1. Introduction
Januse Kinase 2 (JAK2) is a gene located on chromosome 9p24.1 [
1] that encodes for a non-receptor tyrosine kinase and is involved in the JAK-STAT pathway [
2,
3] that is fundamental in hematopoiesis [
4,
5]. An alteration in this gene can lead to many blood diseases like Myeloproliferative Neoplasms (MPNs). In MPNs an abnormal proliferation of one or more terminal myeloid cell lines in the peripheral blood gives rise to the pathology. In particular, polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF) are the classic MPNs [
6]. 95% PV patients and approximately half of the patients with essential ET and MF [
5] present JAK2 V617F mutation, a somatic mutation, due to the substitution of valine to phenylalanine at codon 617 of JAK2 with gain of function [
1,
3]. Consequently, JAK2 V617F mutation screening became a cornerstone in the molecular diagnostic approach for MPNs.
Hematopoietic cells with JAK2V617F mutation are transformed into cytokine-independent growth. Therefore, processes like tumorigenesis, tumor progression and the resulting inflammation are promoted [
1].
Recently finding indicates that JAK2V617F is associated with a specific haplotype, the germline GGCC (46/1) haplotype [
1,
7], found in approximately 45% of the overall population [
8,
9,
10].
This haplotype includes a set of genetic variations distributed along chromosome 9p.24.1, that covers JAK2, INSL6 and INSL4 genes [
1]. The last two genes are not normally transcribed in the hematopoietic system [
11].
The "GGCC" part includes the JAK2 gene region between intron 10 and intron 15, that is characterized by four single nucleotide polymorphisms (SNPs) that replace three thymidines (T) and one cytosine (C) by tow guanosines (G) and two cytosines (rs3780367:G, rs10974944:G, rs12343867:C, and rs1159782:C). All these SNPs are in complete linkage disequilibrium, thus they are always inherited together [
12].
In particular, research activities carried out in Europe, Japan, China, North America and Brazil have demonstrated that all MPNs patients, particularly those who carry the JAK2V617F mutation, have a higher frequency of the variant allele rs10974944 (G) than the control group. [
10,
13,
14,
15,
16,
17]. JAK2 G allele (rs10974944) is characterized by the substitution of a cytosine with a guanosine [
18], thus the C allele is the common allele, whereas the G allele is the variant that represents the risk allele for MPNs. In fact, this haplotype has also been individualized as one of the factors that increases the risk of familial MPNs by more than five times [
1].
The frequency of the JAK2 haplotypeGGCC_46/1 in the healthy population is about 24%, but in patients with JAK2 V617F mutation it was found in 40–80% of the cases [
19].
C-allele has been shown to be associated with ET [
20], while a prevalence of heterozygous haplotype C/G was demonstrated in patients with PMF and PV [
21].
This type of disorder generally tends to become chronic. Treatment strategies aim to alleviate symptoms, reduce the risk of thrombotic events, and prevent disease progression. In details, current approaches include a cytoreductive therapy using hydroxyurea (ATC: L01XX05), and the use of Ruxolitinib, a selective inhibitor of JAK1 and JAK2 [
22].
The first one is an antimetabolite that inhibits DNA synthesis and may kill cancer cells or make them easier to kill with radiation therapy (National Cancer Institute) and is used as first line treatment. Instead, the second one acts on the JAK-STAT pathway, by reducing the phosphorylation and leading to a reduced cellular proliferation and to the induction of apoptosis [
23]. Ruxolitinb is recommended when patients become refractory to hydroxyurea, as demonstrated by an Hct higher than 45% [
24] and symptomatic splenomegaly [
22] or intolerant.
Interestingly, some studies link certain JAK2 haplotypes to laboratory (increased platelet, leucocyte, hematocrit, and hemoglobin counts) and clinical (splenomegaly, splanchnic vein thrombosis, and Budd-Chari syndrome) results indicative of MPNs, but there is a lack of studies about the association between the haplotype tagged by rs10974944 single nucleotide polymorphism in JAK2 V617F-positive patients and their response to the onco-drugs used to treat the different MPNs. Thus, the aim of this work is to discover any association between haplotype tagged by rs10974944 single nucleotide polymorphism in JAK2 V617F-positive patients and their response to onco-drugs, in a cohort of patients selected by the Hematology Laboratory of “V.Fazzi'' hospital in Lecce.
2. Materials and Methods
2.1. Selection of Patients
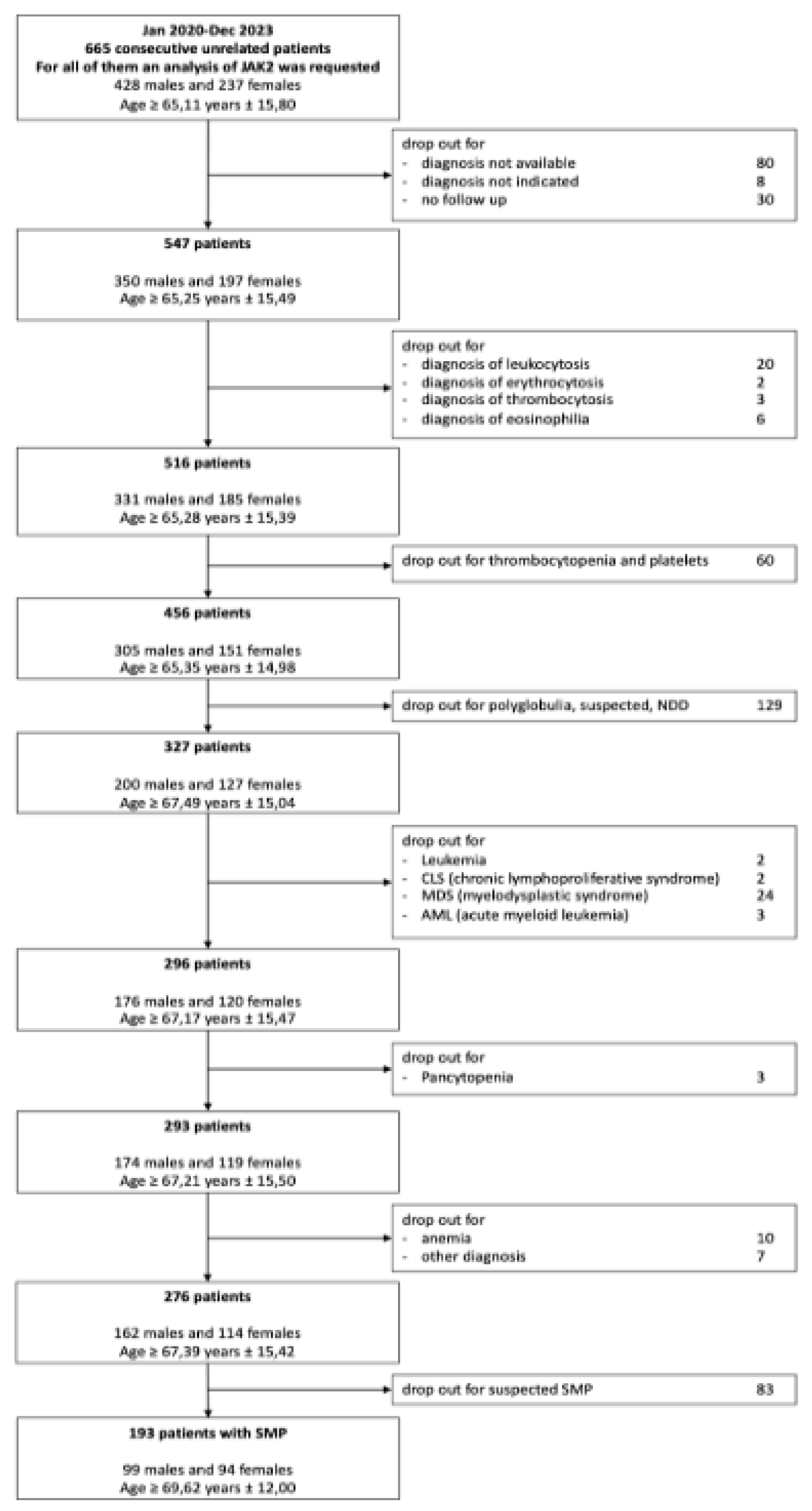
665 samples were collected by the hematology laboratory of “V. Fazzi Hospital (LE) from January 2020 to December 2023. These patients were previously diagnosed for JAK2 V617F mutation. Among them, we selected only patients with confirmed diagnosis of Myeloproliferative Syndrome (MPS), while we excluded individuals with no follow up available for many reasons (consultancy, occasional medical examination, patients who have changed hospitals…) and those with not enough data. Between the 547 remaining patients, we also excluded those with leukocytosis, erythrocytosis, thrombocytosis and eosinophilia. Moreover, patients with thrombocytopenia, different types of polyglobulia, pancytopenia and anemia were also excluded. In the end, individuals with leukemia, Chronic Lymphoproliferative Syndrome (CLS), Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML) were excluded. As a result, 276 patients were obtained. Regardless, 83 patients had no confirmed diagnosis of MPNs and were excluded. Among the 193 remaining patients, 91 were unclassifiable, thus only 102 patients with certain diagnosis of MPNs were considered for this study.
Since there are three main classes of pathologies grouped under the name of MPNs, we analyzed the distribution of JAK2 V617F positive and negative patients in these three categories.
As a result, we selected for our study: 19 patients with polycythemia vera, 18 with primary myelofibrosis and 13 with essential thrombocythemia. Samples analyzed include for the major part peripheral blood samples and just a few from bone marrow.
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of NOVARTIS PHARMACEUTICALS. ClinicalTrials.gov Identifier: NCT05548062, Novartis Reference Number: CINC424BIT01.
2.2. DNA Extraction from Fresh Blood Samples with NucleoSpin DX Blood*
The NucleoSpin DX Blood* kit was used for the isolation and purification of genomic DNA from human whole blood samples for subsequent in vitro diagnostic purposes, following the supplier’s instructions. After the extraction, DNA is quantified with a spectrophotometer. Sample extracted can be stored at -20°C.
2.3. JAK2 MutaQuant Analysis
JAK2 mutation V617F was detected with multiplex PCR using kit ipsogen® JAK2 MutaQuant® (QIAGEN). It is a quantitative in vitro test for the accurate detection and quantification of the JAK2 V617F/G1849T allele starting from genomic DNA extracted from peripheral blood of patients with suspected MPN. PCR mix was prepared in multiwell PCR plates and put in 7500 Fast Dx Real-Time PCR Instrument (Applied Biosystems). Patients tested positive to V617F mutation were selected for the following phase.
2.4. PCR-RFLP Assay
For JAK2 rs10974944 SNP screening it has been used a PCR-RFLP assay developed by Trifa et al. [
25], who developed a simple and inexpensive PCR-RFLP assay for studying the JAK2 rs10974944 SNP, a constituent of the putative JAK2V617F-predisposing haplotype. The primer sequences were as follows: Primer Rev 5’-CTGCTTGCTAGTGGGTGAAT-3’ (Eurofins Genomics), Primer Fw 5’-CAAGGGTCAACTGTAGTACATAA-3’ (Eurofins Genomics).
The PCR reactions were set up in a 50 μL reaction volume, with the following composition: 44 μL PCR Master- Mix containing HotStartTaq® 5 units/μL (QUIAGEN), MgCl2 25 mM (QUIAGEN), dNTPs mix 10 mM (DNA diagnostic A/S), 5 μL of each forward and reverse primer, PCR Buffer 10 X (QUIAGEN), H2O DNase-RNase free (Sigma-Aldrich) and 150 ng of genomic DNA.
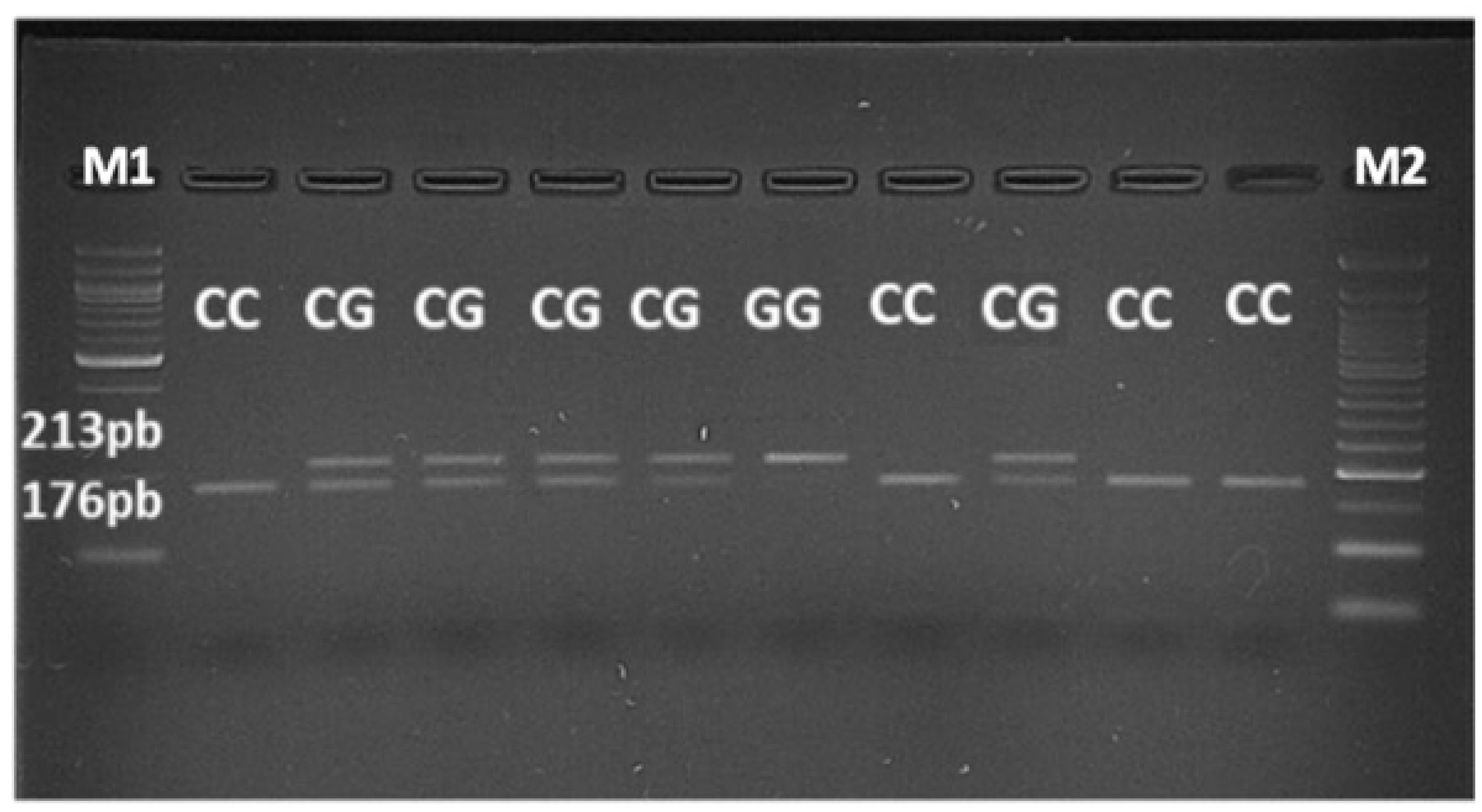
The amplification procedure consisted of an initial activation step at 95 °C for 15 min. In this step HotStartTaq was activated by the heating step. Then 3 step cycling were performed as follow: denaturation at 94 °C for 1 min, annealing at 52°C for 1 min (this step must be performed approximately 5°C below Tm of primers) and final extension at 72°C for 1.30 min. This 3 step cycling was repeated 35 times. At the end, there was a final extension at 72°C for 10 min. In this way, a 243- bp amplicon was obtained, which was then incubated overnight (for 16 hours) at 37 °C with 5 U of the restriction endonuclease MboI (Thermo Scientific), according to manufacturer’s specifications. To visualize the fragments, 3% agarose gel was run, stained with Nucleic Acid Stain 20.000X-1mL (EuroClone) and put in a GelDoc (Uvitec).
If the common C allele was present, the 243-bp amplicon contained 3 restriction sites for MboI, giving rise to 4 fragments after digestion, as follows: 176, 37, 23 and 7 bp. In the presence of the G allele, one of the MboI restriction sites was abolished, such that the digestion with MboI would produce 3 fragments of 213, 23 and 7 bp. The fragments of 23 and 7 bp were not seen on the gel, but the delineation of the C and G alleles was made based on the difference between the fragments of 213 and 176 bp.
2.5. Statistical Analysis
For dichotomous variables, differences between the groups were tested using the Fisher exact test with R project for statistical computing. While Pearson χ2 test was used for categorical variables, always with R project for statistical computing.
Two-sided p values <0,01 were set as the threshold for statistical significance.
3. Results
3.1. Prevalence and Correlations of JAK2–V617F Mutation in MPNs Diseases
A flow diagram illustrating the screening process for patient selection is presented in
Figure 1.
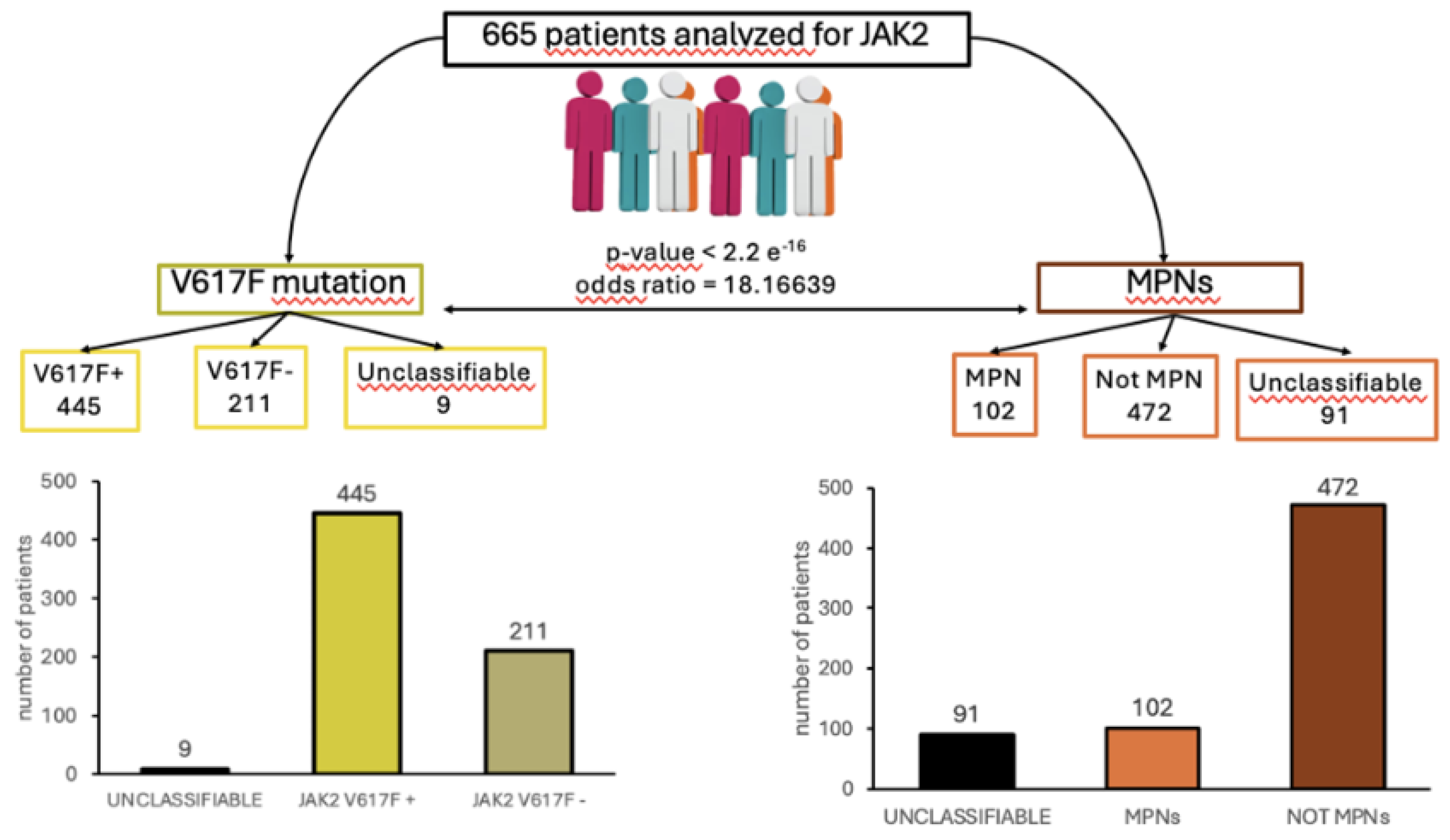
To demonstrate the presence of a correlation between JAK2V617F mutation and the predisposition to MPNs, 665 patient samples were collected from the hematology laboratory of “V. Fazzi" Hospital (LE) and analyzed for their distribution according to JAK2 gene mutation and MPNs diagnosis. Among these patients, 445 tested positive for the JAK2V617F mutation, while 211 had a wildtype form of the gene (
Figure 2A). The remaining patients (9) were undermined after the molecular biology analysis, due to technical and sampling issues, and were not considered in this study. Among the 665 patients tested for JAK2 mutation, 102 patients were diagnosed with myeloproliferative syndrome, while 472 patients included cases of polyglobulia, thrombocytopenia or thrombocytosis, anemia, myeloid or lymphoid leukemia, eosinophilia, leukocytosis, myelodysplasia, or suspected myeloproliferative syndromes not yet classified in a specific clinical picture (Figure 2B). We excluded 91 cases with unclassifiable diagnosis and those in which the diagnosis was not indicated in the medical records. Analyzing the correlation between JAK2V617F mutation positive patients and patients with diagnosis of MPNs, a statistically significant correlation was found, as demonstrated by the p-value lower than 2,2 e-16, calculated with Fisher analysis. Of the 102 MPNs patients involved, 41 (40%) had ET, 25 (25%) had PV while 36 (35%) had PMF (
Figure 3B). JAK2-V617F mutation was found in 69 (69,6%) of the patients assessed (
Figure 3A). This mutation was present in 25 out of 41 patients diagnosed with ET (61%; p-value= 0.07294), 23 out of 25 patients diagnosed with PV (92%; p-value= 0,009964), and 25 out of 36 patients diagnosed with PMF (69%; p-value= 0,8192) (
Figure 3B), showing how JAK2V6171F mutation is highly present in all the MPNs diseases with a prevalence in PV. All together these data confirmed the presence of a strong correlation between the JAK2 V617F mutation and MPNs in the group of patients analyzed. Moreover, the analyses of the correlation among JAK2 V617F mutation and each type of MPNs showed a statistically significant correlation in patients affected by PV but not in patients affected with ET and PMF. Thus, while JAK2V617F mutation could be used as a diagnostic marker for PV, this is not true for ET and PMF, where it could be used only to support diagnosis.
3.2. JAK2 Hapoltype 46/1 Distribution in MPNs Patients Tested Positive for JAK2 V617F Mutation
Subsequently, the 102 patients with a diagnosis of myeloproliferative disorder were tested for the presence of JAK2 haplotype 46/1. From this group, patients subjected to analysis in 2020 were excluded, due to samples lack of availability.
From this selection process, 50 samples were found suitable for the analysis, and they included 19 cases of PV, 18 cases of PMF and 13 cases of ET. From all these patients, a blood sample has been collected and DNA has been extracted. A RFLP-PCR assay has been performed based on the protocol developed by Trifa et al. [
25].
After a restriction enzyme digestion with MboI enzyme, a gel electrophoresis analysis has been performed to test if JAK2 46/1 haplotype exists in patients. Thus, JAK2 rs10974944 SNP was used as tag SNP to identify the haplotype. The aim of this analysis was to evaluate the presence of the rs10974944 (G) allele, but also the heterozygous condition (G/C) and the common allele (C/C) (
Figure 4).
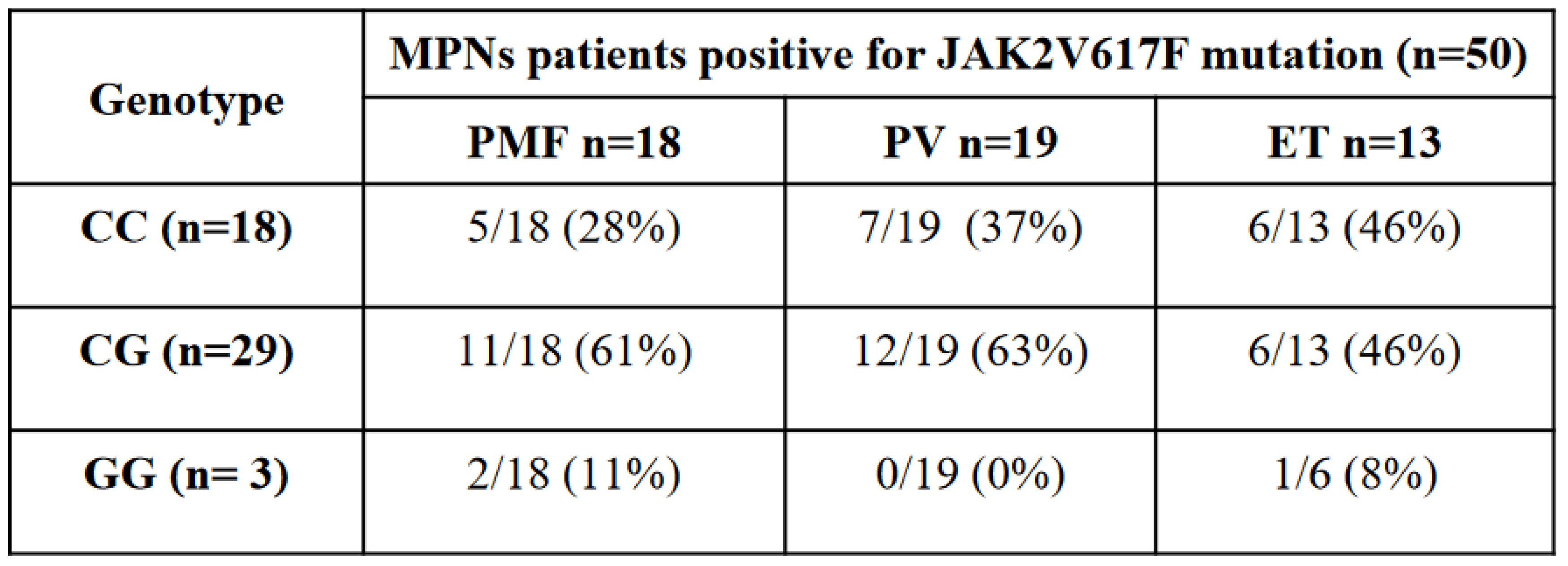
As shown in
Table 1, the C/G genotype was more common in JAK2 V617F positive patients compared to the C/C common allele. Indeed, we found that C/G genotype was present in 58% of JAK2 V617F positive patients with respect to 36% of patients with the C/C common allele and only the 6% patients with the G allele.
By considering the distribution of JAK2 rs10974944 SNP genotypes into the three different MPNs analyzed (TE, PMF, PV), we found that the C/G genotype was prevalent in patients with myelofibrosis or with polycythemia vera, where it represented respectively 61% and 63% of total cases, with respect to 46% of essential thrombocythemia cases (Tab1). Instead, the C/C common allele was found in 46% of ET cases, followed by 37% cases of PV and 28% of PMF (Table1). The JAK2 rs10974944 G/G genotype was present in only 11% of myelofibrosis cases and in 8% of essential thrombocythemia patients.
Table 1 This table shows 50 MPNs patients tested positive for the JAK2V617F mutation classified based on their genotype and on the MPN subtypes.
All together these data suggest that the heterozygous condition (C/G) is the most frequent in JAK2 V617F tested positive patients (58%) and that this genotype is predominantly associated with PMF (61%) and PV (63%). Instead, C/C genotype is more associated with ET (46%) and G/G genotype with PMF (11%).
3.3. JAK2 Haplotype 46/1 Association with Therapy Response in MPNs Patients Positive for JAK2V617F Mutation
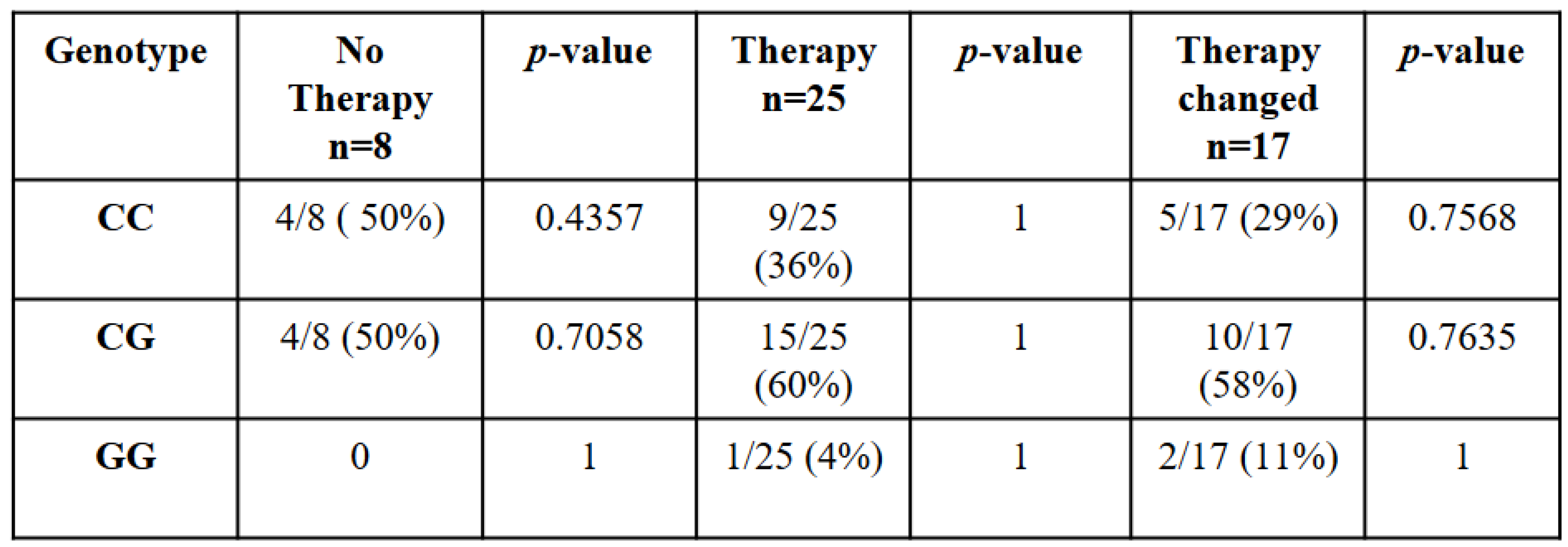
In this work, we studied for the first time the association between JAK2 haplotypeGGCC_46/1 and patients’ response to therapy. According to data reported in patients’ medical records, all 50 patients with JAK2 haplotype GGCC_46/1 were divided into three categories: those who do not do therapy, those who do not undergo change in therapy and those who change therapy.
The analysis of JAK2 haplotypeGGCC_46/1 showed how the common allele (C/C) was present in 50% of subjects that did not do therapy, in 36% of subjects that did therapy without changes and 29% of subjects that changed therapy due to drug resistance (
Table 2). Instead, in the heterozygous condition (C/G) 60% of patients did therapy without changes, 58% of patients presented drug resistance and 50% of subjects did not do any therapy (
Table 2). Finally, the G/G genotype in 4% of patients was associated with effective first line treatment without necessity of changes, while in 11% of patients was associated with the development of onco-drug resistance. Instead, we did not find G/G genotype in patients that did not need therapy (
Table 2). Analyzing the correlation among each genotype and each type of patients’ response to therapy, we found that there was no statistically significant correlation. In particular, no genotype correlated positively with the uprising onco-drug resistance, although 58% of the subjects analyzed that underwent changes in therapy had the C/G genotype, compared to 29% with the C/C common genotype and 11% with the G/G genotype.
Table 2 This table shows 50 MPNs patients tested positive for the JAK2V617F mutation classified based on their genotype and their response to therapy. p value < 0,01 is statistically significance.
3.4. JAK2 haplotype 46/1 Association with Onco-Drug Resistance Symptoms in JAK2V617F Positive MPNs Patients
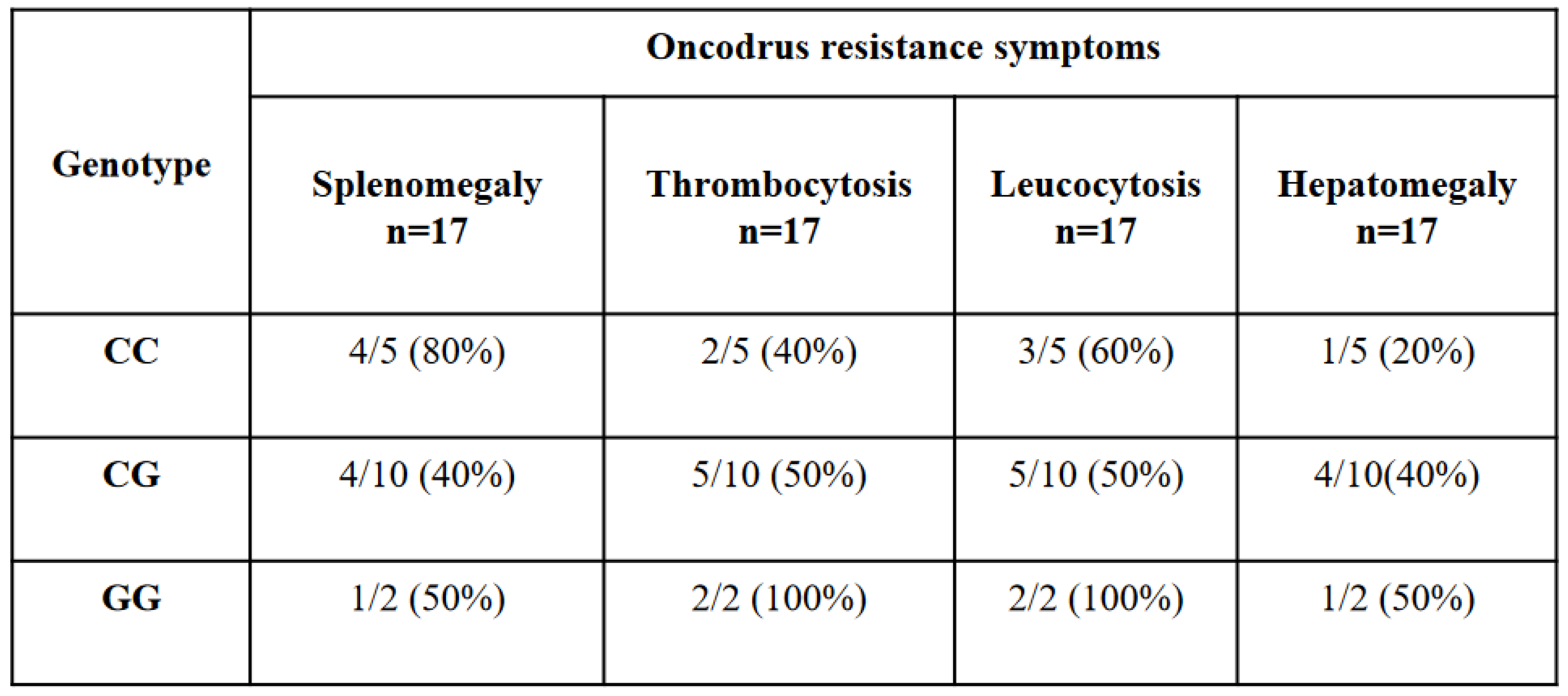
We decided to evaluate, only in the 17 patients who developed a drug resistance, if there was a correlation between the presence of JAK2 46/1 haplotype and some clinical parameters that could lead to onco-drug resistance: splenomegaly, leukocytosis, thrombocytosis and hepatomegaly.
We found that 80% of patients with (C/C) genotype presented splenomegaly, 60% and 40% of patients presented leukocytosis and thrombocytosis respectively, and only 20% of the cases presented hepatomegaly (
Table 3).
On the other hand, patients with heterozygous (C/G) condition displayed in about half of the cases all the symptoms related to onco-drug resistance. In particular, we found thrombocytosis and leukocytosis in 50% of the cases and splenomegaly and hepatomegaly in 40% of the cases (Table3). G/G genotype was represented by few patients because of the reduced frequency of the allele in the general population. Anyway, it is interesting to observe that they presented thrombocytosis and leukocytosis in 100% of the cases and splenomegaly and hepatomegaly in 50% of the cases (
Table 3). These data could suggest how subjects with heterozygous condition (C/G) and those with (G/G) genotype in at least 50% of the cases have the possibility to develop all the related symptoms of onco-drug resistance, although an in-depth analysis in a larger population is recommended.
Table 3.
association of JAK2 rs10974944 SNP genotype frequencies with oncodrugs resistance symptoms.
Table 3.
association of JAK2 rs10974944 SNP genotype frequencies with oncodrugs resistance symptoms.
Table 3) This table shows 17 MPNs patients tested positive for the JAK2V617F mutation classified based on their genotype and their oncodrug resistance symtoms. p value < 0,01 is statistically significance.
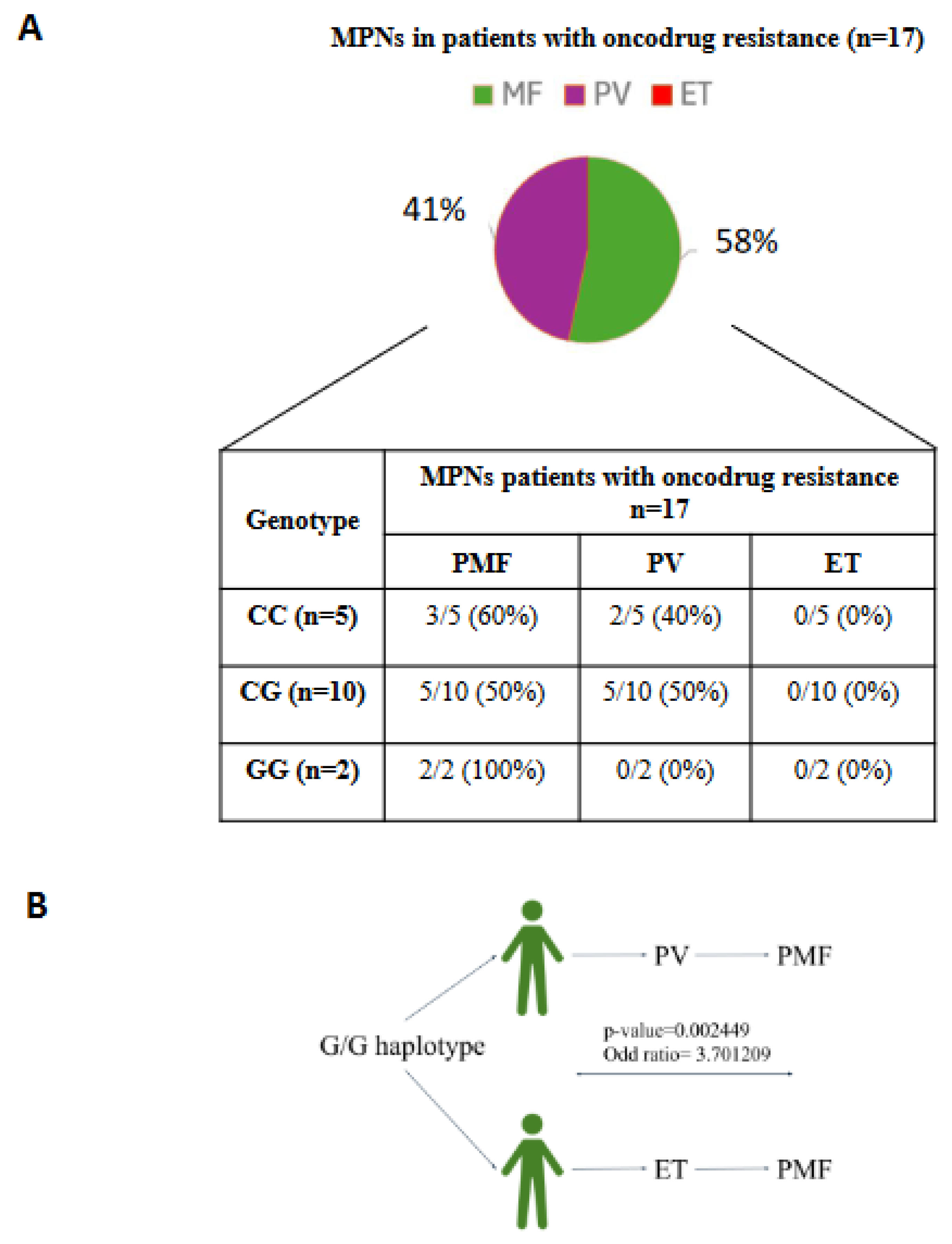
3.5. JAK2 Haplotype 46/1 Contribution in MPNs Patients with Drug Resistance
On a number of 17 patients analyzed with onco-drug resistance, 58% suffered from PMF and 41% of PV, while there weren’t cases of ET. Thus, considering the role of the G allele, as a risk factor, in the outcome of MPNs we analyzed JAK2 rs1097944 SNP genotype contribution.
According to the genotype frequencies, we found that the C/C common allele was present in 60% of patients with PMF and in 40% of patients with PV while the heterozygous condition C/G showed the same percentage (50 %) in both diseases (Figure 5). Instead, we found that subjects with G/G allele were affected only by PMF and that this condition was an evolution of different types of MPNs. Analyzing the other genotypes, 0% of patients with C/C or C/G allele, affected by PMF, presented this condition. Fisher analysis revealed statistically significant correlation between the G/G allele and the evolution of different kind of MPNs towards PMF, with a p-value= 0.002449 and an odd ratio of 3.701209, suggesting how the G/G allele of JAK2 haplotype 46/1 could be useful for the prediction of the disease severity.
To conclude, these data suggested how patients with onco-drug resistance were mostly affected by PMF and PV. Moreover, while in these subjects, C/C and C/G genotypes were not statistically associated with a particular MPNs disorder, the G/G condition could contribute to the development of PMF starting from other MPNs..
4. Discussion
Myeloproliferative neoplasms (MPN) are a group of bone marrow diseases with an excessive cell production, starting from a precursor of the myeloid lineage. It is widely believed that somatic acquisition of genetic aberrations may be one of the pathogenic mechanisms of the MPNs [
15,
26]. The identification of JAK2 V617F mutation represented an important step in the understanding of the molecular mechanisms involved in MPN diseases, although these remain not completely understood.
Among the 665 patients selected by the hematology Laboratory of “V.Fazzi'' hospital in Lecce, 102 received a confirmed diagnosis of Myeloproliferative Syndrome and 70% of these patients tested positive for JAK2 V617F mutation.
By Fisher analysis a statistically significant association between the JAK2V617F mutation and MPNs was found with a p-value lower than 2,2 e-16 and an odd ratio of 18,17 suggesting that these patients have a 18,17 times higher risk to develop myeloproliferative disorders, compared to JAK2V617F negative patients. This finding corroborates previous studies showing the association between JAK2V617F mutation and the predisposition to MPNs [
1,
27]. In detail, we observed a prevalence of JAK2 V617F mutation in patients with PV (92%), followed by PMF (69%) and ET (61%) [
5,
7,
28]. A prevalence of JAK2 V617F positive cases was previously reported in subjects affected by PV, since it is directly associated with the specific pathogenesis of this hematologic malignancy and plays a key role in the constitutive activation of the JAK-STAT pathway [
9].
Recently, different research groups reported for the first time that JAK2V617F mutation tends to occur in a specific haplotype, called 46/1 haplotype (GGCC haplotype). The presence of the JAK2 haplotypeGGCC_46/1 increases the risk of acquiring JAK2 V617F mutation from two to three times [
1], making itself a valid biomarker for monitoring MPNs patients.
Ascertained that there was an association between JAK2V617F mutation and MPN in our cohort of patients, we evaluated JAK2 haplotype distribution.
We used rs10974944 SNP variant to detect JAK2 46/1 haplotype in our patients tested positive for JAK2 V617F mutation. In fact, it has been shown that this SNP is particularly associated MPNs patients tested positive for the mutation V617F [
10,
13,
14,
15,
16,
17].
We found that C/G genotype is present in 58% of patients with JAK2V617F mutation with respect to 36% that instead presented the C/C common allele and to the 6% that had the G allele. Our results are in agreement with data reported in literature that demonstrate that the C/G genotype is more common in JAK2 V617F positive patients compared to C/C [
10,
30] and G/G genotype, that is the least represented [
32].
Moreover, we found that the heterozygous condition of the haplotype 46/1 was prevalent associated with the development of PV (63%) and PMF (61%) in V617F positive patients.
Although different studies have associated the haplotype 46/1 with certain clinical and laboratory findings [
1], especially for subjects affected by PV [
15] no one considers a possible correlation between the haplotype 46/1 and the development of drug resistance. As a consequence, here, we collected medical records of the 50 patients analyzed in order to determine changes in therapy due to drug resistance. Patients were divided into three main categories: those who do not do therapy, those who do not undergo change in therapy and those who change therapy. For each category we evaluated the JAKrs10974944 genotype frequencies and its relation to the patient's therapy response.Although no statistically significant association was found between the presence of the JAK2 haplotype 46/1 variants and the different patients’ therapy response, in patients with onco-drug resistance we observed a prevalence of heterozygous (C/G) genotype (58%). About half of the cases with heterozygous condition developed all the related symptoms to onco-drug resistance: thrombocytosis, leukocytosis, splenomegaly and hepatomegaly.
Furthermore, these patients were mainly affected by PMF (58%) and PV (41%), while no cases of ET were detected. The absence of ET cases, in subjects with onco-drug resistance, could be explained considering that normally JAK2 V617F mutation in these patients causes not only a high risk of thrombosis, but may also promote the progression to PV or PMF [
31]. Moreover, ET patients are often treated simply by aspirin that is associated with a cytoreductive drug only in subjects with high risk.
Besides the prevalence of the heterozygous genotype in subjects with onco-drug resistance, an important finding came out from the analysis of the G/G genotype, even if it was present in only 11% of patients. Indeed, G allele is the most studied, since it represents the risk allele for MPNs [
13,
32]. We found that subjects with G/G allele were affected by PMF and in 50 % of the cases presented all the related symptoms of onco-drug resistance. Analyzing the clinical history of these patients, we discovered that their PMF was an evolution of different types of MPNs and that 0% of patients with C/C or C/G allele, affected by PMF, presented this condition. Fisher analysis revealed statistically significant correlation between the G/G allele and the evolution of different kind of MPNs towards PMF, with a p-value= 0.002449 and an odd ratio of 3.701209, thus suggesting how the G/G allele of JAK2 haplotype 46/1 could be useful for the prediction of the disease severity. In another study, it has been shown that the 46/1 haplotype could predispose PV patients to bone marrow fibrosis [
33].
Thus, despite the narrow cardinality of the sample analyzed, we could assume that the presence of G allele is a risk factor for the evolution of polycythemia vera and essential thrombocythemia in myelofibrosis.
Thus, even if the absence of a correlation among the JAK2 haplotypeGGCC_46/1 and onco-drug resistance in patients with C/G allele, the presence of the G/G allele seems to be related to a predisposition for MPNs evolution to myelofibrosis condition and in the development of clinical parameters that could cause onco-drug resistance. Considering the narrow cardinality of the studied sample, we believe that current efforts should be aimed at shedding light on the clinical role of JAK2 haplotypeGGCC_46/1, through an in-depth analysis, perhaps expanding the sample under study. We believe that the integration of a comprehensive genetic profiling into treatment decisions marks a significant advance in MPN management, influencing disease progression, prognosis, and treatment efficacy.
Author Contributions
“Conceptualization, M.P.; S.S.; D.S.; M.M. and N.DR.; methodology, M.P.; S.S.; A.T.; R.M.; validation, M.P.; S.S. and D.S.; formal analysis, M.P.; S.S.; A.T. and G.L.; investigation M.P. and S.S.; resources, M.M. and N.DR.; data curation, M.P.; S.S.; R.M.; D.S.; G.L.; writing—original draft preparation, M.P. and S.S.; writing—review and editing, M.P. ;S.S.; A.T.; G.L.; R.M.; D.S.; M.M. and N.DR.; supervision, S.S.; D.S.; M.M. and N.DR.; project administration, M.M. and N.DR.; funding acquisition, M.M. and N.DR.
Funding
This research was funded by ALESSIA PALLARA foundation.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of NOVARTIS PHARMACEUTICALS. ClinicalTrials.gov Identifier: NCT05548062, Novartis Reference Number: CINC424BIT01.
Informed Consent Statement
“Informed consent was obtained from all subjects involved in the study.”.
Acknowledgments
The authors are highly thankful to Alessia Pallara foundation for providing financial support for this study. Also we duly acknowledge i) Dott. Antonio Danieli, Laboratory of General Physiology, University of Salento, for its technical support; ii) All medical stuff from the Hematology and Stem Cell Transplant Unit, Onco-Hematological Department, who contributed to this work with their data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paes J, Silva GAV, Tarragô AM, Mourão LPS. The Contribution of JAK2 46/1 Haplotype in the Predisposition to Myeloproliferative Neoplasms. Int J Mol Sci. 2022 Oct 20;23(20):12582. [CrossRef]
- Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021 Nov 26;6(1):402. [CrossRef]
- Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007 Sep;7(9):673-83. [CrossRef]
- Yamaoka K, Saharinen P, Pesu M, Holt VE 3rd, Silvennoinen O, O'Shea JJ. The Janus kinases (Jaks). Genome Biol. 2004;5(12):253. [CrossRef]
- Staerk J, Constantinescu SN. The JAK-STAT pathway and hematopoietic stem cells from the JAK2 V617F perspective. JAKSTAT. 2012 Jul 1;1(3):184-90. [CrossRef]
- Josil J, Thuillier E, Chambrun L, Plo I. Décryptage de l’histoire naturelle des néoplasmes myéloprolifératifs grâce à une approche d’arbres phylogénétiques [Natural history of myeloproliferative neoplasms by a phylogenetic tree-based approach]. Med Sci (Paris). 2024 Feb;40(2):209-211. French. [CrossRef]
- Tefferi A, Lasho TL, Mudireddy M, Finke CM, Hanson CA, Ketterling RP, Gangat N, Pardanani A. The germline JAK2 GGCC (46/1) haplotype and survival among 414 molecularly-annotated patients with primary myelofibrosis. Am J Hematol. 2019 Mar;94(3):299-305. [CrossRef]
- Jones AV, Chase A, Silver RT, Oscier D, Zoi K, Wang YL, Cario H, Pahl HL, Collins A, Reiter A, Grand F, Cross NC. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009 Apr;41(4):446-9. [CrossRef]
- Olcaydu D, Harutyunyan A, Jäger R, Berg T, Gisslinger B, Pabinger I, Gisslinger H, Kralovics R. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009 Apr;41(4):450-4. 10.1038/ng.341. Epub 2009 Mar 15. PMID: 19287385. Paes J, Silva GAV, Tarragô AM, Mourão LPS. The Contribution of JAK2 46/1 Haplotype in the Predisposition to Myeloproliferative Neoplasms. Int J Mol Sci. 2022 Oct 20;23(20):12582. https://doi.org/10.3390/ijms232012582.
- Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, Bass A, Marubayashi S, Heguy A, Garcia-Manero G, Kantarjian H, Offit K, Stone RM, Gilliland DG, Klein RJ, Levine RL. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009 Apr;41(4):455-9. [CrossRef]
- Vannucchi AM, Guglielmelli P. The JAK2 46/1 (GGCC) MPN-predisposing haplotype: A risky haplotype, after all. Am J Hematol. 2019 Mar;94(3):283-285. [CrossRef]
- Anelli L, Zagaria A, Specchia G, Albano F. The JAK2 GGCC (46/1) Haplotype in Myeloproliferative Neoplasms: Causal or Random? Int J Mol Sci. 2018 Apr 11;19(4):1152. [CrossRef]
- Pagliarini-e-Silva S, Santos BC, Pereira EM, Ferreira ME, Baraldi EC, Sell AM, Visentainer JE. Evaluation of the association between the JAK2 46/1 haplotype and chronic myeloproliferative neoplasms in a Brazilian population. Clinics (Sao Paulo). 2013 Jan;68(1):5- 9. [CrossRef]
- Macedo LC, Santos BC, Pagliarini-e-Silva S, Pagnano KB, Rodrigues C, Quintero FC, Ferreira ME, Baraldi EC, Ambrosio-Albuquerque EP, Sell AM, Visentainer JE. JAK2 46/1 haplotype is associated with JAK2 V617F--positive myeloproliferative neoplasms in Brazilian patients. Int J Lab Hematol. 2015 Oct;37(5):654-60. [CrossRef]
- Ohyashiki JH, Yoneta M, Hisatomi H, Iwabuchi T, Umezu T, Ohyashiki K. The C allele of JAK2 rs4495487 is an additional candidate locus that contributes to myeloproliferative neoplasm predisposition in the Japanese population. BMC Med Genet. 2012 Jan 17;13:6. [CrossRef]
- Li SL, Zhang PJ, Sun GX, Lu ZJ. The JAK2 46/1 haplotype (GGCC) in myeloproliferative neoplasms and splanchnic vein thrombosis: a pooled analysis of 26 observational studies. Ann Hematol. 2014 Nov;93(11):1845-52. [CrossRef]
- Koh SP, Yip SP, Lee KK, Chan CC, Lau SM, Kho CS, Lau CK, Lin SY, Lau YM, Wong LG, Au KL, Wong KF, Chu RW, Yu PH, Chow EY, Leung KF, Tsoi WC, Yung BY. Genetic association between germline JAK2 polymorphisms and myeloproliferative neoplasms in Hong Kong Chinese population: a case-control study. BMC Genet. 2014 Dec 20;15:147. [CrossRef]
- Zhang X, Hu T, Wu Z, Kang Z, Liu W, Guan M. The JAK2 46/1 haplotype is a risk factor for myeloproliferative neoplasms in Chinese patients. Int J Hematol. 2012 Nov;96(5):611-6. [CrossRef]
- Andrikovics H, Nahajevszky S, Koszarska M, Meggyesi N, Bors A, Halm G, Lueff S, Lovas N, Matrai Z, Csomor J, Rasonyi R, Egyed M, Varkonyi J, Mikala G, Sipos A, Kozma A, Adam E, Fekete S, Masszi T, Tordai A. JAK2 46/1 haplotype analysis in myeloproliferative neoplasms and acute myeloid leukemia. Leukemia. 2010 Oct;24(10):1809-13. [CrossRef]
- Pardanani A, Lasho TL, Finke CM, Gangat N, Wolanskyj AP, Hanson CA, Tefferi A. The JAK2 46/1 haplotype confers susceptibility to essential thrombocythemia regardless of JAK2V617F mutational status-clinical correlates in a study of 226 consecutive patients. Leukemia. 2010 Jan;24(1):110-4. [CrossRef]
- Guglielmelli P, Biamonte F, Spolverini A, Pieri L, Isgrò A, Antonioli E, Pancrazzi A, Bosi A, Barosi G, Vannucchi AM. Frequency and clinical correlates of JAK2 46/1 (GGCC) haplotype in primary myelofibrosis. Leukemia. 2010 Aug;24(8):1533-7. [CrossRef]
- Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, Catalano JV, Deininger M, Miller C, Silver RT, Talpaz M, Winton EF, Harvey JH Jr, Arcasoy MO, Hexner E, Lyons RM, Paquette R, Raza A, Vaddi K, Erickson-Viitanen S, Koumenis IL, Sun W, Sandor V, Kantarjian HM. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012 Mar 1;366(9):799-807. [CrossRef]
- Passamonti F, Maffioli M. The role of JAK2 inhibitors in MPNs 7 years after approval. Blood. 2018 May 31;131(22):2426-2435. [CrossRef]
- Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, Harrison C, Hasselbalch HC, Hehlmann R, Hoffman R, Kiladjian JJ, Kröger N, Mesa R, McMullin MF, Pardanani A, Passamonti F, Vannucchi AM, Reiter A, Silver RT, Verstovsek S, Tefferi A; European LeukemiaNet. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011 Feb 20;29(6):761-70. [CrossRef]
- Trifa AP, Cucuianu A, Popp RA. Development of a reliable PCR-RFLP assay for investigation of the JAK2 rs10974944 SNP, which might predispose to the acquisition of somatic mutation JAK2(V617F). Acta Haematol. 2010;123(2):84-7. [CrossRef]
- Abdel-Wahab O, Levine R. The spliceosome as an indicted conspirator in myeloid malignancies. Cancer Cell. 2011 Oct 18;20(4):420-3. [CrossRef]
- Duletić AN, Dekanić A, Hadzisejdić I, Kusen I, Matusan-Ilijas K, Grohovac D, Grahovac B, Jonjić N. JAK2-v617F mutation is associated with clinical and laboratory features of myeloproliferative neoplasms. Coll Antropol. 2012 Sep;36(3):859-65.
- Kucine N. Myeloproliferative Neoplasms in Children, Adolescents, and Young Adults. Curr Hematol Malig Rep. 2020 Apr;15(2):141-148. [CrossRef]
- Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2020 Dec;95(12):1599-1613. [CrossRef]
- Ngoc NT, Hau BB, Vuong NB, Xuan NT. JAK2 rs10974944 is associated with both V617F- positive and negative myeloproliferative neoplasms in a Vietnamese population: A potential genetic marker. Mol Genet Genomic Med. 2022 Oct;10(10):e2044. [CrossRef]
- Rumi E, Cazzola M. Diagnosis, risk stratification, and response evaluation in classical myeloproliferative neoplasms. Blood. 2017 Feb 9;129(6):680-692. [CrossRef]
- Trifa AP, Cucuianu A, Petrov L, Urian L, Militaru MS, Dima D, Pop IV, Popp RA. The G allele of the JAK2 rs10974944 SNP, part of JAK2 46/1 haplotype, is strongly associated with JAK2 V617F-positive myeloproliferative neoplasms. Ann Hematol. 2010 Oct;89(10):979-83. [CrossRef]
- Tefferi A, Vainchenker W. Myeloproliferative neoplasms: molecular pathophysiology, essential clinical understanding, and treatment strategies. J Clin Oncol. 2011 Feb 10;29(5):573-82. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).