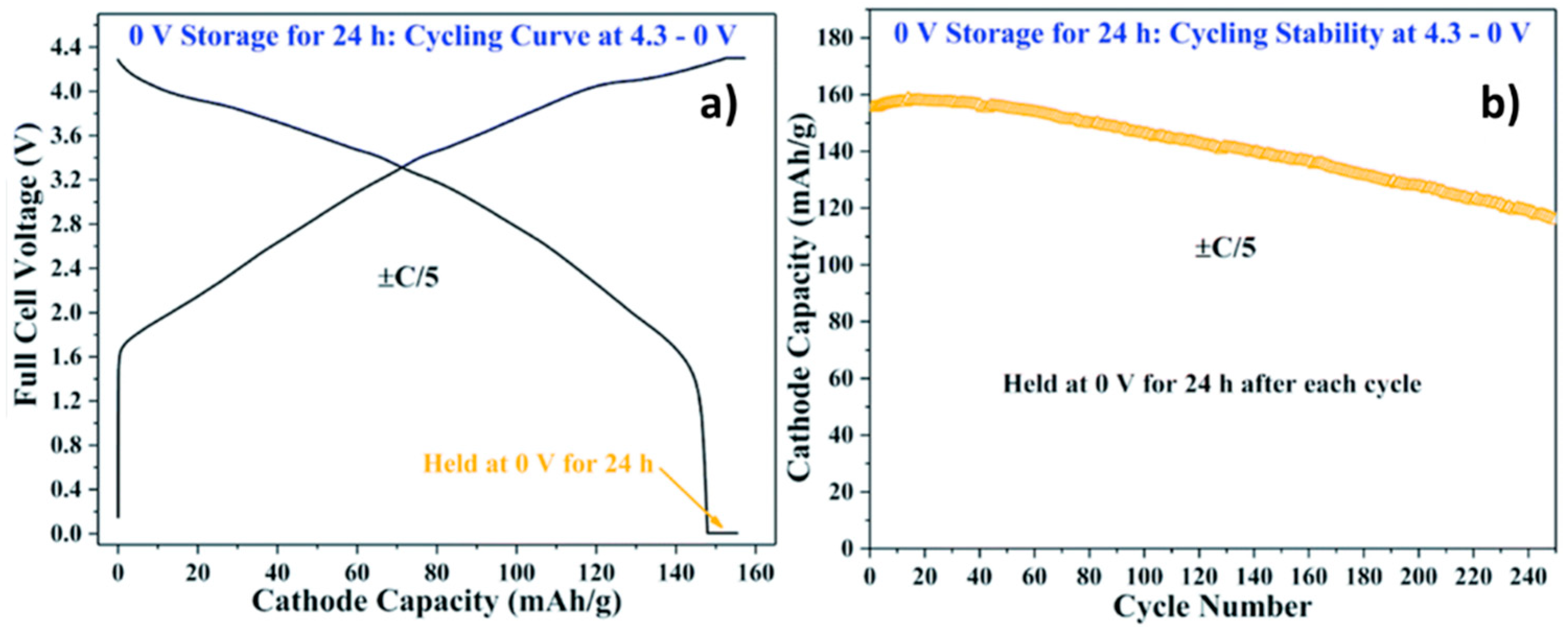
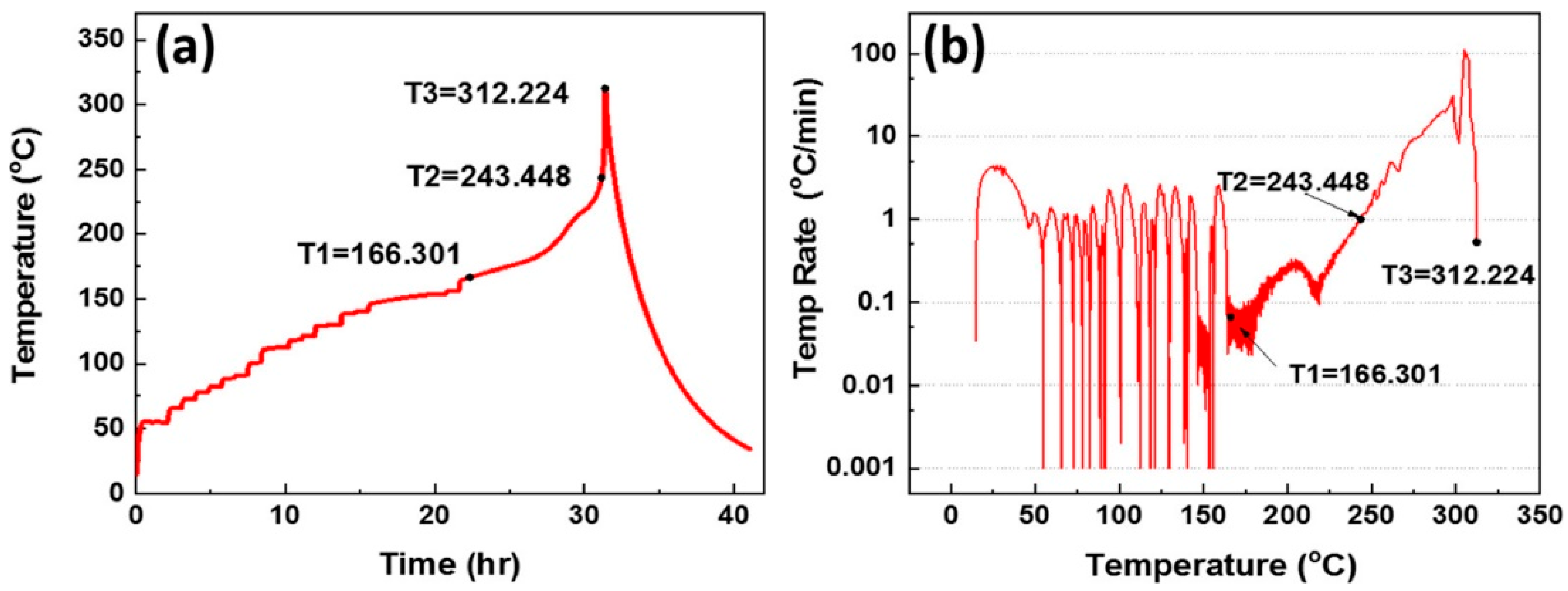
3. Is Zero-Volt Storage Possible for SiBs and What Are the Added Safety Gains?
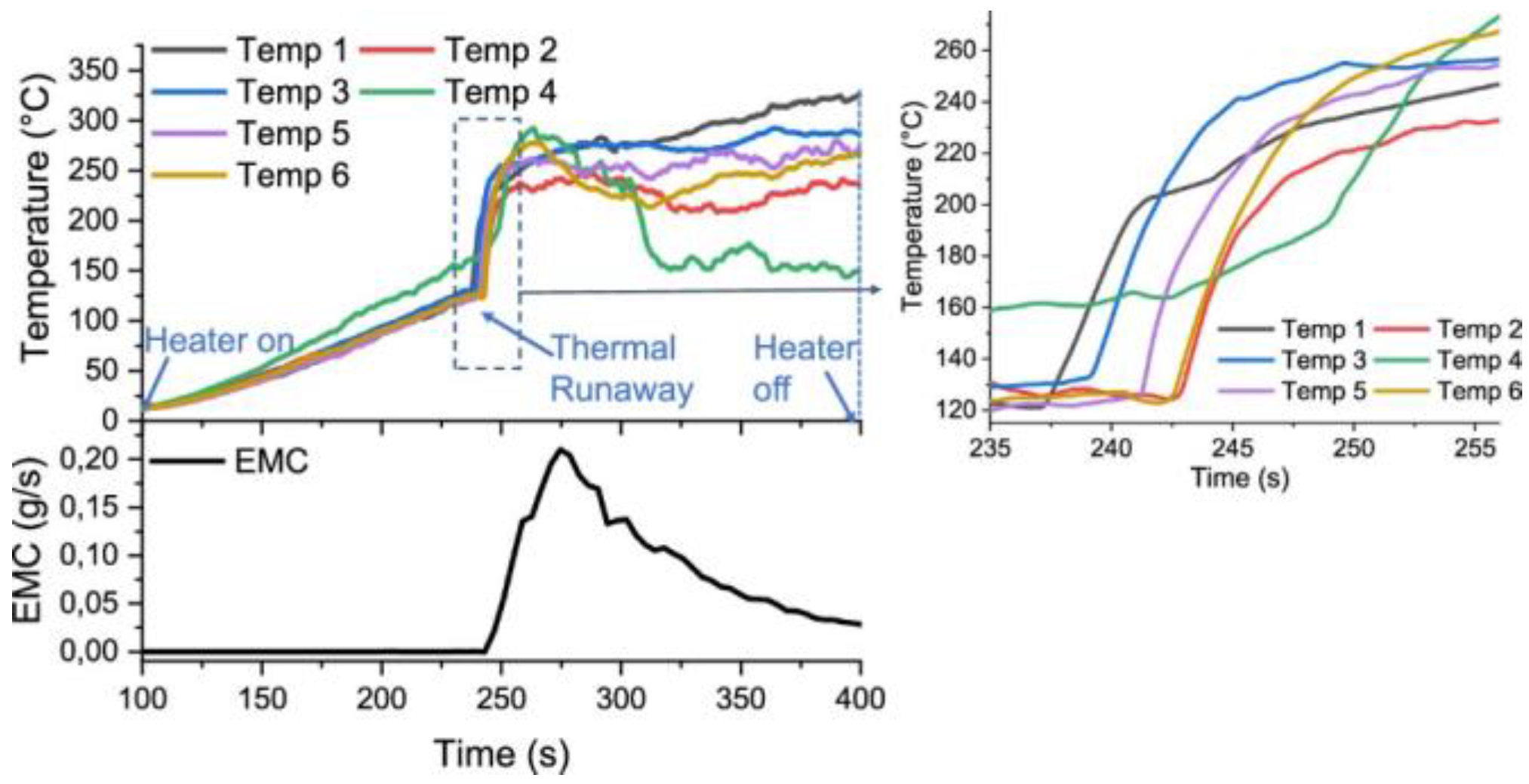
4. Comparative Thermal Studies of SiBs versus LiBs
4.1. Component Level
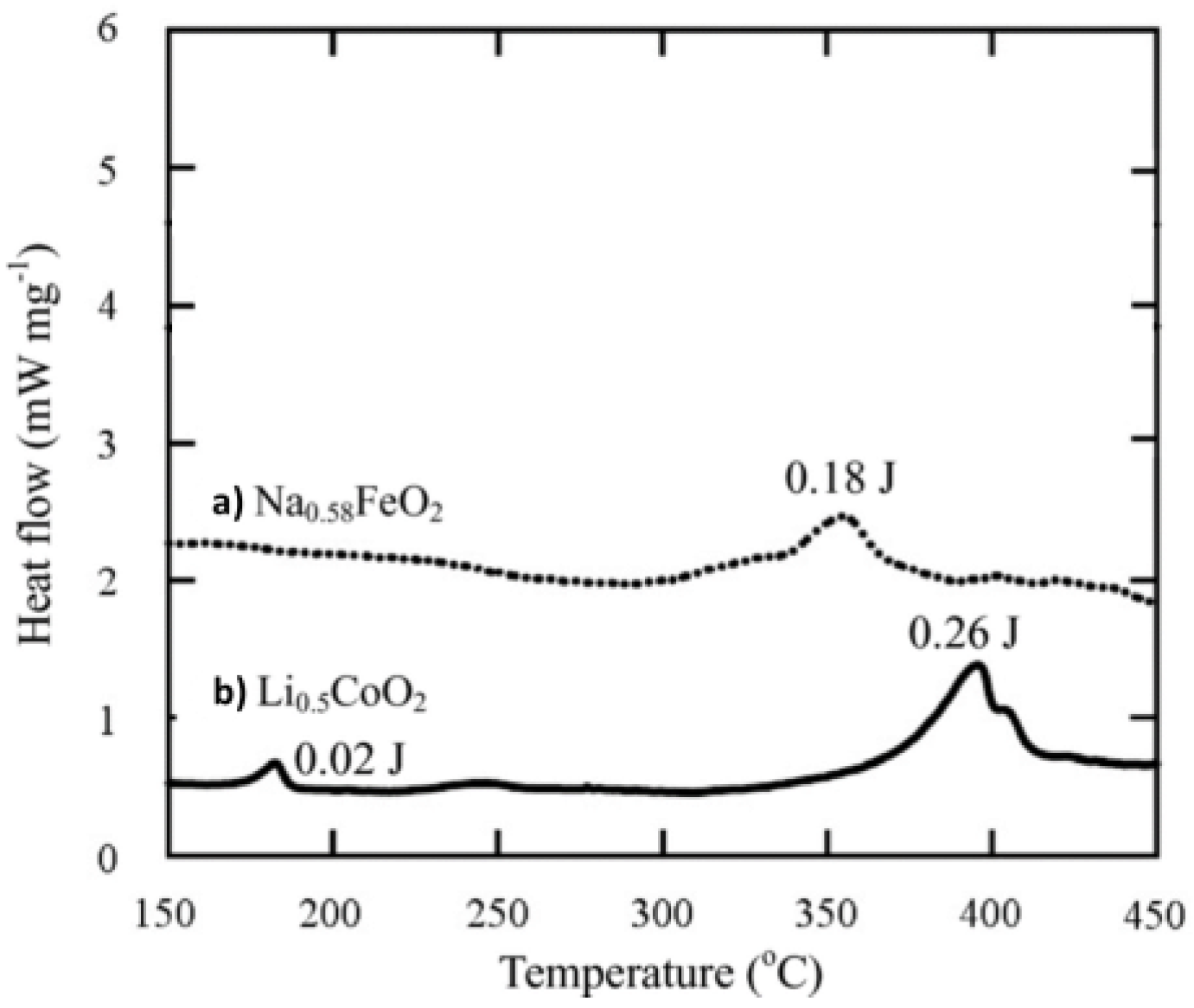
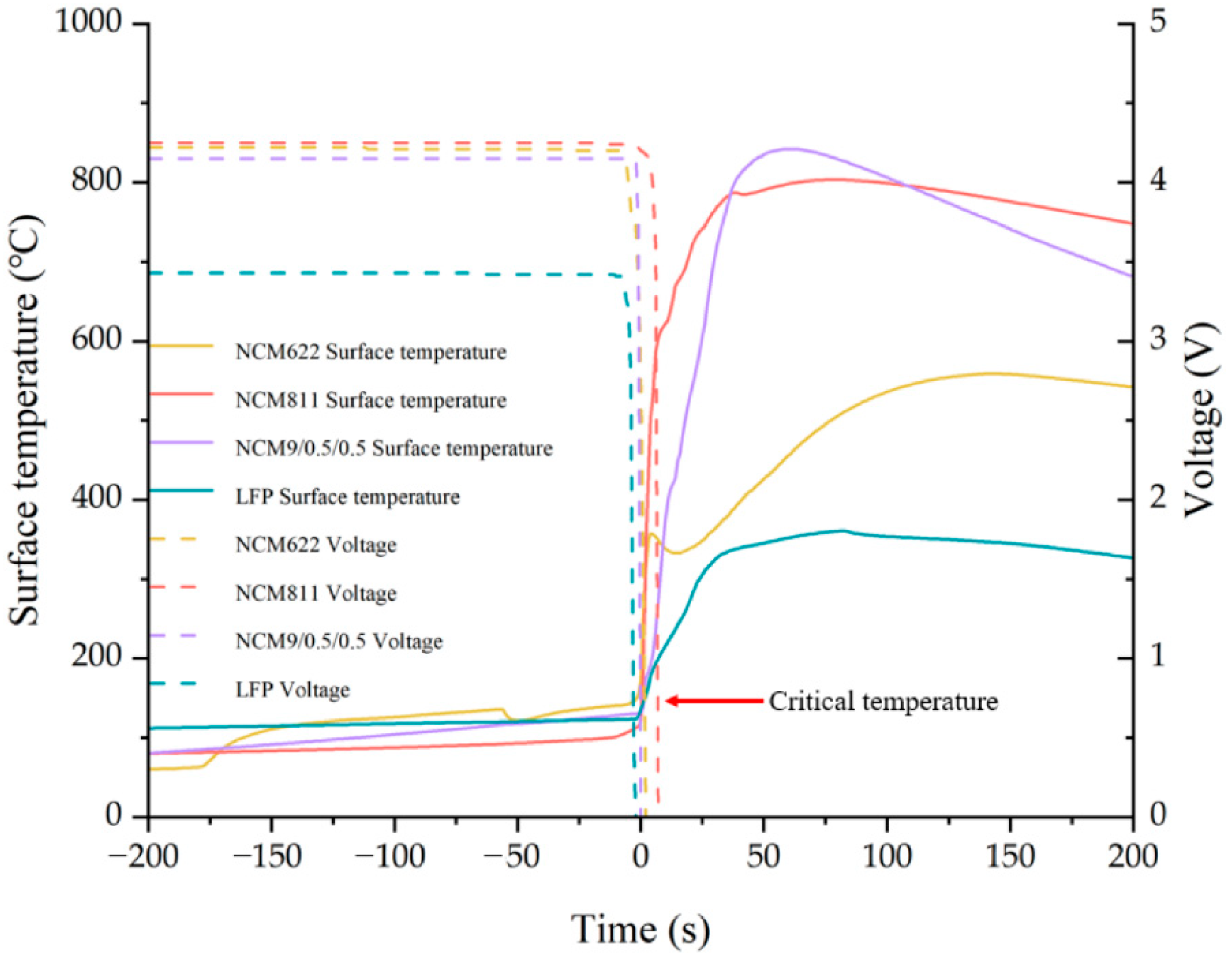
4.1.1. Cathode Material
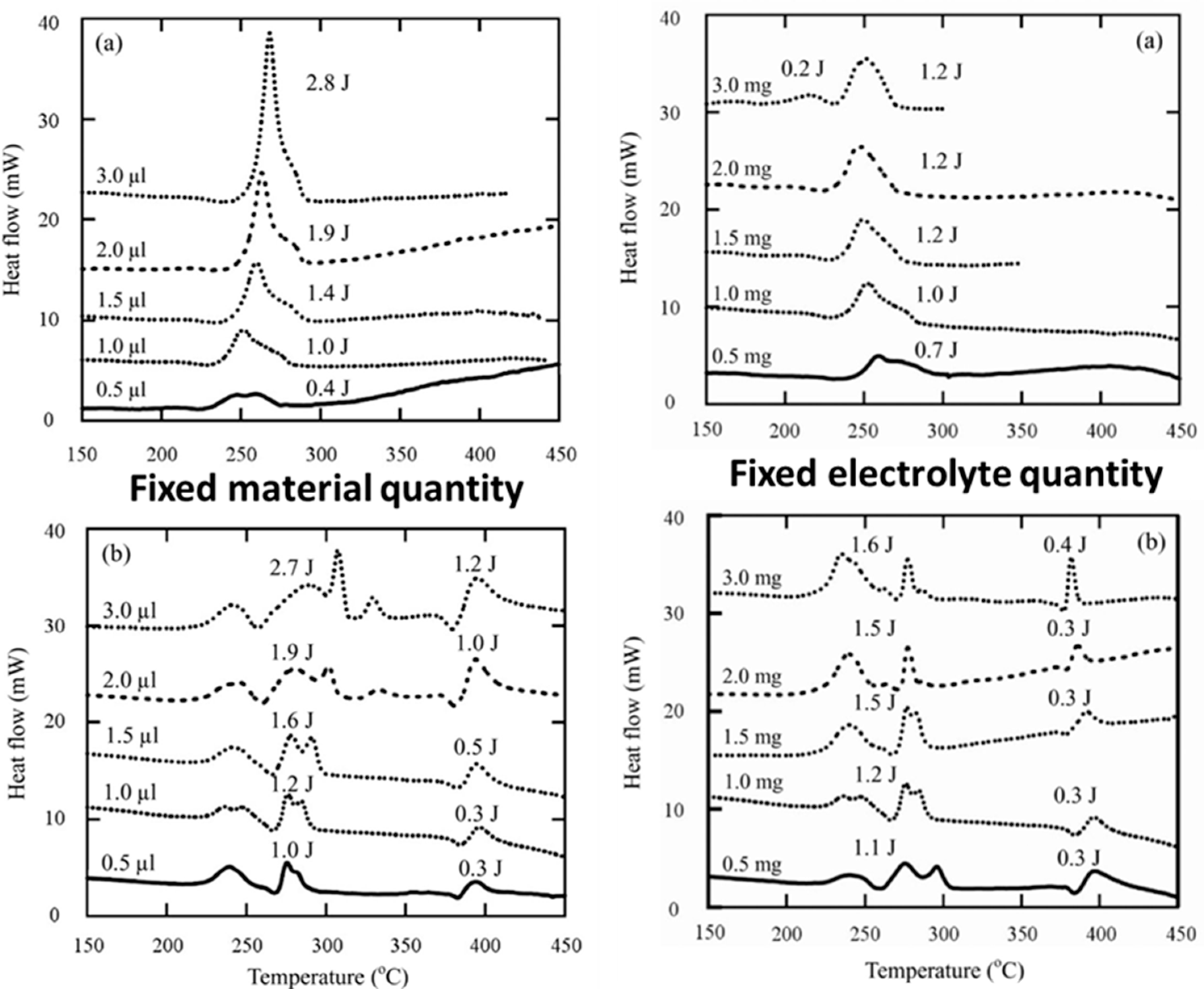
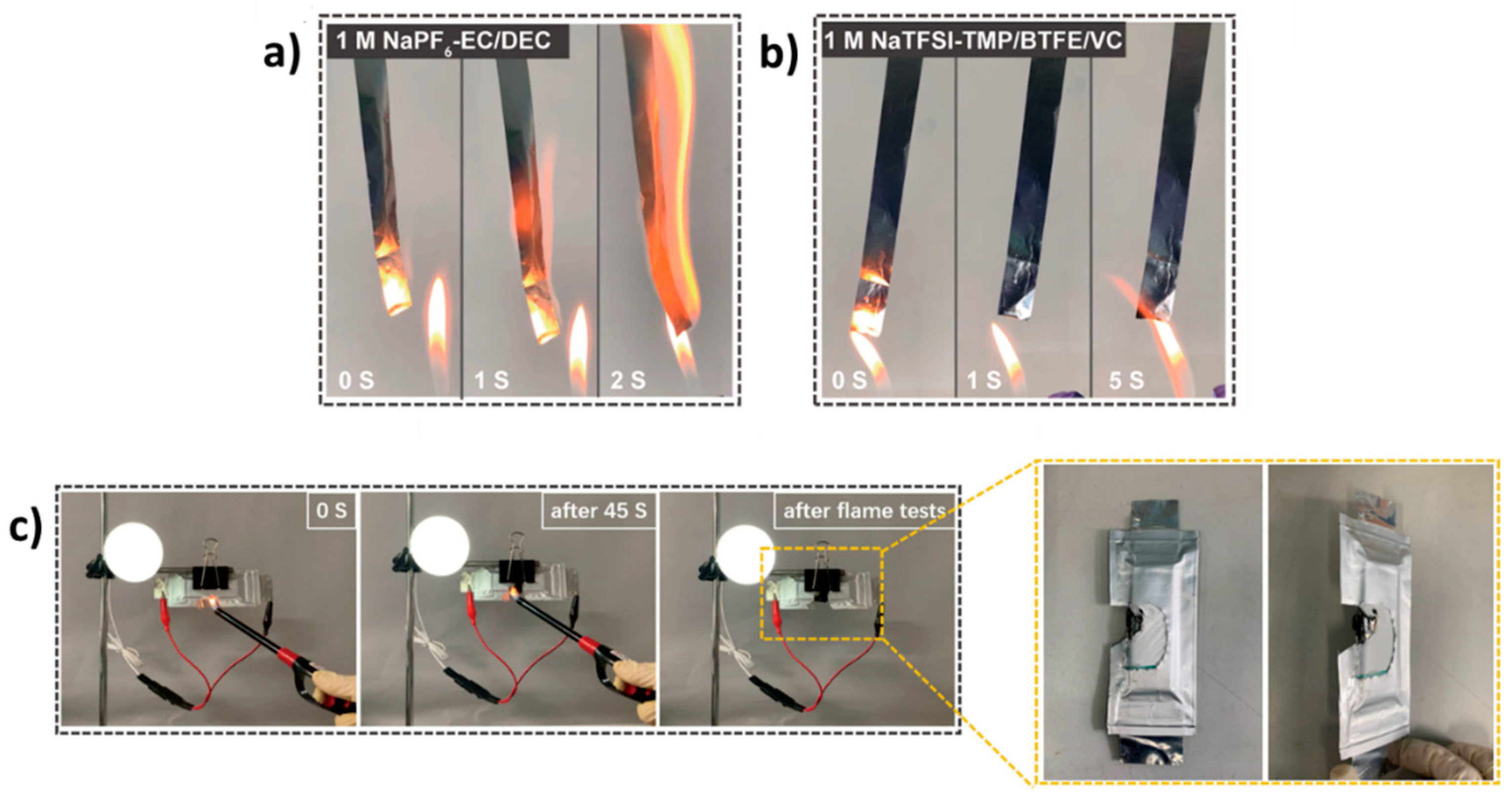
4.1.2. Electrolytes
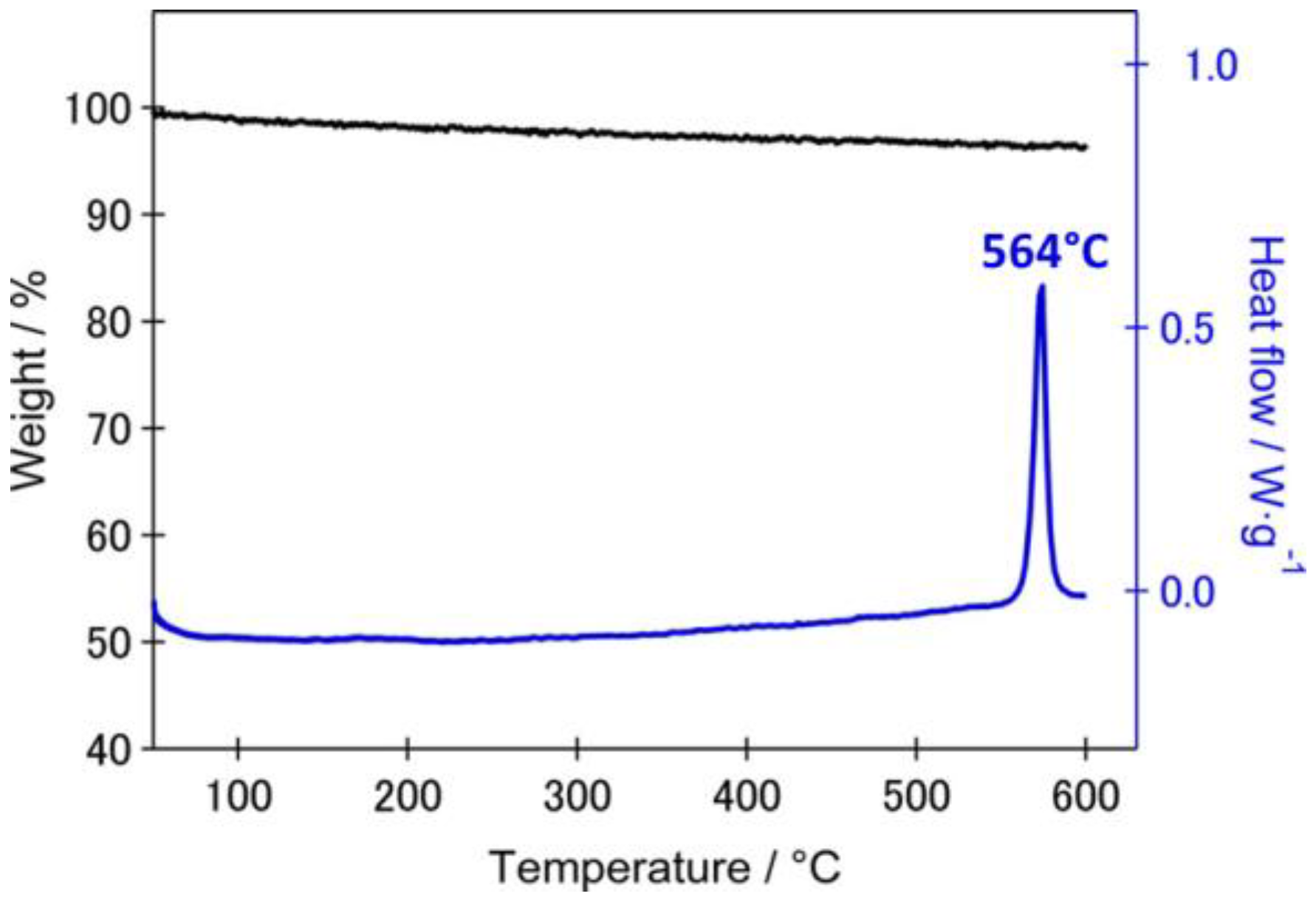
4.2. Full Cell Level
5. Role of the Separator in Battery Safety
6. Conclusion and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of Current Development in Electrical Energy Storage Technologies and the Application Potential in Power System Operation. Applied Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Mitali, J.; Dhinakaran, S.; Mohamad, A.A. Energy Storage Systems: A Review. Energy Storage and Saving 2022, 1, 166–216. [Google Scholar] [CrossRef]
- Kurzweil, P. Gaston Planté and His Invention of the Lead–Acid Battery—The Genesis of the First Practical Rechargeable Battery. Journal of Power Sources 2010, 195, 4424–4434. [Google Scholar] [CrossRef]
- Zhu, X.; Li, L.; Sun, X.; Yang, D.; Gao, L.; Liu, J.; Kumar, R.V.; Yang, J. Preparation of Basic Lead Oxide from Spent Lead Acid Battery Paste via Chemical Conversion. Hydrometallurgy 2012, 117–118, 24–31. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Zhang, X.; Liu, S. Study on the Environmental Risk Assessment of Lead-Acid Batteries. Procedia Environmental Sciences 2016, 31, 873–879. [Google Scholar] [CrossRef]
- World Health Organization, Recycling Used Lead-Acid Batteries: Health Considerations; World Health Organization: Geneva, 2017; ISBN 978-92-4-151285-5.
- Bernard, P. SECONDARY BATTERIES – NICKEL SYSTEMS | Nickel–Cadmium: Sealed. In Encyclopedia of Electrochemical Power Sources; Elsevier, 2009; pp. 459–481 ISBN 978-0-444-52745-5.
- EU Bans Nickel-Cadmium Batteries in Portable Devices by 2025: Impact on Emergency Lighting. Available online: https://www.etaplighting.com/en/blog/end-of-cadmium-batteries-portable-applications (accessed on 16 April 2024).
- Maxianova, K.; Rusche, T.M. Restriction of Hazardous Substances: On the Need for and the Limits of Comitology. Rev EC Int Env Law 2006, 15, 202–210. [Google Scholar] [CrossRef]
- Bernard, P.; Lippert, M. Nickel–Cadmium and Nickel–Metal Hydride Battery Energy Storage. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier, 2015; pp. 223–251 ISBN 978-0-444-62616-5.
- Ikoma, M.; Yuasa, S.; Yuasa, K.; Kaida, S.; Matsumoto, I.; Iwakura, C. Charge Characteristics of Sealed-Type Nickel/Metal-Hydride Battery. Journal of Alloys and Compounds 1998, 267, 252–256. [Google Scholar] [CrossRef]
- Winn, D.A.; Shemilt, J.M.; Steele, B.C.H. Titanium Disulphide: A Solid Solution Electrode for Sodium and Lithium. Materials Research Bulletin 1976, 11, 559–566. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef]
- Cairns, E.J.; Shimotake, H. High-Temperature Batteries: Research in High-Temperature Electrochemistry Reveals Compact, Powerful Energy-Storage Cells. Science 1969, 164, 1347–1355. [Google Scholar] [CrossRef]
- Nishi, Y. Lithium Ion Secondary Batteries; Past 10 Years and the Future. Journal of Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal Runaway Mechanism of Lithium Ion Battery for Electric Vehicles: A Review. Energy Storage Materials 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Lyu, Y.; Wu, X.; Wang, K.; Feng, Z.; Cheng, T.; Liu, Y.; Wang, M.; Chen, R.; Xu, L.; Zhou, J.; et al. An Overview on the Advances of LiCoO 2 Cathodes for Lithium-Ion Batteries. Advanced Energy Materials 2021, 11, 2000982. [Google Scholar] [CrossRef]
- Cho, J.; Kim, Y.J.; Park, B. Novel LiCoO 2 Cathode Material with Al 2 O 3 Coating for a Li Ion Cell. Chem. Mater. 2000, 12, 3788–3791. [Google Scholar] [CrossRef]
- Jo, M.; Hong, Y.-S.; Choo, J.; Cho, J. Effect of LiCoO[Sub 2] Cathode Nanoparticle Size on High Rate Performance for Li-Ion Batteries. J. Electrochem. Soc. 2009, 156, A430. [Google Scholar] [CrossRef]
- Ahangari, M.; Szalai, B.; Lujan, J.; Zhou, M.; Luo, H. Advancements and Challenges in High-Capacity Ni-Rich Cathode Materials for Lithium-Ion Batteries. Materials 2024, 17, 801. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zheng, J.; Wang, C.; Gu, M. Designing Principle for Ni-Rich Cathode Materials with High Energy Density for Practical Applications. Nano Energy 2018, 49, 434–452. [Google Scholar] [CrossRef]
- Zhang, S.S. Problems and Their Origins of Ni-Rich Layered Oxide Cathode Materials. Energy Storage Materials 2020, 24, 247–254. [Google Scholar] [CrossRef]
- Chakraborty, A.; Kunnikuruvan, S.; Kumar, S.; Markovsky, B.; Aurbach, D.; Dixit, M.; Major, D.T. Layered Cathode Materials for Lithium-Ion Batteries: Review of Computational Studies on LiNi 1– x – y Co x Mn y O 2 and LiNi 1– x – y Co x Al y O 2. Chem. Mater. 2020, 32, 915–952. [Google Scholar] [CrossRef]
- Vitins, G.; West, K. Lithium Intercalation into Layered LiMnO2. J. Electrochem. Soc. 1997, 144, 2587–2592. [Google Scholar] [CrossRef]
- Berg, H.; Göransson, K.; Noläng, B.; Thomas, J.O. Electronic Structure and Stability of the LixMn2O4 (0 < x < 2) System. J. Mater. Chem. 1999, 9, 2813–2820. [Google Scholar] [CrossRef]
- Cheruku, R.; Kruthika, G.; Govindaraj, G.; Vijayan, L. Electrical Relaxation Studies of Olivine Type Nanocrystalline LiMPO4 (M=Ni, Mn and Co) Materials. Journal of Physics and Chemistry of Solids 2015, 86, 27–35. [Google Scholar] [CrossRef]
- Manthiram, A. A Reflection on Lithium-Ion Battery Cathode Chemistry. Nat Commun 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Benoit, C.; Franger, S. Chemistry and Electrochemistry of Lithium Iron Phosphate. J Solid State Electrochem 2008, 12, 987–993. [Google Scholar] [CrossRef]
- Dahn, J.; Fuller, E.; Obrovac, M.; Vonsacken, U. Thermal Stability of LixCoO2, LixNiO2 and λ-MnO2 and Consequences for the Safety of Li-Ion Cells. Solid State Ionics 1994, 69, 265–270. [Google Scholar] [CrossRef]
- Bak, S.-M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.-J.; Kim, K.-B.; Chung, K.Y.; Yang, X.-Q.; Nam, K.-W. Structural Changes and Thermal Stability of Charged LiNi x Mn y Co z O 2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef] [PubMed]
- Rumble, C.; Conry, T.E.; Doeff, M.; Cairns, E.J.; Penner-Hahn, J.E.; Deb, A. Structural and Electrochemical Investigation of Li(Ni[Sub 0.4]Co[Sub 0.15]Al[Sub 0.05]Mn[Sub 0.4])O[Sub 2] Cathode Material. J. Electrochem. Soc. 2010, 157, A1317. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Liu, R.; Xu, X.-M.; Zeng, C.-S.; Shen, X.-B.; Gu, Y.-J. The Different Roles of Ni2+/Ni3+, Ni3+/Ni4+, and Mn3+/Mn4+ in Li-Rich Layered Nanostructured LiNi Mn1-O2·Li2MnO3 with High Capacity. International Journal of Electrochemical Science 2021, 16, 210425. [Google Scholar] [CrossRef]
- Schafzahl, L.; Mahne, N.; Schafzahl, B.; Wilkening, M.; Slugovc, C.; Borisov, S.M.; Freunberger, S.A. Singlet Oxygen during Cycling of the Aprotic Sodium-O2 Battery. Angew Chem Int Ed Engl 2017, 56, 15728–15732. [Google Scholar] [CrossRef]
- Kalyani, P.; Kalaiselvi, N. Various Aspects of LiNiO 2 Chemistry: A Review. Science and Technology of Advanced Materials 2005, 6, 689–703. [Google Scholar] [CrossRef]
- Salomez, B.; Grugeon, S.; Armand, M.; Tran-Van, P.; Laruelle, S. Review—Gassing Mechanisms in Lithium-Ion Battery. J. Electrochem. Soc. 2023, 170, 050537. [Google Scholar] [CrossRef]
- Electrochemical Devices for Energy Storage Applications; Kebede, M.A., Ezema, F.I., Eds.; 1st ed.; CRC Press, 2019; ISBN 978-0-367-85511-6.
- Mao, N.; Gadkari, S.; Wang, Z.; Zhang, T.; Bai, J.; Cai, Q. A Comparative Analysis of Lithium-Ion Batteries with Different Cathodes under Overheating and Nail Penetration Conditions. Energy 2023, 278, 128027. [Google Scholar] [CrossRef]
- Brand, M.; Gläser, S.; Geder, J.; Menacher, S.; Obpacher, S.; Jossen, A.; Quinger, D. Electrical Safety of Commercial Li-Ion Cells Based on NMC and NCA Technology Compared to LFP Technology. WEVJ 2013, 6, 572–580. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Resendiz, E.G.; Nwophoke, S.M.; Khanna, S.; James, C.; Brown, S.F. Review of Gas Emissions from Lithium-Ion Battery Thermal Runaway Failure — Considering Toxic and Flammable Compounds. Journal of Energy Storage 2024, 87, 111288. [Google Scholar] [CrossRef]
- Azuma, H.; Imoto, H.; Yamada, S.; Sekai, K. Advanced Carbon Anode Materials for Lithium Ion Cells. Journal of Power Sources 1999, 81–82, 1–7. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material – Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustainable Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Deng, J.; Gou, Y.; Fang, J.; Cui, H.; Zhao, Y.; Cao, M. Carbon-Based Materials for Fast Charging Lithium-Ion Batteries. Carbon 2021, 183, 721–734. [Google Scholar] [CrossRef]
- Yoon, T.; Milien, M.S.; Parimalam, B.S.; Lucht, B.L. Thermal Decomposition of the Solid Electrolyte Interphase (SEI) on Silicon Electrodes for Lithium Ion Batteries. Chem. Mater. 2017, 29, 3237–3245. [Google Scholar] [CrossRef]
- Sick, N.; Krätzig, O.; Eshetu, G.G.; Figgemeier, E. A Review of the Publication and Patent Landscape of Anode Materials for Lithium Ion Batteries. Journal of Energy Storage 2021, 43, 103231. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, D.; Qilu, *!!! REPLACE !!!*; Duo, X.; Sheng, X. A Review of Spinel Lithium Titanate (Li4Ti5O12) as Electrode Material for Advanced Energy Storage Devices. Ceramics International 2021, 47, 5870–5895. [Google Scholar] [CrossRef]
- Belharouak, I.; Koenig, G.M.; Amine, K. Electrochemistry and Safety of Li4Ti5O12 and Graphite Anodes Paired with LiMn2O4 for Hybrid Electric Vehicle Li-Ion Battery Applications. Journal of Power Sources 2011, 196, 10344–10350. [Google Scholar] [CrossRef]
- Desai, P.; Forero-Saboya, J.; Meunier, V.; Rousse, G.; Deschamps, M.; Abakumov, A.M.; Tarascon, J.-M.; Mariyappan, S. Mastering the Synergy between Na3V2(PO4)2F3 Electrode and Electrolyte: A Must for Na-Ion Cells. Energy Storage Materials 2023, 57, 102–117. [Google Scholar] [CrossRef]
- Tournevis sans Fil DEXTER Sodium 3.6 V 0.7 Ah | Leroy Merlin. Available online: https://www.leroymerlin.fr/produits/outillage/outillage-electroportatif/visseuse-et-tournevis-electrique/visseuse/tournevis-sans-fil-dexter-sodium-3-6-v-0-7-ah-86650845.html (accessed on 8 July 2024).
- Barker, J.; Heap, R.J.; Roche, N.; Tan, C.; Sayers, R.; Whitley, J.; Liu, Y. High Energy Density Na-Ion Battery Technology. Meet. Abstr. 2016; MA2016-03, 796–796. [Google Scholar] [CrossRef]
- Faradion Transport Applications. Available online: https://faradion.co.uk/applications/transport-applications/ (accessed on 14 June 2023).
- Gupta, Y.; Siwatch, P.; Karwasra, R.; Sharma, K.; Tripathi, S.K. Recent Progress of Layered Structured P2- and O3- Type Transition Metal Oxides as Cathode Material for Sodium-Ion Batteries. Renewable and Sustainable Energy Reviews 2024, 192, 114167. [Google Scholar] [CrossRef]
- Wang, P.-F.; You, Y.; Yin, Y.-X.; Guo, Y.-G. Layered Oxide Cathodes for Sodium-Ion Batteries: Phase Transition, Air Stability, and Performance. Adv. Energy Mater. 2018, 8, 1701912. [Google Scholar] [CrossRef]
- Grépin, E.; Jacquet, Q.; Moiseev, I.A.; Iadecola, A.; Rousse, G.; Avdeev, M.; Abakumov, A.M.; Tarascon, J.-M.; Mariyappan, S. Mastering the Synthesis of High Na-Content, Moisture-Stable Layered Oxide Cathode for Na-Ion Batteries. Journal of Power Sources 2024, 613, 234962. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, W.; Li, K.; Zhu, X. Na-Deficient P2-Type Layered Oxide Cathodes for Practical Sodium-Ion Batteries. Microstructures 2024, 4. [Google Scholar] [CrossRef]
- Ni, Q.; Bai, Y.; Wu, F.; Wu, C. Polyanion-Type Electrode Materials for Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1600275. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, J.; Yang, C.; Hu, Y.-S. Polyanionic Cathode Materials for Practical Na-Ion Batteries toward High Energy Density and Long Cycle Life. ACS Cent. Sci. 2023, 9, 1721–1736. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, M.; Zhang, Z.; Lai, Y.; Ma, J. Engineering of Polyanion Type Cathode Materials for Sodium-Ion Batteries: Toward Higher Energy/Power Density. Adv Funct Materials 2020, 30, 2000473. [Google Scholar] [CrossRef]
- Lv, Z.; Ling, M.; Yue, M.; Li, X.; Song, M.; Zheng, Q.; Zhang, H. Vanadium-Based Polyanionic Compounds as Cathode Materials for Sodium-Ion Batteries: Toward High-Energy and High-Power Applications. Journal of Energy Chemistry 2021, 55, 361–390. [Google Scholar] [CrossRef]
- He, M.; Davis, R.; Chartouni, D.; Johnson, M.; Abplanalp, M.; Troendle, P.; Suetterlin, R.-P. Assessment of the First Commercial Prussian Blue Based Sodium-Ion Battery. Journal of Power Sources 2022, 548, 232036. [Google Scholar] [CrossRef]
- Sodium-Ion Batteries & Sustainable Energy | Natron Energy. Available online: https://natron.energy/ (accessed on 8 July 2024).
- Bie, X.; Kubota, K.; Hosaka, T.; Chihara, K.; Komaba, S. Synthesis and Electrochemical Properties of Na-Rich Prussian Blue Analogues Containing Mn, Fe, Co, and Fe for Na-Ion Batteries. Journal of Power Sources 2018, 378, 322–330. [Google Scholar] [CrossRef]
- Han, J.; Lin, Y.; Yang, Y.; Zuo, D.; Wang, C.; Liu, X. Dominant Role of M Element on the Stability and Properties of Prussian Blue Analogues NaxMFe(CN)6 (M = 3d Transition Metal) as Cathode Material for the Sodium-Ion Batteries. Journal of Alloys and Compounds 2021, 870, 159533. [Google Scholar] [CrossRef]
- Ojwang, D.O.; Häggström, L.; Ericsson, T.; Ångström, J.; Brant, W.R. Influence of Sodium Content on the Thermal Behavior of Low Vacancy Prussian White Cathode Material. Dalton Trans. 2020, 49, 3570–3579. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xiao, J.; Zhao, H.; Li, J.; Zhang, G.; Zhang, D.; Guo, X.; Gao, H.; Wang, Y.; Chen, J.; et al. Prussian Blue Analogues for Sodium-Ion Battery Cathodes: A Review of Mechanistic Insights, Current Challenges, and Future Pathways. Small 2024, 2401957. [Google Scholar] [CrossRef]
- Perveen, T.; Siddiq, M.; Shahzad, N.; Ihsan, R.; Ahmad, A.; Shahzad, M.I. Prospects in Anode Materials for Sodium Ion Batteries - A Review. Renewable and Sustainable Energy Reviews 2020, 119, 109549. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard Carbons for Sodium-Ion Batteries: Structure, Analysis, Sustainability, and Electrochemistry. Materials Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Yan, C.; Yuan, H.; Park, H.S.; Huang, J.-Q. Perspective on the Critical Role of Interface for Advanced Batteries. Journal of Energy Chemistry 2020, 47, 217–220. [Google Scholar] [CrossRef]
- Winter, M. The Solid Electrolyte Interphase – The Most Important and the Least Understood Solid Electrolyte in Rechargeable Li Batteries. Zeitschrift für Physikalische Chemie 2009, 223, 1395–1406. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, F.; Liu, X.; Yang, S.; Ma, J. Formation and Modification of Cathode Electrolyte Interphase: A Mini Review. Electrochemistry Communications 2021, 122, 106870. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Diemant, T.; Hekmatfar, M.; Grugeon, S.; Behm, R.J.; Laruelle, S.; Armand, M.; Passerini, S. Impact of the Electrolyte Salt Anion on the Solid Electrolyte Interphase Formation in Sodium Ion Batteries. Nano Energy 2019, 55, 327–340. [Google Scholar] [CrossRef]
- Ma, L.A.; Naylor, A.J.; Nyholm, L.; Younesi, R. Strategies for Mitigating Dissolution of Solid Electrolyte Interphases in Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 4855–4863. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, R.; Brandell, D.; Younesi, R. Solubility of the Solid Electrolyte Interphase (SEI) in Sodium Ion Batteries. ACS Energy Lett. 2016, 1, 1173–1178. [Google Scholar] [CrossRef]
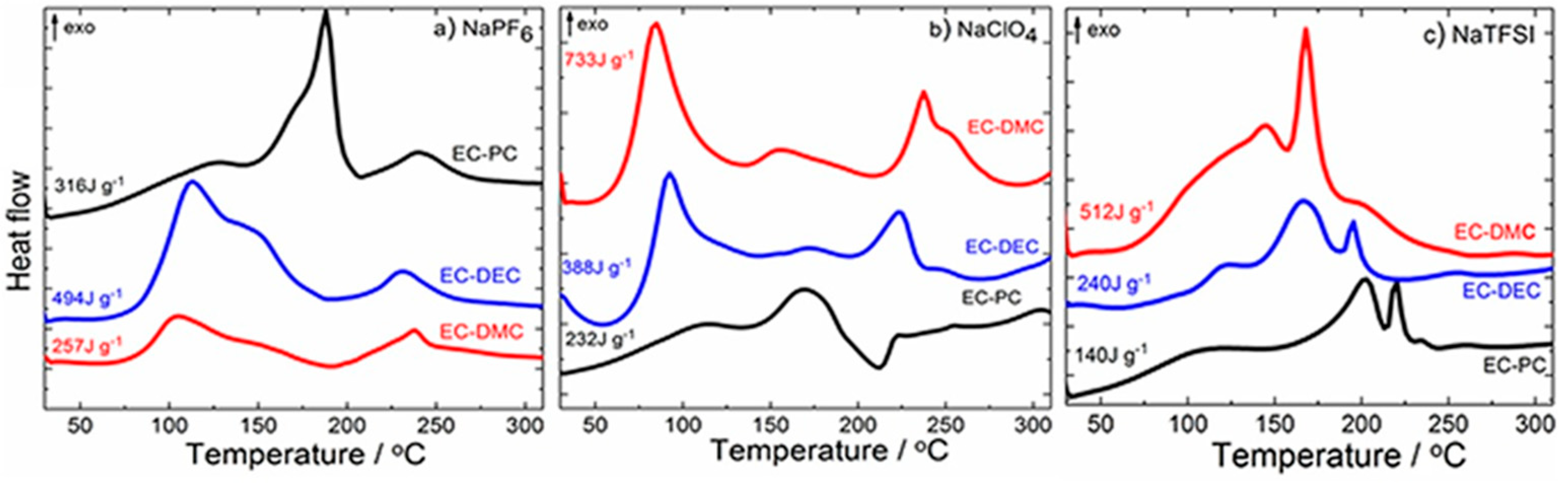
- Eshetu, G.G.; Grugeon, S.; Kim, H.; Jeong, S.; Wu, L.; Gachot, G.; Laruelle, S.; Armand, M.; Passerini, S. Comprehensive Insights into the Reactivity of Electrolytes Based on Sodium Ions. ChemSusChem 2016, 9, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Liaw, H.-J.; Liou, Y.-R.; Liu, P.-H.; Chen, H.-Y.; Shu, C.-M. Increased Flammability Hazard When Ionic Liquid [C6mim][Cl] Is Exposed to High Temperatures. Journal of Hazardous Materials 2019, 367, 407–417. [Google Scholar] [CrossRef] [PubMed]
- 01 - Home | EV Fire Safe. Available online: https://www.evfiresafe.com/ (accessed on 22 April 2024).
- EPRI BESS Failure Incident Database. Available online: https://storagewiki.epri.com/index.php/BESS_Failure_Incident_Database (accessed on 29 July 2024).
- Marlair Guy; Lecocq Amandine; Bordes Arnaud; Christensen Paul; Truchot Benjamin Key Learnings From Recent Lithium-Ion Battery Incidents That Have Impacted e-Mobility and Energy Storage Fast Growing Markets. Chemical Engineering Transactions 2022, 90, 643–648. [CrossRef]
- CALCE and the University of Maryland Safety. Available online: https://web.calce.umd.edu/batteries/safety.html (accessed on 20 September 2023).
- Park Taejon Renault-Samsung’s Electric Vehicle Catches Fire Due to Ignition from Bonnet. Available online: https://english.etnews.com/20160127200001 (accessed on 20 September 2023).
- CTIF International Association of Fire and Rescue Services Large Explosion and Fire at French Lithium Battery Warehouse. Available online: https://ctif.org/news/large-explosion-and-fire-french-lithium-battery-warehouse (accessed on 20 September 2023).
- South Korea: Exploding Lithium Batteries Spark Deadly Factory Fire. Available online: https://www.bbc.com/news/articles/crgggmeyjj7o (accessed on 9 July 2024).
- DESTINY PhD Programme Marie Skłodowska-Curie Actions COFUND. Available online: https://www.destiny-phd.eu/topic-22---2 (accessed on 22 April 2024).
- Regulation - 2023/1542 - EN - EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2023/1542/oj (accessed on 22 April 2024).
- Official Journal of the European Union REGULATION (EU) 2023/1542 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 12 July 2023 Concerning Batteries and Waste Batteries, Amending Directive 2008/98/EC and Regulation (EU) 2019/1020 and Repealing Directive 2006/66/EC.
- Patil, A.; Patil, V.; Wook Shin, D.; Choi, J.-W.; Paik, D.-S.; Yoon, S.-J. Issue and Challenges Facing Rechargeable Thin Film Lithium Batteries. Materials Research Bulletin 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Wei, F.; Zhang, J.-G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The State of Understanding of the Lithium-Ion-Battery Graphite Solid Electrolyte Interphase (SEI) and Its Relationship to Formation Cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Hausbrand, R. Electronic Energy Levels at Li-Ion Cathode–Liquid Electrolyte Interfaces: Concepts, Experimental Insights, and Perspectives. The Journal of Chemical Physics 2020, 152, 180902. [Google Scholar] [CrossRef]
- Abe, K.; Yoshitake, H.; Kitakura, T.; Hattori, T.; Wang, H.; Yoshio, M. Additives-Containing Functional Electrolytes for Suppressing Electrolyte Decomposition in Lithium-Ion Batteries. Electrochimica Acta 2004, 49, 4613–4622. [Google Scholar] [CrossRef]
- Cometto, C.; Yan, G.; Mariyappan, S.; Tarascon, J.-M. Means of Using Cyclic Voltammetry to Rapidly Design a Stable DMC-Based Electrolyte for Na-Ion Batteries. J. Electrochem. Soc. 2019, 166, A3723–A3730. [Google Scholar] [CrossRef]
- Zhang, L.; Tsolakidou, C.; Mariyappan, S.; Tarascon, J.-M.; Trabesinger, S. Unraveling Gas Evolution in Sodium Batteries by Online Electrochemical Mass Spectrometry. Energy Storage Materials 2021, 42, 12–21. [Google Scholar] [CrossRef]
- Yan, G.; Reeves, K.; Foix, D.; Li, Z.; Cometto, C.; Mariyappan, S.; Salanne, M.; Tarascon, J. A New Electrolyte Formulation for Securing High Temperature Cycling and Storage Performances of Na-Ion Batteries. Adv. Energy Mater. 2019, 9, 1901431. [Google Scholar] [CrossRef]
- Tran, M.-K.; Mevawalla, A.; Aziz, A.; Panchal, S.; Xie, Y.; Fowler, M. A Review of Lithium-Ion Battery Thermal Runaway Modeling and Diagnosis Approaches. Processes 2022, 10, 1192. [Google Scholar] [CrossRef]
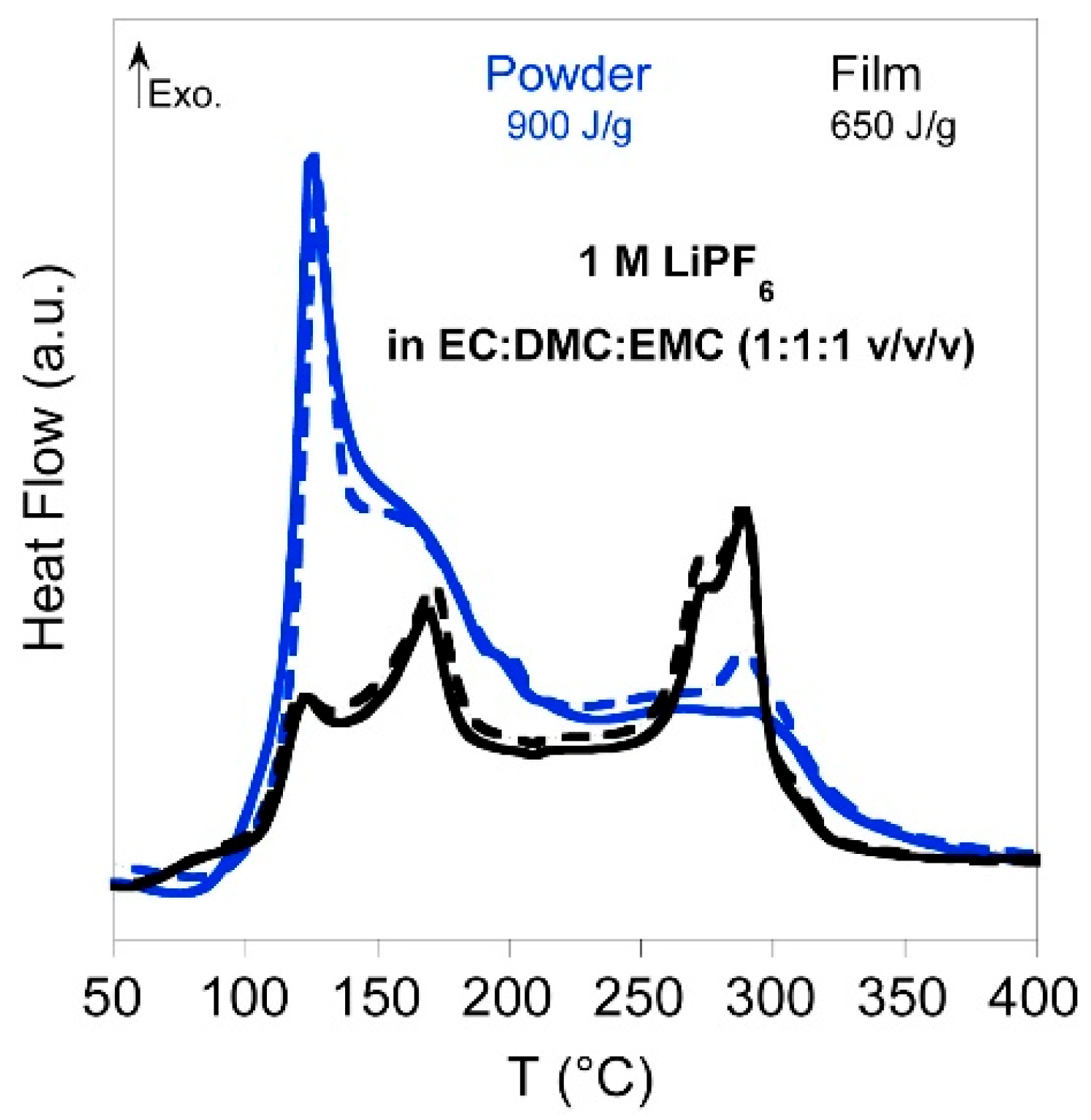
- Forestier, C.; Grugeon, S.; Davoisne, C.; Lecocq, A.; Marlair, G.; Armand, M.; Sannier, L.; Laruelle, S. Graphite Electrode Thermal Behavior and Solid Electrolyte Interphase Investigations: Role of State-of-the-Art Binders, Carbonate Additives and Lithium Bis(Fluorosulfonyl)Imide Salt. Journal of Power Sources 2016, 330, 186–194. [Google Scholar] [CrossRef]
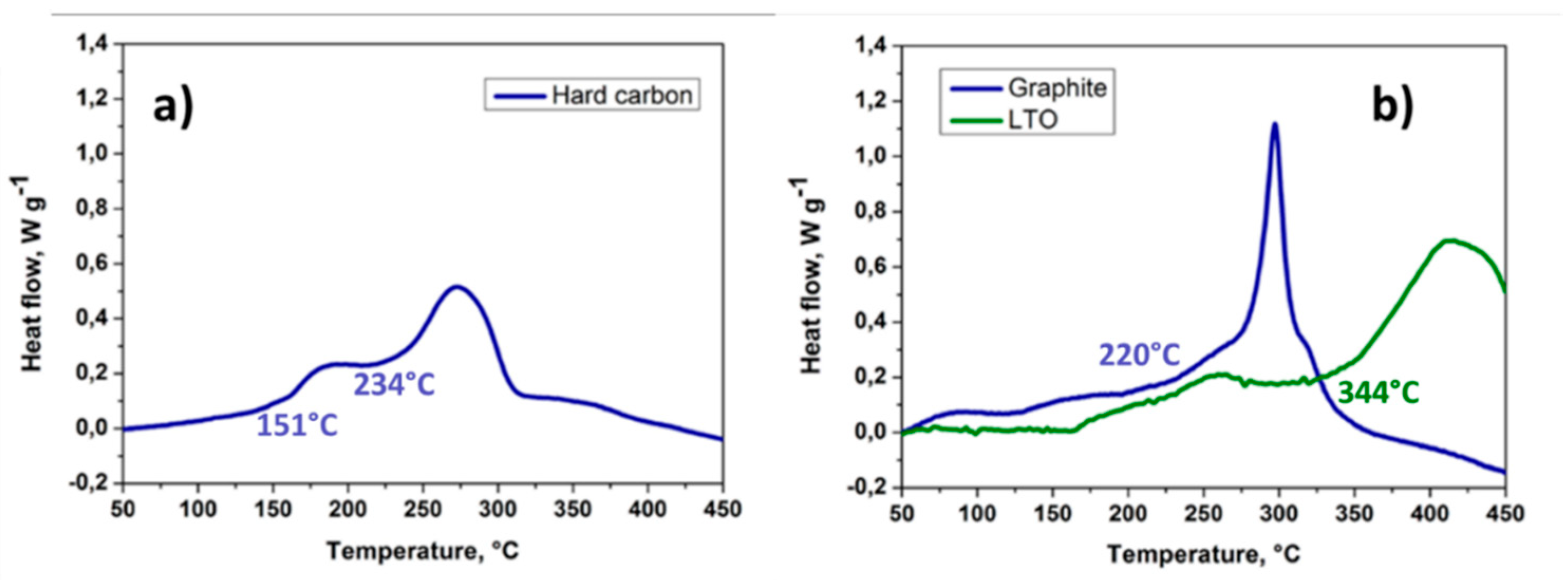
- Samigullin, R.R.; Drozhzhin, O.A.; Antipov, E.V. Comparative Study of the Thermal Stability of Electrode Materials for Li-Ion and Na-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 14–19. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Fabrication of Li4Ti5O12 (LTO) as Anode Material for Li-Ion Batteries. Micromachines 2024, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Shakourian-Fard, M.; Kamath, G.; Smith, K.; Xiong, H.; Sankaranarayanan, S.K.R.S. Trends in Na-Ion Solvation with Alkyl-Carbonate Electrolytes for Sodium-Ion Batteries: Insights from First-Principles Calculations. J. Phys. Chem. C 2015, 119, 22747–22759. [Google Scholar] [CrossRef]
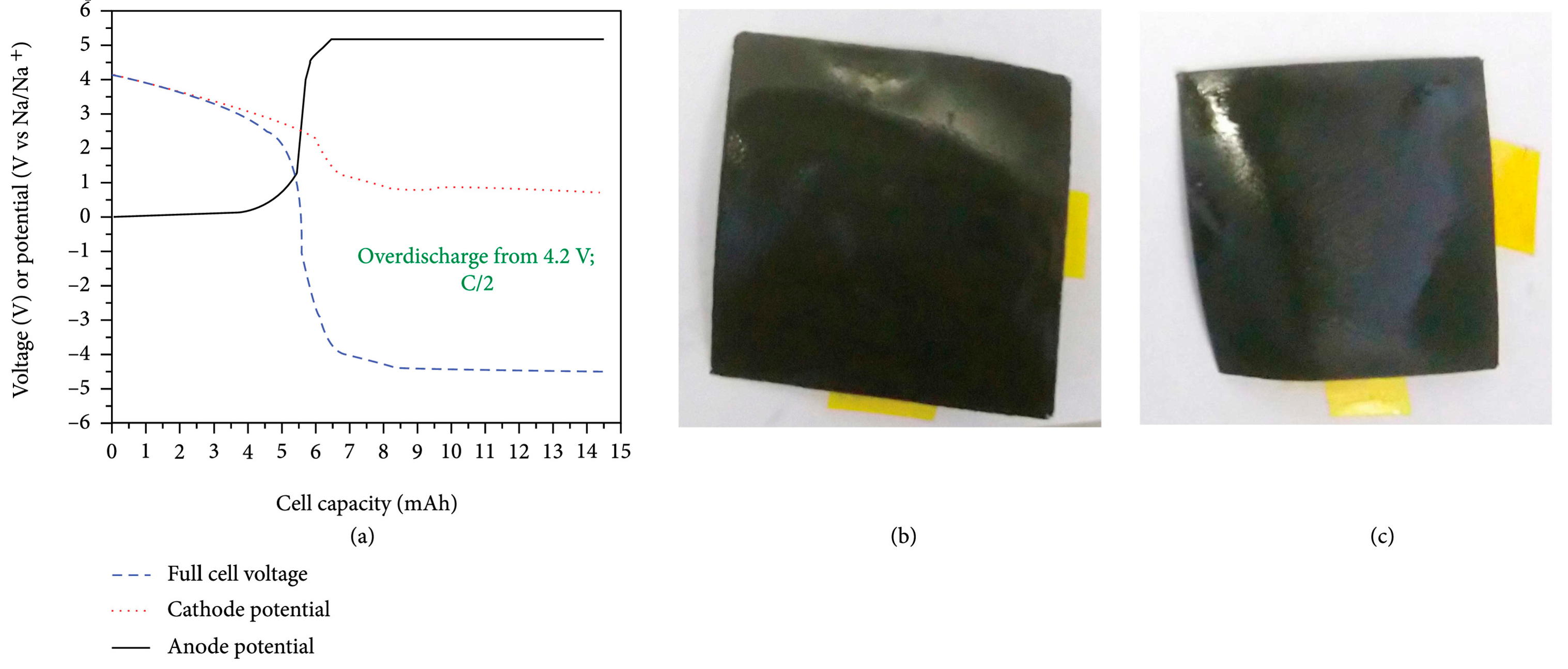
- Zhou, H.; Fear, C.; Jeevarajan, J.A.; Mukherjee, P.P. State-of-Electrode (SOE) Analytics of Lithium-Ion Cells under Overdischarge Extremes. Energy Storage Materials 2023, 54, 60–74. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Börner, M.; Streipert, B.; Meister, P.; Wagner, R.; Cekic Laskovic, I.; Winter, M. Lithium Ion Battery Cells under Abusive Discharge Conditions: Electrode Potential Development and Interactions between Positive and Negative Electrode. Journal of Power Sources 2017, 362, 278–282. [Google Scholar] [CrossRef]
- Flügel, M.; Waldmann, T.; Kasper, M.; Wohlfahrt-Mehrens, M. Detection of Copper Deposition on Anodes of Over-Discharged Lithium Ion Cells by GD-OES Depth Profiling. ChemPhysChem 2020, 21, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Rudola, A.; Wright, C.J.; Barker, J. Reviewing the Safe Shipping of Lithium-Ion and Sodium-Ion Cells: A Materials Chemistry Perspective. Energy Mater Adv 2021, 2021, 9798460. [Google Scholar] [CrossRef]
- Rudola, A.; Rennie, A.J.R.; Heap, R.; Meysami, S.S.; Lowbridge, A.; Mazzali, F.; Sayers, R.; Wright, C.J.; Barker, J. Commercialisation of High Energy Density Sodium-Ion Batteries: Faradion’s Journey and Outlook. J. Mater. Chem. A 2021, 9, 8279–8302. [Google Scholar] [CrossRef]
- Huo, H.; Xing, Y.; Pecht, M.; Züger, B.J.; Khare, N.; Vezzini, A. Safety Requirements for Transportation of Lithium Batteries. Energies 2017, 10, 793. [Google Scholar] [CrossRef]
- DSV Class 9A Lithium Batteries Dangerous Goods. Available online: https://www.dsv.com/en-gb/our-solutions/modes-of-transport/value-added-services/transporting-dangerous-goods/9-classes-of-dangerous-goods/class-9a-lithium-batteries (accessed on 19 September 2023).
- He, X.; Hu, Z.; Restuccia, F.; Fang, J.; Rein, G. Experimental Study of the Effect of the State of Charge on Self-Heating Ignition of Large Ensembles of Lithium-Ion Batteries in Storage. Applied Thermal Engineering 2022, 212, 118621. [Google Scholar] [CrossRef]
- ICAO. International Civil Aviation Organization Techincal Instructions for the Safe Transport of Dangerous Goods by Air (Doc 9284) 2015-2016 Edition.
- IATA. 2024 Lithium Battery Guidance Document Transport of Lithium Metal and Lithium Ion Batteries 2024.
- D. Brennan REDUCED CHARGE FOR VEHICLES POWERED BY LITHIUM ION, LITHIUM METAL OR SODIUM ION BATTERIES. In Proceedings of the DANGEROUS GOODS PANEL (DGP) TWENTY-NINTH MEETING; ICAO: Montréal, November 13 2023.
- United Nations New York and Geneva, 2023 Recommendations on the Transport of Dangerous Goods Model Regulations Volume I.
- UN/SCETDG/55/INF.38 Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals. In Proceedings of the Sub-Committee of Experts on the Transport of Dangerous Goods Fifty-fifth session; Geneva, June 27 2019.
- Desai, P.; Huang, J.; Foix, D.; Tarascon, J.-M.; Mariyappan, S. Zero Volt Storage of Na-Ion Batteries: Performance Dependence on Cell Chemistry! Journal of Power Sources 2022, 551, 232177. [Google Scholar] [CrossRef]
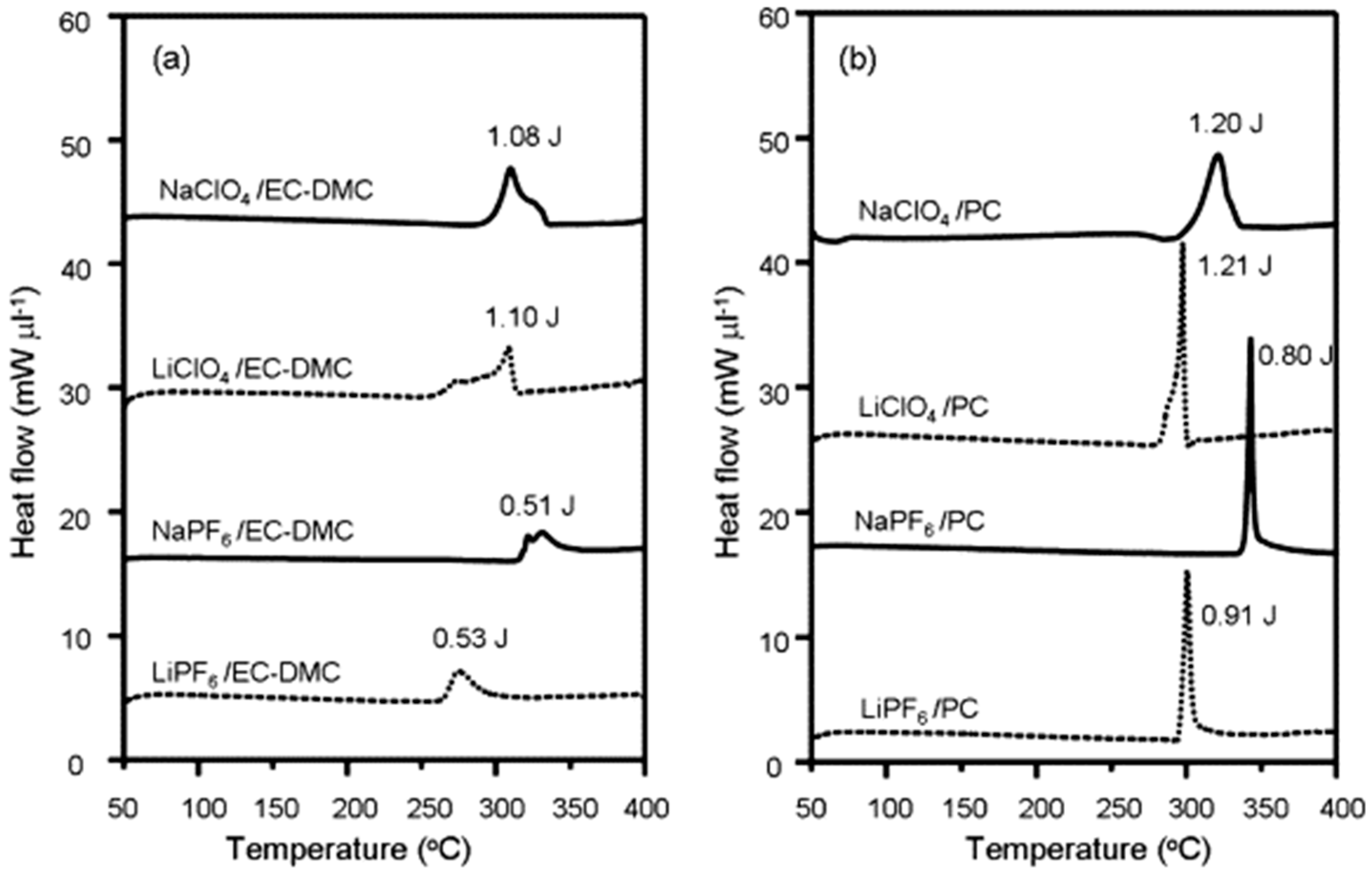
- Zhao, J.; Zhao, L.; Dimov, N.; Okada, S.; Nishida, T. Electrochemical and Thermal Properties of α-NaFeO 2 Cathode for Na-Ion Batteries. J. Electrochem. Soc. 2013, 160, A3077–A3081. [Google Scholar] [CrossRef]
- Baba, Y. Thermal Stability of LixCoO2 Cathode for Lithium Ion Battery. Solid State Ionics 2002, 148, 311–316. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Dahn, J.R. The Reaction of Charged Cathodes with Nonaqueous Solvents and Electrolytes: I. Li[Sub 0.5]CoO[Sub 2]. J. Electrochem. Soc. 2001, 148, A1205. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Dahn, J.R. The Reactions of Li[Sub 0.5]CoO[Sub 2] with Nonaqueous Solvents at Elevated Temperatures. J. Electrochem. Soc. 2002, 149, A912. [Google Scholar] [CrossRef]
- Hossain, M.S.; Furusawa, T.; Sato, M. Hydrothermal Synthesis, Characterization and Thermal Stability Studies of α-Fe2O3 Hollow Microspheres. Advanced Powder Technology 2022, 33, 103797. [Google Scholar] [CrossRef]
- Darezereshki, E.; Bakhtiari, F.; Alizadeh, M.; Behrad Vakylabad, A.; Ranjbar, M. Direct Thermal Decomposition Synthesis and Characterization of Hematite (α-Fe2O3) Nanoparticles. Materials Science in Semiconductor Processing 2012, 15, 91–97. [Google Scholar] [CrossRef]
- Barpanda, P.; Liu, G.; Ling, C.D.; Tamaru, M.; Avdeev, M.; Chung, S.-C.; Yamada, Y.; Yamada, A. Na 2 FeP 2 O 7 : A Safe Cathode for Rechargeable Sodium-Ion Batteries. Chem. Mater. 2013, 25, 3480–3487. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Z.; Indris, S.; Hua, W.; Casati, N.P.M.; Tayal, A.; Darma, M.S.D.; Wang, G.; Liu, Y.; Wu, C.; et al. The Structural Origin of Enhanced Stability of Na3.32Fe2.11Ca0.23(P2O7)2 Cathode for Na-Ion Batteries. Nano Energy 2021, 79, 105417. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, Y.; Jo, E.; Chung, K.Y.; Choi, W.; Kim, S.M.; Chang, W. Investigation of Thermal Stability of P2–Na x CoO 2 Cathode Materials for Sodium Ion Batteries Using Real-Time Electron Microscopy. ACS Appl. Mater. Interfaces 2017, 9, 18883–18888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, L.; Chihara, K.; Okada, S.; Yamaki, J.; Matsumoto, S.; Kuze, S.; Nakane, K. Electrochemical and Thermal Properties of Hard Carbon-Type Anodes for Na-Ion Batteries. Journal of Power Sources 2013, 244, 752–757. [Google Scholar] [CrossRef]
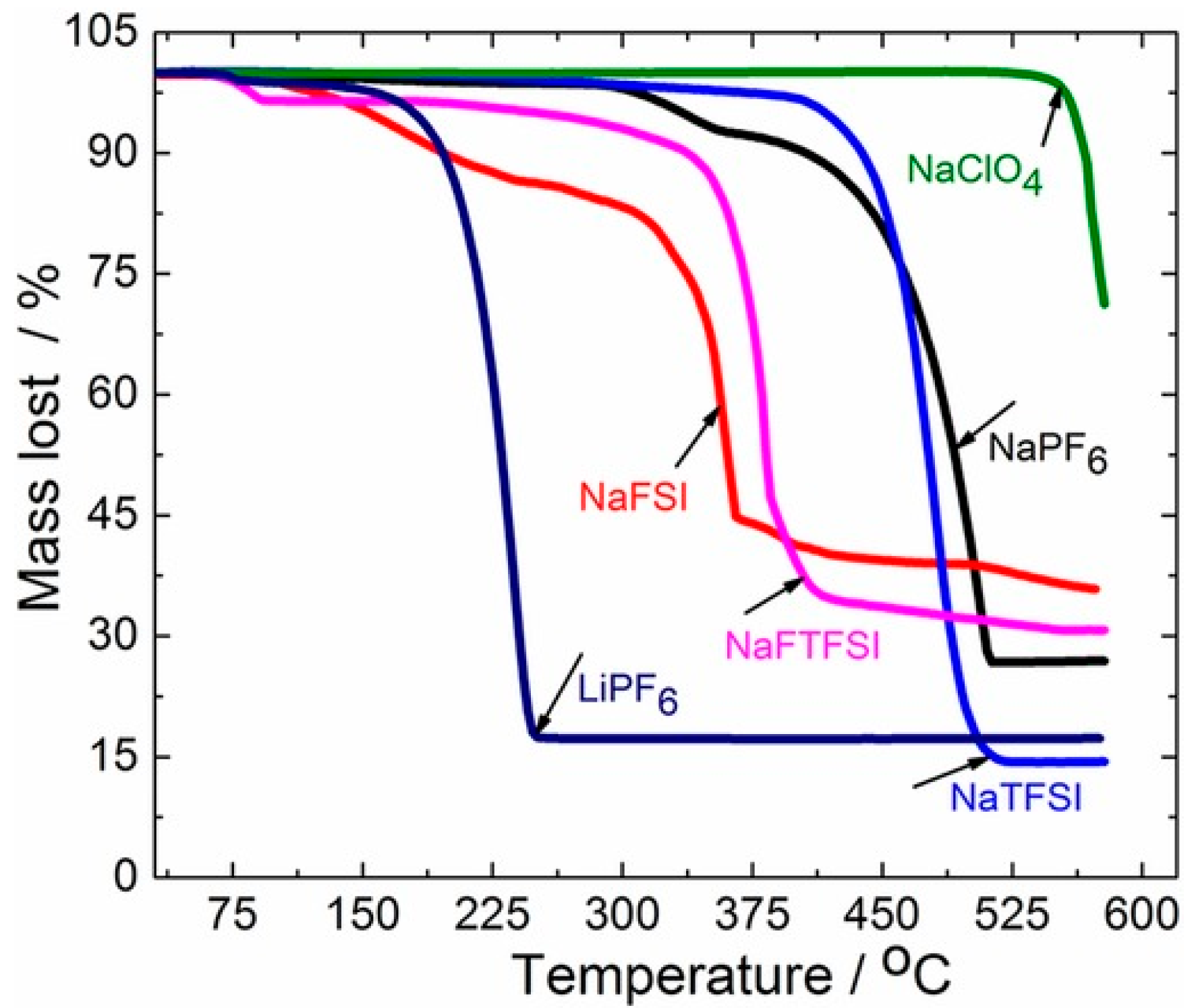
- SODIUM PERCHLORATE | CAMEO Chemicals | NOAA. Available online: https://cameochemicals.noaa.gov/chemical/1514 (accessed on 26 April 2024).
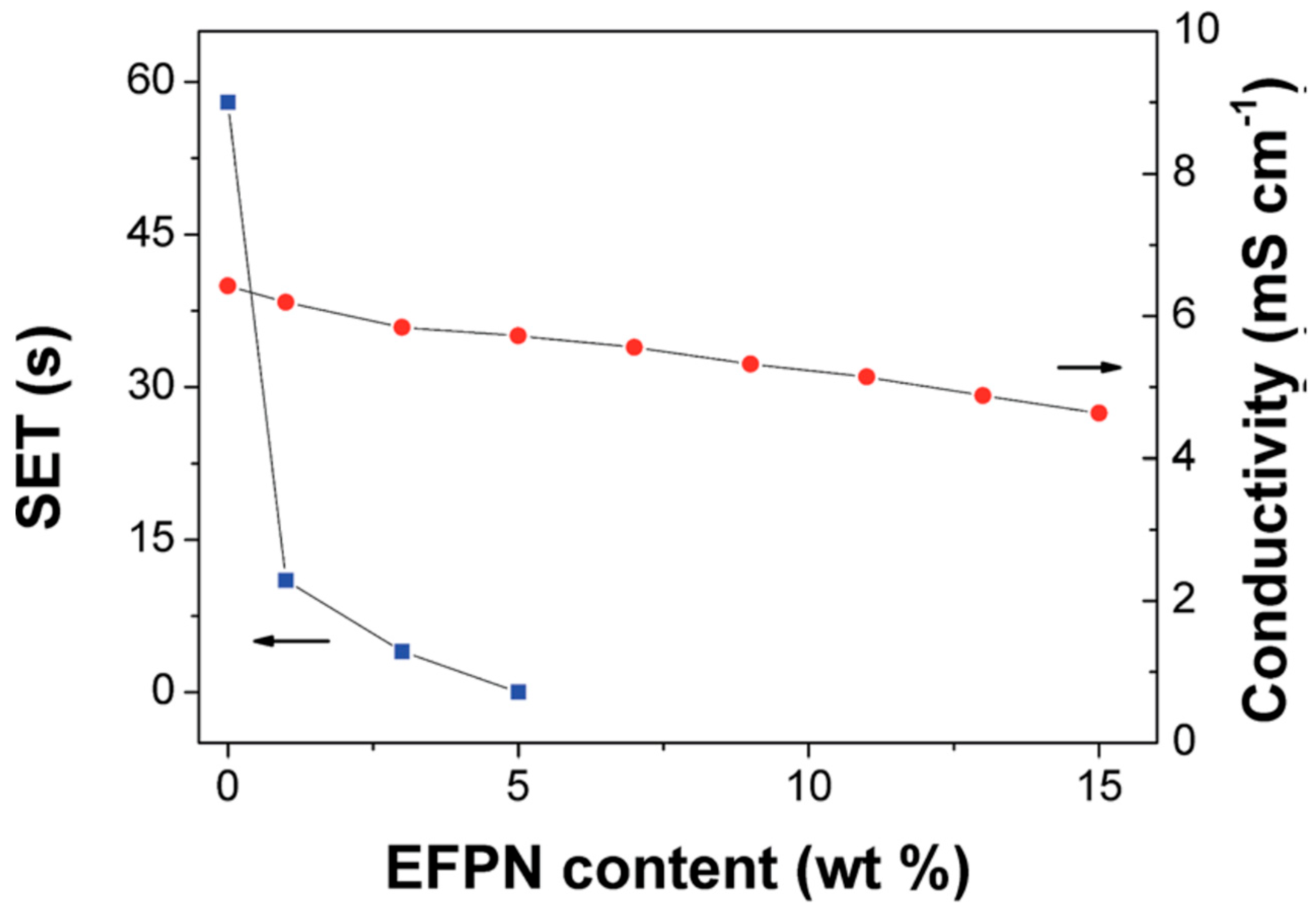
- Feng, J.; An, Y.; Ci, L.; Xiong, S. Nonflammable Electrolyte for Safer Non-Aqueous Sodium Batteries. J. Mater. Chem. A 2015, 3, 14539–14544. [Google Scholar] [CrossRef]
- Tribouilloy Benoit; Paillery Esteban; Binotto Ghislain; Marlair Guy Flammability of Halogenated Liquids: Flash Points Limits. Chemical Engineering Transactions 2022, 90, 529–534. [CrossRef]
- Yang, Z.; He, J.; Lai, W.; Peng, J.; Liu, X.; He, X.; Guo, X.; Li, L.; Qiao, Y.; Ma, J.; et al. Fire-Retardant, Stable-Cycling and High-Safety Sodium Ion Battery. Angewandte Chemie 2021, 133, 27292–27300. [Google Scholar] [CrossRef]
- Bordes, A.; Marlair, G.; Zantman, A.; Chesnaye, A.; Lore, P.-A.L.; Lecocq, A. Safety Evaluation of a Sodium-Ion Cell: Assessment of Vent Gas Emissions under Thermal Runaway. ACS Energy Lett. 2022, 7, 3386–3391. [Google Scholar] [CrossRef]
- Bordes, A.; Marlair, G.; Zantman, A.; Herreyre, S.; Papin, A.; Desprez, P.; Lecocq, A. New Insight on the Risk Profile Pertaining to Lithium-Ion Batteries under Thermal Runaway as Affected by System Modularity and Subsequent Oxidation Regime. Journal of Energy Storage 2022, 52, 104790. [Google Scholar] [CrossRef]
- Fernandes, Y.; Bry, A.; De Persis, S. Identification and Quantification of Gases Emitted during Abuse Tests by Overcharge of a Commercial Li-Ion Battery. Journal of Power Sources 2018, 389, 106–119. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Li, M.; Li, Y.; Li, C.; Zhang, Y.; Chen, S.; Shen, H.; Qian, F.; Feng, X.; et al. Experimental Study on Thermal Runaway Behavior of Lithium-Ion Battery and Analysis of Combustible Limit of Gas Production. Batteries 2022, 8, 250. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Davidson, J.N.; Brown, S.F. Assessment of Thermal Runaway in Commercial Lithium Iron Phosphate Cells Due to Overheating in an Oven Test. Energy Procedia 2018, 151, 74–78. [Google Scholar] [CrossRef]
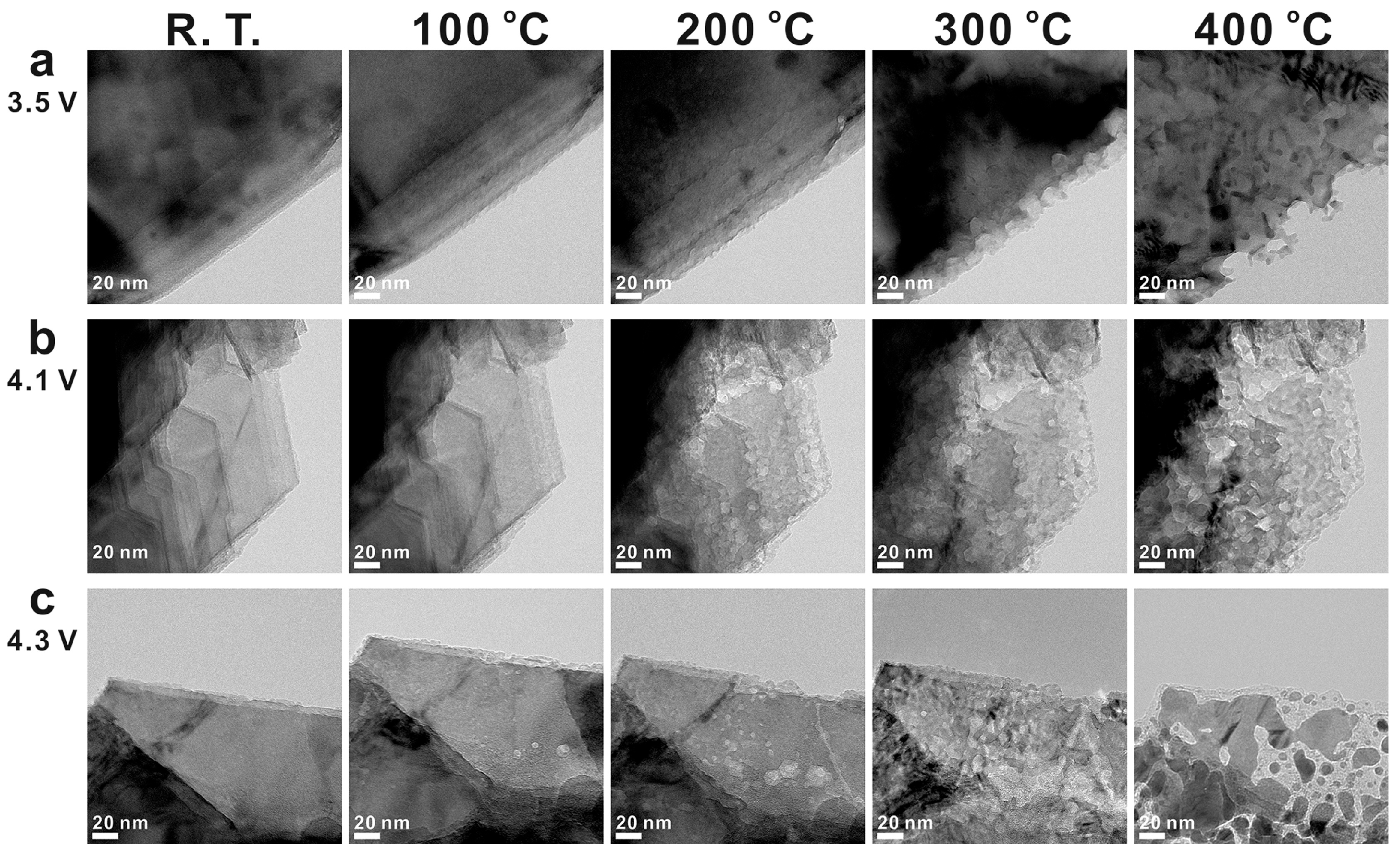
- Xie, Y.; Xu, G.-L.; Che, H.; Wang, H.; Yang, K.; Yang, X.; Guo, F.; Ren, Y.; Chen, Z.; Amine, K.; et al. Probing Thermal and Chemical Stability of Na x Ni 1/3 Fe 1/3 Mn 1/3 O 2 Cathode Material toward Safe Sodium-Ion Batteries. Chem. Mater. 2018, 30, 4909–4918. [Google Scholar] [CrossRef]
- Townsend, D.I.; Tou, J.C. Thermal Hazard Evaluation by an Accelerating Rate Calorimeter. Thermochimica Acta 1980, 37, 1–30. [Google Scholar] [CrossRef]
- Jhu, C.-Y.; Wang, Y.-W.; Wen, C.-Y.; Shu, C.-M. Thermal Runaway Potential of LiCoO2 and Li(Ni1/3Co1/3Mn1/3)O2 Batteries Determined with Adiabatic Calorimetry Methodology. Applied Energy 2012, 100, 127–131. [Google Scholar] [CrossRef]
- Lei, B.; Zhao, W.; Ziebert, C.; Uhlmann, N.; Rohde, M.; Seifert, H. Experimental Analysis of Thermal Runaway in 18650 Cylindrical Li-Ion Cells Using an Accelerating Rate Calorimeter. Batteries 2017, 3, 14. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.-S.; Qi, X.; Rong, X.; Li, H.; Huang, X.; Chen, L. Advanced Sodium-Ion Batteries Using Superior Low Cost Pyrolyzed Anthracite Anode: Towards Practical Applications. Energy Storage Materials 2016, 5, 191–197. [Google Scholar] [CrossRef]
- Rosalind, E. Franklin Crystallite Growth in Graphitizing and Non-Graphitizing Carbons. Proc. R. Soc. Lond. A 1951, 209, 196–218. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Choi, J.U.; Yoon, C.S.; Yashiro, H.; Sun, Y.-K. Resolving the Degradation Pathways of the O3-Type Layered Oxide Cathode Surface through the Nano-Scale Aluminum Oxide Coating for High-Energy Density Sodium-Ion Batteries. J. Mater. Chem. A 2017, 5, 23671–23680. [Google Scholar] [CrossRef]
- Pan, Y.; Chou, S.; Liu, H.K.; Dou, S.X. Functional Membrane Separators for Next-Generation High-Energy Rechargeable Batteries. National Science Review 2017, 4, 917–933. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. (John) Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Li, A.; Yuen, A.C.Y.; Wang, W.; De Cachinho Cordeiro, I.M.; Wang, C.; Chen, T.B.Y.; Zhang, J.; Chan, Q.N.; Yeoh, G.H. A Review on Lithium-Ion Battery Separators towards Enhanced Safety Performances and Modelling Approaches. Molecules 2021, 26, 478. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, C.J. The Role of Separators in Lithium-Ion Cell Safety. Interface magazine 2012, 21, 61–65. [Google Scholar] [CrossRef]
- Zhou, H.; Fear, C.; Parekh, M.; Gray, F.; Fleetwood, J.; Adams, T.; Tomar, V.; Pol, V.G.; Mukherjee, P.P. The Role of Separator Thermal Stability in Safety Characteristics of Lithium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 090521. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for Lithium-Ion Batteries: A Review on the Production Processes and Recent Developments. Energy Technology 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Zu, C.; Li, J.; Cai, B.; Qiu, J.; Zhao, Y.; Yang, Q.; Li, H.; Yu, H. Separators with Reactive Metal Oxide Coatings for Dendrite-Free Lithium Metal Anodes. Journal of Power Sources 2023, 555, 232336. [Google Scholar] [CrossRef]
- Ho, V.; Nguyen, B.T.D.; Thi, H.Y.N.; Kim, J.F.; Mun, J. Poly(Dopamine) Surface-modified Polyethylene Separator with Electrolyte-philic Characteristics for Enhancing the Performance of Sodium-ion Batteries. Intl J of Energy Research 2022, 46, 5177–5188. [Google Scholar] [CrossRef]
- Kim, J.I.; Heo, J.; Park, J.H. Tailored Metal Oxide Thin Film on Polyethylene Separators for Sodium-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1965–A1969. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




