1. Introduction
In the past years, fossil resources have been used for energy and material production purposes without considering their limited reserves [
1]. Their overuse has caused severe concerns such as global warming and natural resources reduction. Renewable organic materials, such as biomasses, have attracted significant attention for the sustainable production of chemicals, fuels and materials with important economic, environmental and social benefits [
2]. It is estimated that 1 kg of biomass raw material can be transformed in 6 MJ of energy or 0.8 kg of chemicals [
2,
3]. Lignocellulosic biomass from agriculture, forestry and urban waste has a huge potential due to the high abundance, renewability, and low cost. It has been assessed that more than 200 value-added compounds can be produced from lignocelluloses and some even at industrial scale through microbial fermentation [
3]. Among the others, perennial ryegrasses (
Lolium perenne) are considered an important resource due to their high and stable biomass yield, which accounts for about 2-20 ton (dry matter)/ha [
4].
Lactic acid (LA) is considered an important platform chemical with a wide range of applications, especially in food, chemical, cosmetic and pharmaceutical markets. More recently, LA has reached the plastic sector for the production of biodegradable and biocompatible polylactic acid (PLA) polymers [
5,
6]. Because of the broad applications areas, the corresponding market is estimated to reach 1960 kt in 2025 [
7,
8]. The production of LA using microbial fermentation covers more than 90% of the total output with high yields up to 90-95 wt% [
8,
9]. The cost of LA depends on the final application and range between 1.38 USD/kg for the lowest purity and 1.59 USD/kg for the higher purity [
10]. However, the feedstock costs account for the 40-70% of the total production cost [
7,
10]. In this view, the exploitation of underutilized materials such as lignocelluloses can contribute to lowering the final price. Several attempts have been made to use lignocellulosic biomass for the LA production.
Table 1 summarizes the main findings over the last years.
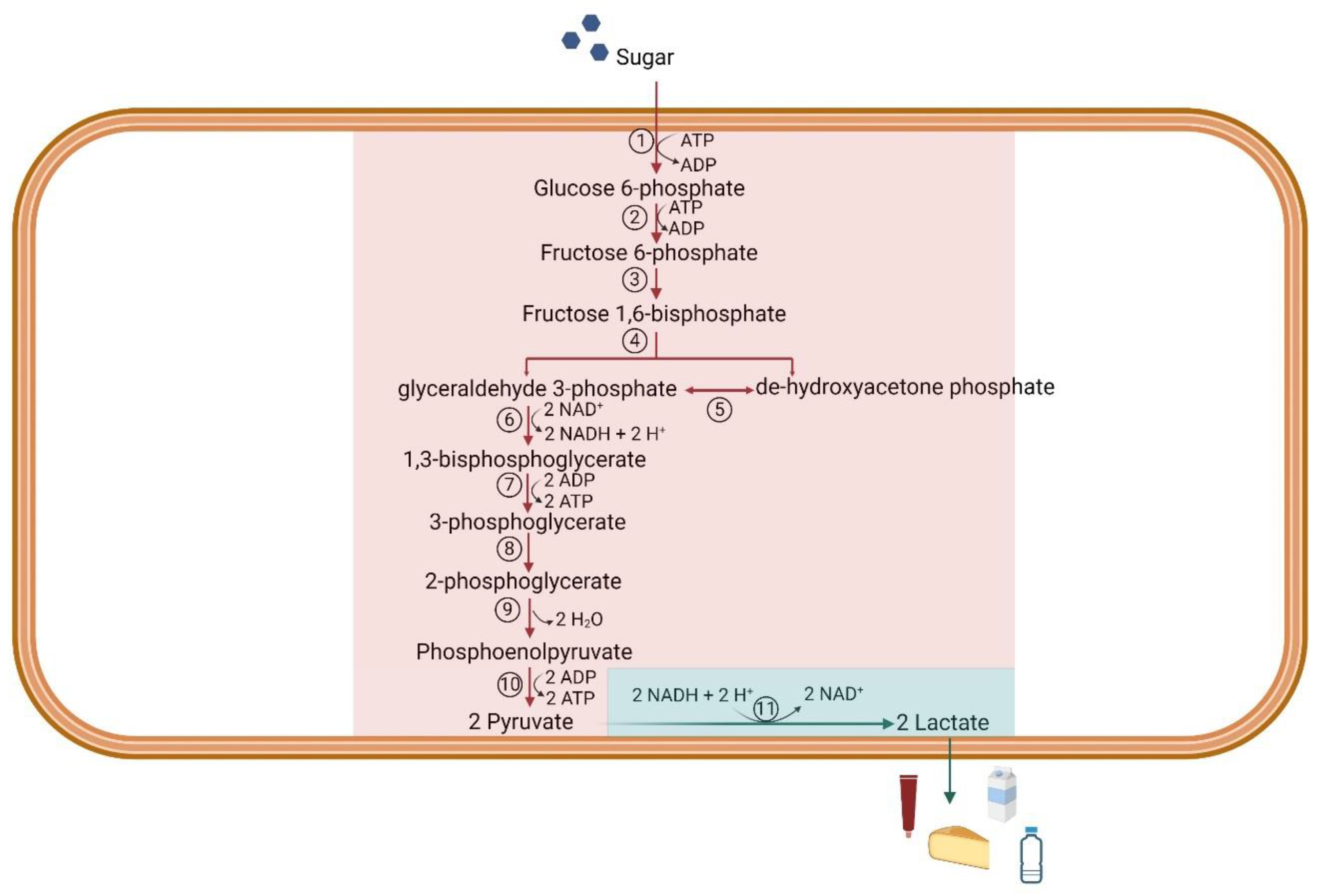
Traditionally, lactic acid bacteria (LABs) are the microorganism of choice for LA production, as they produce LA as major product using homo- or heterofermentation, and are recognize as safe [
31]. Homolactic bacteria such as
Lactobacillus,
Lactococcus and
Enterococcus produce two molecules of LA per mol of sugar with a theoretical yield of 1 g/g (
Figure 1), whereas heterolactic bacteria yield 0.5-0.6 g of LA per g of sugar [
32]. Some LABs are defined facultative heterolactic since they metabolize hexoses and pentoses by producing LA and by-products (acetic acid, ethanol and formic acid) [
33].
Since homolactic bacteria can reach yield close to the maximum theoretical one, they are more suited for the industrial production [
31]. Several key parameters influence LA production, including sugar concentration, nutrients availability, pH, temperature and acid stress [
32]. Specifically, the LA release can lead to product inhibition due to the pH reduction, which in turn affects the membrane potential. Therefore, maintaining an optimal pH in the fermentation medium is essential [
32]. Regarding the nutrients, LABs have complex nutrients requirement, as they have limited capacity to synthetize molecules for their growth. For instance, nitrogen must be supplemented in the fermentation media as yeast extract, peptone or meat extract since LABs are unable to synthetize essential nitrogen-base substances such as amino acids, purines, pyrimidines and co-factors [
34,
35]. Both mineral elements and vitamins are crucial in many enzymatic reactions, in membrane transport processes, and in molecules and structural complexes [
35,
36,
37]. However, the addition of those elements increases the overall cost of LA production [42,43].
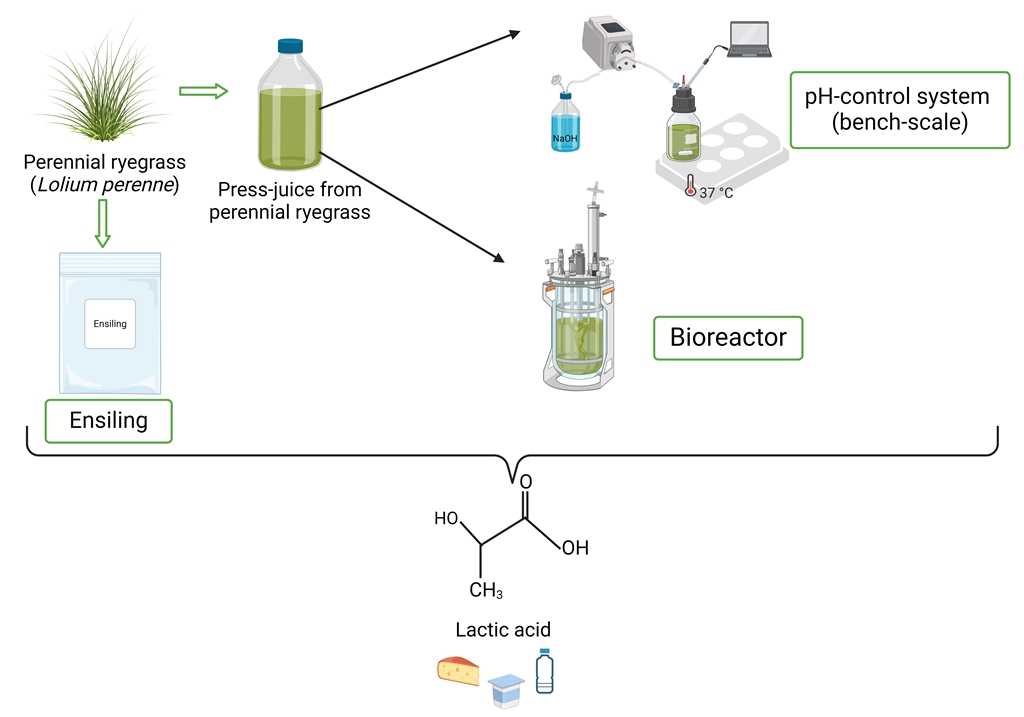
The aim of this work was to compare the ability of the homofermentive L. delbrueckii subsp. lactis to produce LA in conventional complex medium and in press-juice from a perennial ryegrass. The whole production process was designed to enhance the sustainability. For this reason, perennial ryegrass was mechanically pretreated using a screw-press to obtain the press-juice [39]. Physical pretreatments are generally employed prior to any other methods, as they aimed to facilitate the enzymatic hydrolysis [40]. Moreover, compared to the other pretreatment procedures, mechanical methods are more eco-friendly as they do not require chemicals or heat [46,47]. Additionally, in a general biorefinery scheme, the monomeric sugars obtained after the saccharification must be recovered. However, the recovery process requires additional energy and power [41]. The use of the screw-pressing bypassed the need for an additional recovery step, as the sugars are already contained in the press-juice and can be immediately use in the fermentation process.
Different juice percentages were tested for online growth measurements to determine the optimal juice concentration for bacterial growth. Then, based on previous findings, the selected press-juice concentration was optimized with glucose, sodium-acetate and Tween 80 to ensure LA production [24]. The experiments in both complex medium and in press-juice medium were firstly performed at bench-scale using a pH-control system to contrast the pH drops in the cultivations. This developed system aimed to eliminate the addition of neutralizing agents that make the downstream process difficult. Then, the experiments in both media were scaled up in bioreactor and various fermentation parameters including sugar uptake and sugar consumption rate, production yield, productivity, and selectivity were compared. Finally, preliminary study on the ensiling process on different varieties was carried out.
2. Materials and Methods
2.1. Raw Material
At the Julius Kühn Institute (Braunschweig, Germany), a field experiment was conducted to produce fresh biomass for investigating its use as a raw material in a biorefinery. The study site is located in Braunschweig at an altitude of 80 metres above sea level. The region has an annual precipitation of 617 mm and an average temperature of 9.1°C. The soil is characterised as silty sand with a topsoil depth of 30 cm. Selected varieties of
Lolium perenne (
Table 1) were cultivated in large-scale plots to produce sufficient biomass for subsequent analysis. In order to obtain representative samples for Near Infrared Spectroscopy (NIRS) quality determination, chopped samples of at least 500 g were collected from each plot for dry matter (DM) determination and subsequent grinding. These samples were dried in an oven at 60°C for 48 hours and uniformly grinded. Quality parameters including crude protein (CP), water soluble carbohydrates (WSC) and crude fibre (CF) were analysed using a Foss NIRSystem 5000 spectrometer (Foss GmbH, Hamburg, Germany). For the biorefinery application, fresh biomass samples were harvested, vacuum sealed and immediately cooled for shipment.
Table 1.
Lolium perenne varieties used in this study with information on ploidy and breeder.
Table 1.
Lolium perenne varieties used in this study with information on ploidy and breeder.
| Variety |
Ploidy |
Breeder |
| Agaska |
2x |
DLF |
| Honroso |
2x |
DSV |
| Arvicola |
4x |
Freudenberger |
| Barmigo |
4x |
Barenbrug |
| Exlosion |
4x |
DSV |
These varieties were selected to encompass a range of genetic diversity and breeding backgrounds. The evaluation aimed to assess biomass yield and quality characteristics under the specified conditions, critical for their potential use in biorefinery processes. The implementation of standardized procedures for sample collection, drying, and analysis ensures the reliability and comparability of the results across different varieties.
2.3 Pretreatment and Press- Juice Preparation
The pretreatment of the samples and the production of the press-juice can be found elsewhere [39]. Briefly, the raw material was mechanically pretreated using a screw press (Angel Juicer 7500, Luba GmbH, Bad Homburg, Germany). The liquid fraction (press-juice) was centrifuged (4500 rpm, 20 min; Z38K, Hermle Labortechnik GmbH, Wehingen, Germany) to remove the remaining solid particles, and filter sterilized (Stericup®, 0.2 µm pore size, MerckMillipore, Massachusetts, USA). The pressed juice (pH 5.3) was analysed in terms of sugars (about 40 g/L), organic nitrogen (about 14.87 g/L), and ions (about 15.75 g/L).
2.2. Microorganism, Preculture and Complex Medium
The bacteria Lactobacillus delbrueckii subsp. lactis DSM 20729 was obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany).
Precultures and the experiments in complex media were carried out using the De Man – Rogosa – Sharpe (MRS) medium with the following composition: casein peptone 10 g/L, meat extract 10 g/L, yeast extract 5 g/L, Tween-80 1 g/L, K2HPO4 2 g/L, sodium-acetate 5 g/L, (NH4)3 citrate 2 g/L, MgSO4 x 7 H2O 0.20 g/L, MnSO4 x H2O 0.05 g/L, glucose or fructose carbon source about 20 g/L. The pH of the medium was adjusted to 6.2 ± 0.1 using 2.5 M NaOH. In all the experiments, the carbon source (glucose or fructose) was prepared and autoclaved (Systec V-150, Systec GmbH & Co. KG, Linden, Germany) separately to avoid the degradation. All the chemicals were purchased from Carl Roth + Co KG (Karslruhe, Germany), except glucose and fructose (Sigma Aldrich, Merck KGaA, Darmstadt, Germany). Precultures were anaerobically grown in 150 mL glass bottles with 100 mL working volume. The bottles were tightly sealed with rubber septa and incubated at 37°C, 150 rpm for approximately 24 hours (Ecotron, Infors, AG, Bottmingen, Switzerland).
2.3. Cultivation in Different Percentage of Press-Juice
The cultivations in different percentages (10, 25, 30, 50, 60, 75 %(v/v)) of press-juice were carried out in 100 mL serum bottles with 50 mL working volume, tightly sealed with rubber septa. Before the inoculation (0.1 OD (optical density)), the bottles were sparged with pure nitrogen. Incubation was carried out at 37°C and 150 rpm in incubator shaker (Ecotron, Infors AG, Bottmingen, Switzerland) equipped with the Cell Growth Quantifier (Scientific Bioprocessing, Aquila Biolabs GmbH, Germany). The system recorded the growth of the bacteria by taking scattered light measurements and the values were directly used to develop the growth curves.
2.4. Bench-Scale Fermentation in Schott Bottle
Bench-scale fermentations were carried out in 150 mL Schott bottles with 100 mL as working volume. Before the inoculation (0.1 OD), the bottles were sparged with pure nitrogen. The pH values were constantly measured using pHenomenal® pH-electrodes (IDP 721, VWR, Darmstadt, Germany) and the Profilux 3.1T aquarium computer with six pH modules (GHL Advanced Technology GmbH & Co. KG, Kaiserslautern, Germany). During the cultivations, the pH value was regulated to 6.8 by automatically adding 2.5 M NaOH as titrant correction agent (accuracy ± 0.1). A heating and stirring plate (MULTI-HS 6, Carl Roth GmbH & Co. KG, Karlsruhe, Germany) was used for temperature control and for mixing of the Schott bottles. The medium temperature in the bottles was set to 37°C, and mixing was carried out at 200 rpm using cylindrical magnetic stirrer bars with a diameter of 8 mm and a length of 20 mm (ROTILABO, Carl Roth GmbH & Co. KG, Karlsruhe, Germany). The experiments in complex medium and the experiments in press-juice medium were conducted over 25 and 28 h, respectively.
2.5. Scaled-Up Fermentation in Bioreactor
Scaled-up fermentations were carried out in 0.5 L BIO-STAT® Qplus controlled bioreactor system (Sartorius AG, Germany). In the experiments performed with complex media, the bioreactors were filled with all the medium components and autoclaved (121°C, 15 min), except for the sugar. Pure nitrogen was sparged overnight to ensure anaerobic condition. Afterward, the sterilized sugar was added aseptically to the reactors, and nitrogen was sparged for one hour to remove any remaining oxygen. In the experiments with 75 %(v/v) press-juice, the bioreactors were sterilized (121°C, 15 min) before the addition of the press-juice. After sterilization, the press-juice was aseptically filled in, and pure nitrogen was sparged overnight. The optimized nutrient mixture was added afterward, and nitrogen was sparged for one hour. In all the experiments, the temperature was set at 37°C, the stirring speed at 150 rpm, the pH at 6.5 by the automatic addition of 2.5 M NaOH with peristaltic pumps. In all the experiments, the reactors were inoculated to 0.1 OD using preculture previously prepared.
2.6. Ensiling of Different Varieties of Lolium perenne Whole Grass Fractions
To obtain lactic acid from the ensiling of Lolium perenne fresh grass, the grass was cut into small pieces. Before ensiling, dry matter was determined using the DBS 60 3 moisture analyzer from KERN (Balingen-Frommern, Germany). For each setup, a vacuum bag was filled with 50 g of fresh mass (FM) of the chosen variety, and 10 mL of a silage additive (diluted with deionized water 1:500) was added. This additive, sourced from Lactosan (Kapfenberg, Austria), consisted of a mixture of the strains Lactobacillus plantarum, Lactobacillus rhamnosus, and Pediococcus pentosaceus. After the addition, the mixture was briefly mixed, and the bag was sealed using the VMKH-300 vacuum sealer. The prepared setups were placed in plastic buckets, purged with nitrogen gas, and sealed. Ensiling was conducted at room temperature for 75 days. To maintain the nitrogen environment inside the buckets, nitrogen was renewed every three days. For sampling, the designated bags were removed from the buckets.
To measure the ensiling products, complete sample-bags were removed from the ensiling and pressed. The press juice obtained was centrifuged at 16,100 rcf for 15 minutes using. Afterward, the samples were diluted to a concentration within the calibration range and filtered through a 0.22 µm pore size polyethersulfone filter.
2.7. Analytical Methods
The analyses of the press-juices were carried out with regard to sugars, protein, amino acids, cations and anions. The protein concentration was determined using Bradford assay (Pierce® Coomassie Bradford Protein Assay kit, Thermo Scientific, Massachusetts, United States) and the Bovine serum albumin as internal standard (Thermo Fisher Scientific, Massachusetts, United States). The absorbances of the calibration curve and of the samples were measured at 595 nm (Cary 60 UV-Vis, Agilent Technologies, USA).
Sugars and lactic acid were analysed by HPLC (High Performance Liquid Chromatography) [Autosempler AS 6.1L and Azura pump P 6.1L (Kneur GmbH, Berlin, Germany. The HPLC was equipped with an Aminex HPX-87H column at 80°C (Bio-Rad, 300x7.8 mm, Hercules, California, USA) and a refractive index detector (RI 101 Shodex, Kawasaki, Japan). The mobile phase was 2.5 mM H2SO4 and the flow rate was set at 0.6 mL/min. The instrument control and the data evaluation were carried out with a Clarity Software system (Data Apex, Prague, Czech Republic). Before the analysis, the samples were diluted to a concentration inside the calibration range using external standards, and then filtered through a 0.22 µm pore size nylon filter (KX Syringe Filter Nylon, Cole Parmer GmbH, Germany).
Amino acids were separated using a resolve C18 column (150x3.9 mm, Waters Corporation, Milford, USA) with a SecurityGuard Cartridge (C18, 4 x 3.0 mm ID, Phenomenex, Torrance, California, USA) set at 30 °C. The detection was performed using Azura photodiode-array detector DAD 2.1L at 230 nm (Knauer GmbH, Berlin, Germany). The analysis included a precolumn derivatization with ortho-Phthaldialdehyde (OPA). The derivatization process is described in the Supplementary. The mobile phase consisted of solvent A (0.025 M sodium-acetate anhydrous and 0.025 M NaH2PO4 monohydrate) and solvent B (50% methanol). The pH value of solvent A was adjusted to 7 with 10 M NaOH, then 21 mL of both tetrahydrofuran and methanol were added. The gradient elution program was as follows: from 0 to 50 min, solvent B changed linearly from 0% to 100%; from 50 to 55 min, solvent B was set as isocratic at 100%; from 55 to 60 min, solvent B changed linearly from 100% to 0%; from 60 to 67 min, solvent B was set as isocratic at 0%. The flow rate was 1.0 mL/min. Samples preparation is described in the Supplementary.
Cations and anions were analyzed by ion chromatography (IC) (930 Compact IC Flex, Metrohm GmbH & Co. KG, Germany) with an inline system for dialysis (930 Compact IC Flex, Metrohm, Filderstadt, Germany) and an IC Conductivity Detector (Metrohom, Filderstadt, Germany). Cations were measured with a cation column (Metrosep C6-250/4.0, Metrohm) using 4 mM HNO3 and 0.7 mM dipicolinic acid as mobile phase at a flow rate of 0.9 mL/min. Anions were measured with an anion column (Metrosep A Supp5-250/4.0, Metrohom) using 1 mM NaHCO3 and 3.2 mM Na2CO3 as mobile phase at a flow rate of 0.7 mL/min. The oven temperature in both cases was 35°C. For each analysis, the samples were diluted to a concentration inside the external calibration range (using 2 mM HNO3 for cation determination).
Cell growth was monitored by optical density measurements at 600 nm (UV spectrophotometer LAMBA Bio+, Perkin Elmer Corporation, Waltham, USA). The samples collected during the fermentations were centrifuged at 14000 rpm for 7 min (Eppendorf, Hamburg, Germany) and the supernatant stored at -20°C for metabolites analysis.
2.8. Data Processing and Evaluation
The percentage of sugar uptake (
Su) and the sugar consumption rate (
rs) were calculated at the time the cells were growing exponentially and followed equation (1) and (2), respectively:
where
Si is the initial sugar(s) concentration(s) and
St is the sugar(s) concentration(s) at time
t.
The selectivity (S) was calculated following equation (3):
The production yield (
YP/S) of LA was expressed as equation (4):
Where
LAf indicates the final LA concentration,
LAi is the starting LA concentration.
The volumetric LA productivity (
QLA) was estimated as (5):
CLA represents the concentration of LA at the end of the fermentation.
The biomass production yield (
YX/S) followed equation (7):
where
Xf indicates the final biomass concentration and
Xi is the initial biomass concentration.
The volumetric cells productivity (
QCELL) was expressed as the ratio between the final biomass concentration (
CCELL) and the time
t, according to (8):
3. Results and Discussion
3.1. Influence of Raw Material
The 2021 harvests were analyzed in terms of yield and quality parameters in order to diversifying their use in a biorefinery view. In particular, DM, CF, CP, and WSC were evaluated and the results are reported in
Table 2.
Agaska exhibited the highest DM yield, followed by Barmigo and Arvicola (
Table 2). This suggests that Agaska is the most productive variety for second cut of year in terms of biomass yield, which is beneficial for processes requiring large quantities of raw material, such as bioenergy production. However, it is important to note that the first cut of the year typically produces more DM than the second cut. In terms of CF content, Agaska also had the highest amount, indicating a robust cell wall structure. This high CF content can be advantageous for producing materials that require high fiber content. Barmigo and Arvicola had slightly lower CF contents, with Arvicola having the lowest, which might make it more suitable for processes requiring lower fiber content and higher digestibility. Arvicola had the highest CP content, making it potentially more valuable for animal feed applications where protein content is critical. Barmigo and Agaska had lower CP contents, suggesting that while Arvicola is less productive in terms of biomass yield, its higher protein content could offer advantages in feed applications. WSC are crucial for fermentation processes. Arvicola again led with the highest WSC content, followed by Barmigo and Agaska. The high WSC content in Arvicola highlights its potential suitability for processes like chemicals and fuels production, where a high sugar content is desirable. Separately, the comparison between Explosion and Honroso varieties harvested in 2022 revealed some differences. Both varieties have similar DM yields, with Honroso slightly lower than Explosion. The CF content was comparable between the two, indicating a robust structure for both varieties. However, Honroso had a higher CP content compared to Explosion, while the WSC content was similar between the two, making both varieties viable options for biorefinery processes. For lactic acid production, both the Explosion and Honroso varieties were particularly suitable due to their high DM yields and adequate carbohydrate content. The large volume of juice required for LA production was obtained by utilizing both replicates.
Table 2.
Yield and quality parameters of Lolium perenne varieties. DM: dry matter; CF: crude fiber; CP: crude proteins; WSC: water soluble carbohydrates.
Table 2.
Yield and quality parameters of Lolium perenne varieties. DM: dry matter; CF: crude fiber; CP: crude proteins; WSC: water soluble carbohydrates.
| Variety |
Cut of the Year |
Harvest Date |
DM
(dt/ha) |
CF
(%) |
CP
(%) |
WSC
(%) |
| Agaska |
2 |
07.07.2021 |
26.76 |
25.73 |
10.81 |
16.15 |
| Arvicola |
2 |
07.07.2021 |
20.79 |
21.52 |
11.34 |
21.41 |
| Barmigo |
2 |
07.07.2021 |
23.55 |
24.76 |
10.04 |
18.37 |
| Explosion |
1 |
03.06.2022 |
51.95 |
25.95 |
5.82 |
22.62 |
| Honroso |
1 |
03.06.2022 |
43.33 |
25.29 |
7.95 |
20.37 |
3.2. Press-Juices Analysis
LABs have high and complex nutrient requests to grow and to produce LA. According to this, the press-juices obtained after the mechanical pretreatment were analyzed to assess the nutrients content in terms of sugars, proteins, amino acids and ions. The results are reported in
Table 3.
Table 3.
Analysis of the different varieties of grass press-juices obtained after the mechanical pretreatment.
Table 3.
Analysis of the different varieties of grass press-juices obtained after the mechanical pretreatment.
| |
Arvicola |
Agaska |
Barmigo |
| Total sugar [g/L] |
23.8 |
39.5 |
28.3 |
| Protein [mg/L] |
559 |
683 |
642 |
| Amino acid [mg/L] |
n. a |
3130.5 |
2560.9 |
| Cations [g/L] |
9.3 |
8.1 |
8.2 |
| Anions [g/L] |
6.0 |
5.9 |
4.8 |
Although the biomass yields of the grass vary significantly, the nutrient contents of the grass press-juices are remarkably similar. According to this, it is possible to stated that the time of harvest should not have a major influence on the production and utilization of the press-juice in fermentation. Specifically, the comparison of the glucose and fructose content in the fibers extract and in the pressed juices showed that most of the monosaccharides were contained in the press-cake (
Table 4). Approx. 31 % of the monosaccharides of
Lolium perenne can be transferred to the pressed juice by pressing (see
Table 4).
Table 4.
Analysis of the available monosaccharides (glucose and fructose) in grass press juices.
Table 4.
Analysis of the available monosaccharides (glucose and fructose) in grass press juices.
| Sample |
Glucose content
[% (m/m)] |
Deviation |
Fructose content
[% (m/m)] |
Deviation |
| Honroso |
2,93 % |
0,41 % |
5,32 % |
1,20 % |
| Explosion |
5,09 % |
0,79 % |
7,56 % |
3,00 % |
3.3. Bench-Scale Fermentation in Complex Medium
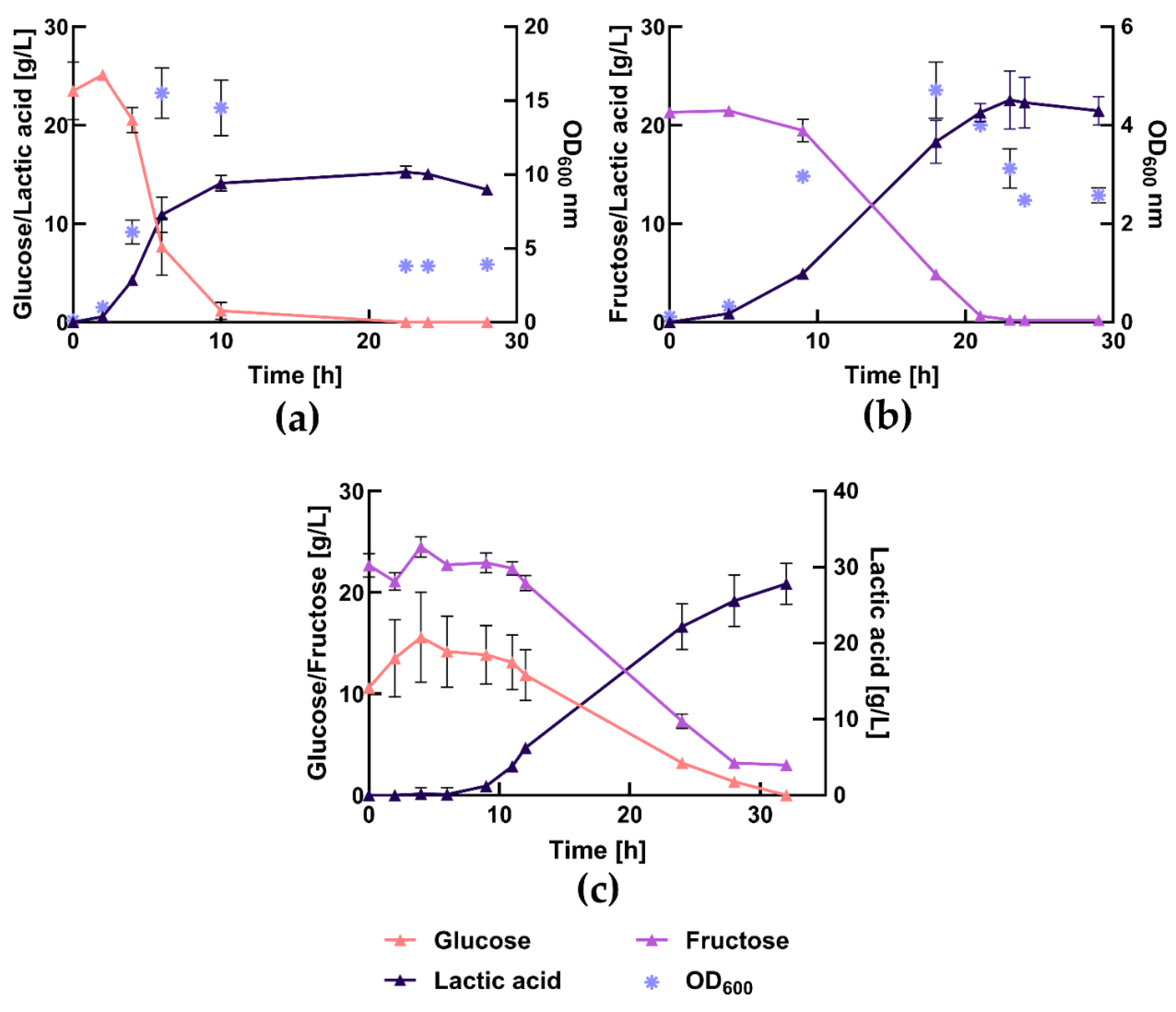
To assess the ability of L. delbrueckii to grow and to produce LA, bench-scale fermentations in MRS medium were initially performed under pH-controlled conditions using NaOH addition. During LA fermentation, the pH of the broth drops below the LA-pKa value (3.8), thus inhibiting the metabolic functions of LABs [32,42]. Typically, neutralizing agents such as Ca(OH)2 or CaCO3 are utilized to adjust the pH in the optimal range (pH 5-7) [35,42]. However, these agents have some disadvantages such as limited LA accumulation and the formation of calcium lactate, which can increase the costs of the downstream processing [42]. Therefore, the pH control is critical fundamental for a successful sustainable LA production.
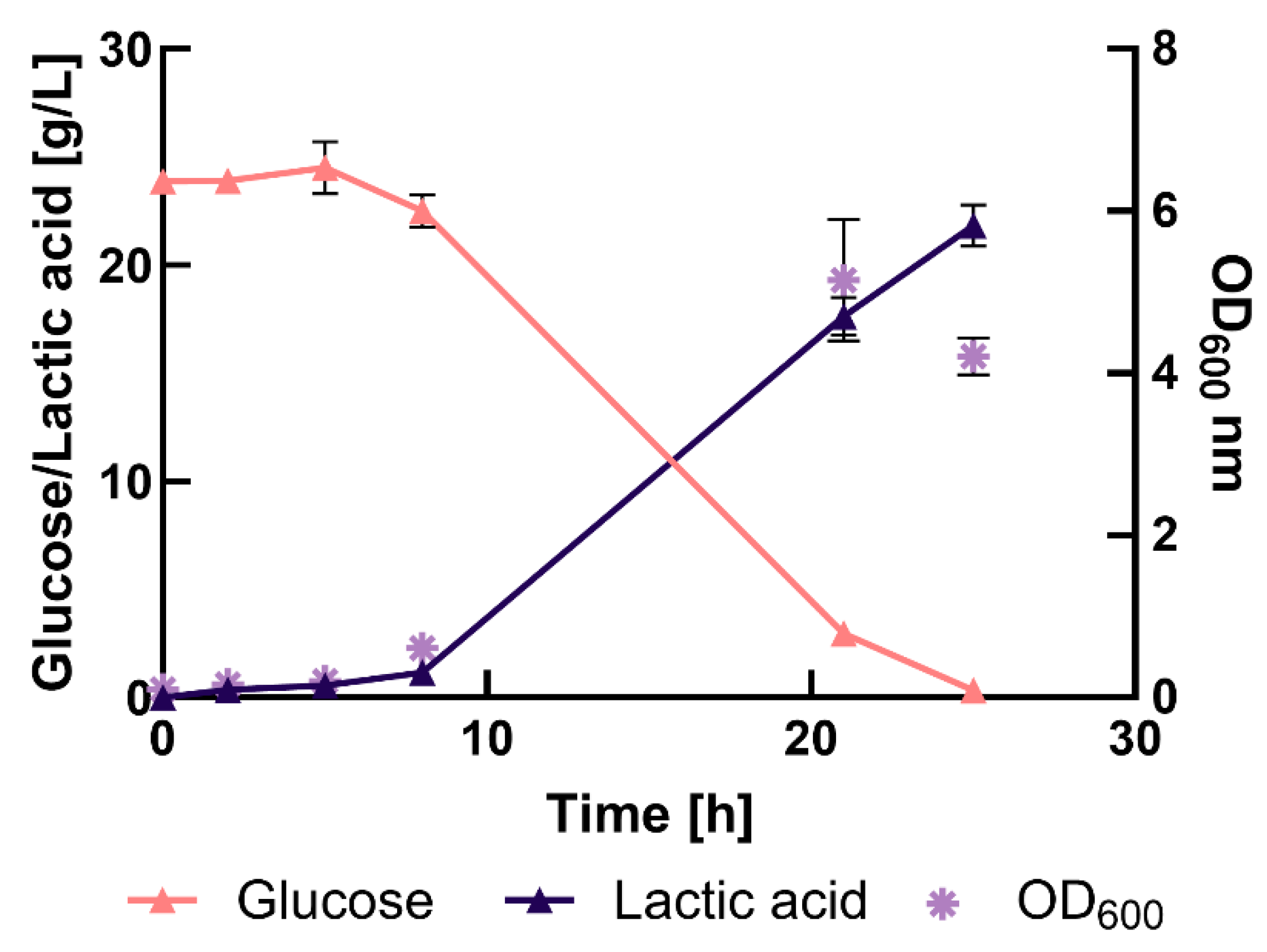
The fermentations in MRS medium lasted for 25 h. Bacterial growth exhibited a lag-phase of approximately 6 h, during which glucose levels remained constant and no LA was produced. The lag-phase was followed by the exponential growth-phase (6 h-21 h) during which glucose was rapidly metabolized (87% respect to the initial glucose concentration), and LA was produced (17.6 ± 0.9 g/L). By the end of the cultivation (25 h), the cell density decreased, the glucose was completely depleted and LA accumulation reached about 22 g/L.
Mussatto et al., showed that pH-controlled experiments yielded better results compared to those without pH control. Using MRS medium with an initial glucose concentration of 50 g/L, the authors achieved about 23.5 g/L of LA [43]. The authors noted that glucose consumption was positively affected by the pH. Si et al., investigated different pH values and found that maintaining pH 6 during LA production in corn straw hydrolysate resulted in the highest LA production [44].
Figure 2.
Bench-scale fermentation in MRS medium with a pH-regulated system. Culture conditions: 200 rpm, 37°C, pH= 6.8 ± 1 (2.5 M NaOH buffer). Error bars indicated deviation standards of the mean (n= 3).
Figure 2.
Bench-scale fermentation in MRS medium with a pH-regulated system. Culture conditions: 200 rpm, 37°C, pH= 6.8 ± 1 (2.5 M NaOH buffer). Error bars indicated deviation standards of the mean (n= 3).
3.4. Cultivation in Different Percentage of Press-Juice
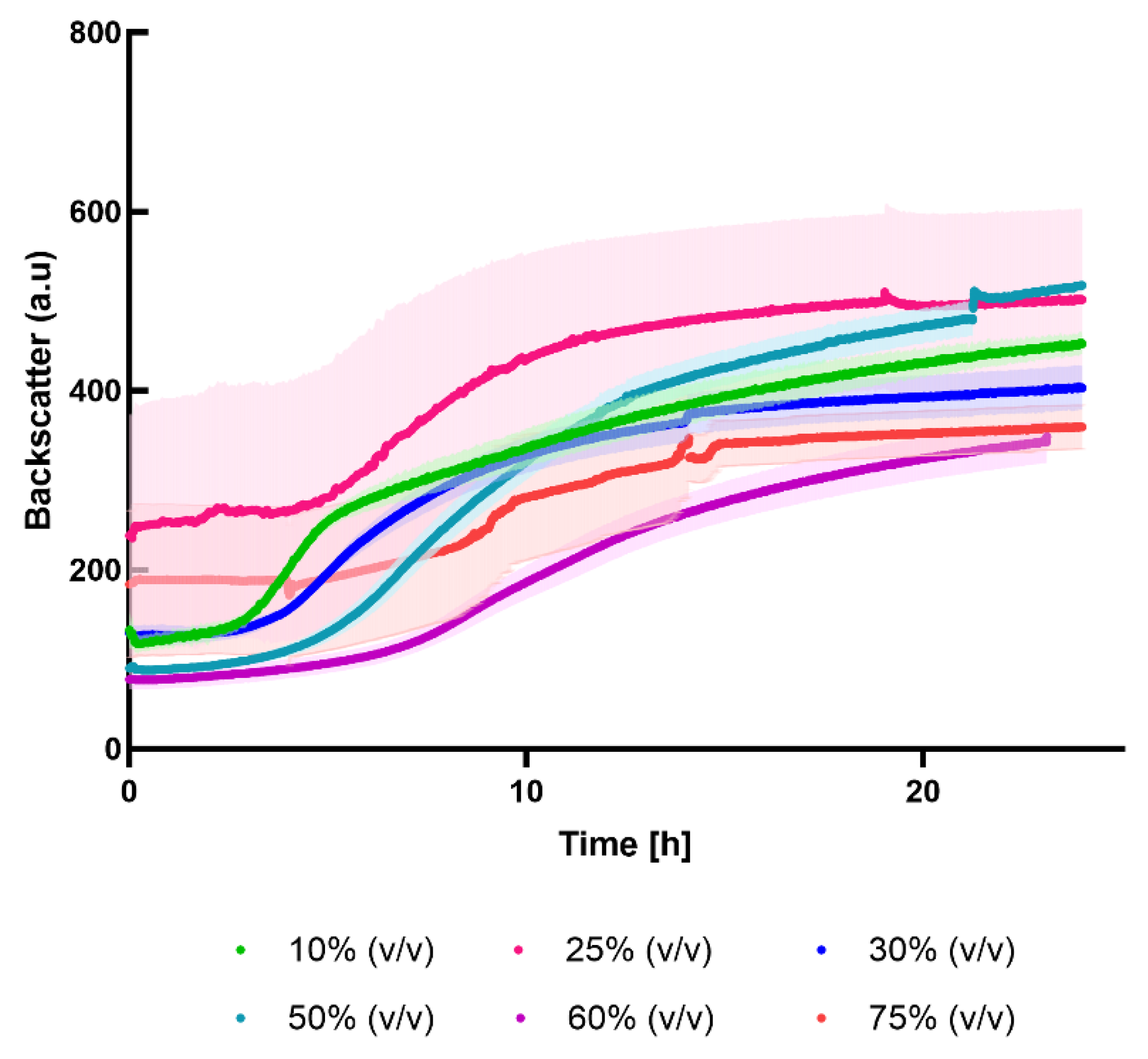
To evaluate if L. delbrueckii could grow in press-juice, cultivations in different juice percentages (10, 25, 30, 50, 75 %(v/v)) were carried out. Since the experiments aimed to indicate bacterial growth, online measurements with CGQ and without pH control were performed.
The results revealed that the LAB grew in all the juice percentages, although in different manner (
Figure 3). At lower juice concentrations, the latency phase was shorter than the other cases. At these juice percentages, more of the 50% of the medium was composed of complex media components. Since the preculture was grown in MRS medium, it is possible to state that the bacteria were not perturbated and thus could adapt faster. At higher juice percentages, the lag-phase took longer due to the change of the medium. As depicted in
Figure 3, the higher the juice amount the longer the lag-phase lasted. This could be due to the differences in nutrients availability. Indeed, LABs have elaborated nutrient requirements because of their limited capability to synthesize vitamins and amino acids from inorganic nitrogen source [35]. Therefore, an optimal fermentation medium should provide all the compounds to sustain the bacterial growth and the metabolic activity. For instance, MRS complex medium includes multiple nitrogen sources (casein peptone, meat extract, and yeast extract) that supply several amino acids, peptides, minerals and vitamins crucial for LABs growth [24,45]. In contrast, press-juice primarily contains nitrogen sources in the form of amino acids and proteins at a usually lower concentration than the complex medium. Furthermore, the use of proteins as a source of nutrient occurs when the preotolysis takes place [46]. According to this, the nutrients in the press-juice are not immediately available for LABs growth and activity.
However, the experiments aimed to establish whether L. delbrueckii could grow in the tested juice percentages to replace as much as possible the expensive complex media components. Based on these findings, 75% (v/v) was selected as the juice percentage for the further experiments.
3.5. Bench-Scale Fermentation Using the Press-Juice
Seventy five percent (v/v) of pressed juice was selected for LA production, aiming to replace expensive complex media components such as yeast extract. Previous studies have demonstrated the high nutrients content of the juice from grass raw materials [47,48], suggesting that the supplement of only the essential nutrients could be economically advantageous.
Previous studies investigated the optimization of the fermentation medium using the brewers’ spent grain liquor. It was analysed the effect strength of each MRS medium components on the LA production, and it was found out that sodium-acetate and Tween 80 had the highest impact [24]. It is reported that sodium-acetate enhances both the final cell density and LA concentration, while Tween 80 supplies long-chain fatty unsatured fatty acids that increase the cell membrane permeability [49,50,51].
Based on these results, 75% (v/v) was supplemented with a mixture consisting of 25% (v/v) glucose, sodium-acetate and Tween 80. Preliminary experiments were performed in Schott bottles equipped with a pH-control system to evaluate the potential of this optimized juice medium for LA production.
The results revealed that both glucose and fructose were consumed during the fermentation, with no discernible preference between the sugars (
Figure 4). This result was in line with previous investigations: Mousavi et al., observed similar sugar utilization patterns in pomegranate juice by different Lactobacilli strains [52]. Costa et al., used a mixture of pear residues and ricotta cheese whey to produce LA, revealing that fructose and glucose were completely metabolized by
L. casei within 24 h. The LA reached 43 g/L after 48 h with a yield of 50.6% on total sugars [23]. Volkmar et al., found out that
L. delbrueckii can consume the glucose and the fructose contained in municipal residues, producing about 17 g/L of LA [53]. Although the cell growth was not monitored due to the brownish colour of the medium, bacterial growth can be inferred from LA release. During the first 6 h of cultivation, the sugars were not metabolized and no LA was produced. Subsequently, exponential LA production was observed over the next 22 supported by a rapidly sugars consumption. By the end of the fermentations, approximately 88% of sugars were consumed and 26.6 ± 1.2 g/L of LA was produced. The results corresponded to a production yield of 0.78 ± 0.03 g
Sugars/g
Lactate. Boakye-Boaten N. et al., produced LA in 90% (v/v) miscanthus press-juice using
L. platarum,
L. brevis and the combination of both in serum bottles. Their results gave 11.7, 10.3 and 10.2 g/L of LA after 48 h of fermentation, respectively [54]. Santamaria-Fernandez M. et al., produced up to 22 g/L of LA from grass clover using
L. salivarius [55]. Si et al., used a pH-control system to produce LA from corn straw hydrolysate. The authors obtained 99.8 g/L of LA, which corresponded to a production yield of 0.67 g
LA/g
Sugars [44]
.
3.6. Comparison between Complex-Medium and Press-Juice in Bioreactor
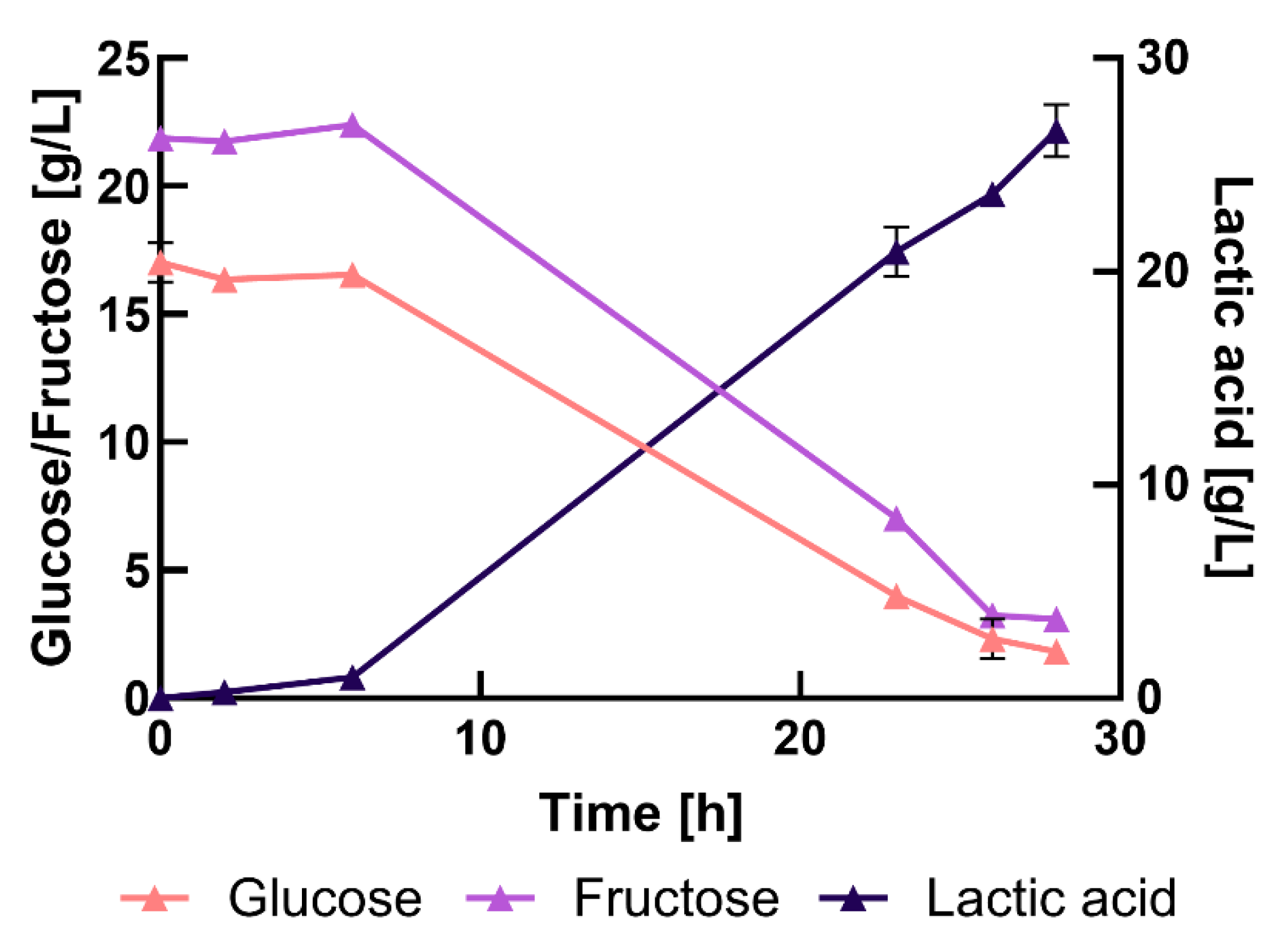
After assessing the possibility of producing LA in a pH-control system using either MRS medium or press-juice, both the experiments were scaled-up in stirred tank bioreactors. In MRS medium, fermentations were carried out using either glucose or fructose as carbon source (
Figure 5 a-b). Specifically, fructose was included to provide a further comparative analysis since it is the main sugar in the press-juice. The experiments with the press-juice were performed as previously described (75% (v/v) press-juice and 25% (v/v) nutrients mixture) (
Figure 5c).
The choice of the carbon source significantly affected the bacterial growth in MRS media. When glucose was used as carbon source, the lag-phase lasted for about 2 h (
Figure 5a). In contrast, when fructose was added in the medium, the lag-phase was longer (about 4 h) (
Figure 5b). In the case of the press-juice, OD measurements were not collected due to the brownish colour of the medium. However, as for the experiments in Schott bottles, information regarding the growth can be deduced from the LA release. In this case, the lag-phase was longer respect to those in complex media, lasting until 8 h (
Figure 5c). During this phase, the cells turned on the metabolic activity, such as the activation of signalling pathways, upregulation of protein assembly, nucleotide metabolism, and other processes necessary for the differentiation and duplication. All these activities result in cell division, which is fully activated during the exponential-growth phase [56,57]. The prolonged lag-phase observed in press-juice could stress the bacteria due to inadequate or insufficient nutrient concentrations. According to Kim J. et al., the nitrogen-to-carbon ratio significantly impacts sugar conversion to LA [
35]. Akermann et al., reported that sodium-acetate and Tween 80 were the medium components that more affected the cultivation of
L. delbrueckii. Hebert E. et al., studied the nutritional requirements of
L. delbrueckii subsp.
lactis and they found out that MnSO
4 and FeSO
4 are essential for the growth, since they act as a cofactor in several enzymatic reactions [
35,45]. It might be that the formulation of the press-juice medium may not be optimal for the bacterial growth. Nonetheless, the environmental changes from the preculture grown in complex medium and the cultivation in press-juice could have impacted the growth. It is also reported that the microbial lag-phase is an adjustment period before active growth begins, and its duration can vary based on environmental conditions [57].
The exponential-growth phase lasted until 10 h and 18 h when glucose and fructose were used, respectively (
Figure 5a-b). Nonetheless, during the whole cultivations, higher cell density was observed with glucose as carbon source. The same result was obtained by Petrut S. et al., who cultivated
L. rhamnosus on different sugars. They found out the glucose gave a higher cell density respect to other sugars such as fructose, sucrose, mannose and arabinose [58]. Chen H. et al., cultivated
L. acidophilus on maltose, glucose, lactose, and whey powder. The authors got higher cells concentration (in Colony Forming Unit/mL) when the bacteria were grown on glucose and whey powder [59]. During the exponential growth phase, most of the glucose and the fructose were consumed when the complex media were used. In particular, about 22.3 ± 3.6 g/L of glucose and about 16.5 ± 0.5 g/L of fructose were metabolized, which corresponding to 94.6 ± 4.5 % and 77.2 ± 1.4 % of sugar utilization (
Table 3). The biomass yield was higher when glucose was used as carbon source (0.17 ± 0.02 g
Biomass/g
Glucose) than with fructose (0.12 ± 0.01 g
Biomass/g
Fructose), suggesting that glucose stimulated more cell growth than LA production. When the 75%(v/v) press-juice was used as fermentation medium, the exponential-phase lasted until 28 h (
Figure 5c), likely due to the higher sugar availability. During this phase, the glucose was totally metabolized, and about 86.8 ± 0.6 % of fructose was consumed. The corresponding LA concentrations were 14.1 ± 0.8 g/L in the experiment in MRS with glucose, 18.3 ± 2.2 g/L in the case of MRS with fructose, and 25.6 ± 3.4 g/L in pressed juice. Extending the fermentation time in complex media led to LA degradation.
The production yield and the productivity are crucial parameters in fermentation processes, and they are summarised in
Table 4. The highest production yield was obtained when in MRS with fructose as carbon source, reachingthe maximum theoretical yield of 1 g/g [32]. However, when 75% (v/v) press-juice was used, the production yield was slightly lower (0.91 ± 0.07 g
LA/g
Sugars). This result was comparable to the one of Chen C. et al., who obtained a yield of 0.93 g
LA/g
Sugars with
L. plantarum 23 using microalgae hydrolysate [60]. Similarly, LA produced from orange peel wastes using
L. delbrueckii subsp.
delbrueckii CECT286, reached a production yield of 0.95 g
LA/g
Sugars [61]. Thus, the results of this experiments are consistent with those reported in literatures.
In terms of productivity (QLA), the highest value was obtained when 75% (v/v) press-juice was used (0.87 ± 0.10 g/Lh). This outperformed other studies, such as Pontes et al., who obtained LA productivity of 0.81 g/Lh using forest and marginal lands lignocellulosic biomass and L. rhamnosus ATCC 7469 [62]. Erliana et al., reported a productivity of 0.69 g/Lh using sugar palm trunk hydrolysate with a combination of L. rhamnosus and L. brevis [63].
Table 4.
Comparison of the fermentation parameters between the bioreactor fermentations in MRS medium (with glucose or fructose) and the bioreactor fermentations in optimized 75 %(v/v) press-juice. Si is the initial sugar(s) concentration, Su and rS were evaluated during the exponential-growth phase.
Table 4.
Comparison of the fermentation parameters between the bioreactor fermentations in MRS medium (with glucose or fructose) and the bioreactor fermentations in optimized 75 %(v/v) press-juice. Si is the initial sugar(s) concentration, Su and rS were evaluated during the exponential-growth phase.
| |
MRS medium with glucose1
|
MRS medium with fructose1
|
75% (v/v) press-juice2
|
| Si [g/L] |
23.5 ± 2.9 |
21.3 ± 0.3 |
33.3 ± 0.84 |
| Sugar(s) remained [g/L] |
0 ± 0.00 |
0.21 ± 00 |
2.9 ± 0.00 |
| Su [%] |
94.6 ± 4.5 |
77.2 ± 1.43 |
102.7 ± 3.5 |
| rS [g/Lh] |
-2.23 ± 0.36 |
-0.91 ± 0.03 |
-1.03 ±0.04 |
| CLA [g/L] |
13.47 ± 0.57 |
21.45 ± 1.45 |
27.81 ± 2.69 |
| S [g/g] |
0.59 ± 0.11 |
1.02 ± 0.08 |
0.84 ± 0.01 |
| YP/S [g/g] |
0.63 ± 0.02 |
1.02 ± 0.08 |
0.91 ± 0.07 |
| QLA [g/L h] |
0.48 ± 0.02 |
0.69 ± 0.00 |
0.87 ± 0.10 |
| Fermentation efficiency [%] |
51.3 ± 1.9 |
101.7 ± 8.4 |
91.4 ± 6.7 |
| YX/S [g/g] |
0.17 ± 0.02 |
0.12 ± 0.01 |
n.a |
| QCELLS [g/L h] |
0.16 ± 0.00 |
0.09 ± 0.01 |
n.a |
3.6. Lactic Acid Yields from Ensiling Whole Grass Fractions of Different Varieties of Lolium perenne
As preliminary study to assess influence of the varieties on ensiling, a classical whole grass ensiling process was conducted for the first projects crops in summer and autumn 2020. LA yields after ensiling the whole grass fraction of
Lolium perenne varieties harvested in 2020 are shown in
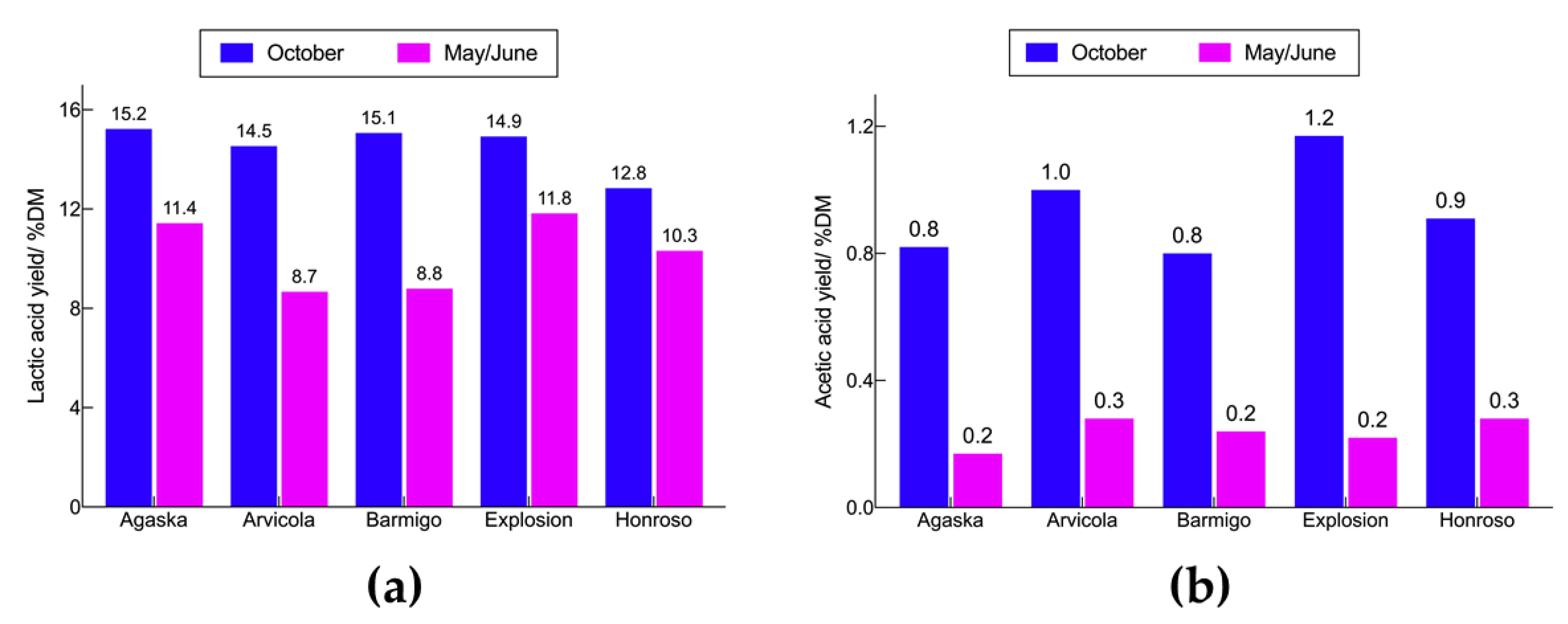
Figure 6a. The pH values dropped rapidly within the first 15 days, stabilizing around pH 4, indicative of a successful fermentation process. The maximum LA yield was reached after 2-3 weeks of ensiling. Samples harvested in October show substantially higher LA yields of up to 15.2% of the grass dry mass after ensiling compared to those harvested in May and June. Among the October-harvested samples, Agaska had the highest LA yield, while Honroso had the lowest with 12.8% DM. For the samples harvested in May and June, Explosion had the highest LA yield at 11.8% DM, while Arvicola had the lowest at 8.6% DM. Among the different
Lolium perenne varieties, Agaska showed the least impact, with a 24% decrease in lactic acid yield when harvested in May/June compared to October, while Barmigo exhibited the greatest reduction, with a 41.7% decrease.
High-quality silages exhibit LA yields of 6-10% based on dry matter. This range was achieved across all varieties and experiments [64]. Notably, the grasses harvested in October even exceeded 10%. The yields obtained in this study are consistent with literature values, with the 15.2% yield of Agaska harvested in October surpassing them. This superior yield positions Agaska as a highly efficient variety for LA production through ensiling. Johnson et al. reported yields of up to approximately 6.5% DM in 16 days with silages in vacuum bags without the addition of a silage additive [65]. Haag et al. achieved yields of 7% DM or untreated silages and silages with a biological silage additive, and up to 13% DM for silages with both biological and chemical additives [66]. Finally, Ren et al. reported yields of around 14% DM for silages with a biological additive [66].
The WSC values for the 2020 crops could not be assessed as precise as in the following years. Given that the WSC contents are assumed to be relatively similar across the varieties, the differences in LA yields are likely due to the inherent genetic variability among the Lolium perenne varieties, impacting their fermentation efficiency. The WSC content may include oligosaccharides that cannot be converted into LA by microorganisms. Factors such as microbial efficiency, nutrient availability, and specific conditions during ensiling also significantly influence LA yields.
The average proportions of the by-product acetic acid during the ensiling are shown in
Figure 6(b). In high-quality silages, the acetic acid content should be between 1% and 3% DM [64]. The upper limit for acetic acid content was maintained across all varieties. The highest acetic acid content was found in the variety Agaska (October) with 2.0% DM, while the lowest was in the variety Agaska May/June at 0.2% DM. Low acetic acid content is desirable in silage because it increases aerobic stability. However, since this work primarily focuses on lactic acid production, falling below the lower limit is not considered negative.
4. Conclusions
In this study, it was demonstrated that the press-juice from the perennial ryegrass can replace or partially substitute expensive media components that are generally used in fermentation processes. The experiments performed in Schott-bottles confirmed that the pH-control system is a fundamental parameter to control in LA production. The utilization of this strategy not only would avoid the addition of neutralizing agents which make the downstream process more complicated, but it would also contribute to reduce the total costs. The fermentation in bioreactors proved that the optimized press-juice medium allowed to produce more than 27 g/L of LA, which corresponded to a production yield of 0.91 ± gLA/gSugars. Overall, the use of press-juice as fermentation medium not only approached the maximum theoretical yield but also provides a high productivity, making it a promising and economically viable substitute to traditional complex media for LA production.
The whole grass fraction of Lolium perenne also showed significant potential for lactic acid production. The Agaska variety, in particular, achieved a lactic acid yield of 15.2% dry mass (DM) when harvested in October, significantly surpassing typical silage yields of 6-10% DM. The study highlights the significant potential of certain Lolium perenne varieties, particularly Agaska and Explosion, for high-efficiency lactic acid production through ensiling. Barmigo and Arvicola also show reasonable efficiency, while Honroso appears less suitable for maximizing lactic acid yields. These findings suggest that selecting appropriate grass varieties is critical for optimizing lactic acid yields of whole grass ensiling. Additionally, the impact of harvest time on ensiling outcomes cannot be overlooked, as autumn harvests tend to produce better lactic acid yields.
Author Contributions
Conceptualization, L.V. and R.U.; methodology, K.K., L.V., N.T. and J.N.H.; validation, L.V.; formal analysis, L.V.; investigation, L.V.; data curation, L.V.; writing—original draft preparation, L.V., J.N.H. and T.G.; writing—review and editing, K.K., N.T., R.U.; visualization, L.V. and T.G.; supervision, R.U.; project administration, K.K., N.T. and R.U.; funding acquisition, K.K., N.T. and R.U.
Figure 1.
Schematic representation of the LA homo-fermentation pathway from sugar to LA. The red box represents the glycolysis, the green box the homolactic fermentation. The numbers in the circles are the enzymes involved in that step: 1) hexokinase, 2) phosphoglucose isomerase, 3) phosphofructokinase, 4) aldolase, 5) triose phosphate isomerase, 6) glyceraldehyde 3-phosphate dehydrogenase, 7) phosphoglycerate kinase, 8) phosphoglyceromutase, 9) enolase, 19) pyruvate kinase, 11) lactate dehydrogenase. The figure was adapted from Pessione A. et al., [38]and it was created on Biorender.
Figure 1.
Schematic representation of the LA homo-fermentation pathway from sugar to LA. The red box represents the glycolysis, the green box the homolactic fermentation. The numbers in the circles are the enzymes involved in that step: 1) hexokinase, 2) phosphoglucose isomerase, 3) phosphofructokinase, 4) aldolase, 5) triose phosphate isomerase, 6) glyceraldehyde 3-phosphate dehydrogenase, 7) phosphoglycerate kinase, 8) phosphoglyceromutase, 9) enolase, 19) pyruvate kinase, 11) lactate dehydrogenase. The figure was adapted from Pessione A. et al., [38]and it was created on Biorender.
Figure 3.
Cultivation of L. delbrueckii in different juice percentages. The experiments were performed using the CGQ to measure online the bacterial growth (37°C, 150 rpm). The cultivations were carried out in triplicates and the standard deviations are shown as the corresponding colour shade.
Figure 3.
Cultivation of L. delbrueckii in different juice percentages. The experiments were performed using the CGQ to measure online the bacterial growth (37°C, 150 rpm). The cultivations were carried out in triplicates and the standard deviations are shown as the corresponding colour shade.
Figure 4.
Bench-scale fermentation in 75% (v/v) press-juice and 25% (v/v) mixture of Glucose/sodium-acetate/Tween-80 (mixture component concentration: glucose 10 g/L, sodium-acetate 5 g/L, Tween-80 1 g/L). The cultivations were performed in Schott-bottles with a pH-regulated system. Culture conditions: 200 rpm, 37°C, pH= 6.8 ± 1 (2.5 M NaOH buffer). Error bars indicated deviation standards of the mean (n= 2).
Figure 4.
Bench-scale fermentation in 75% (v/v) press-juice and 25% (v/v) mixture of Glucose/sodium-acetate/Tween-80 (mixture component concentration: glucose 10 g/L, sodium-acetate 5 g/L, Tween-80 1 g/L). The cultivations were performed in Schott-bottles with a pH-regulated system. Culture conditions: 200 rpm, 37°C, pH= 6.8 ± 1 (2.5 M NaOH buffer). Error bars indicated deviation standards of the mean (n= 2).
Figure 5.
Bioreactor fermentations in MRS medium with glucose (a) (n=3), MRS medium with fructose (b) (n=2), and 75% (v/v) press-juice (c) (n=2). In all the experiments, the cultivations were carried out anaerobically, at 37°C, 150 rpm, pH 6.5.
Figure 5.
Bioreactor fermentations in MRS medium with glucose (a) (n=3), MRS medium with fructose (b) (n=2), and 75% (v/v) press-juice (c) (n=2). In all the experiments, the cultivations were carried out anaerobically, at 37°C, 150 rpm, pH 6.5.
Figure 6.
Lactic acid (a) and acetic acid (b) yields from ensiling different varieties of Lolium perenne (room temperature, 14 days).
Figure 6.
Lactic acid (a) and acetic acid (b) yields from ensiling different varieties of Lolium perenne (room temperature, 14 days).
Table 1.
Microorganism, feedstock, type of cultivation used for LA production over the last years.
Table 1.
Microorganism, feedstock, type of cultivation used for LA production over the last years.
| Microorganism |
Feedstock |
Cultivation |
Yield [g/g] |
Ref. |
|
L. rhamnosus LA-04-01a
|
Defatted rice brain hydrolysate |
Batch |
0.95 |
[11] |
| Continuous |
0.98 |
|
L. paracasei 7BLa
|
Wood chips |
Fed-Batch |
0.96 |
[12] |
| Rice straw |
0.97 |
|
L. casei G-02a
|
Jerusalem artichoke |
Fed-Batch (SSF) |
0.96 |
[13] |
|
L. agilis LPB 56b
|
Soybean vinasse |
Batch |
0.85 |
[14] |
|
L. plantarum NCIMB 8826a
|
Delignified hardwood pulp |
Batch (SSF) |
0.88 |
[15] |
|
L. paracasei LA104a
|
Curcuma longa waste |
Batch (SSF) |
0.69 |
[16] |
|
L. coryniformis ATCC 25600b
|
0.65 |
|
L. pentosus FL0421a
|
Corn stover |
Fed-batch (SSF) |
0.66 |
[17] |
|
L. pentosus DSM20314a
|
Wheat bran |
Batch |
0.73 |
[18] |
|
L. delbrueckii NBRC 3202b
|
Cassava fibrous waste |
Batch |
0.50 |
[19] |
|
L. rhamosus ATCC 7469a
|
Lignocellulosic mixture |
Batch (SSF) |
0.97 |
[20] |
|
L. delbrueckii subsp. bulgarius ATCC 11842b
|
Beechwood hydrolysaete |
Batch (SSF) |
0.69 |
[21] |
| Pine hydrolysate |
0.40 |
|
L. delbrueckii DSM 20074b
|
Household bio-waste |
Batch |
0.65 |
[22] |
|
L. casei DSM 20011a
|
Agro-industrial waste |
Batch |
0.78 |
[23] |
|
L. delbrueckii subsp. lactis DSMZ 20072b
|
Brewers‘ spent grain |
Batch |
0.89 |
[24] |
|
L. brevis MTCC 4460c
|
Cottonseed cake |
Batch (SSF) |
0.22 |
[25] |
| Wheat straw |
0.49 |
| Sugarcane bagasse |
0.52 |
|
L. buchneri NRRL B-30929c
|
Elephant grass liquor |
Batch |
0.50 |
[26] |
|
B. coagulans A107b
|
Tapioca starch hydrolysate |
Continuous |
0.80 |
[7] |
|
B. coagulans ADb
|
Corn stover hydrolysate |
Continuous |
0.95 |
[27] |
|
B. coagulans LA204b
|
Corncob |
Fed-Batch (SSF) |
0.77 |
[28] |
|
B. coagulans LA1507b
|
Sweet sorghum bagasse |
Open-Fed-batch (SSF) |
0.44 |
[31] |
|
E. faecalis RKY1b
|
Molasses |
Batch |
0.95 |
[29] |
|
E. faecium WH51-1b
|
Corn steep water effluent |
Batch |
0.89 |
[30] |